Lipid Nanoparticles Loading Steroidal Alkaloids of Tomatoes Affect Neuroblastoma Cell Viability in an In Vitro Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. α-TM-SLN and TD-SLN Preparation
2.3. α-TM-SLN and TD-SLN Characterization
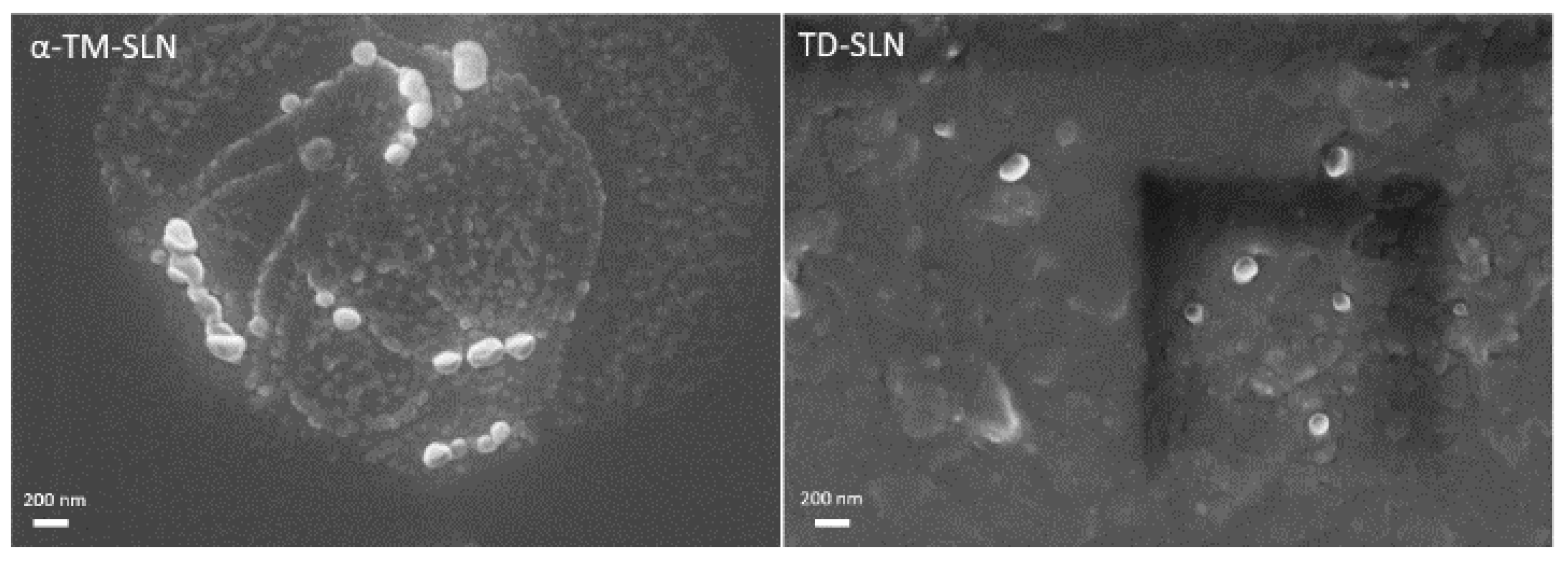
