Decreased Penetration Mechanism of Ranitidine Due to Application of Sodium Sulfobutyl Ether-β-Cyclodextrin
Abstract
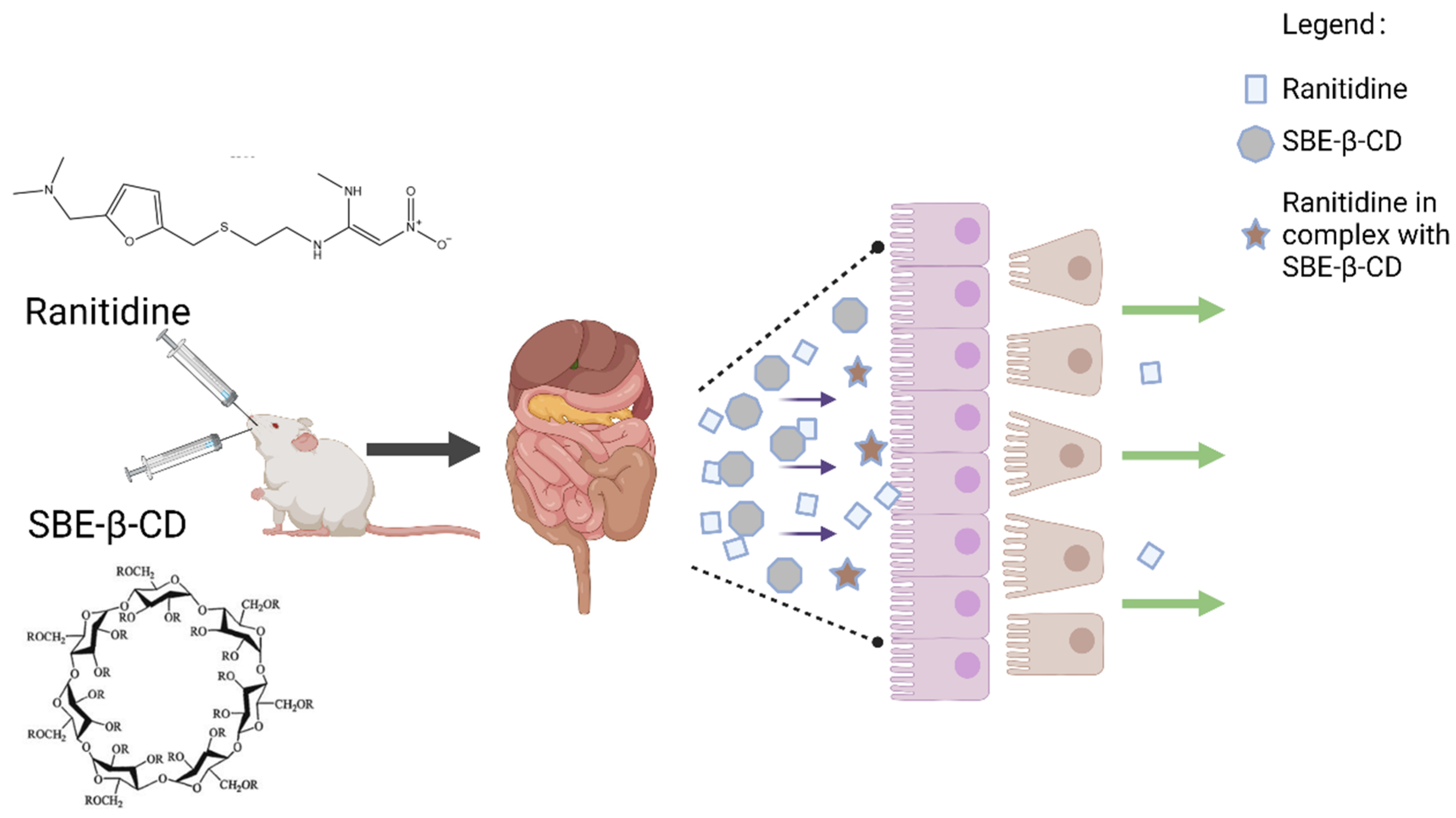
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
2.1.2. Animals
2.2. Methods
2.2.1. Preparation of Working Solutions
- (1)
- Preparation of donor chamber buffers
- (2)
- Preparation of reception chamber buffers
- (3)
- Preparation of working solutions in the donor room
2.2.2. Parallel Artificial Membrane Permeability Assay
- (1)
- Measurement of Ranitidine concentration
- (2)
- Two-way conversion experiment
2.2.3. Caco-2 Cell Experiment
- (1)
- Determination of ranitidine concentration using UPLC/MS/MS
- (2)
- preparation of working solution for caco-2 cell experiment
- (3)
- Caco-2 cell culture
- (4)
- Measurement of transepithelial transport of ranitidine
2.2.4. Pharmacokinetic Study of Ranitidine in Rats
- (1)
- Determination of ranitidine concentration in rat plasma
- (2)
- Pharmacokinetic study of ranitidine in rats
2.2.5. Zeta Potential Determination
3. Results
3.1. In Vitro Analysis of Inhibitory Effect of Ranitidine by SBE-β-CD
3.1.1. Parallel Artificial Membrane Permeability Assay
- (1)
- Measurement of Ranitidine concentration
- (2)
- Measurement of transepithelial transport of ranitidine by Pampa assay
3.1.2. Results of Transepithelial Transport of Ranitidine by Caco-2 Cells
3.2. In Vivo Analysis of Inhibitory Effect of Ranitidine by SBE-β-CD
3.3. Mechanism of Inhibition of Ranitidine Permeability In Vivo by SBE-β-CD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donovan, D.H.O.; De Fusco, C.; Kuhnke, L.; Reichel, A. Trends in Molecular Properties, Bioavailability, and Permeability across the Bayer Compound Collection. J. Med. Chem. 2023, 66, 2347–2360. [Google Scholar] [CrossRef]
- Garg, A.; Garg, R. A Comprehensive Review on Recent Advances and Considerations for the Selection of Cell-based In-vitro Techniques for the Assessment of Permeability of Drug Molecules. Curr. Drug Deliv. 2023, 20, 526–544. [Google Scholar] [CrossRef]
- Jacobsen, A.-C.; Visentin, S.; Butnarasu, C.; Stein, P.C.; di Cagno, M.P. Commercially Available Cell-Free Permeability Tests for Industrial Drug Development: Increased Sustainability through Reduction of In Vivo Studies. Pharmaceutics 2023, 15, 592. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, Z.; Tu, Y.; Hu, W.; Xie, C.; Ye, L. Experimental and computational models to investigate intestinal drug permeability and metabolism. Xenobiotica 2023, 53, 25–45. [Google Scholar] [CrossRef]
