Celecoxib/Cyclodextrin Eye Drop Microsuspensions: Evaluation of In Vitro Cytotoxicity and Anti-VEGF Efficacy for Retinal Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterizations of CCB Eye Drop Microsuspensions (MS)
2.3. Hemolysis Activity
2.4. Irritation Study by Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM)
2.5. In Vitro Cytotoxicity Study
2.5.1. Short-Time Exposure (STE)
2.5.2. Cell Viability (CV) Test
2.6. Anti-VEGF Activity
2.6.1. Hypoxia Exposure
2.6.2. Western Blot
2.6.3. Real-Time Polymerase Chain Reaction (RT-PCR)
2.7. Ex Vivo Porcine Transscleral Permeation of CCB
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of CCB Eye Drop MS
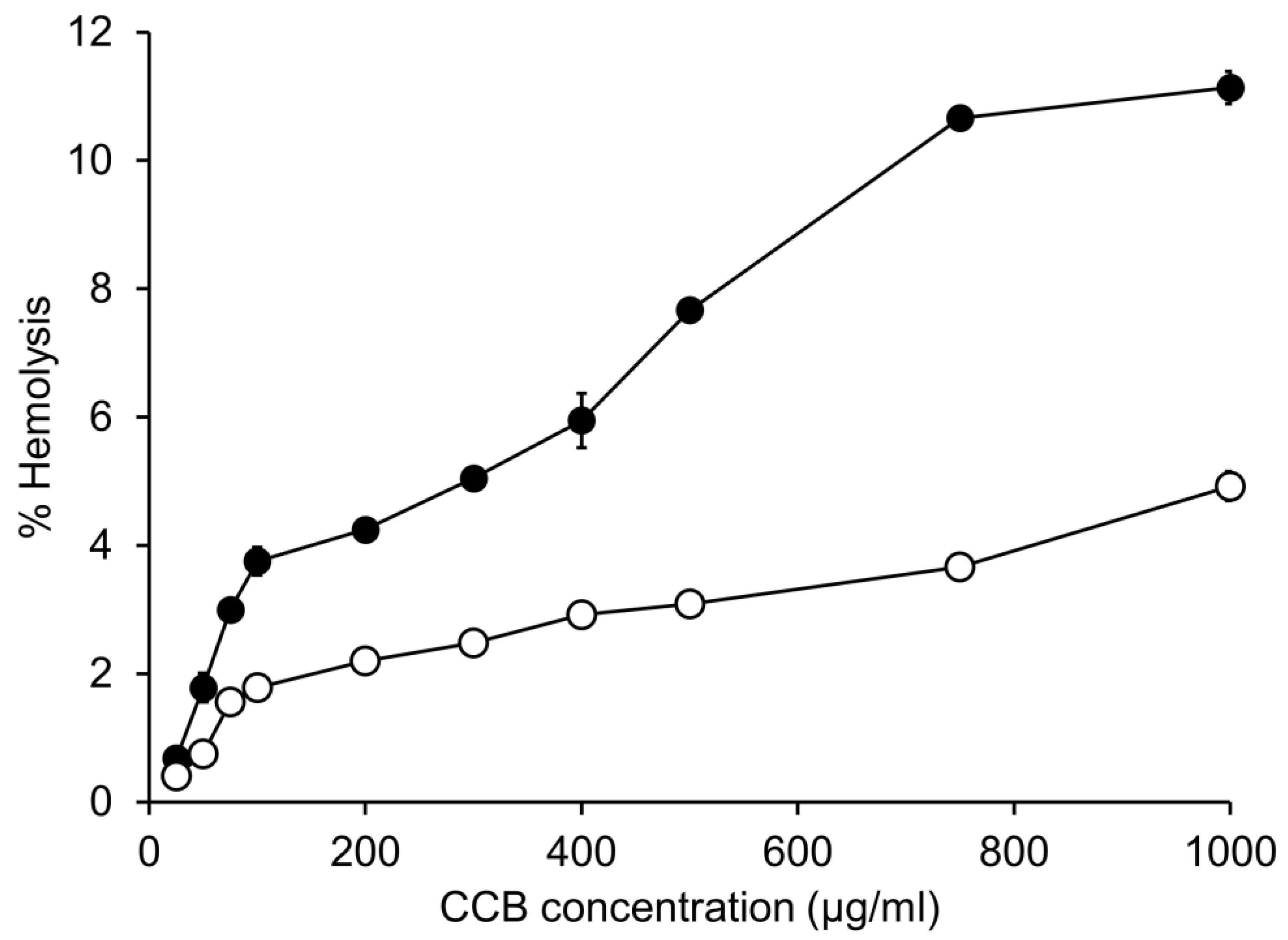
3.2. In Vitro Hemolytic Activity
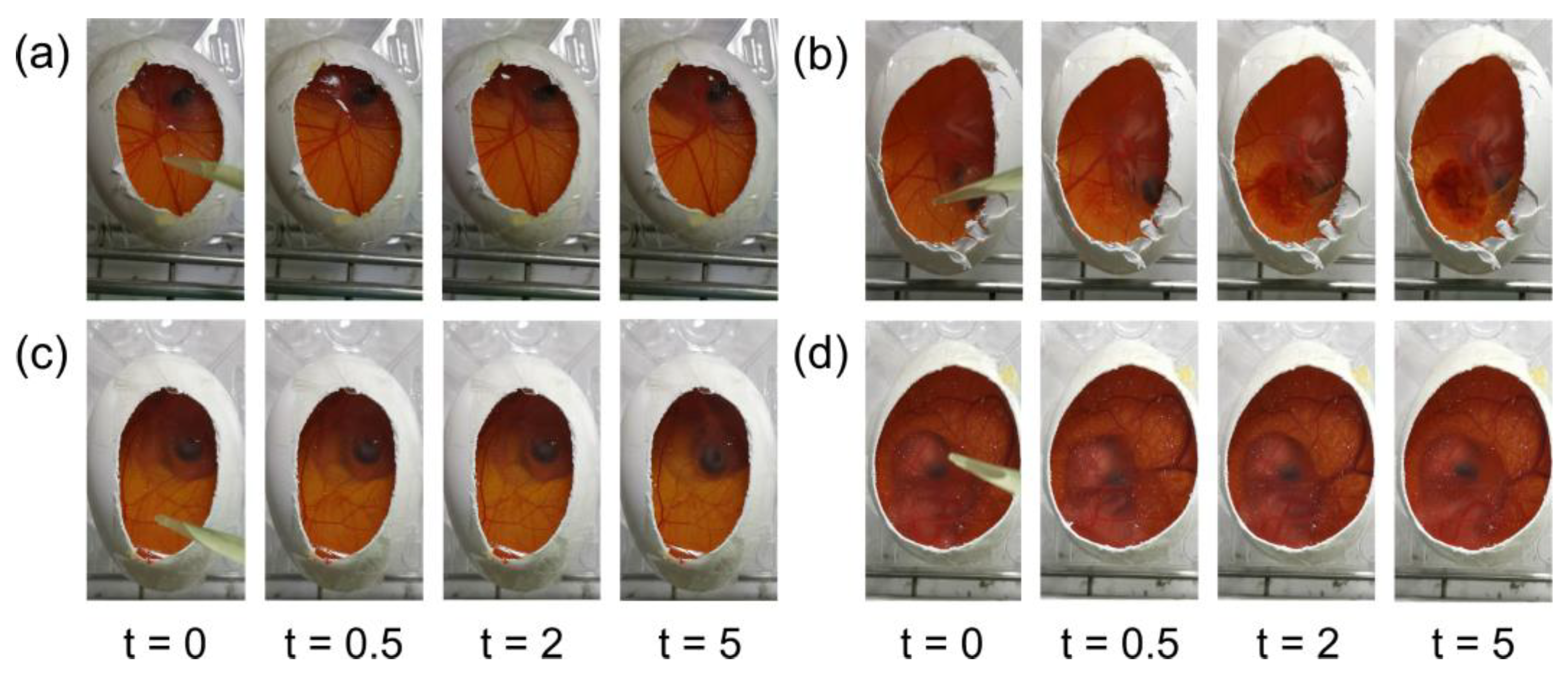
3.3. Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM)
3.4. Short-Time Exposure (STE) Test
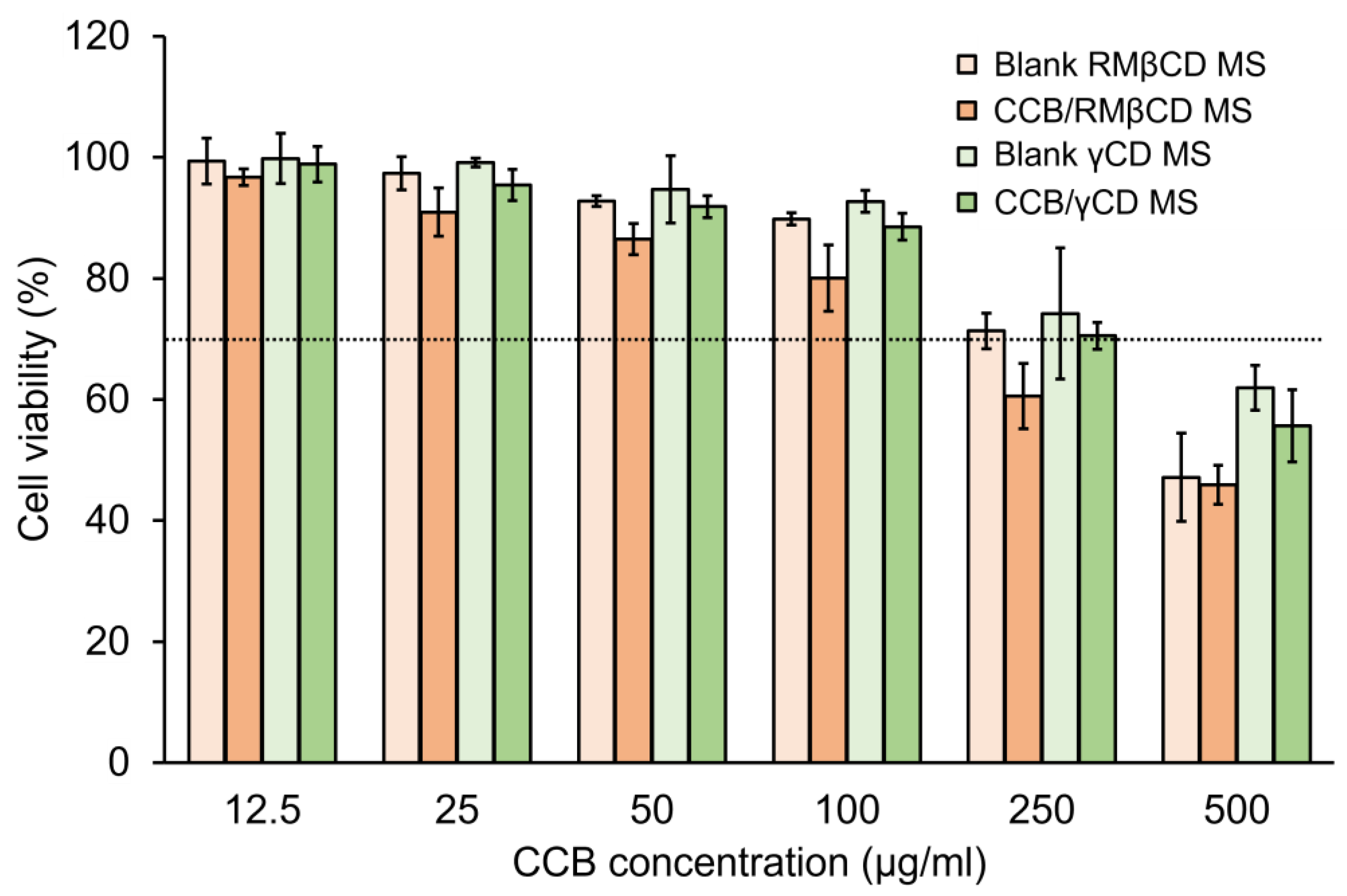
3.5. Cell Viability (CV) Test
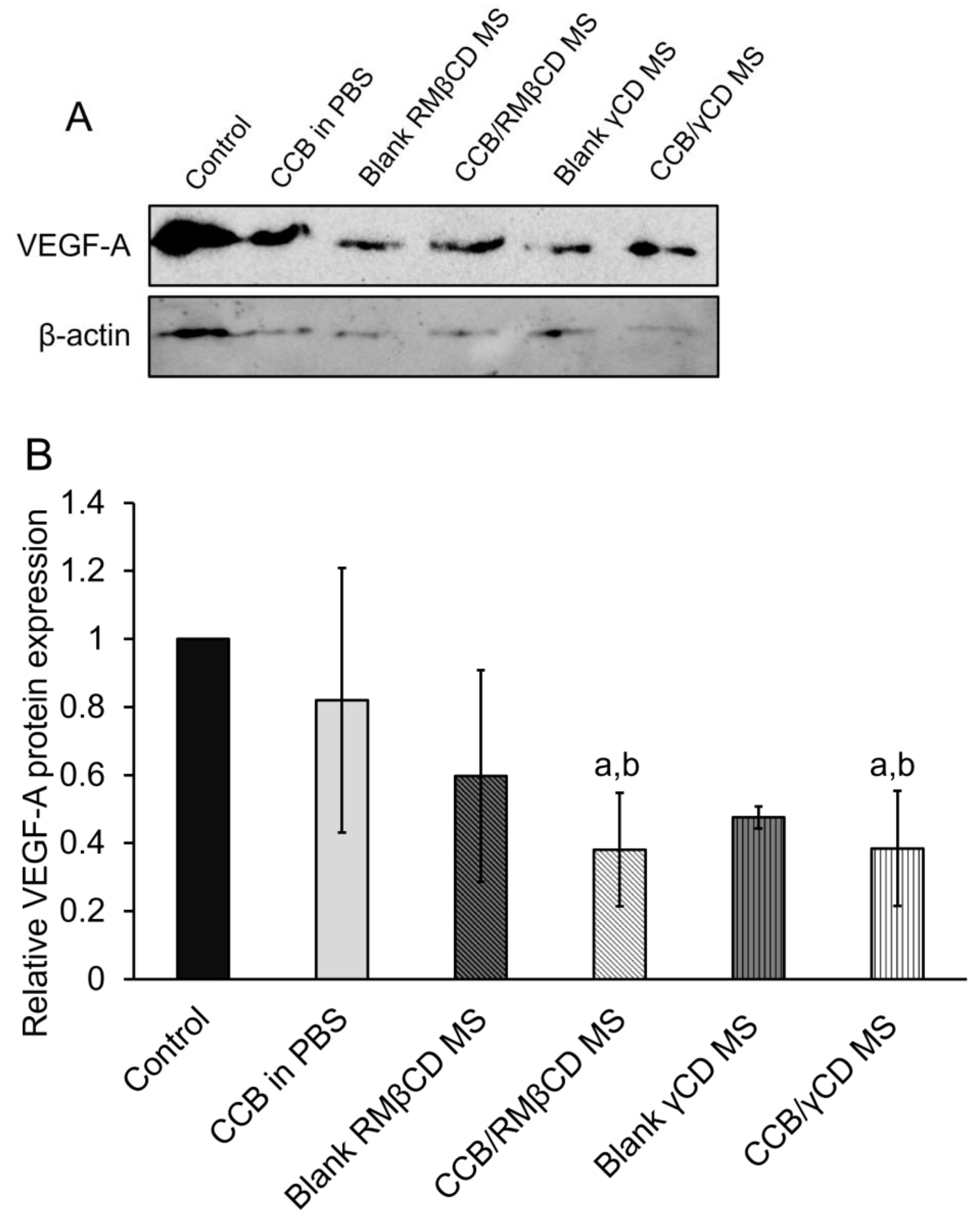
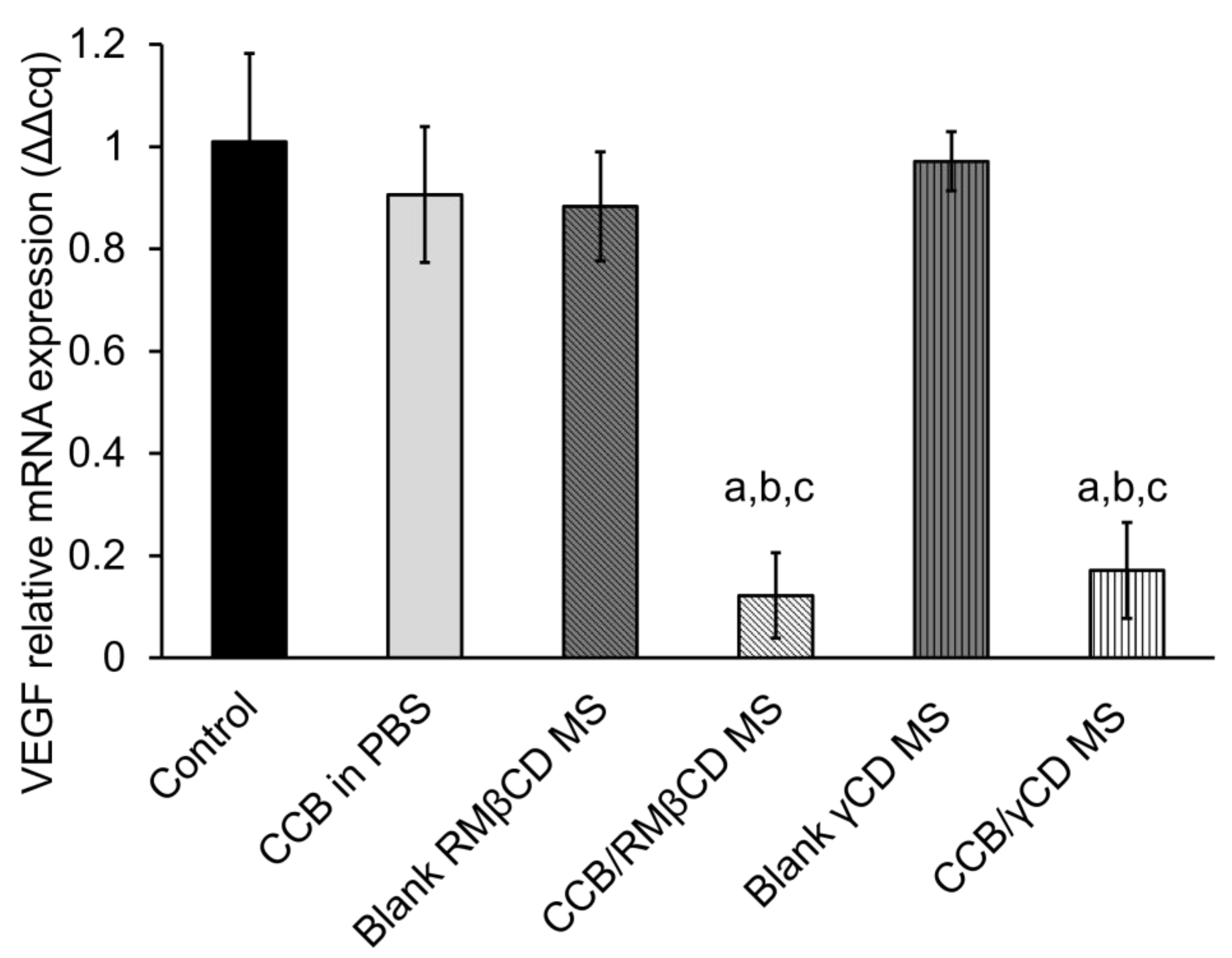
3.6. Western Blotting Assay and Real-Time Polymerase Chain Reaction (RT-PCR)
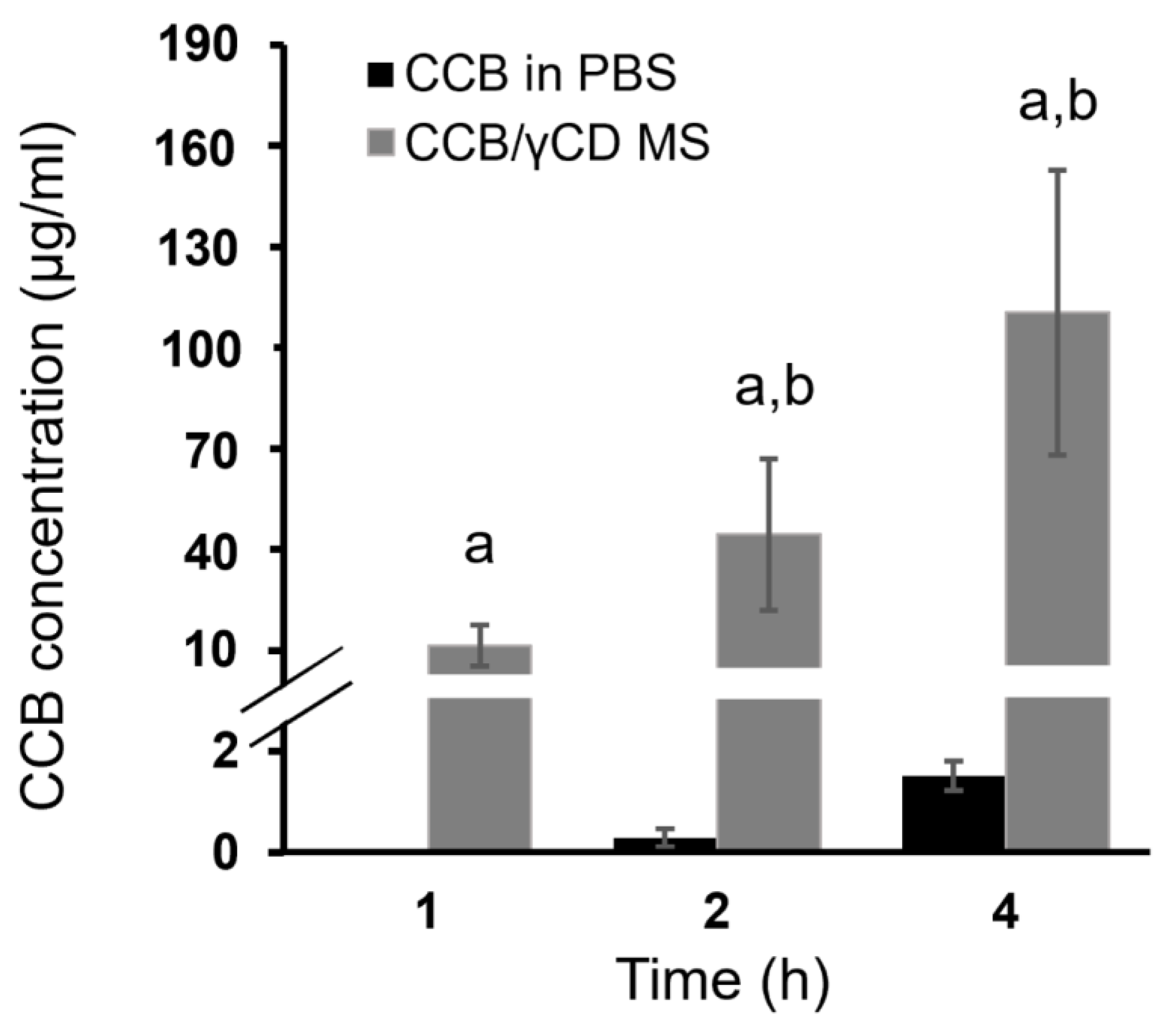
3.7. Ex Vivo Transscleral Diffusion Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of age-related macular degeneration in europe: The past and the future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012, 60, 428. [Google Scholar] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Amrite, A.; Pugazhenthi, V.; Cheruvu, N.; Kompella, U. Delivery of celecoxib for treating diseases of the eye: Influence of pigment and diabetes. Expert Opin. Drug Deliv. 