Challenges Related to Acquisition of Physiological Data for Physiologically Based Pharmacokinetic (PBPK) Models in Postpartum, Lactating Women and Breastfed Infants—A Contribution from the ConcePTION Project
Abstract
:1. Introduction
2. Postpartum Maternal Weight Retention
3. Human Milk Intake and Composition
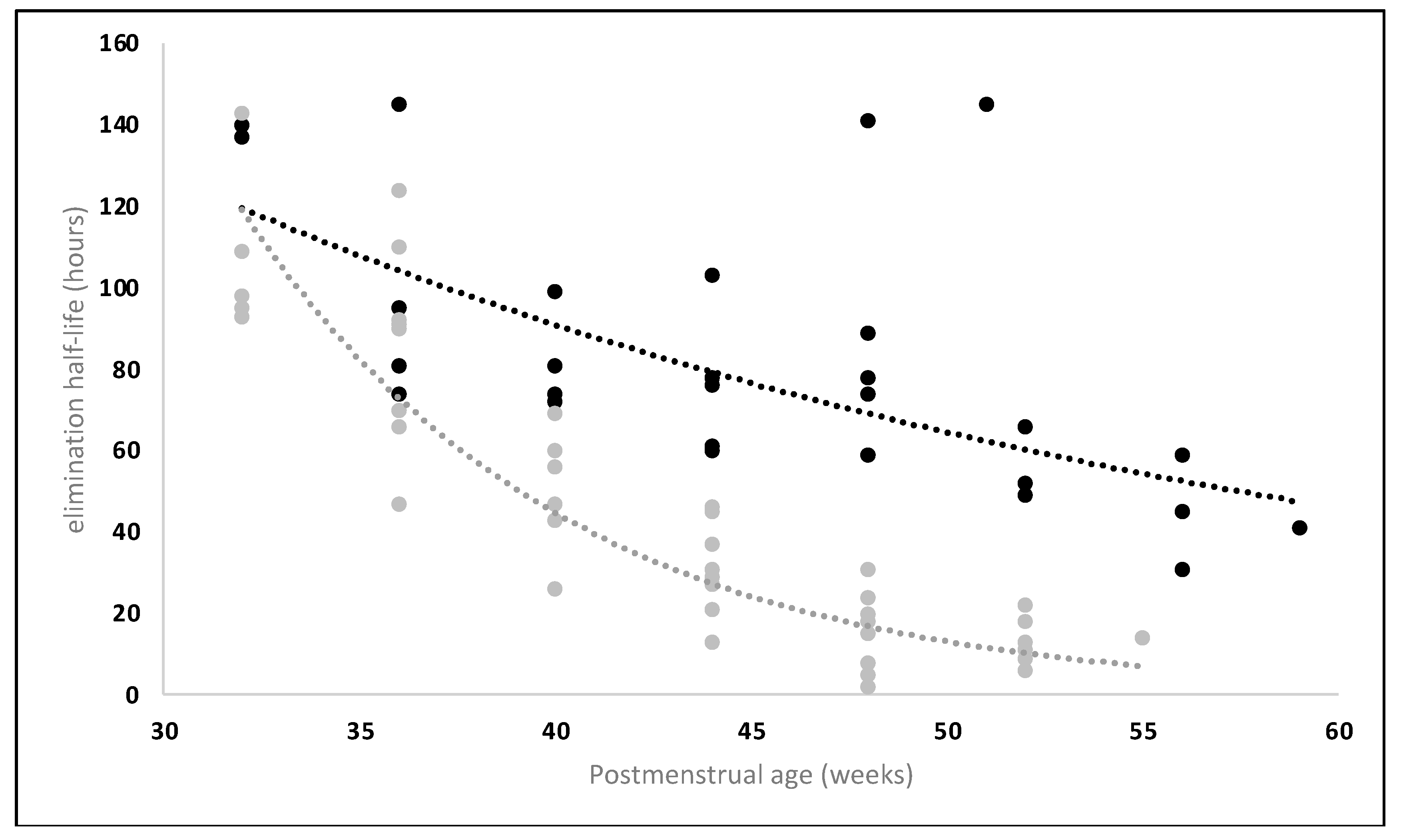
4. The Specific Physiology of Breastfed Infants
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP). Guideline on the Reporting of Physiologically Based Pharmacokinetic (PBPK) Modelling and Simulation; European Medicines Agency: London, UK, 2018. [Google Scholar]
- Smits, A.; de Cock, P.; Vermeulen, A.; Allegaert, K. Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation in Neonatal Drug Development: How Clinicians Can Contribute. Expert. Opin. Drug Metab. Toxicol. 2019, 15, 25–34. [Google Scholar] [CrossRef]
- FDA. Physiologically Based Pharmacokinetic Analyses—Format and Content: Guidance for Industry; FDA: Rockville, MD, USA, 2018; pp. 1–6. [Google Scholar]
- Leong, R.; Vieira, M.L.T.; Zhao, P.; Mulugeta, Y.; Lee, C.S.; Huang, S.M.; Burckart, G.J. Regulatory Experience with Physiologically Based Pharmacokinetic Modeling for Pediatric Drug Trials. Clin. Pharmacol. Ther. 2012, 91, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.A.; Adiwidjaja, J.; Sjöstedt, N.; Yang, K.; Beaudoin, J.J.; Spires, J.; Siler, S.Q.; Neuhoff, S.; Brouwer, K.L.R. Considerations