- Ruiz-Picazo, A.; Gonzalez-Alvarez, M.; Gonzalez-Alvarez, I.; Bermejo, M. Effect of Common Excipients on Intestinal Drug Absorption in Wistar Rats. Mol. Pharm. 2020, 17, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Picazo, A.; Lozoya-Agullo, I.; González-Álvarez, I.; Bermejo, M.; González-Álvarez, M. Effect of excipients on oral absorption process according to the different gastrointestinal segments. Expert Opin. Drug Deliv. 2020, 18, 1005–1024. [Google Scholar] [CrossRef]
- Takizawa, Y.; Goto, N.; Furuya, T.; Hayashi, M. Influene of Pharmaceutical Excipients on the Membrane Transport of a P-glycoprotein Substrate in the Rat Small Intestine. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Deepanjan, D.; Panchal, D.S.; Venuganti, V.V.K. Transdermal Delivery of Vancomycin Hydrochloride: Influence of Chemical and Physical Permeation Enhancers. Int. J. Pharm. 2021, 609, 120663. [Google Scholar]
- Sadashivaiah, R.; Satheesha Babu, B.K.; Rohith, G. A Comparative Evaluation of Permeation Enhancers for Ropinirole Hydrochloride a Bcs Class Iii Drug to Be Formulated as Transdermal Drug Delivery System. Int. J. Pharm. Sci. Res. 2020, 11, 6149–6156. [Google Scholar]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef]
- Li, S.K.; Chantasart, D. Skin Permeation Enhancement in Aqueous Solution: Correlation with Equilibrium Enhancer Concentration and Octanol/Water Partition Coefficient. J. Pharm. Sci. 2018, 108, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Pawar, B.M.; Rahman, S.N.R.; Pawde, D.M.; Goswami, A.; Shunmugaperumal, T. Orally Administered Drug Solubility-Enhancing Formulations: Lesson Learnt from Optimum Solubility-Permeability Balance. Aaps. Pharmscitech. 2021, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Feitosa, R.; Souza Ribeiro Costa, J.; van Vliet Lima, M.; Sawa Akioka Ishikawa, E.; Cogo Müller, K.; Bonin Okasaki, F.; Sabadini, E.; Garnero, C.; Longhi, M.R.; Lavayen, V.; et al. Supramolecular Arrangement of Doxycycline with Sulfobutylether-β-Cyclodextrin: Impact on Nanostructuration with Chitosan, Drug Degradation and Antimicrobial Potency. Pharmaceutics 2023, 15, 1285. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.E.M.; Almaiman, A.A.; Alshehade, S.A.; Alsalemi, W.; Kamran, S.; Suliman, F.O.; Alshawsh, M.A. Characterization of Thymoquinone-Sulfobutylether-Beta-Cyclodextrin Inclusion Complex for Anticancer Applications. Molecules 2023, 28, 4096. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Zagami, R.; De Plano, L.M.; Burduja, N.; Guglielmino, S.P.P.; Scolaro, L.M.; Mazzaglia, A. Antimicrobial and Antibiofilm Photodynamic Action of Photosensitizing Nanoassemblies Based on Sulfobutylether-β-Cyclodextrin. Molecules 2023, 28, 2493. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cui, H.; Guo, X.; Dong, Q.; You, X.; Guo, X.; Qin, S.; Jia, L. Enantioseparation by Zeolitic Imidazolate Framework-8-Silica Hybrid Monolithic Column with Sulfobutylether-Beta-Cyclodextrin as a Chiral Additive in Capillary Electrochromatography. Microchimica Acta 2023, 190, 315. [Google Scholar] [CrossRef]