2010, 7, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Kliffen, M.; Sharma, H.S.; Mooy, C.M.; Kerkvliet, S.; de Jong, P.T. Increased expression of angiogenic growth factors in age-related maculopathy. Br. J. Ophthalmol. 1997, 81, 154–162. [Google Scholar] [CrossRef]
- Adamis, A.P.; Miller, J.W.; Bernal, M.-T.; D’Amico, D.J.; Folkman, J.; Yeo, T.-K.; Yeo, K.-T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 1994, 118, 445–450. [Google Scholar] [CrossRef]
- Freund, K.B.; Yannuzzi, L.A.; Sorenson, J.A. Age-related macular degeneration and choroidal neovascularization. Am. J. Ophthalmol. 1993, 115, 786–791. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Amador, A.G.; Zinman, B. Diabetic retinopathy and diabetic macular edema: Pathophysiology, screening, and novel therapies. Diabetes Care 2003, 26, 2653–2664. [Google Scholar] [CrossRef]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.-E.; Wilson, C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef]
- Lu, L.; Jiang, Y.; Jaganathan, R.; Hao, Y. Current advances in pharmacotherapy and technology for diabetic retinopathy: A systematic review. J. Ophthalmol. 2018, 2018, 1694187. [Google Scholar]
- Rusciano, D.; Bagnoli, P. Pharmacotherapy and nutritional supplements for neovascular eye diseases. Medicina 2023, 59, 1334. [Google Scholar] [CrossRef] [PubMed]