for Physiologically Based Modeling in Liver Disease: From Nonalcoholic Fatty Liver (NAFL) to Nonalcoholic Steatohepatitis (NASH). Clin. Pharmacol. Ther. 2022, 113, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Isoherranen, N. Novel Mechanistic PBPK Model to Predict Renal Clearance in Varying Stages of CKD by Incorporating Tubular Adaptation and Dynamic Passive Reabsorption. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 571–583. [Google Scholar] [CrossRef]
- Claassen, K.; Thelen, K.; Coboeken, K.; Gaub, T.; Lippert, J.; Allegaert, K.; Willmann, S. Development of a Physiologically-Based Pharmacokinetic Model for Preterm Neonates: Evaluation with In Vivo Data. Curr. Pharm. Des. 2015, 21, 5688–5698. [Google Scholar] [CrossRef]
- Edginton, A.N.; Schmitt, W.; Willmann, S. Development and Evaluation of a Generic Physiologically Based Pharmacokinetic Model for Children. Clin. Pharmacokinet. 2006, 45, 1013–1034. [Google Scholar] [CrossRef] [PubMed]
- Abduljalil, K.; Pansari, A.; Ning, J.; Jamei, M. Prediction of Drug Concentrations in Milk during Breastfeeding, Integrating Predictive Algorithms within a Physiologically-Based Pharmacokinetic Model. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 878–889. [Google Scholar] [CrossRef]
- Breastfeeding. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed on 29 November 2021).