- Kovacs, T.; Kurtan, K.; Varga, Z.; Nagy, P.; Panyi, G.; Zakany, F. Veklury® (Remdesivir) Formulations Inhibit Initial Membrane-Coupled Events of Sars-Cov-2 Infection Due to Their Sulfobutylether-Beta-Cyclodextrin Content. Br. J. Pharmacol. 2023, 180, 2064–2084. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Liang, H.; Huang, X.; Meng, N.; Zhou, N. Silver Nanoparticles Based on Sulfobutylether-Beta-Cyclodextrin Functionalized Graphene Oxide Nanocomposite: Synthesized, Characterization, and Antibacterial Activity. Colloids Surf. B-Biointerfaces 2023, 221, 113009. [Google Scholar] [CrossRef]
- Das, S.K.; Kahali, N.; Bose, A.; Khanam, J. Physicochemical Characterization and in Vitro Dissolution Performance of Ibuprofen-Captisol® (Sulfobutylether Sodium Salt of Beta-Cd) Inclusion Complexes. J. Mol. Liq. 2018, 261, 239–249. [Google Scholar] [CrossRef]
- Khurana, R.; Kakatkar, A.S.; Chatterjee, S.; Barooah, N.; Kunwar, A.; Bhasikuttan, A.C.; Mohanty, J. Supramolecular Nanorods of (N-Methylpyridyl) Porphyrin With Captisol: Effective Photosensitizer for Anti-bacterial and Anti-tumor Activities. Front. Chem. 2019, 7, 452. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Kanabar, D.D.; Patel, K.; Ayehunie, S.; Muth, A.; Gupta, V. Enhanced Solubility, Stability, Permeation and Anti-Cancer Efficacy of Celastrol-Β-Cyclodextrin Inclusion Complex. J. Mol. Liq. 2020, 318, 113936. [Google Scholar] [CrossRef]
- Niu, Y.; Zhou, L.; Wang, H.; Dai, J.; Bao, Y.; Hou, B.; Yin, Q. Enhancing the Water Solubility of 9-Fluorenone Using Cyclodextrin Inclusions: A Green Approach for the Environmental Remediation of OPAHs. Crystals 2023, 13, 775. [Google Scholar] [CrossRef]
- Petitprez, J.; Legrand, F.-X.; Tams, C.; Pipkin, J.D.; Antle, V.; Kfoury, M.; Fourmentin, S. Huge Solubility Increase of Poorly Water-Soluble Pharmaceuticals by Sulfobutylether-Beta-Cyclodextrin Complexation in a Low-Melting Mixture. Environ. Chem. Lett. 2022, 20, 1561–1568. [Google Scholar] [CrossRef]
- Szabó, Z.-I.; Gál, R.; Gáll, Z.; Vancea, S.; Rédai, E.; Fülöp, I.; Sipos, E.; Donáth-Nagy, G.; Noszál, B.; Tóth, G. Cyclodextrin complexation improves aqueous solubility of the antiepileptic drug, rufinamide: Solution and solid state characterization of compound-cyclodextrin binary systems. J. Incl. Phenom. Macrocycl. Chem. 2017, 88, 43–52. [Google Scholar] [CrossRef]