- Ayalasomayajula, S.P.; Kompella, U.B. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur. J. Pharmacol. 2003, 458, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Raghava, S.; Hammond, M.; Kompella, U.B. Periocular routes for retinal drug delivery. Expert Opin. Drug Deliv. 2004, 1, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Geroski, D.H.; Prausnitz, M.R.; Edelhauser, H.F. Drug delivery through the sclera: Effects of thickness, hydration, and sustained release systems. Exp. Eye Res. 2004, 78, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Kulsirachote, P.; Loftsson, T. Cyclodextrin solubilization of celecoxib: Solid and solution state characterization. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 75–88. [Google Scholar] [CrossRef]
- Jansook, P.; Kulsirachote, P.; Asasutjarit, R.; Loftsson, T. Development of celecoxib eye drop solution and microsuspension: A comparative investigation of binary and ternary cyclodextrin complexes. Carbohydr. Polym. 2019, 225, 115209. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Maw, P.D.; Soe, H.M.S.H.; Chuangchunsong, R.; Saiborisuth, K.; Payonitikarn, N.; Autthateinchai, R.; Pruksakorn, P. Development of amphotericin B nanosuspensions for fungal keratitis therapy: Effect of self-assembled γ-cyclodextrin. J. Pharm. Investig. 2020, 50, 513–525. [Google Scholar] [CrossRef]