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J. Breastfeeding and Maternal Health Outcomes: A Systematic Review and Meta-Analysis. Acta Paediatr. Int. J. Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef]
- Krause, K.M.; Lovelady, C.A.; Peterson, B.L.; Chowdhury, N.; Stbye, T. Effect of Breast-Feeding on Weight Retention at 3 and 6 Months Postpartum: Data from the North Carolina WIC Programme. Public Health Nutr. 2010, 13, 2019–2026. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st Century: Epidemiology, Mechanisms, and Lifelong Effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Yeung, C.H.T.; Fong, S.; Malik, P.R.V.; Edginton, A.N. Quantifying Breast Milk Intake by Term and Preterm Infants for Input into Paediatric Physiologically Based Pharmacokinetic Models. Matern. Child Nutr. 2020, 16, e12938. [Google Scholar] [CrossRef]
- Saha, M.R.; Ryan, K.; Amir, L.H. Postpartum Women’s Use of Medicines and Breastfeeding Practices: A Systematic Review. Int. Breastfeed. J. 2015, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and Pregnancy: Mechanisms of Short Term and Long Term Adverse Consequences for Mother and Child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- Lende, M.; Rijhsinghani, A. Gestational Diabetes: Overview with Emphasis on Medical Management. Int. J. Environ. Res. Public Health 2020, 17, 9573. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.O. Drugs in Lactation. Pharm. Res. 2018, 35, 45. [Google Scholar] [CrossRef]
- Bogaerts, A.; De Baetselier, E.; Ameye, L.; Dilles, T.; Van Rompaey, B.; Devlieger, R. Postpartum Weight Trajectories in Overweight and Lean Women. Midwifery 2017, 49, 134–141. [Google Scholar] [CrossRef]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines; Rasmussen, K.; Yaktine, A. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009; ISBN 9780309131131. [Google Scholar]
- Technical Advisory Group on Gestational Weight Gain (TAG-GWG). Available online: https://www.who.int/groups/technical-advisory-group-on-gestational-weight-gain-(tag-gwg) (accessed on 26 July 2023).
- Bijlholt, M.; Ameye, L.; van Uytsel, H.; Devlieger, R.; Bogaerts, A. Evolution of Postpartum Weight and Body Composition after Excessive Gestational Weight Gain: The Role of Lifestyle Behaviors—Data from the INTER-ACT Control Group. Int. J. Environ. Res. Public Health 2021, 18, 6344. [Google Scholar] [CrossRef]
- Nehring, I.; Schmoll, S.; Beyerlein, A.; Hauner, H.; Von Kries, R. Gestational Weight Gain and Long-Term Postpartum Weight Retention: A Meta-Analysis. Am. J. Clin. Nutr. 2011, 94, 1225–1231. [Google Scholar] [CrossRef]
- McDowell, M.; Cain, M.A.; Brumley, J. Excessive Gestational Weight Gain. J. Midwifery Womens Health 2019, 64, 46–54. [Google Scholar] [CrossRef]
- Versele, V.; Stas, L.; Aerenhouts, D.; Deliens, T.; Clarys, P.; Gucciardo, L.; Bogaerts, A.; Devlieger, R. Changes in Maternal and Paternal Body Composition during the Transition to Parenthood (TRANSPARENTS). Obesity 2022, 31, 225–233. [Google Scholar] [CrossRef]
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational Diabetes Mellitus: An Updated Overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef]
- Rosenbaum, D.L.; Gillen, M.M.; Markey, C.H. The Importance of Sleep and Parity in Understanding Changes in Weight and Breastfeeding Behavior among Postpartum Women. Appetite 2022, 170, 105889. [Google Scholar] [CrossRef] [PubMed]
