Novel Nanoprobe with Combined Ultrasonography/Chemical Exchange Saturation Transfer Magnetic Resonance Imaging for Precise Diagnosis of Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
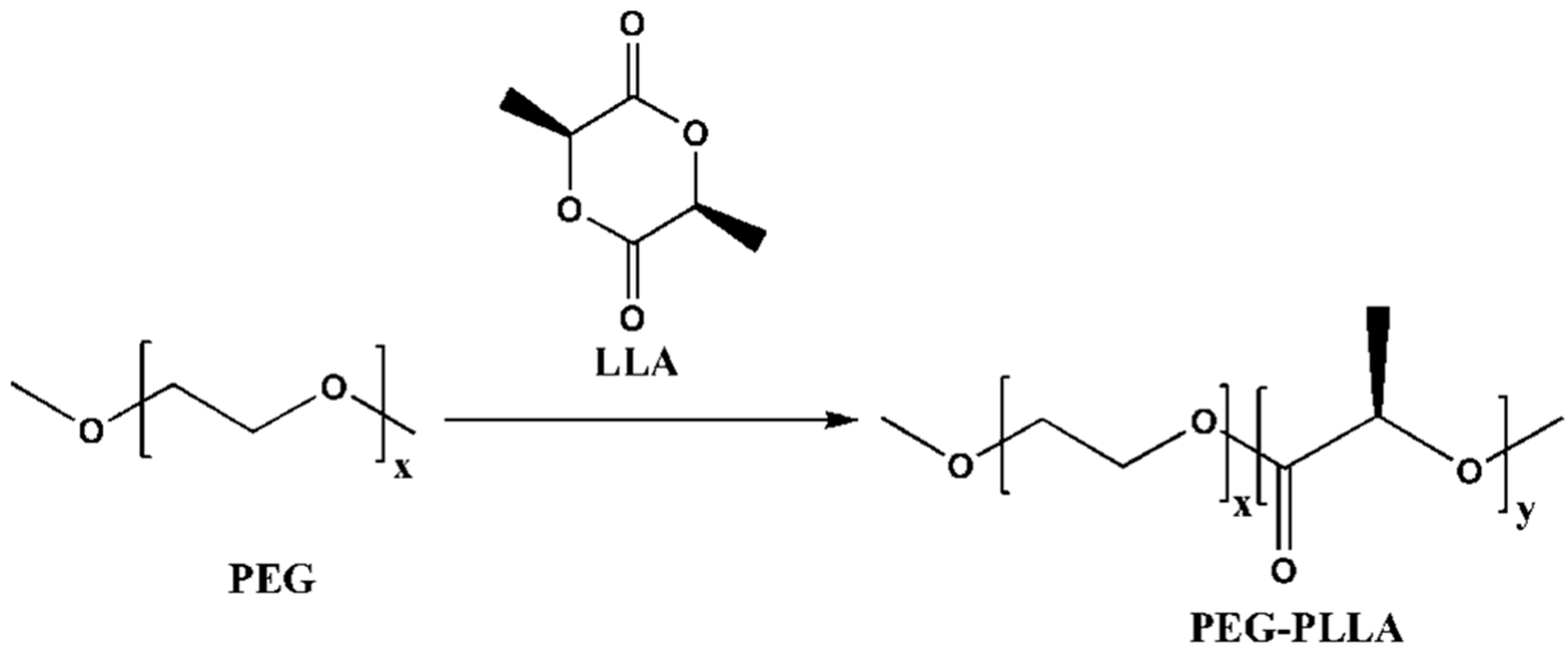
2.2. The Synthesis of PEG-PLLA Diblock Copolymer
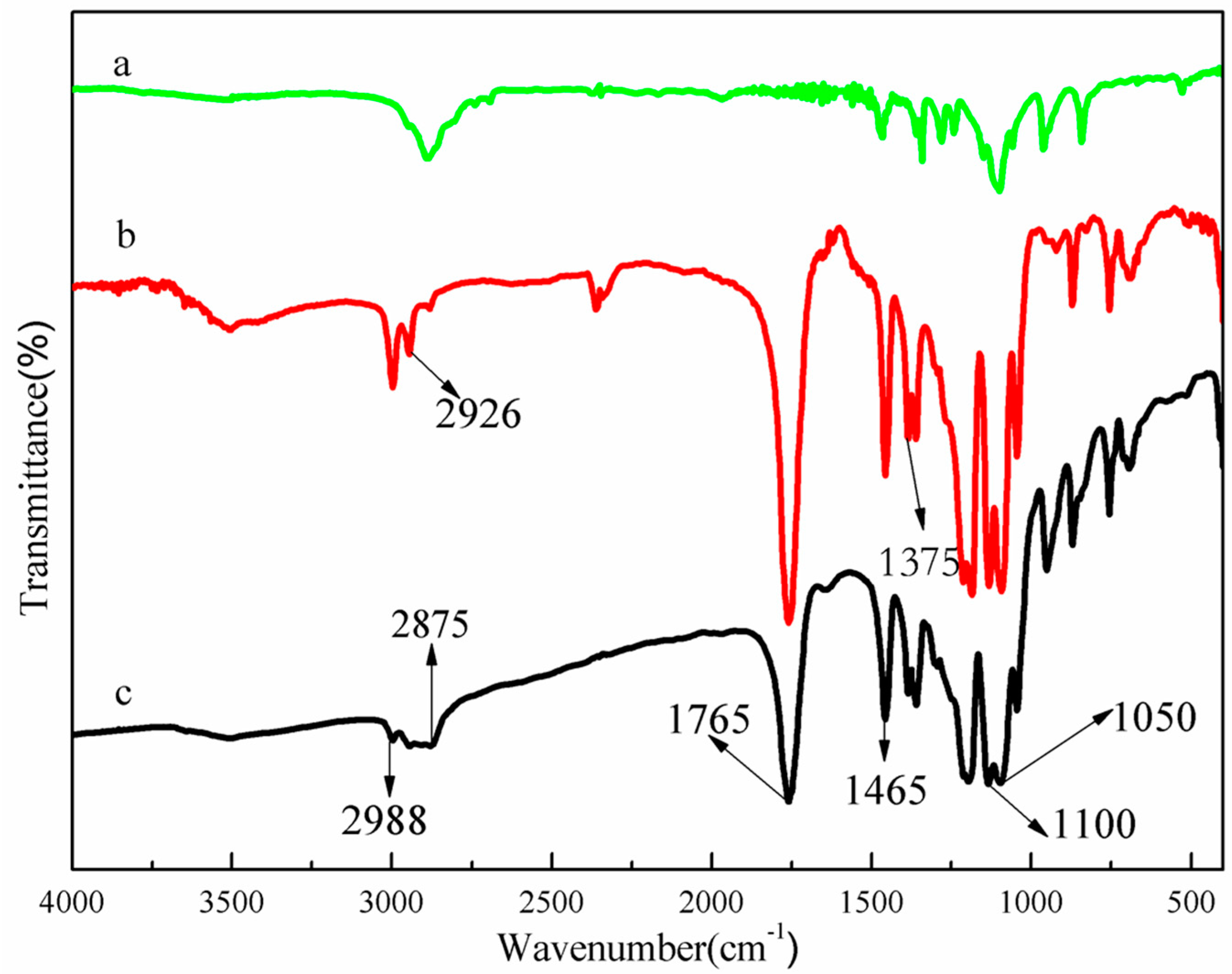
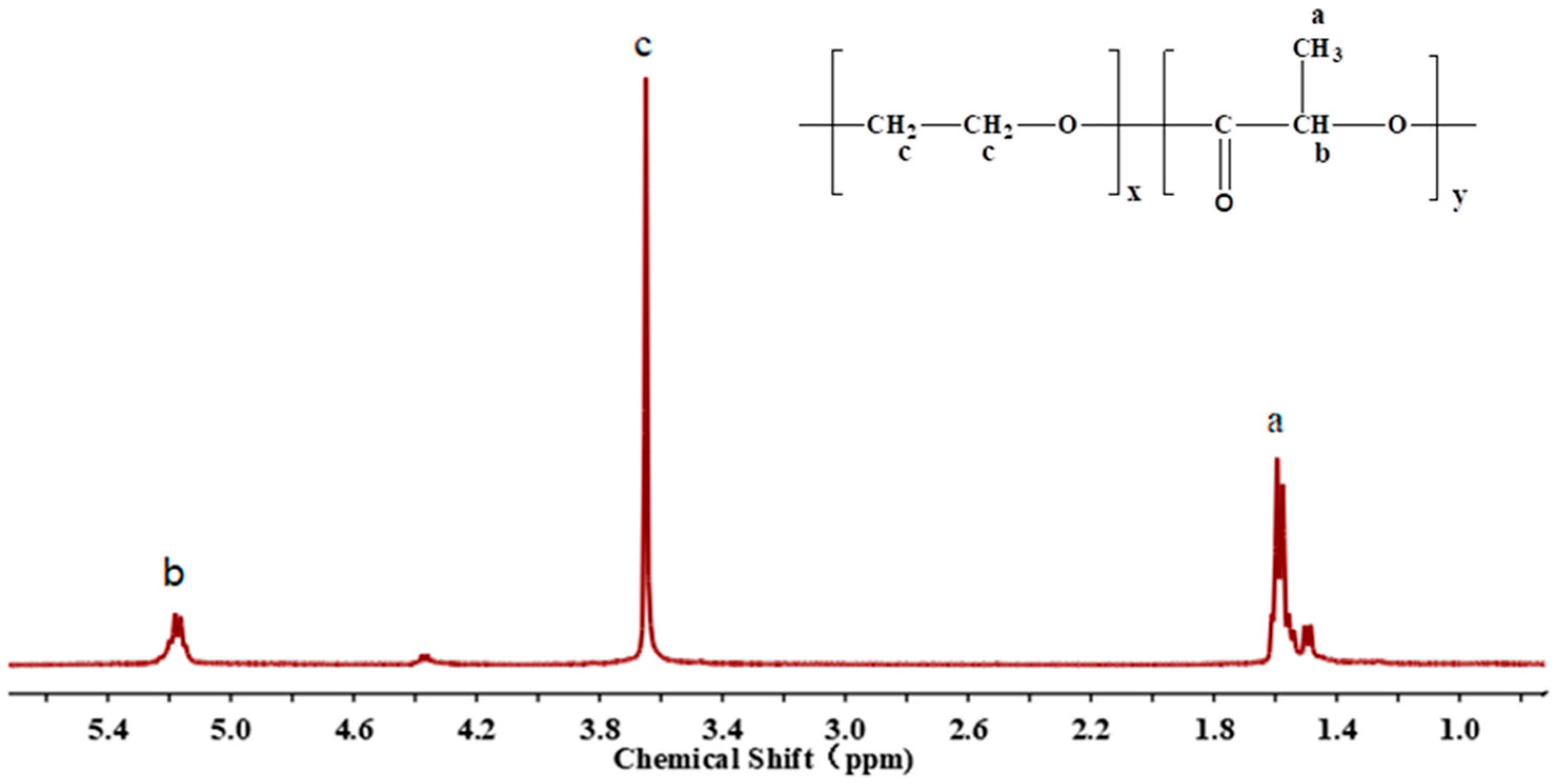
2.3. Structural Characterization of PEG-PLLA Diblock Copolymer
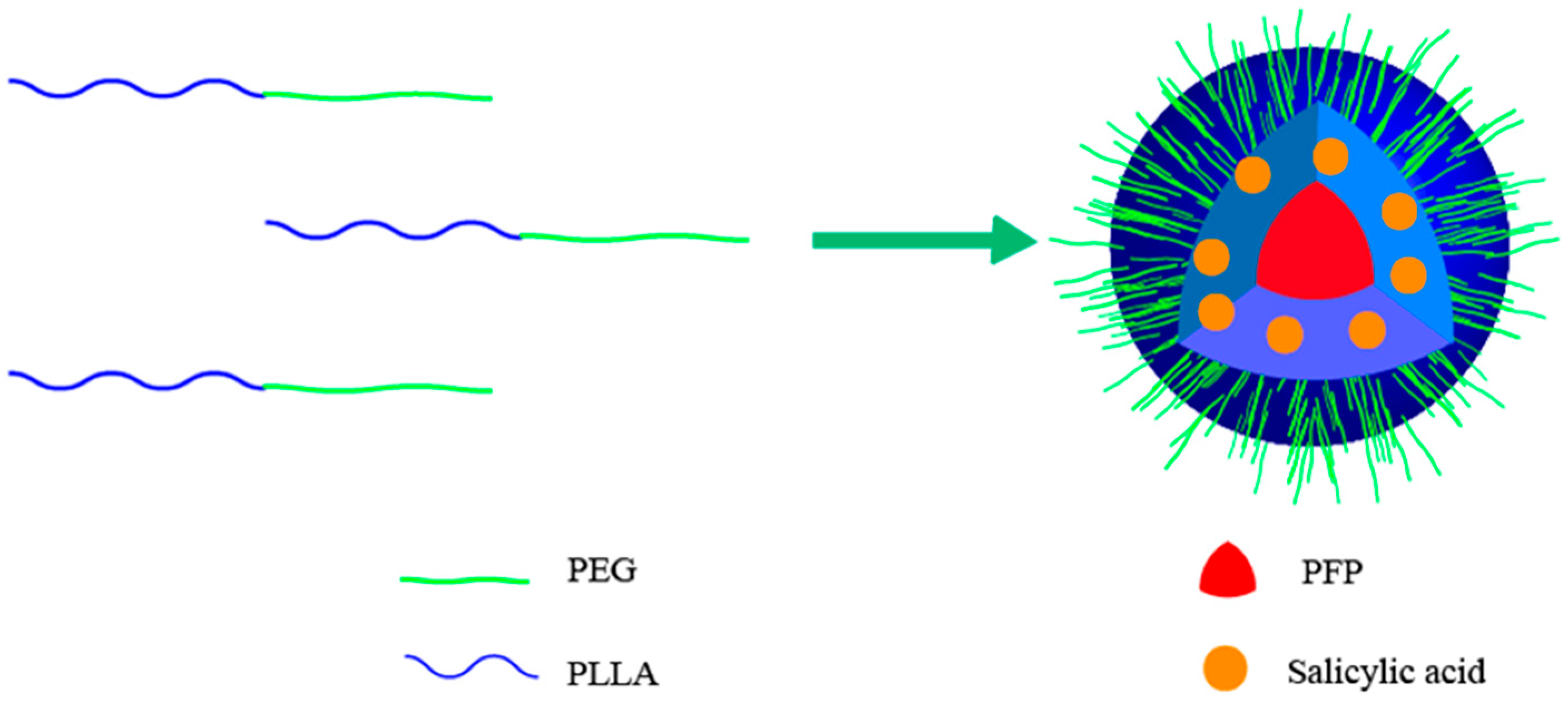
2.4. Preparation of PEG-PLLA Nanoparticles Encapsulating Salicylic Acid and Perfluoropentane
2.5. Characterization of PEG-PLLA Nanoparticles Encapsulating Salicylic Acid and Perfluoropentane
2.6. Cell Toxicity Assay
2.7. Cell Migration Test
2.8. Hemolysis
2.9. Ultrasound Imaging In Vitro
2.10. Animal Care
2.11. Ultrasound Imaging In Vivo
2.12. CEST MR Imaging In Vitro
2.13. CEST MR Imaging In Vivo
2.14. Statistical Verification
3. Results and Discussion
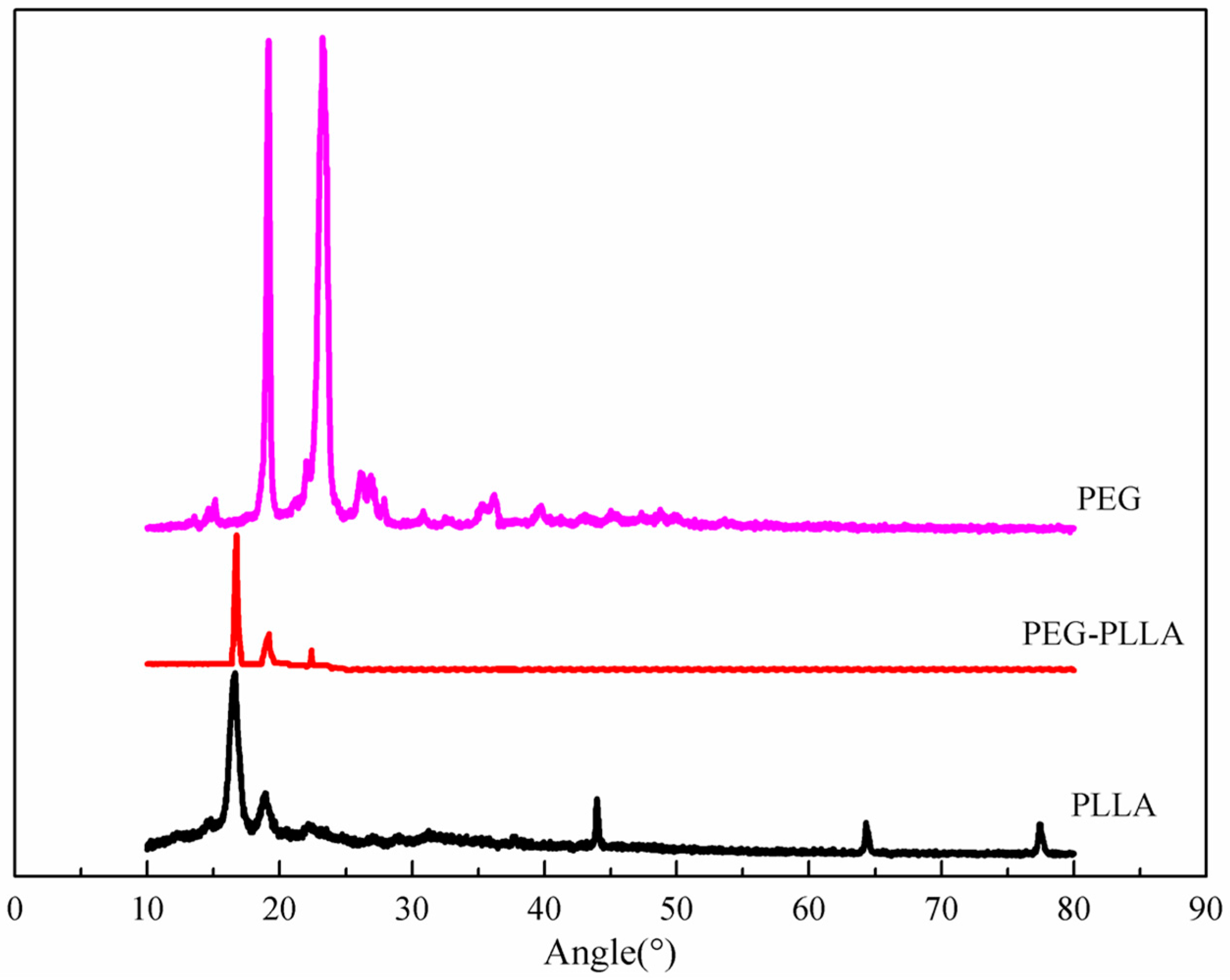
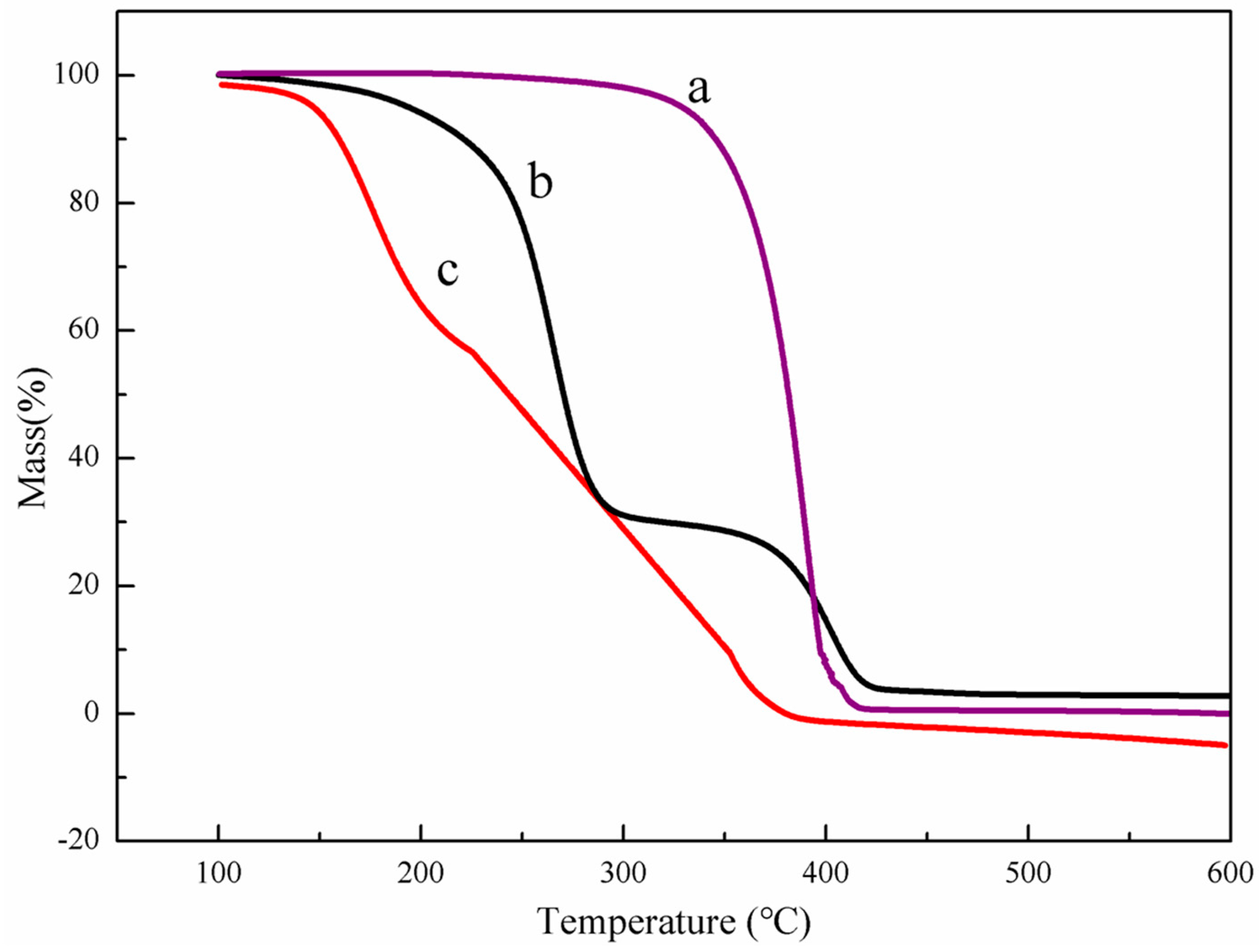
3.1. Characteristics of PEG-PLLA Copolymer
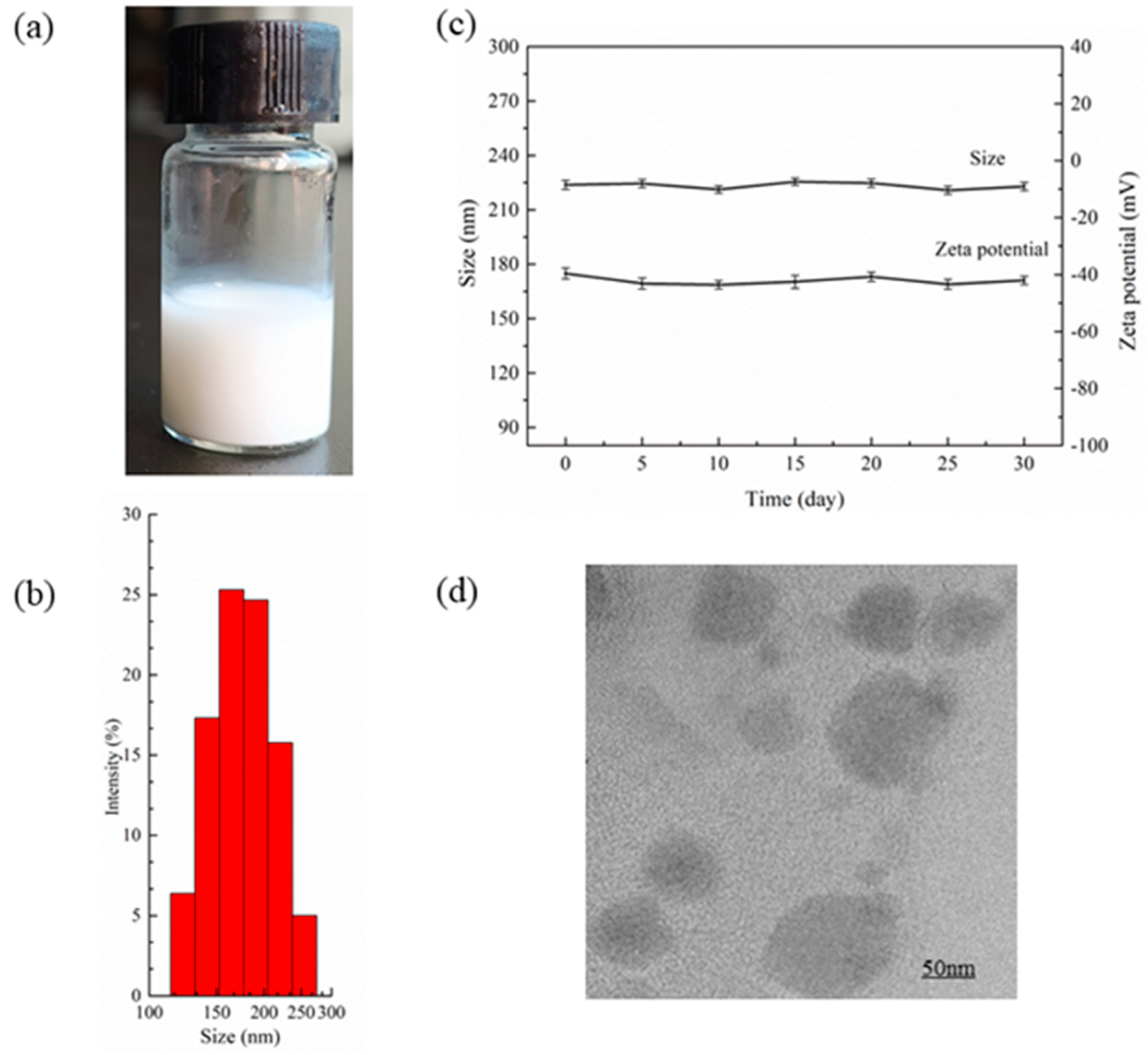
3.2. Measurement of PEG-PLLA@SA·PFP Nanoparticle Encapsulating Salicylic Acid/PFP Characteristics
3.3. Cytotoxicity Analysis
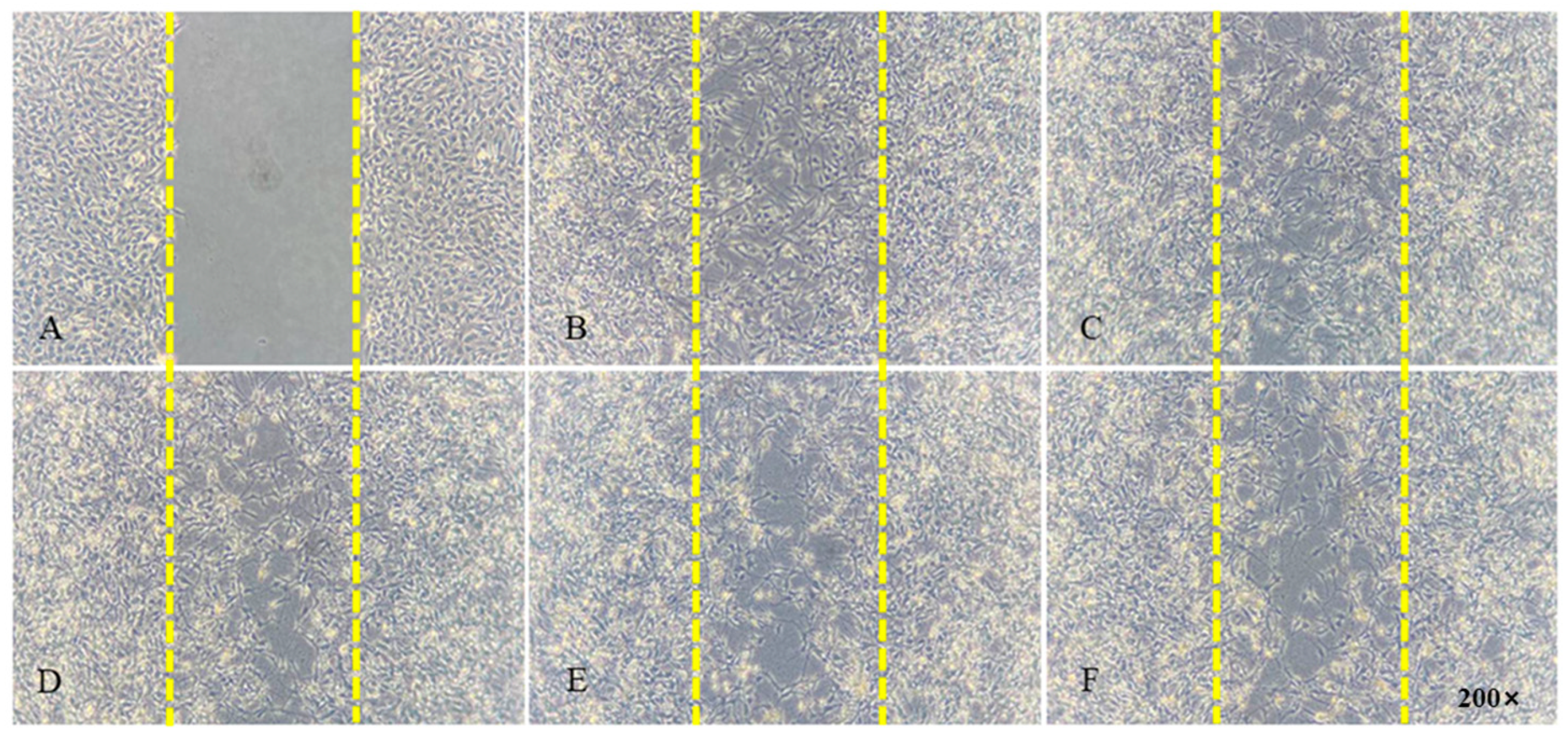
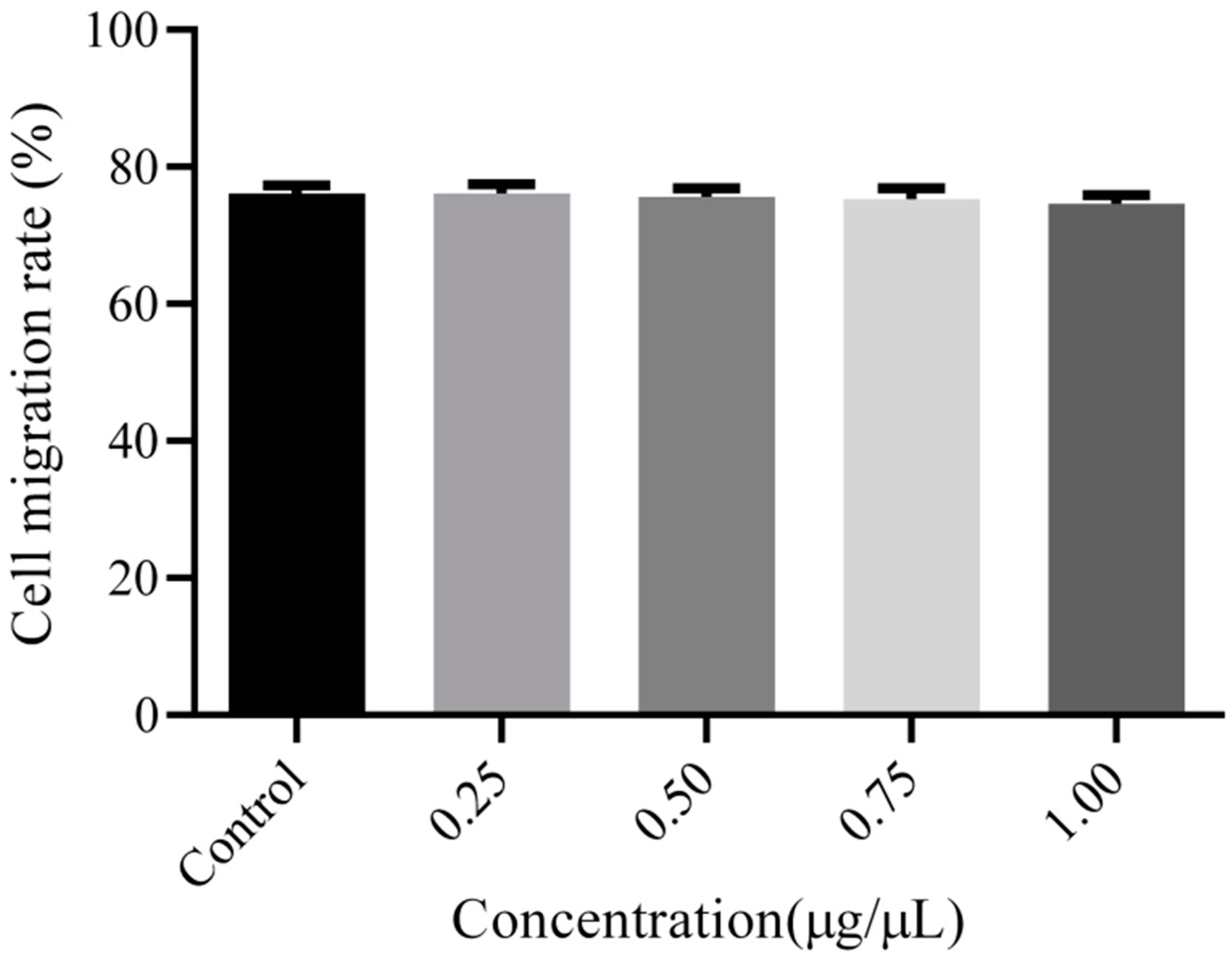
3.4. Cell Migration Analysis
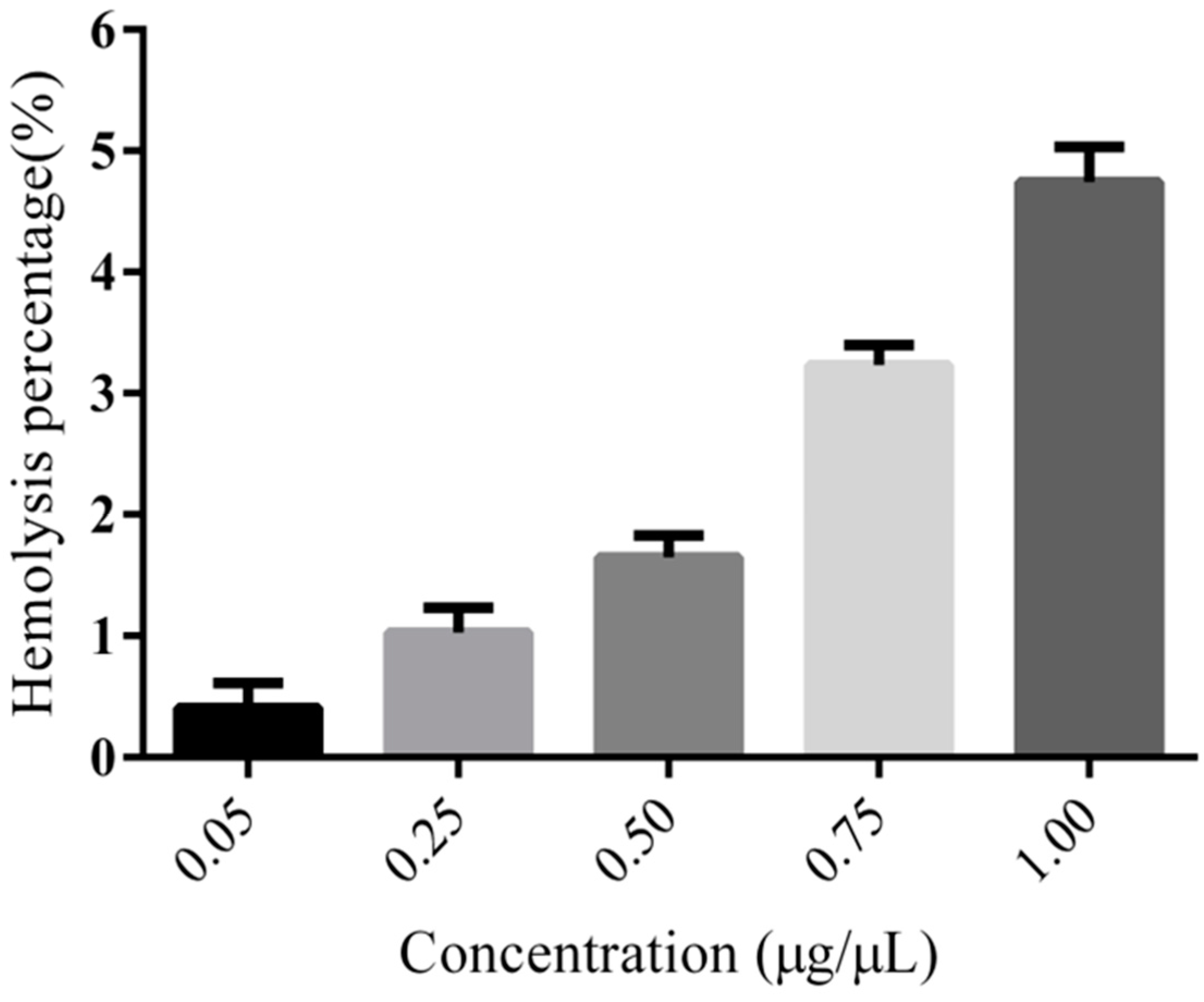
3.5. Hemolysis Assay of PEG-PLLA
3.6. Ultrasound and Heat-Triggered Vaporization of Perfluoropentane
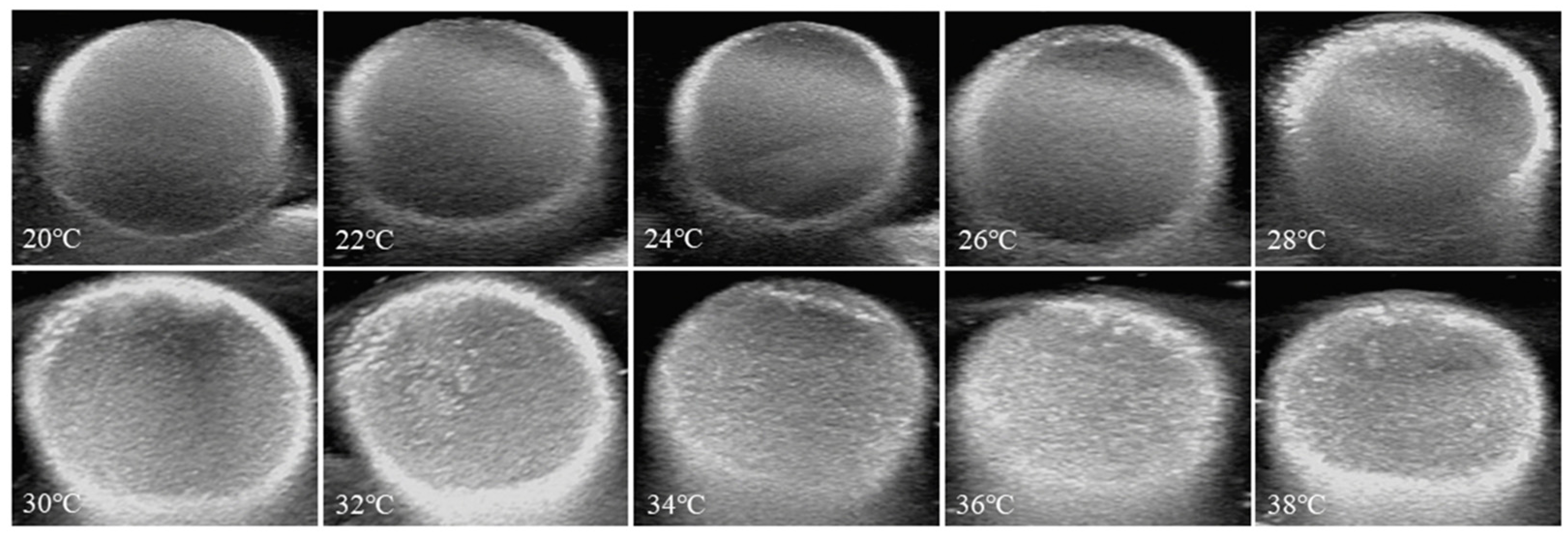
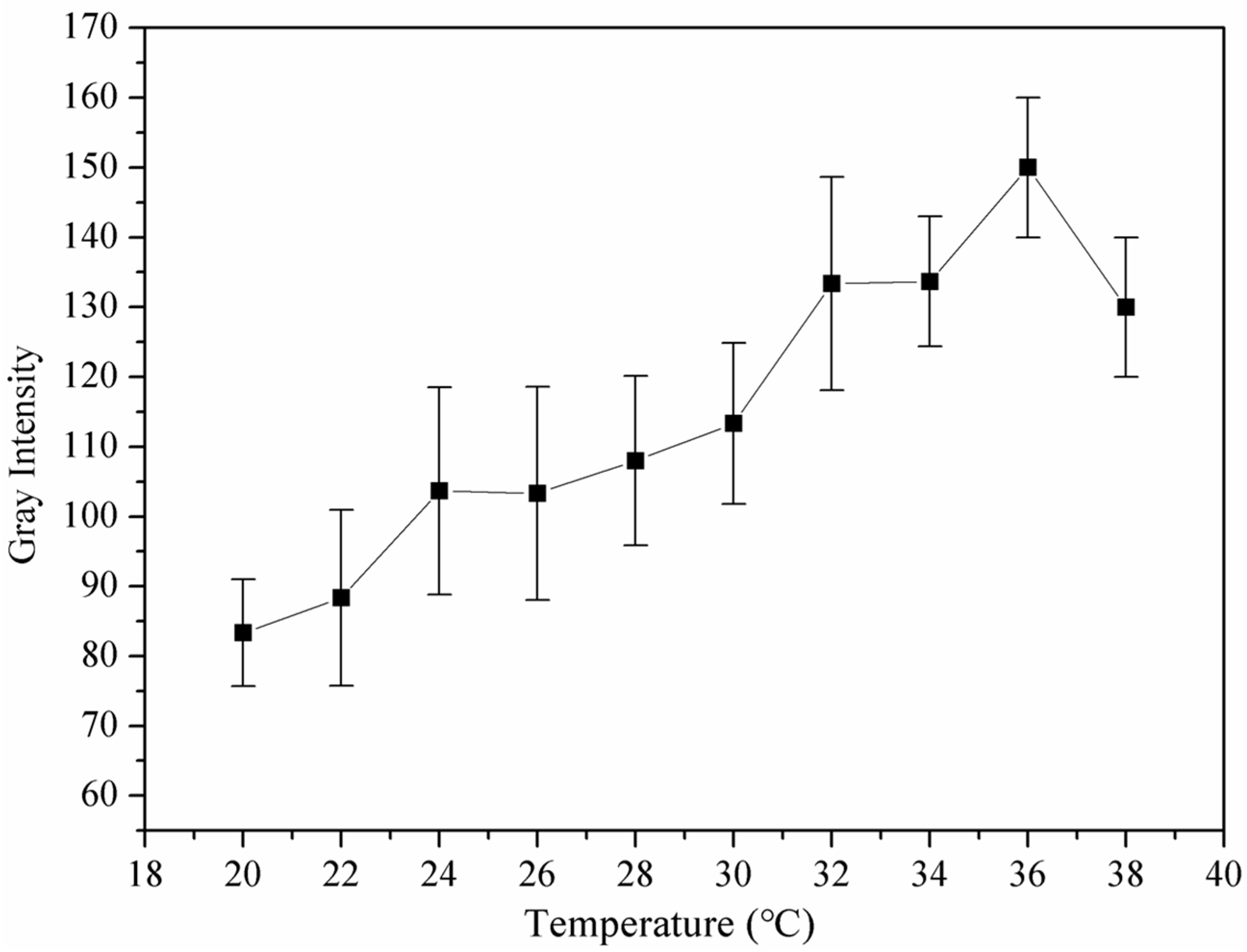
3.7. Echogenicity of Nanoparticles In Vitro at 37 °C
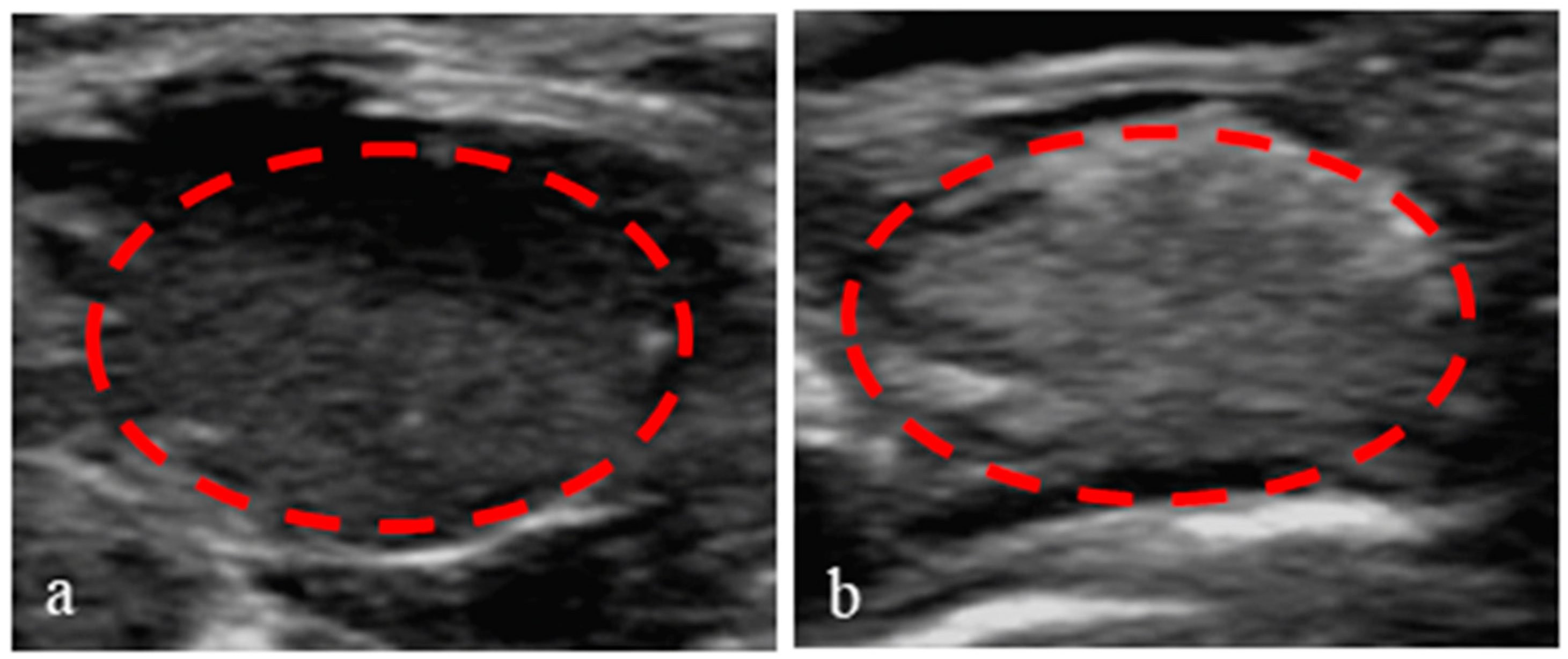
3.8. Application of Nanoparticles in Ultrasound-Enhanced Imaging of Tumors
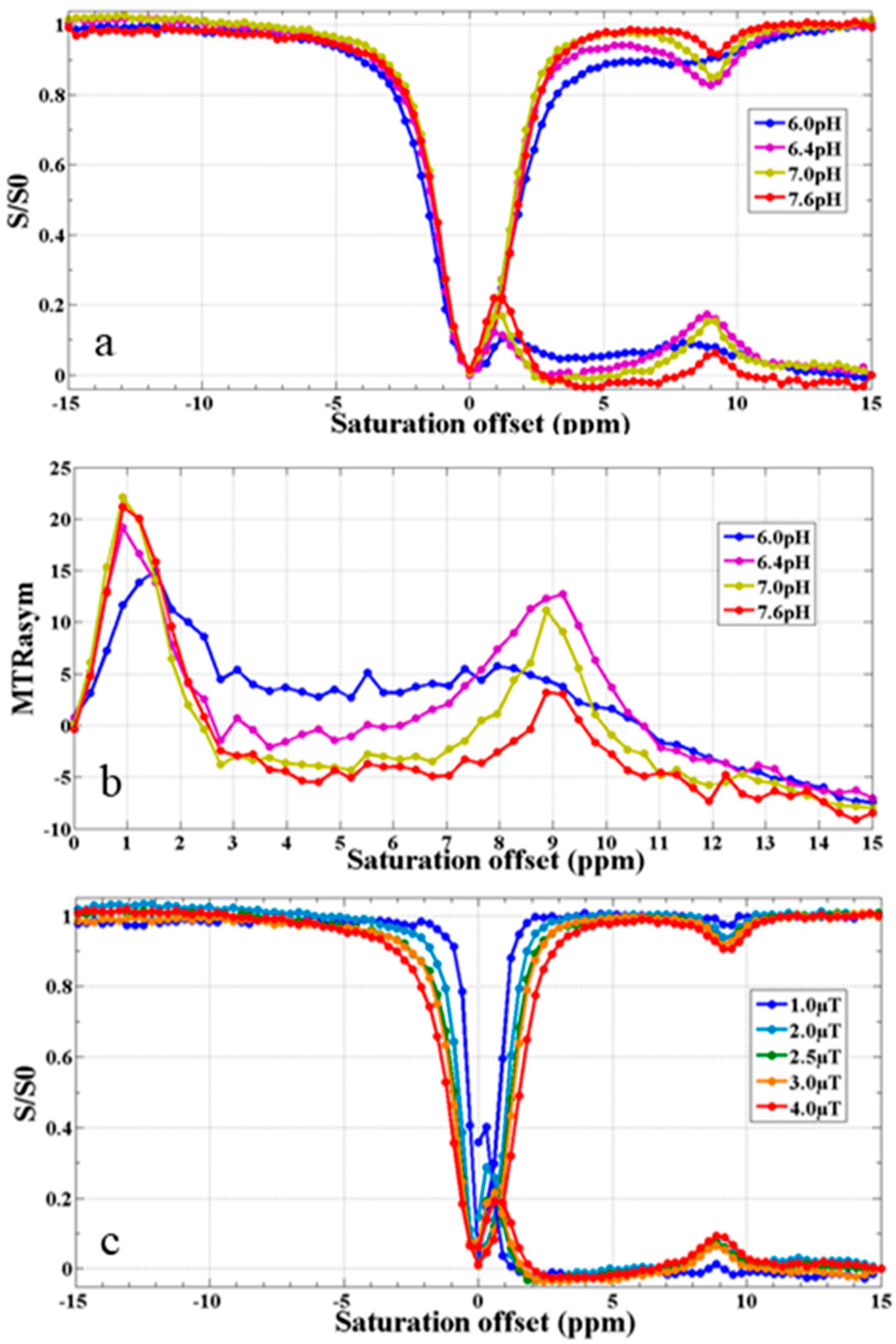
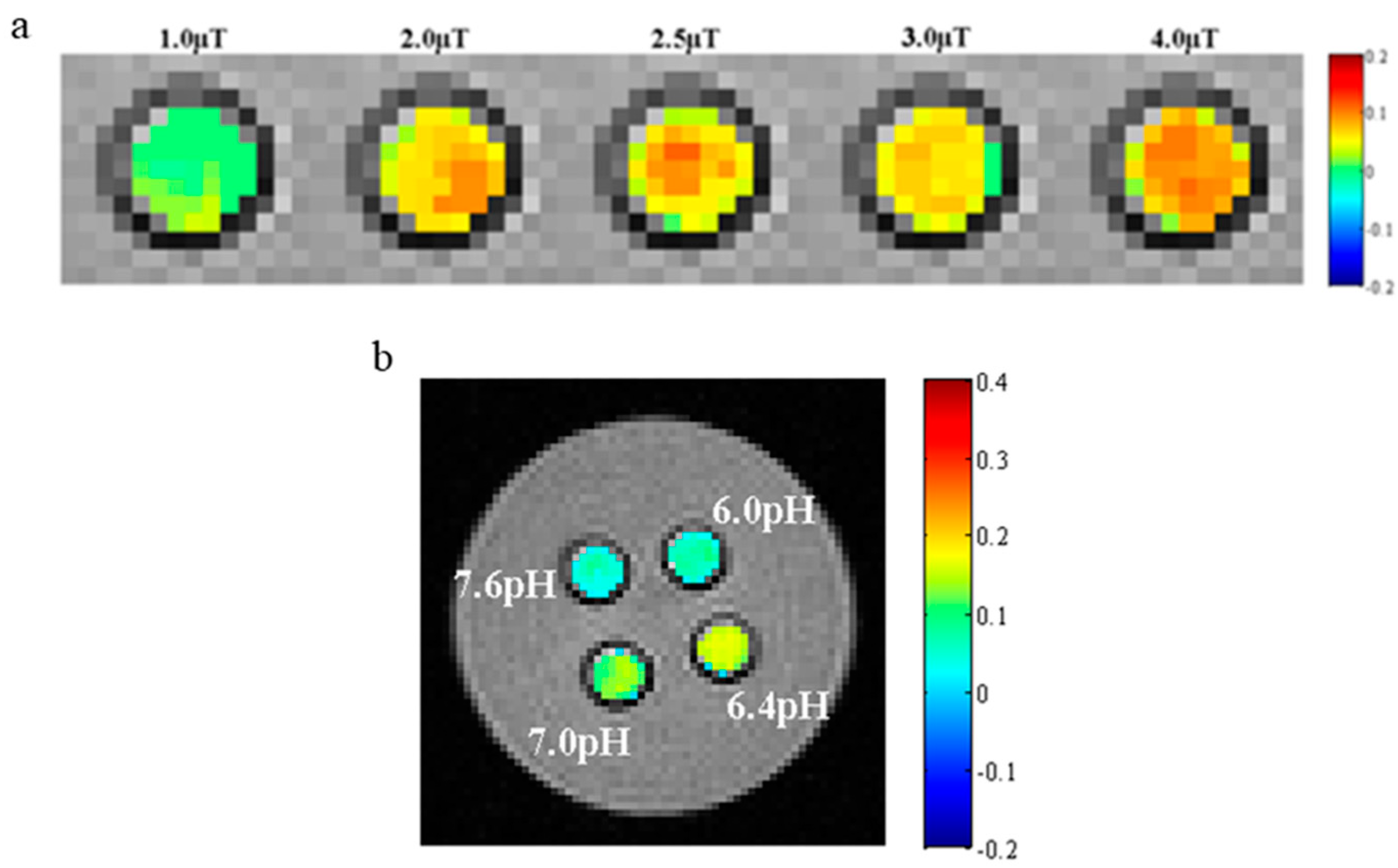
3.9. CEST MR Imaging In Vitro