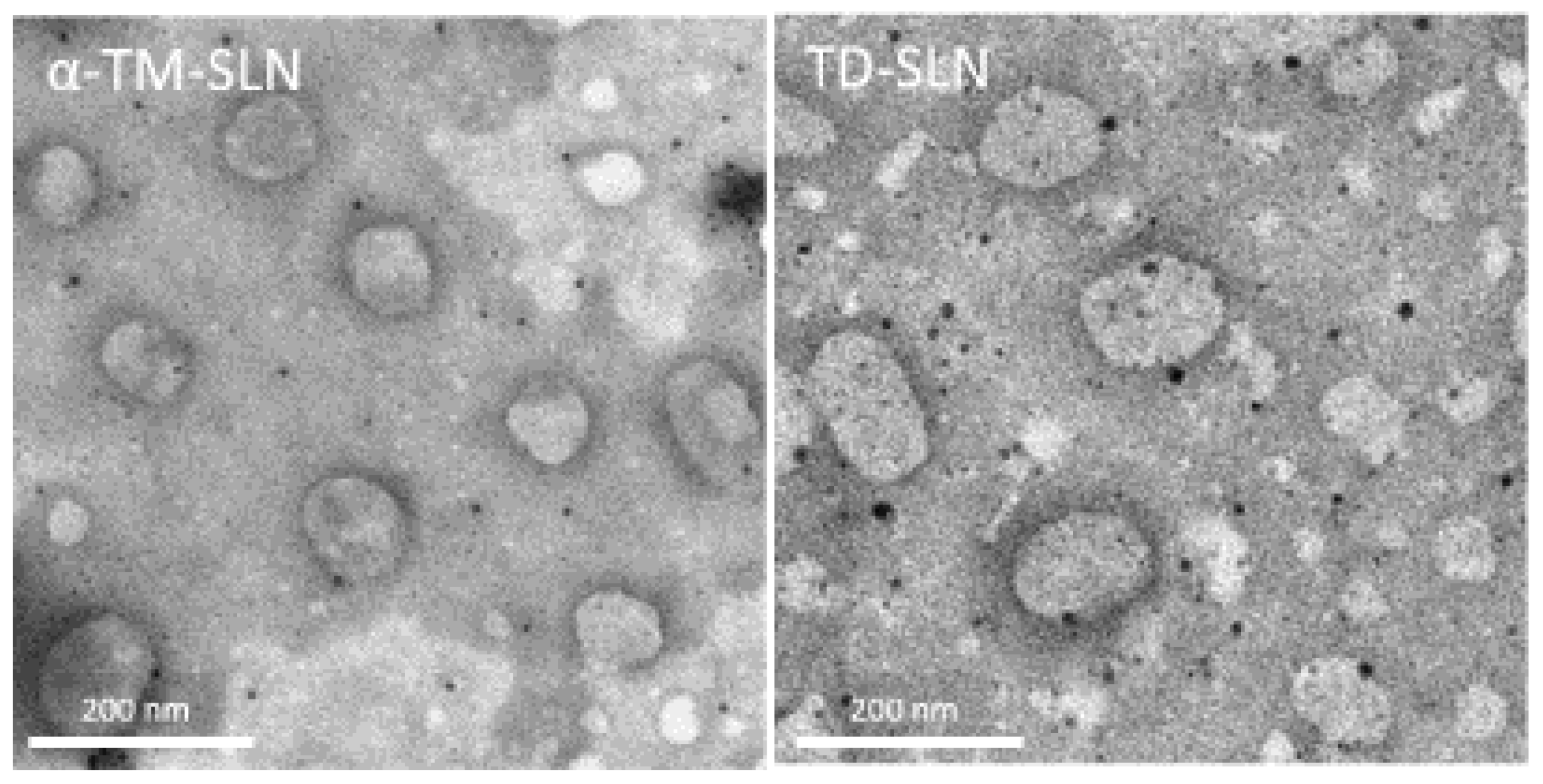
2.4. α-TM-SLN and TD-SLN Morphology
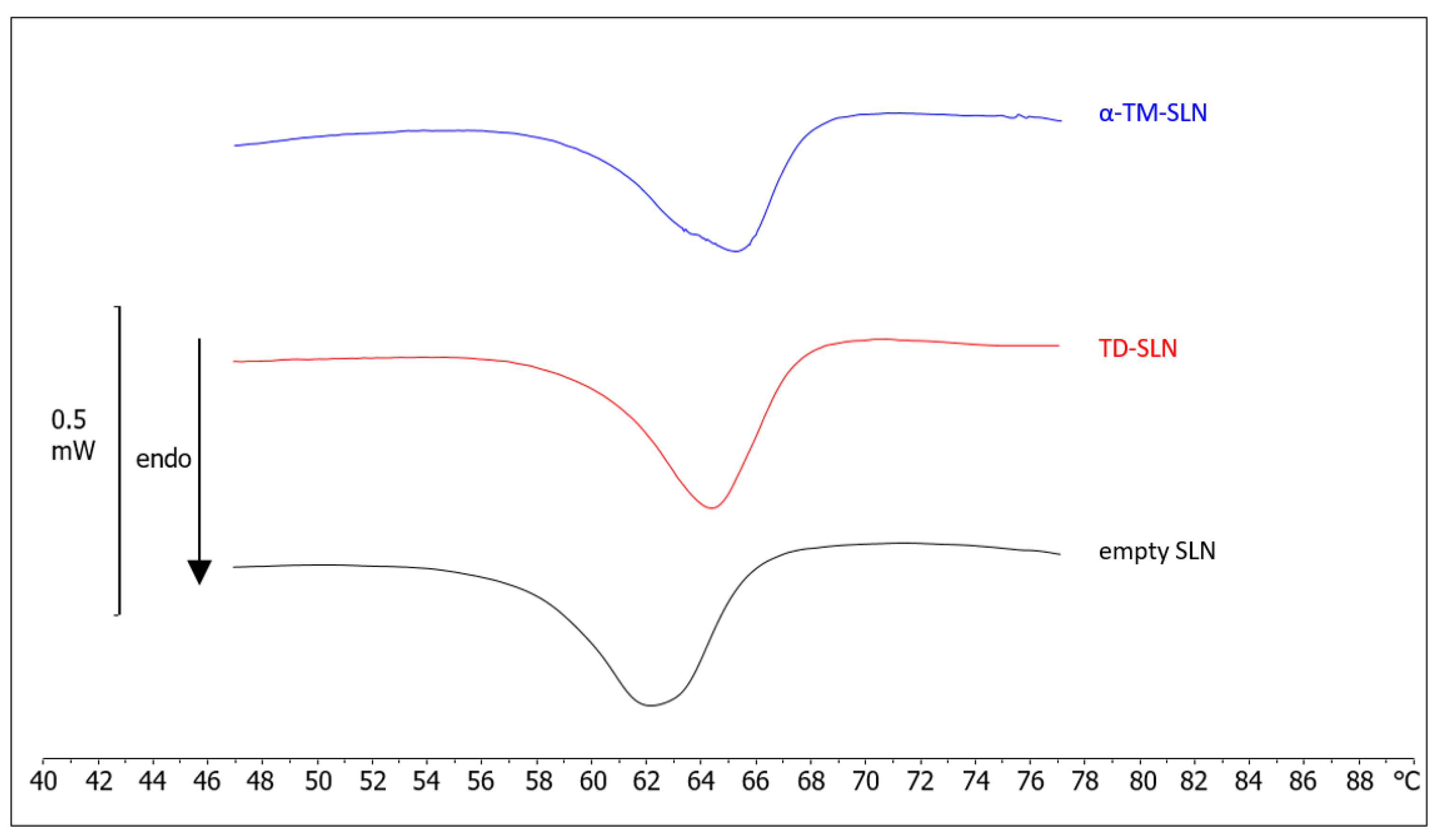
2.5. Differential Scanning Calorimetry (DSC)
- Heating from 25 °C to 85 °C, heating rate: 2 °C/min.
- Cooling from 85 °C to 25 °C, cooling rate: 4 °C/min.