- Lambrinou, C.P.; Karaglani, E.; Manios, Y. Breastfeeding and Postpartum Weight Loss. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 413–417. [Google Scholar] [CrossRef]
- Antonakou, A.; Papoutsis, D.; Panou, I.; Chiou, A.; Matalas, A.L. Role of Exclusive Breastfeeding in Energy Balance and Weight Loss during the First Six Months Postpartum. Clin. Exp. Obs. Gynecol. 2013, 40, 485–488. [Google Scholar]
- Borschel, M.W.; Phd, R.D.; Kirksey, A.; Hannemann, R.E. Evaluation of Test-Weighing for the Assessment of Milk Volume Intake of Formula-Fed Infants and Its Application to Breast-Fed Infants. Am. J. Clin. Nutr. 1986, 43, 367–373. [Google Scholar] [CrossRef]
- Savenije, O.E.M.; Brand, P.L.P. Accuracy and Precision of Test Weighing to Assess Milk Intake in Newborn Infants. Arch. Dis. Child. -Fetal Neonatal Ed. 2006, 91, F330-2. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Stable Isotope Technique to Assess Intake of Human Milk in Breastfed Infants; IAEA Human Health Series No. 7; IAEA: Vienna, Austria, 2010; pp. 1–66. [Google Scholar]
- Tongchom, W.; Pongcharoen, T.; Judprasong, K.; Udomkesmalee, E.; Kriengsinyos, W.; Winichagoon, P. Human Milk Intake of Thai Breastfed Infants During the First 6 Months Using the Dose-to-Mother Deuterium Dilution Method. Food Nutr. Bull. 2020, 41, 343–354. [Google Scholar] [CrossRef]
- Kushner, D.J.; Baker, A.; Dunstall, T.G. Pharmacological Uses and Perspectives of Heavy Water and Deuterated Compounds. Can. J. Physiol. Pharmacol. 1999, 77, 79–88. [Google Scholar] [CrossRef]
- Anderson, P.O.; Manoguerra, A.S.; Valdés, V. A Review of Adverse Reactions in Infants from Medications in Breastmilk. Clin. Pediatr. 2016, 55, 236–244. [Google Scholar] [CrossRef]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P.E. Volume and Frequency of Breastfeedings and Fat Content of Breast Milk throughout the Day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef]
- Oras, P.; Blomqvist, Y.T.; Nyqvist, K.H.; Gradin, M.; Rubertsson, C.; Hellström-Westas, L.; Funkquist, E.-L. Breastfeeding Patterns in Preterm Infants Born at 28-33 Gestational Weeks. J. Hum. Lact. 2015, 31, 377–385. [Google Scholar] [CrossRef]
- Pressly, M.A.; Schmidt, S.; Guinn, D.; Liu, Z.; Ceresa, C.; Samuels, S.; Madabushi, R.; Florian, J.; Fletcher, E.P. Informing a Comprehensive Risk Assessment of Infant Drug Exposure From Human Milk: Application of a Physiologically Based Pharmacokinetic Lactation Model for Sotalol. J. Clin. Pharmacol. 2023, 63, S106–S116. [Google Scholar] [CrossRef] [PubMed]
- Gourley, G.R.; Arend, R.A. Beta-Glucuronidase and Hyperbilirubinaemia in Breast-Fed and Formula-Fed Babies. Lancet 1986, 1, 644–646. [Google Scholar] [CrossRef]
- Ren, Q.; Zhou, Y.; Zhang, W.; Tian, Y.; Sun, H.; Zhao, X.; Xu, Y.; Jiang, S. Longitudinal Changes in the Bioactive Proteins in Human Milk of the Chinese Population: A Systematic Review. Food Sci. Nutr. 2021, 9, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, M. Enzymes in Human Milk. In Handbook of Milk Composition; Elsevier: Amsterdam, The Netherlands, 1995; pp. 388–427. [Google Scholar]