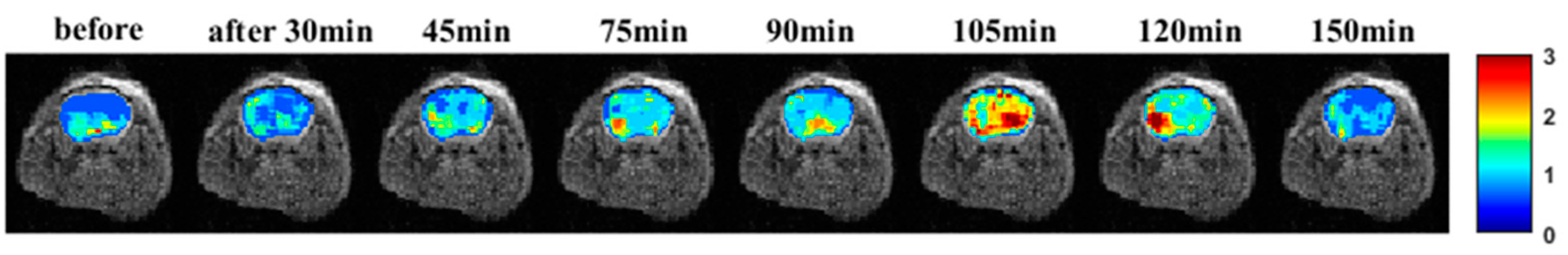
3.10. CEST MR Imaging In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Liu, K.; Wang, Y.; Zhang, C.; Shi, M.; Wang, P.; Shen, L.; Xia, J.; Ye, L.; Shi, X.; et al. A multifunctional low-generation dendrimer-based nanoprobe for the targeted dual mode MR/CT imaging of orthotopic brain gliomas. J. Mater. Chem. B 2019, 7, 3639–3643. [Google Scholar] [CrossRef]
- Liu, R.; Tang, J.; Xu, Y.; Dai, Z. Bioluminescence imaging of inflammation in vivo based on bioluminescence and fluorescence resonance energy transfer using nanobubble ultrasound contrast agent. ACS Nano 2019, 13, 5124–5132. [Google Scholar] [CrossRef]
- Guvener, N.; Appold, L.; de Lorenzi, F.; Golombek, S.K.; Rizzo, L.Y.; Lammers, T.; Kiessling, F. Recent advances in ultrasound-based diagnosis and therapy with micro- and nanometer-sized formulations. Methods 2017, 130, 4–13. [Google Scholar] [CrossRef]
- Gao, Y.; Hernandez, C.; Yuan, H.X.; Lilly, J.; Kota, P.; Zhou, H.; Wu, H.; Exner, A.A. Ultrasound molecular imaging of ovarian cancer with CA-125 targeted nanobubble contrast agents. Nanomedicine 2017, 13, 2159–2168. [Google Scholar] [CrossRef]
- Hernandez, C.; Nieves, L.; de Leon, A.C.; Advincula, R.; Exner, A.A. Role of surface tension in gas nanobubble stability under ultrasound. ACS Appl. Mater. Interfaces 2018, 10, 9949–9956. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, L.; Xu, F.; He, L.; Ye, L.; Huang, L.; Zhang, L.; Luo, B. Construction of novel amphiphilic chitosan-polylactide graft copolymer nanodroplets for contrast enhanced ultrasound tumor imaging. J. Biomater. Appl. 2021, 36, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Baspinar, Y.; Erel-Akbaba, G.; Kotmakci, M.; Akbaba, H. Development and characterization of nanobubbles containing paclitaxel and survivin inhibitor YM155 against lung cancer. Int. J. Pharm. 