- Christaki, S.; Spanidi, E.; Panagiotidou, E.; Athanasopoulou, S.; Kyriakoudi, A.; Mourtzinos, I.; Gardikis, K. Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceuticals 2023, 16, 1274. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Miller, J.M. The Solubility–Permeability Interplay and Its Implications in Formulation Design and Development for Poorly Soluble Drugs. AAPS J. 2012, 14, 244–251. [Google Scholar] [CrossRef]
- Fine-Shamir, N.; Beig, A.; Dahan, A. Adequate Formulation Approach for Oral Chemotherapy: Etoposide Solubility, Permeability, and Overall Bioavailability from Cosolvent- Vs. Vitamin E Tpgs-Based Delivery Systems. Int. J. Pharm. 2021, 597, 120295. [Google Scholar] [CrossRef]
- Porat, D.; Dahan, A. Active intestinal drug absorption and the solubility-permeability interplay. Int. J. Pharm. 2018, 537, 84–93. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Biedrzycki, G.; Wolszczak-Biedrzycka, B.; Dorf, J.; Michalak, D.; Żendzian-Piotrowska, M.; Zalewska, A.; Maciejczyk, M. Antioxidant and Anti-Glycation Potential of H2 Receptor Antagonists—In Vitro Studies and a Systematic Literature Review. Pharmaceuticals 2023, 16, 1273. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Q.; Zhang, Y.; Ding, J.; Yang, M.; Yang, L.; Wang, W.; Cui, P.; Dai, Z.; Ma, L. Preparation and Evaluation of Liposomes Containing Ethanol and Propylene Glycol as Carriers for Nicotine. Curr. Drug Deliv. 2024, 21, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Metry, M.; Polli, J.E. Evaluation of Excipient Risk in BCS Class I and III Biowaivers. AAPS J. 2022, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sinha, V.R.; Dahiya, L.; Singh, G.; Sarwal, A. Impact of cyclodextrin derivatives on systemic release of duloxetine HCl via buccal route. Drug Dev. Ind. Pharm. 2020, 46, 931–945. [Google Scholar] [CrossRef] [PubMed]




| Papp (A-B, cm/s) | Papp (A-B, Mean ± SD, n = 4) | |
|---|---|---|
| A-B | 2.1760 × 10−5 | (2.39 ± 0.267) × 10−5 |
| 2.8514 × 10−5 | ||
| 2.2886 × 10−5 | ||
| 2.2605 × 10−5 | ||
| B-A | 2.60281 × 10−5 | (2.60 ± 0.152) × 10−5 |
| 2.4513 × 10−5 | ||
| 2.84091 × 10−5 | ||
| 2.48918 × 10−5 | ||
| 0.12% | 1.33189 × 10−5 | (1.19 ± 0.143) × 10−5 |
| 1.20058 × 10−5 | ||
| 1.13492 × 10−5 | ||
| 1.07864 × 10−5 | ||
| 0.36% | 8.16017 × 10−6 | (6.87 ± 1.30) × 10−6 |
| 4.87734 × 10−6 | ||
| 6.56566 × 10−6 | ||
| 7.87879 × 10−6 | ||
| 3.6% | 7.50361 × 10−6 | (6.42 ± 1.15) × 10−6 |
| 4.97114 × 10−6 | ||
| 5.62771 × 10−6 | ||
| 7.59740 × 10−6 |
| PK Parameters | Unit | Ranitidine Only | Ranitidine and 0.12% SBE-β-CD | Ranitidine and 0.36% SBE-β-CD | Ranitidine and 3.6% SBE-β-CD |