- Freed, L.M.; Berkow, S.E.; Hamosh, P.; York, C.M.; Mehta, N.R.; Hamosh, M. Lipases in Human Milk: Effect of Gestational Age and Length of Lactation on Enzyme Activity. J. Am. Coll. Nutr. 1989, 8, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human Milk Composition. Nutrients and Bioactive Factors. Pediatr. Clin. North. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Morriss, F.H.; Brewer, E.D.; Spedale, S.B.; Riddle, L.; Temple, D.M.; Caprioli, R.M.; West, M.S. Relationship of Human Milk PH During Course of Lactation to Concentrations of Citrate and Fatty Acids. Pediatrics 1986, 78, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human Breast Milk: A Review on Its Composition and Bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Koletzko, B. Human Milk Lipids. Ann. Nutr. Metab. 2017, 69, 28–40. [Google Scholar] [CrossRef]
- Allegaert, K.; Simons, S.H.P.; Tibboel, D.; Krekels, E.H.; Knibbe, C.A.; van den Anker, J.N. Non-Maturational Covariates for Dynamic Systems Pharmacology Models in Neonates, Infants, and Children: Filling the Gaps beyond Developmental Pharmacology. Eur. J. Pharm. Sci. 2017, 109, S27–S31. [Google Scholar] [CrossRef]
- Anderson, B.J.; Woollard, G.A.; Holford, N.H.G. A Model for Size and Age Changes in the Pharmacokinetics of Paracetamol in Neonates, Infants and Children. Br. J. Clin. Pharmacol. 2000, 50, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.J.; Vajjah, P.; Abduljalil, K.; Jamei, M.; Rostami-Hodjegan, A.; Tucker, G.T.; Johnson, T.N. Does Age Affect Gastric Emptying Time? A Model-Based Meta-Analysis of Data from Premature Neonates through to Adults. Biopharm. Drug Dispos. 2015, 36, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Staelens, S.; Van Den Driessche, M.; Barclay, D.; Carrié-Faessler, A.L.; Haschke, F.; Verbeke, K.; Vandebroek, H.; Allegaert, K.; Van Overmeire, B.; Van Damme, M.; et al. Gastric Emptying in Healthy Newborns Fed an Intact Protein Formula, a Partially and an Extensively Hydrolysed Formula. Clin. Nutr. 2008, 27, 264–268. [Google Scholar] [CrossRef]
- Johnson, T.N.; Bonner, J.J.; Tucker, G.T.; Turner, D.B.; Jamei, M. Development and Applications of a Physiologically-Based Model of Paediatric Oral Drug Absorption. Eur. J. Pharm. Sci. 2018, 115, 57–67. [Google Scholar] [CrossRef]
- Davanzo, R.; Cannioto, Z.; Ronfani, L.; Monasta, L.; Demarini, S. Breastfeeding and Neonatal Weight Loss in Healthy Term Infants. J. Hum. Lact. 2013, 29, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Roggero, P.; Giannì, M.L.; Orsi, A.; Piemontese, P.; Amato, O.; Moioli, C.; Mosca, F. Neonatal Period: Body Composition Changes in Breast-Fed Full-Term Newborns. Neonatology 2010, 97, 139–143. [Google Scholar] [CrossRef]
- Macdonald, P.D. Neonatal Weight Loss in Breast and Formula Fed Infants. Arch. Dis. Child. -Fetal Neonatal Ed. 2003, 88, F472–F476. [Google Scholar] [CrossRef]
- Wilbaux, M.; Kasser, S.; Gromann, J.; Mancino, I.; Coscia, T.; Lapaire, O.; van den Anker, J.N.; Pfister, M.; Wellmann, S. Personalized Weight Change Prediction in the First Week of Life. Clin. Nutr. 2019, 38, 689–696. [Google Scholar] [CrossRef]
- de Onis, M.; Garza, C.; Habicht, J.P. Time for a New Growth Reference. Pediatrics 1997, 100, E8. [Google Scholar] [CrossRef]