2019, 566, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Hu, K.; Yu, H.; Zhou, L.; Song, L.; Zhang, Y.; Shan, X.; Liu, J.; Gu, N. A Functional Iron Oxide Nanoparticles Modified with PLA-PEG-DG as Tumor-Targeted MRI Contrast Agent. Pharma. Res. 2017, 34, 1683–1692. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zhang, Q.; Li, T.; Yang, M.; Yao, Q.; Xie, X.; Hu, H.Y. Gadolinium-Labeled Aminoglycoside and Its Potential Application as a Bacteria-Targeting Magnetic Resonance Imaging Contrast Agent. Anal. Chem. 2018, 90, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Wang, S.; Rao, R.; Liu, X.; Xu, H.; Wu, Y.; Yang, X.; Liu, W. Conjugation magnetic PAEEP-PLLA nanoparticles with lactoferrin as a specific targeting MRI contrast agent for detection of brain glioma in rats. Nanoscale Res. Lett. 2016, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Ding, J.; Chen, J.; Zhao, Z.; He, L.; Wang, H.; Huang, S.; Luo, B. Citric acid coated ultrasmall superparamagnetic iron oxide nanoparticles conjugated with lactoferrin for targeted negative MR imaging of glioma. J. Biomater. Appl. 2021, 36, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Liu, X.; Hu, H.; Li, H.; Yu, L.; Geng, D.; Chen, Y.; Zhang, J. Dual-targeting and excretable ultrasmall SPIONs for T1-weighted positive MR imaging of intracranial glioblastoma cells by targeting the lipoprotein receptor-related protein. J. Mater. Chem. 2020, 8, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, S.; Hu, L.; Feng, S.; Wu, Z.; Liu, S.; Duan, S.; Chen, Z.; Zhou, C.; Zhao, X. Imaging Characteristics of USPIO Nanoparticles (<5 nm) as MR Contrast Agent In Vitro and in the Liver of Rats. Contrast Media Mol. Imag. 2019, 2019, 3687537. [Google Scholar]