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). ICCVAM-Recommended Test Method Protocol: Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Test Method; Tech. Rep. 10-7553; NIH Publication: Research Triangle Park, NC, USA, 2010. [Google Scholar]
- Luepke, N. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Takahashi, Y.; Koike, M.; Honda, H.; Ito, Y.; Sakaguchi, H.; Suzuki, H.; Nishiyama, N. Development of the short time exposure (STE) test: An in vitro eye irritation test using SIRC cells. Toxicol. In Vitro 2008, 22, 760–770. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Valenti, D.; Escribano, E.; Hillaireau, H.; Fadda, A.M.; Fattal, E. Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. Int. J. Pharm. 2017, 525, 203–210. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Managit, C.; Phanaksri, T.; Treesuppharat, W.; Fuongfuchat, A. Formulation development and in vitro evaluation of transferrin-conjugated liposomes as a carrier of ganciclovir targeting the retina. Int. J. Pharm. 2020, 577, 119084. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.M.; Saint-Geniez, M.; Walshe, T.; Zahr, A.; D’Amore, P.A. Expression and role of VEGF in the adult retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9478–9487. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, F.; Cervellati, C.; Romani, A.; Cremonini, E.; Sticozzi, C.; Belmonte, G.; Pessina, F.; Valacchi, G. Hypoxia induces cell damage via oxidative stress in retinal epithelial cells. Free Radic. Res. 2014, 48, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Puddu, A.; Sanguineti, R.; Traverso, C.E.; Viviani, G.L.; Nicolò, M. Response to anti-VEGF-A treatment of retinal pigment epithelial cells in vitro. Eur. J. Ophthalmol. 2016, 26, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Soe, H.M.S.H.; Maw, P.D.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Tacrolimus/hydroxypropyl-β-cyclodextrin-loaded nanoemulsions stabilized by Zein-Soluplus® nanoparticles for retinal diseases. J. Drug Deliv. Sci. Technol. 2023, 88, 104936. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef]
- Kramer, I.; Haber, M.; Duis, A. Requirements concerning antiseptics for periorbital, orbital and intraorbital application: Formulation requirements for the ophthalmic use of antiseptics. In Antiseptic Prophylaxis and Therapy in Ocular Infections; Kramer, A., Behrens-Baumann, W., Eds.; Karger Publishers Inc.: Basel, Switzerland, 2002; pp. 85–116. [Google Scholar]
- Aldrich, D.S.; Bach, C.M.; Brown, W.; Chambers, W.; Fleitman, J.; Hunt, D.; Marques, M.R.C.; Mille, Y.; Mitra, A.K.; Platzer, S.M.; et al. Ophthalmic Preparations. 2003. Available online: http://www.triphasepharmasolutions.com/Resources/USP%20Ophthalmic%20Preparations.pdf (accessed on 18 January 2023).
- Nagyová, B.; Tiffany, J.M. Components responsible for the surface tension of human tears. Curr. Eye Res. 1999, 19, 4–11. [Google Scholar] [CrossRef]