- Roggero, P.; Gianni`, M.L.; Gianni`, G.; Orsi, A.; Piemontese, P.; Amato, O.; Liotto, N.; Morlacchi, L.; Taroni, F.; Fields, D.A.; et al. Quality of Growth in Exclusively Breast-Fed Infants in the First Six Months of Life: An Italian Study. Pediatr. Res. 2010, 68, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Giannì, M.L.; Roggero, P.; Orsi, A.; Piemontese, P.; Garbarino, F.; Bracco, B.; Garavaglia, E.; Agosti, M.; Mosca, F. Body Composition Changes in the First 6 Months of Life According to Method of Feeding. J. Hum. Lact. 2014, 30, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Huang, I.F.; Chen, Y.C.; Chen, P.H.; Yang, L.Y. Differences in Serum Biochemistry between Breast-Fed and Formula-Fed Infants. J. Chin. Med. Assoc. 2011, 74, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.H.; Ott, P.; Juul, A.; Skakkebaek, N.E.; Michaelsen, K.F. Does Breast Feeding Influence Liver Biochemistry? J. Pediatr. Gastroenterol. Nutr. 2003, 37, 559–565. [Google Scholar] [CrossRef]
- Blake, M.J.; Abdel-Rahman, S.M.; Pearce, R.E.; Leeder, J.S.; Kearns, G.L. Effect of Diet on the Development of Drug Metabolism by Cytochrome P-450 Enzymes in Healthy Infants. Pediatr. Res. 2006, 60, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Le Guennec, J.-C.; Billon, B. Delay in Caffeine Elimination in Breast-Fed Infants. Pediatrics 1987, 79, 264–268. [Google Scholar] [CrossRef]
- Tsunoda, S.M.; Gonzales, C.; Jarmusch, A.K.; Momper, J.D.; Ma, J.D. Contribution of the Gut Microbiome to Drug Disposition, Pharmacokinetic and Pharmacodynamic Variability. Clin. Pharmacokinet. 2021, 60, 971–984. [Google Scholar] [CrossRef]
- Nauwelaerts, N.; Macente, J.; Deferm, N.; Bonan, R.H.; Huang, M.C.; Van Neste, M.; Bibi, D.; Badee, J.; Martins, F.S.; Smits, A.; et al. Generic Workflow to Predict Medicine Concentrations in Human Milk Using Physiologically-Based Pharmacokinetic (PBPK) Modelling—A Contribution from the ConcePTION Project. Pharmaceutics 2023, 15, 1469. [Google Scholar] [CrossRef]
- Dallmann, A.; Himstedt, A.; Solodenko, J.; Ince, I.; Hempel, G.; Eissing, T. Integration of Physiological Changes during the Postpartum Period into a PBPK Framework and Prediction of Amoxicillin Disposition before and Shortly after Delivery. J. Pharmacokinet. Pharmacodyn. 2020, 47, 341–359. [Google Scholar] [CrossRef]
- Job, K.M.; Dallmann, A.; Parry, S.; Saade, G.; Haas, D.M.; Hughes, B.; Berens, P.; Chen, J.Y.; Fu, C.; Humphrey, K.; et al. Development of a Generic Physiologically-Based Pharmacokinetic Model for Lactation and Prediction of Maternal and Infant Exposure to Ondansetron via Breast Milk. Clin. Pharmacol. Ther. 2022, 111, 1111–1120. [Google Scholar] [CrossRef]
- Ganguly, S.; Edginton, A.N.; Gerhart, J.G.; Cohen-Wolkowiez, M.; Greenberg, R.G.; Gonzalez, D.; Benjamin, D.K.; Hornik, C.; Zimmerman, K.; Kennel, P.; et al. Physiologically Based Pharmacokinetic Modeling of Meropenem in Preterm and Term Infants. Clin. Pharmacokinet. 2021, 60, 1591–1604. [Google Scholar] [CrossRef]
- Haidich, A.B. Meta-Analysis in Medical Research. Hippokratia 2010, 14, 29–37. [Google Scholar]