- Lai, J.H.C.; Liu, J.; Yang, T.; Huang, J.; Liu, Y.; Chen, Z.; Lee, Y.; Leung, G.K.K.; Chan, K.W.Y. Chemical exchange saturation transfer magnetic resonance imaging for longitudinal assessment of intracerebral hemorrhage and deferoxamine treatment at 3T in a mouse model. Stroke 2022, 54, 255–264. [Google Scholar] [CrossRef]
- Anemone, A.; Capozza, M.; Arena, F.; Zullino, S.; Bardini, P.; Terreno, E.; Longo, D.L.; Aime, S. In vitro and in vivo comparison of MRI chemical exchange saturation transfer (CEST) properties between native glucose and 3-O-Methyl-D-glucose in a murine tumor model. NMR Biomed. 2021, 34, e4602. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Song, X.; Chan, K.W.; McMahon, M.T. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 2013, 26, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wen, Z.; Ahn, S.S.; Cai, K.; Paech, D.; Eberhart, C.G.; Zhou, J. Applications of chemical exchange saturation transfer magnetic resonance imaging in identifying genetic markers in gliomas. NMR Biomed. 2022, 36, e4731. [Google Scholar] [CrossRef]
- Liu, J.; Chu, C.; Zhang, J.; Bie, C.; Chen, L.; Aafreen, S.; Xu, J.; Kamson, D.O.; van Zijl, P.C.M.; Walczak, P.; et al. Label-free assessment of mannitol accumulation following osmotic blood-brain barrier opening using chemical exchange saturation transfer magnetic resonance imaging. Pharmaceutics 2022, 14, 2519. [Google Scholar] [CrossRef]
- Jia, Y.; Geng, K.; Cheng, Y.; Li, Y.; Chen, Y.; Wu, R. Nanomedicine particles associated with chemical exchange saturation tTransfer contrast agents in biomedical applications. Front. Chem. 2020, 8, 326. [Google Scholar] [CrossRef]