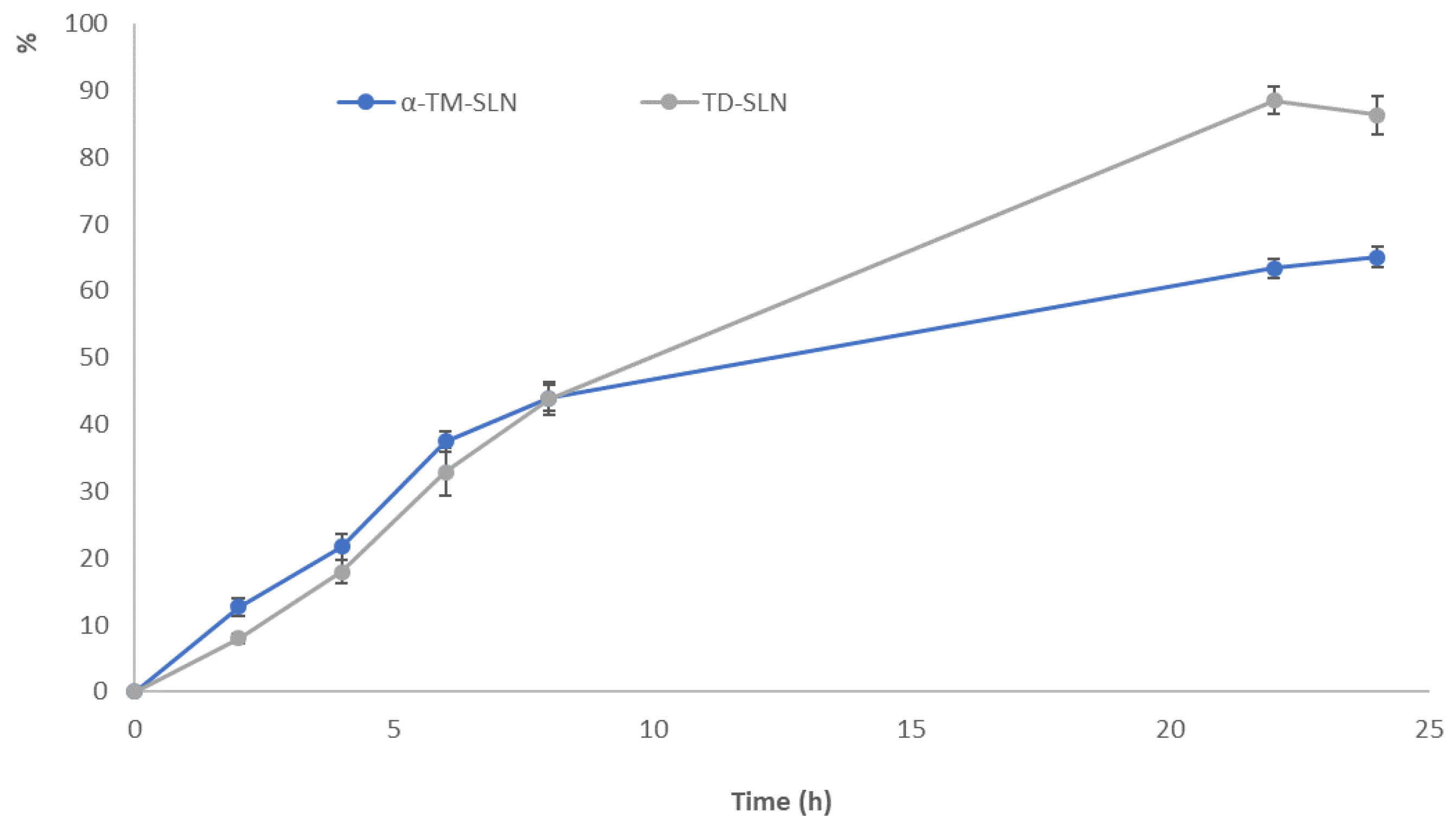
2.6. In Vitro Release Study
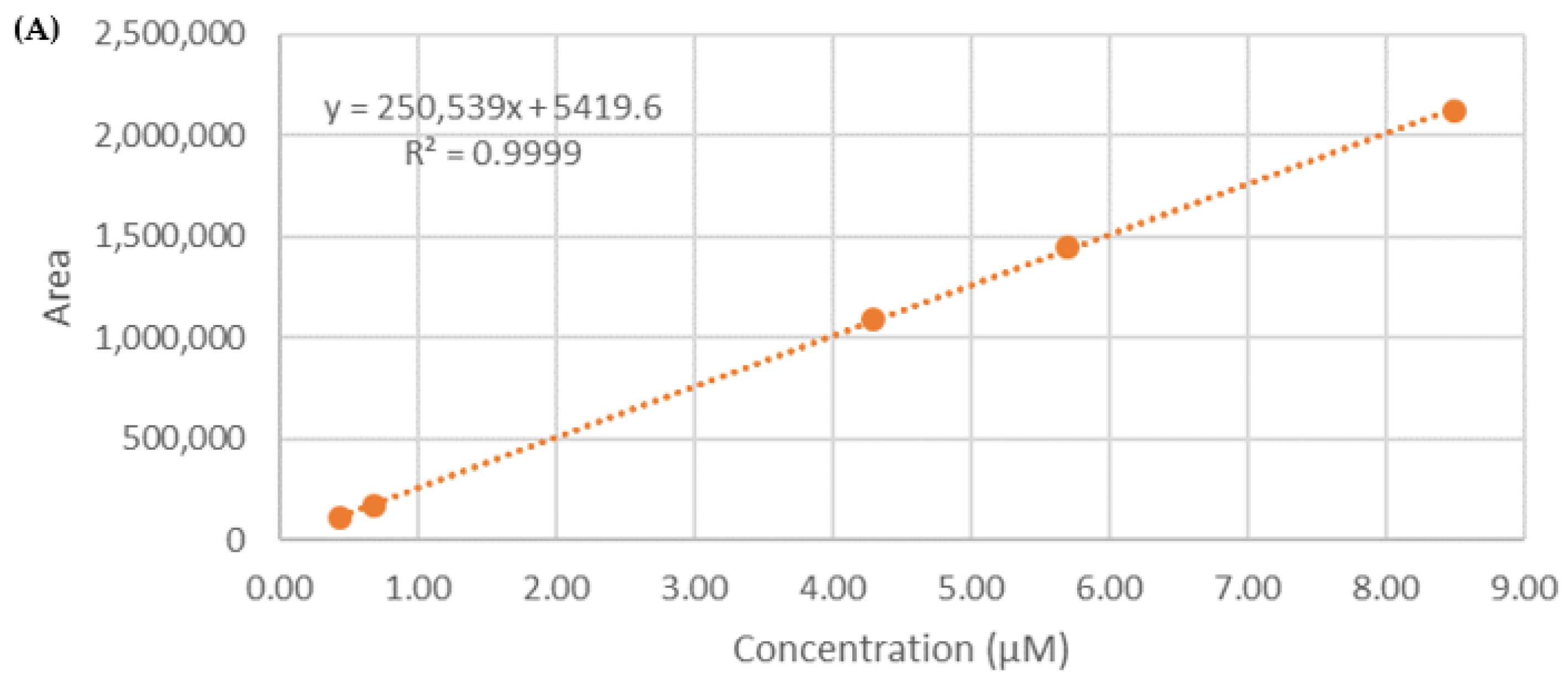
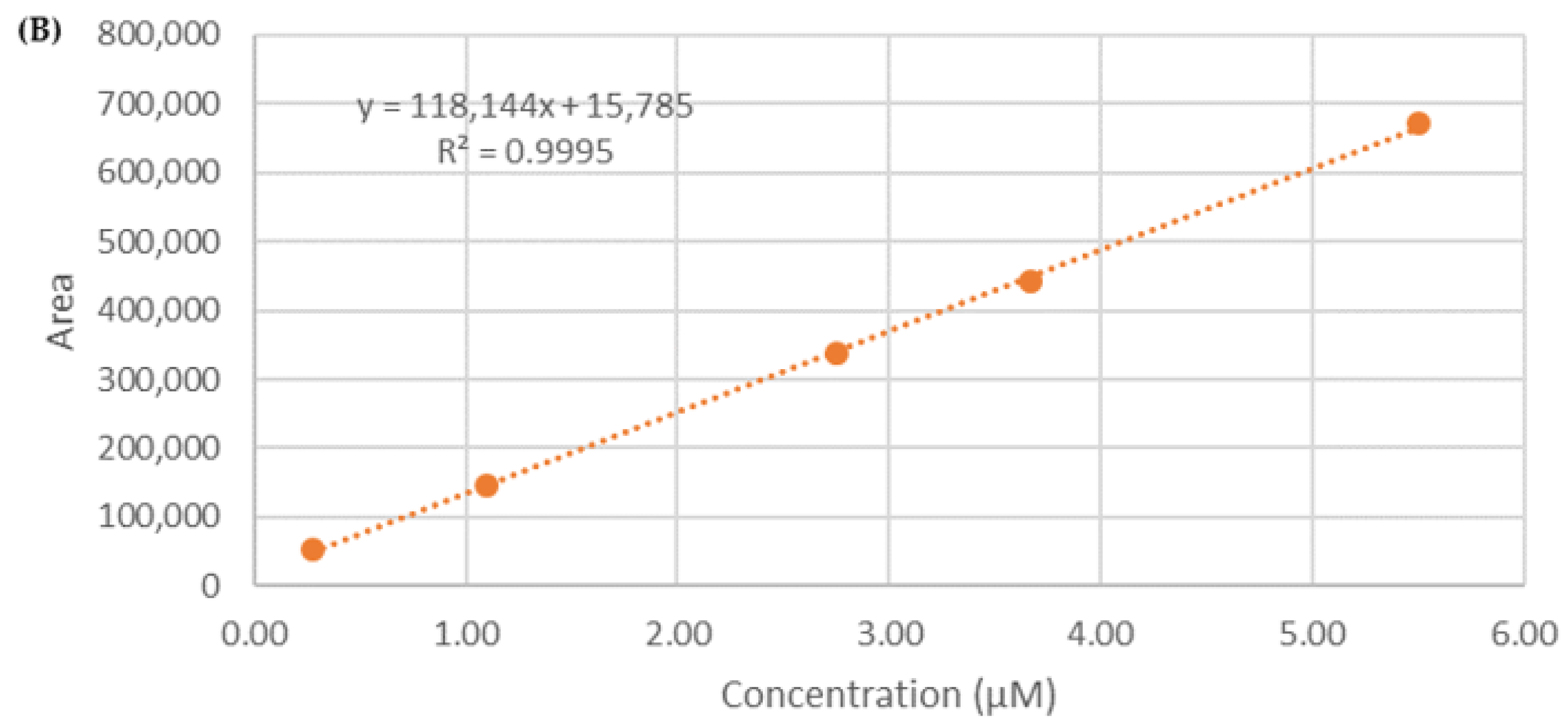
2.7. Calibration Curve
2.8. Encapsulation Efficiency (EE%)
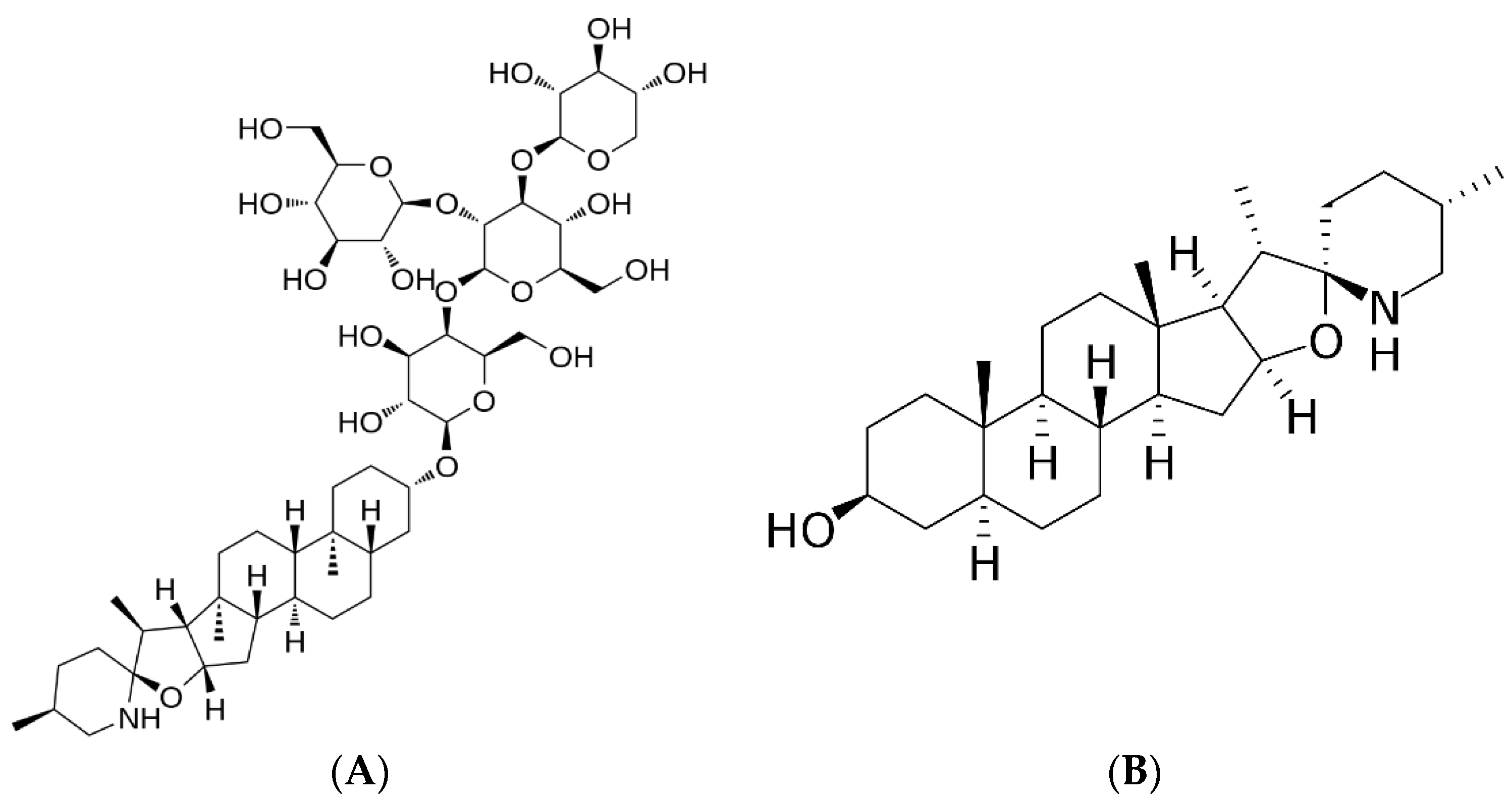
2.8.1. Mass Spectrometric Analysis for Quantification of α-TM and TD in SLN Formulations
2.8.2. Determination of Entrapment Efficiency (EE)
2.9. In Vitro Study on α-TM-SLN and TD-SLN
2.9.1. Primary OEC Cultures
2.9.2. SH-SY5Y Cell Line Cultures
2.9.3. Treatment of Cells
2.9.4. MTT Assay
2.10. Statistical Analysis
3. Results
3.1. Characterization of α-TM-SLN and TD-SLN
3.2. Morphology of α-TM-SLN and TD-SLN
3.3. Differential Scanning Calorimetry (DSC)
3.4. Determination of Entrapment Efficiency (EE%)
3.5. In Vitro Release Study
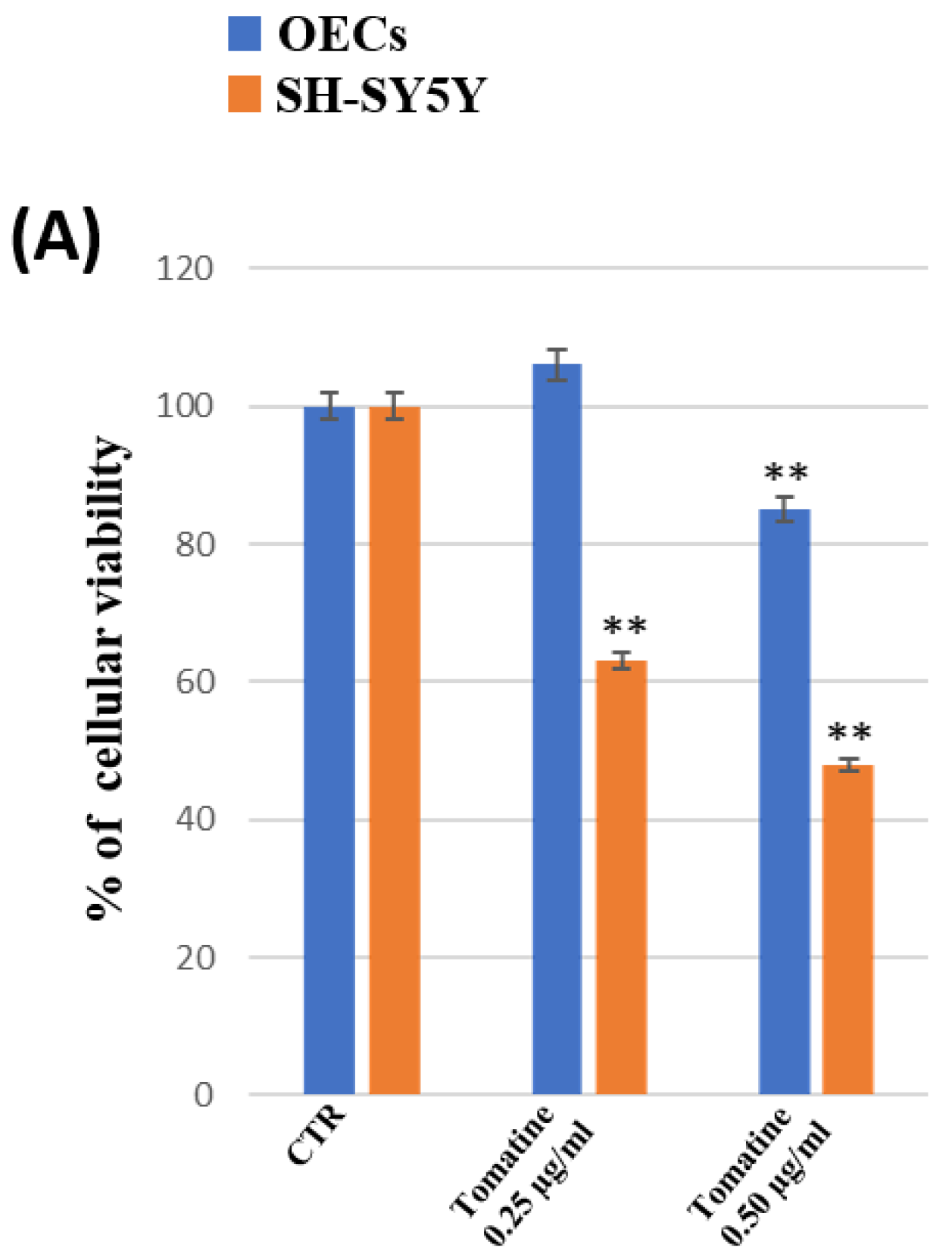
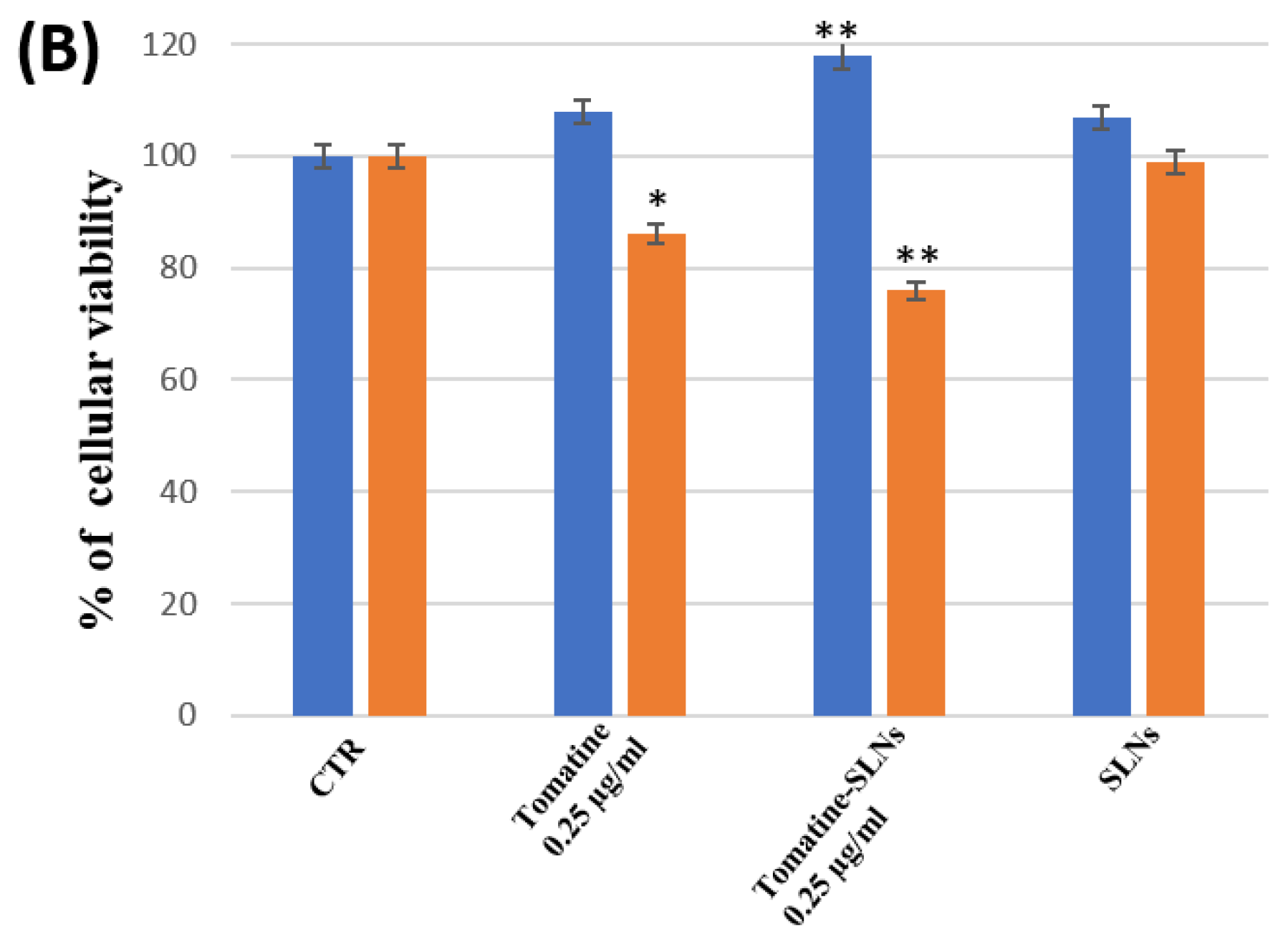
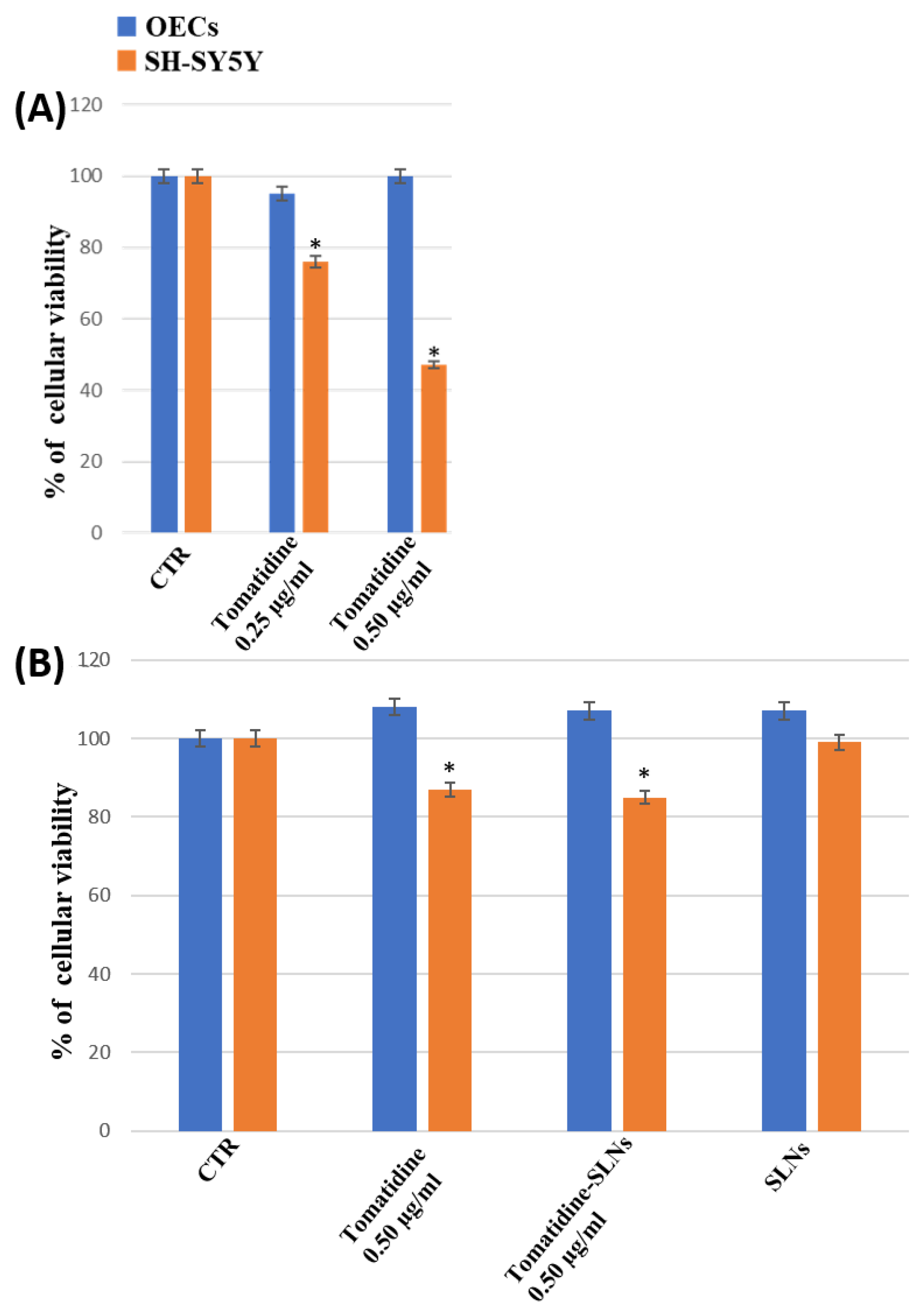
3.6. Percentage of Cell Viability of OEC and SH-SY5Y Cultures
4. Discussion
Limitation Section
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alsaffar, A.A. Sustainable diets: The interaction between food industry, nutrition, health and the environment. Food Sci. Technol. Int. 2016, 22, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Antón, J.M.; Rubio-Andrada, L.; Celemín-Pedroche, M.S.; Ruíz-Peñalver, S.M. From the circular economy to the sustainable development goals in the European Union: An empirical comparison. Int. Environ. Agreem. 