- Quinney, S.K.; Bies, R.R.; Grannis, S.J.; Bartlett, C.W.; Mendonca, E.; Rogerson, C.M.; Backes, C.H.; Shah, D.K.; Tillman, E.M.; Costantine, M.M.; et al. The MPRINT Hub Data, Model, Knowledge and Research Coordination Center: Bridging the Gap in Maternal–Pediatric Therapeutics Research through Data Integration and Pharmacometrics. Pharmacotherapy 2023, 43, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Peeters, M.Y.; Beleyn, B.; Smits, A.; Kulo, A.; van Calsteren, K.; Deprest, J.; de Hoon, J.; Knibbe, C.A.J. Paracetamol Pharmacokinetics and Metabolism in Young Women. BMC Anesth. 2015, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gockenbach, M.; Grimstein, M.; Sachs, H.C.; Mirochnick, M.; Struble, K.; Belew, Y.; Wang, J.; Capparelli, E.V.; Best, B.M.; et al. Characterization of Plasma Protein Alterations in Pregnant and Postpartum Individuals Living With HIV to Support Physiologically-Based Pharmacokinetic Model Development. Front. Pediatr. 2021, 9, 721059. [Google Scholar] [CrossRef] [PubMed]
- FDA. Clinical Lactation Studies: Considerations for Study Design Guidance for Industry Draft Guidance; FDA: Rockville, MD, USA, 2019; pp. 1–4. [Google Scholar]
- Allegaert, K.; Abbasi, M.Y.; Annaert, P.; Olafuyi, O. Current and Future Physiologically Based Pharmacokinetic (PBPK) Modeling Approaches to Optimize Pharmacotherapy in Preterm Neonates. Expert. Opin. Drug Metab. Toxicol. 2022, 18, 301–312. [Google Scholar] [CrossRef] [PubMed]
| Compound | Postnatal Age (Weeks) | Formula-Fed Infants | Breastfed Infants |
|---|---|---|---|
| 2–3 | <0.01 | <0.01 | |
| (1,7 MX + 1-MX)/caffeine | 4–6 | <0.01 | <0.01 |
| urinary molar ratio | 8–10 | 0.08 | <0.01 |
| 12–15 | 0.48 | 0.04 | |
| 16–20 | 0.89 | 0.14 | |
| 24–30 | 3.44 | 0.46 | |
| 2–3 | 0.25 | 0.16 | |
| 3-HM/dextromethorphan | 4–6 | 0.41 | 0.32 |
| urinary molar ratio | 8–10 | 0.92 | 0.62 |
| 12–15 | 1.22 | 0.73 | |
| 16–20 | 1.01 | 0.94 | |
| 24–30 | 1.21 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Neste, M.; Bogaerts, A.; Nauwelaerts, N.; Macente, J.; Smits, A.; Annaert, P.; Allegaert, K. Challenges Related to Acquisition of Physiological Data for Physiologically Based Pharmacokinetic (PBPK) Models in Postpartum, Lactating Women and Breastfed Infants—A Contribution from the ConcePTION Project. Pharmaceutics 2023, 15, 2618. https://doi.org/10.3390/pharmaceutics15112618
Van Neste M, Bogaerts A, Nauwelaerts N, Macente J, Smits A, Annaert P, Allegaert K. Challenges Related to Acquisition of Physiological Data for Physiologically Based Pharmacokinetic (PBPK) Models in Postpartum, Lactating Women and Breastfed Infants—A Contribution from the ConcePTION Project. Pharmaceutics. 2023; 15(11):2618. https://doi.org/10.3390/pharmaceutics15112618
Chicago/Turabian StyleVan Neste, Martje, Annick Bogaerts, Nina Nauwelaerts, Julia Macente, Anne Smits, Pieter Annaert, and Karel Allegaert. 2023. "Challenges Related to Acquisition of Physiological Data for Physiologically Based Pharmacokinetic (PBPK) Models in Postpartum, Lactating Women and Breastfed Infants—A Contribution from the ConcePTION Project" Pharmaceutics 15, no. 11: 2618. https://doi.org/10.3390/pharmaceutics15112618
APA StyleVan Neste, M., Bogaerts, A., Nauwelaerts, N., Macente, J., Smits, A., Annaert, P., & Allegaert, K. (2023). Challenges Related to Acquisition of Physiological Data for Physiologically Based Pharmacokinetic (PBPK) Models in Postpartum, Lactating Women and Breastfed Infants—A Contribution from the ConcePTION Project. Pharmaceutics, 15(11), 2618. https://doi.org/10.3390/pharmaceutics15112618