- Han, Z.; Liu, G. CEST MRI trackable nanoparticle drug delivery systems. Biomed. Mater. 2021, 16, 024103. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, Y.; Geng, K.; Cheng, Y.; Li, Y.; Qiu, J.; Huang, H.; Wang, R.; Zhang, Y.; Wu, R. Glutamate Chemical Exchange Saturation Transfer (GluCEST) Magnetic Resonance Imaging in Pre-clinical and Clinical Applications for Encephalitis. Front. Neurosci. 2020, 14, 750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X.; Wang, Y.; Wang, B.; Zhang, L.; Wei, X.; He, X. Application of a Markov chain Monte Carlo method for robust quantification in chemical exchange saturation transfer magnetic resonance imaging. Quant. Imag. Med. Surg. 2022, 12, 5140–5155. [Google Scholar] [CrossRef] [PubMed]
- Haris, M.; Nanga, R.P.R.; Singh, A.; Cai, K.; Kogan, F.; Hariharan, H.; Reddy, R. Exchange rates of creatine kinase metabolites: Feasibility of imaging creatine by chemical exchange saturation transfer MRI. NMR Biomed. 2012, 25, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Balaban, R.S. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST). Magn. Reson. Med. 2000, 44, 799–802. [Google Scholar] [CrossRef]
- Kombala, C.J.; Kotrotsou, A.; Schuler, F.W.; de la Cerda, J.; Ma, J.C.; Zhang, S.; Pagel, M.D. Development of a nanoscale chemical exchange saturation transfer magnetic resonance imaging contrast agent that measures pH. ACS Nano 2021, 15, 20678–20688. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Huang, J.; Han, X.; Cheu, J.W.S.; Liu, Y.; Law, L.H.; Lai, J.H.C.; Li, J.; Park, S.W.; Wong, C.C.L.; et al. Monitor tumor pHe and response longitudinally during treatment using CEST MRI-detectable alginate microbeads. ACS Appl. Mater. Interfaces 2022, 14, 54401–54410. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Z.; Park, S.W.; Lai, J.H.C.; Chan, K.W.Y. Molecular imaging of brain tumors and drug delivery using CEST MRI: Promises and challenges. Pharmaceutics 2022, 14, 451. [Google Scholar] [CrossRef]
- Kombala, C.J.; Lokugama, S.D.; Kotrotsou, A.; Li, T.; Pollard, A.C.; Pagel, M.D. Simultaneous evaluations of pH and enzyme activity with a CEST MRI contrast agent. ACS Sensors 2021, 6, 4535–4544. [Google Scholar] [CrossRef]