2022, 22, 67–95. [Google Scholar] [CrossRef]
- Albizzati, P.F.; Tonini, D.; Astrup, T.F. A Quantitative Sustainability Assessment of Food Waste Management in the European Union. Environ. Sci. Technol. 2021, 55, 16099–16109. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Chang, S.K.; Bolling, B.; Oh, W.Y.; Shahidi, F. Specialty seeds: Nutrients, bioactives, bioavailability, and health benefits: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2382–2427. [Google Scholar] [CrossRef] [PubMed]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Santonocito, D.; Musumeci, T.; Cardile, V.; Graziano, A.C.E.; Salerno, L.; Raciti, G.; Crascì, L.; Panico, A.M.; Puglisi, G. Nanotechnological Approach to Increase the Antioxidant and Cytotoxic Efficacy of Crocin and Crocetin. Planta Med. 2019, 85, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz Jde, J.; López-Mata, M.A.; DelToro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, Flavonoid, Tomatine, and Tomatidine Contents and Antioxidant and Antimicrobial Activities of Extracts of Tomato Plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The steroidal alkaloids α-tomatine and tomatidine: Panorama of their mode of action and pharmacological properties. Steroids 2021, 176, 108933. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.M.; Ramalingam, S. Antioxidant potentials of skin, pulp, and seed fractions of commercially important tomato cultivars. Food Sci. Biotechnol. 2011, 20, 15–21. [Google Scholar] [CrossRef]
- Chiu, F.L.; Lin, J.K. Tomatidine inhibits iNOS and COX-2 through suppression of NF-kappaB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008, 582, 2407–2412. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, J.B.; Kozukue, N.; Kim, H.J.; Nishitani, Y.; Zhang, L.; Mizuno, M.; Levin, C.E.; Friedman, M. Structure-activity relationships of α-, β(1)-, γ-, and δ-tomatine and tomatidine against human breast (MDA-MB-231), gastric (KATO-III), and prostate (PC3) cancer cells. J. Agric. Food Chem. 2012, 60, 3891–3899. [Google Scholar] [CrossRef] [PubMed]
- Guay, I.; Boulanger, S.; Isabelle, C.; Brouillette, E.; Chagnon, F.; Bouarab, K.; Marsault, E.; Malouin, F. Tomatidine and analog FC04-100 possess bactericidal activities against Listeria, Bacillus and Staphylococcus spp. BMC Pharmacol. Toxicol. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Wong, P.F.; He, H.; Hooper, J.D.; Mustafa, M.R. Alpha-tomatine attenuation of in vivo growth of subcutaneous and orthotopic xenograft tumors of human prostate carcinoma PC-3 cells is accompanied by inactivation of nuclear factor-kappa B signaling. PLoS ONE 2013, 8, e57708. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Picerno, P.; Mencherini, T.; Villecco, F.; D’Ursi, A.M.; Aquino, R.P.; Lauro, M.R. Flavonoid microparticles by spray-drying: Influence of enhancers of the dissolution rate on properties and stability. J. Food Eng. 2011, 103, 188–196. [Google Scholar] [CrossRef]
- Santonocito, D.; Vivero-Lopez, M.; Lauro, M.R.; Torrisi, C.; Castelli, F.; Sarpietro, M.G.; Puglia, C. Design of Nanotechnological Carriers for Ocular Delivery of Mangiferin: Preformulation Study. Molecules 2022, 27, 1328. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Romero, C.A.; Montoya-Inzunza, L.A.; Contreras-Angulo, L.A.; Heredia, J.B.; Gutiérrez-Grijalva, E.P. Solanum Fruits: Phytochemicals, Bioaccessibility and Bioavailability, and Their Relationship with Their Health-Promoting Effects. Front. Nutr. 2021, 8, 790582. [Google Scholar] [CrossRef] [PubMed]