|---|---|---|---|---|---|
| Tmax | h | 0.83 ± 0.29 | 1.17 ± 0.76 | 1.33 ± 0.58 | 1.50 ± 0.87 |
| Cmax | μg/mL | 0.73 ± 0.18 | 0.064 ± 0.009 * | 0.30 ± 0.05 * | 0.13 ± 0.007 * |
| t1/2 | h | 6 ± 6 | 5 ± 2 | 1 ± 0 | 2 ± 1 |
| AUCall | h·μg/mL | 3.6 ± 1.2 | 0.3 ± 0.1 * | 1.3 ± 0.2 | 0.5 ± 0.1 * |
| AUC_Extrap | % | 27 ± 30 | 31 ± 19 | 4 ± 3 | 18 ± 12 |
| Vz | L/kg | 19.8 ± 10.8 | 191 ± 17 *** | 20.5 ± 8.8 | 82.0 ± 24.6 * |
| CL | L/h/kg | 3.07 ± 1.84 | 35.4 ± 20.5 | 10.3 ± 1.9 * | 27.2 ± 12.4 |
| AUMClast | h·h·μg/mL | 14.1 ± 5.5 | 1.0 ± 0.4 | 3.6 ± 0.5 | 1.1 ± 0.4 * |
| MRTlast | h | 4 ± 1 | 3 ± 0 | 3 ± 0 | 3 ± 0 |
| Zeta Potential (mV) | p-Value of Levene’s Test for Equality of Variances | p-Value of T Test for Equality of Means | |
|---|---|---|---|
| Ranitidine | −21.40 ± 6.90 | / | / |
| 0.06% SBE-β-CD | −5.09 ± 2.02 | 0.5988 | 0.0057 ** |
| 0.06% SBE-β-CD + Ranitidine | −16.60 ± 3.09 | ||
| 0.12% SBE-β-CD | −3.66 ± 2.12 | 0.6235 | 0.0029 ** |
| 0.12% SBE-β-CD + Ranitidine | −17.90 ± 3.15 | ||
| 0.24% SBE-β-CD | −0.52 ± −0.52 | 0.1210 | 0.0021 ** |
| 0.24% SBE-β-CD + Ranitidine | −16.6 ± −16.6 | ||
| 0.36% SBE-β-CD | −7.02 ± 5.29 | 0.8090 | 0.1108 |
| 0.36% SBE-β-CD + Ranitidine | −15.10 ± 4.36 | ||
| 0.72% SBE-β-CD | −2.50 ± 0.53 | 0.0076 | 0.0203 |
| 0.72% SBE-β-CD + Ranitidine | −21.00 ± 8.58 | ||
| 1.44% SBE-β-CD | −5.20 ± 3.55 | 0.9211 | 0.2148 |
| 1.44% SBE-β-CD + Ranitidine | −9.31 ± 3.28 | ||
| 3.6% SBE-β-CD | −4.36 ± 1.41 | 0.3447 | 0.0504 |
| 3.6% SBE-β-CD + Ranitidine | −9.79 ± 3.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Zhang, J.; Huang, J.; Wang, X.; Yang, H.; Jin, Q. Decreased Penetration Mechanism of Ranitidine Due to Application of Sodium Sulfobutyl Ether-β-Cyclodextrin. Pharmaceutics 2023, 15, 2593. https://doi.org/10.3390/pharmaceutics15112593
Yang R, Zhang J, Huang J, Wang X, Yang H, Jin Q. Decreased Penetration Mechanism of Ranitidine Due to Application of Sodium Sulfobutyl Ether-β-Cyclodextrin. Pharmaceutics. 2023; 15(11):2593. https://doi.org/10.3390/pharmaceutics15112593
Chicago/Turabian StyleYang, Rui, Jing Zhang, Jiaqi Huang, Xiaofeng Wang, Huiying Yang, and Qingri Jin. 2023. "Decreased Penetration Mechanism of Ranitidine Due to Application of Sodium Sulfobutyl Ether-β-Cyclodextrin" Pharmaceutics 15, no. 11: 2593. https://doi.org/10.3390/pharmaceutics15112593
APA StyleYang, R., Zhang, J., Huang, J., Wang, X., Yang, H., & Jin, Q. (2023). Decreased Penetration Mechanism of Ranitidine Due to Application of Sodium Sulfobutyl Ether-β-Cyclodextrin. Pharmaceutics, 15(11), 2593. https://doi.org/10.3390/pharmaceutics15112593