- Aime, S.; Castelli, D.D.; Terreno, E. Novel pH-reporter MRI contrast agents. Angew. Chem. Int. Ed. Engl. 2002, 41, 4334–4336. [Google Scholar] [CrossRef]
- Aime, S.; Barge, A.; Castelli, D.D.; Fedeli, F.; Mortillaro, A.; Nielsen, F.U.; Terreno, E. Paramagnetic lanthanide (III) complexes as pH-sensitive chemical exchange saturation transfer (CEST) contrast agents for MRI applications. Magn. Reson. Med. 2002, 47, 639–648. [Google Scholar] [CrossRef]
- Delli Castelli, D.; Ferrauto, G.; Cutrin, J.C.; Terreno, E.; Aime, S. In vivo maps of extracellular pH in murine melanoma by CEST-MRI. Magn. Reson. Med. 2014, 71, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, L.R.; Randtke, E.A.; High, R.A.; Jones, K.M.; Howison, C.M.; Pagel, M.D. A comparison of exogenous and endogenous CEST MRI methods for evaluating in vivo pH. Magn. Reson. Med. 2018, 79, 2766–2772. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.Z. Quasi-steady-state chemical exchange saturation transfer (QUASS CEST) MRI analysis enables T1 normalized CEST quantification—Insight into T1 contribution to CEST measurement. J. Magn. Reson. 2021, 329, 107022. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, W.G.; Oskolkov, N.; Song, X.; Lal, B.; Yang, X.; Pomper, M.; Laterra, J.; Nimmagadda, S.; McMahon, M.T. Salicylic acid conjugated dendrimers are a tunable, high perfprmance CEST MRI Nanoplatform. Nano Lett. 2016, 16, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, X.; Li, Y.; Liu, G.; Banerjee, S.R.; Pomper, M.G.; McMahon, M.T. Salicylic acid and analogues as diaCEST MRI contrast agents with highly shifted exchangeable proton frequencies. Angew. Chem. 2013, 52, 8116–8119. [Google Scholar] [CrossRef]