- Liparulo, A.; Esposito, R.; Santonocito, D.; Muñoz-Ramírez, A.; Spaziano, G.; Bruno, F.; Xiao, J.; Puglia, C.; Filosa, R.; Berrino, L.; et al. Formulation and Characterization of Solid Lipid Nanoparticles Loading RF22-c, a Potent and Selective 5-LO Inhibitor, in a Monocrotaline-Induced Model of Pulmonary Hypertension. Front. Pharmacol. 2020, 11, 83. [Google Scholar] [CrossRef]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Khare, T.; Palakurthi, S.S.; Shah, B.M.; Palakurthi, S.; Khare, S. Natural Product-Based Nanomedicine in Treatment of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2020, 21, 3956. [Google Scholar] [CrossRef]
- Urbán-Morlán, Z.; Ganem-Rondero, A.; Melgoza-Contreras, L.M.; Escobar-Chávez, J.J.; Nava-Arzaluz, M.G.; Quintanar-Guerrero, D. Preparation and characterization of solid lipid nanoparticles containing cyclosporine by the emulsification-diffusion method. Int. J. Nanomed. 2010, 5, 611–620. [Google Scholar]
- Kawashima, Y.; Niwa, T.; Handa, T.; Takeuchi, H.; Iwamoto, T.; Itoh, Y. Preparation of prolonged-release spherical micro-matrix of ibuprofen with acrylic polymer by the emulsion-solvent diffusion method for improving bioavailability. Chem. Pharm. Bull. 1989, 37, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Shaikh, F.; Gupta, A.; Tripathi, P.; Yadav, J.S. A Recent Update: Solid Lipid Nanoparticles for Effective Drug Delivery. Adv. Pharm. Bull. 2022, 12, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Torrisi, C.; Cardullo, N.; Muccilli, V.; La