- Hotujac Grgurević, M.; Juretić, M.; Hafner, A.; Lovrić, J.; Pepić, I. Tear fluid-eye drops compatibility assessment using surface tension. Drug Dev. Ind. Pharm. 2017, 43, 275–282. [Google Scholar] [CrossRef]
- Szejtli, J. Past, present and futute of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.M.; Cairns, D. The hen’s egg chorioallantoic membrane (HET-CAM) test to predict the ophthalmic irritation potential of a cysteamine-containing gel: Quantification using Photoshop® and ImageJ. Int. J. Pharm. 2015, 490, 1–8. [Google Scholar] [CrossRef]
- Romagna, G.; Allifranchini, E.; Bocchietto, E.; Todeschi, S.; Esposito, M.; Farsalinos, K.E. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): Comparison with tobacco cigarette smoke extract. Inhal. Toxicol. 2013, 25, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-W.; Hailian, Q.; Na, Y.; Kang, B.-C.; Yoon, J.-H.; Cho, E.-Y.; Lee, M.; Kim, D.-E.; Bae, S.; Seok, S.H.; et al. Exploration and comparison of in vitro eye irritation tests with the ISO standard in vivo rabbit test for the evaluation of the ocular irritancy of contact lenses. Toxicol. In Vitro 2016, 37, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.A.; Avery, R.L.; Foley, E.D.; Aiello, L.P.; Smith, L.E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. USA 1995, 92, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control. Release 2020, 326, 419–441. [Google Scholar] [CrossRef]