- Cazals, Y. Auditory sensori-neural alterations induced by salicylate. Prog. Neurobiol. 2006, 62, 583–631. [Google Scholar] [CrossRef] [PubMed]
- Adelman, A.; Freeman, S.; Paz, Z.; Sohmer, H. Salicylic acid injection before noise exposure reduces perm anent threshold shif. Audiol. Neurootol. 2008, 13, 266–272. [Google Scholar] [CrossRef]
- Yu, N.; Zhu, M.L.; Johnson, B.; Liu, Y.P.; Jones, R.O.; Zhao, H.B. Prestin up·regulation in chronic salicylate (aspirin)administration: An implication of functional dependence of prestin expression. Cell. Mol. Life Sci. 2008, 65, 2407–2418. [Google Scholar] [CrossRef]
- Chuan, D.; Wang, Y.; Fan, R.; Zhou, L.; Chen, H.; Xu, J.; Guo, G. Fabrication and properties of a biomimetic dura matter substitute based on stereocomplex poly(Lactic Acid) nanofibers. Int. J. Nanomed. 2020, 15, 3729–3740. [Google Scholar] [CrossRef]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M.; Seyedabadi, M. A new approach in compatibilization of the poly(lactic acid)/thermoplastic starch (PLA/TPS) blends. Carbohydr. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef]
- Maadani, A.M.; Salahinejad, E. Performance comparison of PLA- and PLGA-coated porous bioceramic scaffolds: Mechanical, biodegradability, bioactivity, delivery and biocompatibility assessments. J. Control. Release 2022, 351, 1–7. [Google Scholar] [CrossRef]
- Jia, Z.; Ma, H.; Liu, J.; Yan, X.; Liu, T.; Cheng, Y.Y.; Li, X.; Wu, S.; Zhang, J.; Song, K. Preparation and characterization of polylactic acid/nano hydroxyapatite/nano hydroxyapatite/human acellular amniotic membrane (PLA/nHAp/HAAM) hybrid scaffold for bone tissue defect repair. Materials 2023, 16, 1937. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Dong, X.; Chen, S.; Lu, W.; Zhang, X.; Chen, W. Confined crystallization, melting behavior and morphology in PEG-b-PLA diblock copolymers: Amorphous versus crystalline PLA. J. Polym. Sci. 2020, 58, 455–465. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, Z.; Chen, J.; He, L.; Wang, H.; Huang, L.; Luo, B. Preparation and ultrasonic imaging investigation of perfluoropentane-filled polylactic acid nanobubbles as a novel targeted ultrasound contrast agent. Front. Mater. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Luo, B.; Zhang, H.; Liu, X.; Rao, R.; Wu, Y.; Liu, W. Novel DiR and SPIO nanoparticles embedded PEG-PLGA nanobubbles as a multimodalimaging contrast agent. Bio-Med. Mater. Eng. 2015, 26 (Suppl. 1), S911–S916. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; He, L.; Yang, L.; Cheng, L.; Zhao, Z.; Luo, B.; Jia, Y. Novel Nanoprobe with Combined Ultrasonography/Chemical Exchange Saturation Transfer Magnetic Resonance Imaging for Precise Diagnosis of Tumors. Pharmaceutics 2023, 15, 2693. https://doi.org/10.3390/pharmaceutics15122693
Ding J, He L, Yang L, Cheng L, Zhao Z, Luo B, Jia Y. Novel Nanoprobe with Combined Ultrasonography/Chemical Exchange Saturation Transfer Magnetic Resonance Imaging for Precise Diagnosis of Tumors. Pharmaceutics. 2023; 15(12):2693. https://doi.org/10.3390/pharmaceutics15122693
Chicago/Turabian StyleDing, Jieqiong, Liu He, Lin Yang, Liyuan Cheng, Zhiwei Zhao, Binhua Luo, and Yanlong Jia. 2023. "Novel Nanoprobe with Combined Ultrasonography/Chemical Exchange Saturation Transfer Magnetic Resonance Imaging for Precise Diagnosis of Tumors" Pharmaceutics 15, no. 12: 2693. https://doi.org/10.3390/pharmaceutics15122693
APA StyleDing, J., He, L., Yang, L., Cheng, L., Zhao, Z., Luo, B., & Jia, Y. (2023). Novel Nanoprobe with Combined Ultrasonography/Chemical Exchange Saturation Transfer Magnetic Resonance Imaging for Precise Diagnosis of Tumors. Pharmaceutics, 15(12), 2693. https://doi.org/10.3390/pharmaceutics15122693