- Sun, Y.-Z.; Cai, N.; Liu, N.-N. Celecoxib Down-Regulates the hypoxia-induced expression of HIF-1α and VEGF through the PI3K/AKT pathway in retinal pigment epithelial cells. Cell. Physiol. Biochem. 2017, 44, 1640–1650. [Google Scholar] [CrossRef]
| Parameters | CCB/RMβCD MS | CCB/γCD MS |
|---|---|---|
| Appearance | Milky white suspension | Milky white suspension |
| pH | 7.40 ± 0.06 | 7.39 ± 0.02 |
| Osmolality (mOsmol/kg) | 299.67 ± 2.08 | 301.00 ± 2.00 |
| Viscosity (cPs) a | 22.76 ± 1.53 | 27.53 ± 1.72 |
| Surface tension (mN/m) a | 45.32 ± 0.15 | 48.35 ± 2.07 |
| Redispersion time (s) | 28.00 ± 2.65 | 20.67 ± 1.15 |
| Total drug content (%) | 102.51 ± 0.07 | 96.34 ± 0.78 |
| Dissolved CCB content (%) | 51.44 ± 4.11 | 1.61 ± 0.12 |
| Concentrations of Test Samples | Test Samples | %CV of SIRC Cells | Criteria for Scoring | Obtained Scores |
|---|---|---|---|---|
| (I) 5% | Blank RMβCD MS | 92.95 ± 1.70 | If CV > 70%: scored = 0 If CV ≤ 70%: scored = 1 | 0 |
| Blank γCD MS | 94.51 ± 2.76 | 0 | ||
| CCB/RMβCD MS | 77.96 ± 4.57 | 0 | ||
| CCB/γCD MS | 84.88 ± 3.15 | 0 | ||
| (II) 0.05% | Blank RMβCD MS | 99.30 ± 1.29 | If CV > 70%: scored = 1 If CV ≤ 70%: scored = 2 | 1 |
| Blank γCD MS | 99.68 ± 1.00 | 1 | ||
| CCB/RMβCD MS | 89.74 ± 2.21 | 1 | ||
| CCB/γCD MS | 91.22 ± 1.41 | 1 | ||
| Total score (I and II) | ||||
| Blank RMβCD MS | 1 | |||
| Blank γCD MS | 1 | |||
| CCB/RMβCD MS | 1 | |||
| CCB/γCD MS | 1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansook, P.; Soe, H.M.S.H.; Asasutjarit, R.; Tun, T.; Hnin, H.M.; Maw, P.D.; Watchararot, T.; Loftsson, T. Celecoxib/Cyclodextrin Eye Drop Microsuspensions: Evaluation of In Vitro Cytotoxicity and Anti-VEGF Efficacy for Retinal Diseases. Pharmaceutics 2023, 15, 2689. https://doi.org/10.3390/pharmaceutics15122689
Jansook P, Soe HMSH, Asasutjarit R, Tun T, Hnin HM, Maw PD, Watchararot T, Loftsson T. Celecoxib/Cyclodextrin Eye Drop Microsuspensions: Evaluation of In Vitro Cytotoxicity and Anti-VEGF Efficacy for Retinal Diseases. Pharmaceutics. 2023; 15(12):2689. https://doi.org/10.3390/pharmaceutics15122689
Chicago/Turabian StyleJansook, Phatsawee, Hay Man Saung Hnin Soe, Rathapon Asasutjarit, Theingi Tun, Hay Marn Hnin, Phyo Darli Maw, Tanapong Watchararot, and Thorsteinn Loftsson. 2023. "Celecoxib/Cyclodextrin Eye Drop Microsuspensions: Evaluation of In Vitro Cytotoxicity and Anti-VEGF Efficacy for Retinal Diseases" Pharmaceutics 15, no. 12: 2689. https://doi.org/10.3390/pharmaceutics15122689
APA StyleJansook, P., Soe, H. M. S. H., Asasutjarit, R., Tun, T., Hnin, H. M., Maw, P. D., Watchararot, T., & Loftsson, T. (2023). Celecoxib/Cyclodextrin Eye Drop Microsuspensions: Evaluation of In Vitro Cytotoxicity and Anti-VEGF Efficacy for Retinal Diseases. Pharmaceutics, 15(12), 2689. https://doi.org/10.3390/pharmaceutics15122689