Mantia, A.; Castelli, F.; Acquaviva, R.; Sarpietro, M.G. Ethyl Protocatechuate Encapsulation in Solid Lipid Nanoparticles: Assessment of Pharmacotechnical Parameters and Preliminary In Vitro Evaluation for Colorectal Cancer Treatment. Pharmaceutics 2023, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.S.; Woodhouse, A.; Fung, S.; Dickson, T.C.; West, A.H.; Vickers, J.C.; Chuah, M.I. Olfactory ensheathing cells promote neurite sprouting of injured axons in vitro by direct cellular contact and secretion of soluble factors. CellMol Life Sci. 2004, 61, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Pellitteri, R.; Catania, M.V.; Bonaccorso, C.M.; Ranno, E.; Dell’Albani, P.; Zaccheo, D. Viability of olfactory ensheathing cells after hypoxia and serum deprivation: Implication for therapeutic transplantation. J. Neurosci. Res. 2014, 92, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Chuah, M.I.; Au, C. Cultures of Ensheathing Cells from Neonatal Rat Olfactory Bulbs. Brain Res. 1993, 601, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Twarużek, M.; Zastempowska, E.; Soszczyńska, E.; Ałtyn, I. The use of in vitro assays for the assessment of cytotoxicity on the example of MTT test. Acta Univ. Lodz. Folia Biol. Oecologica 2018, 14, 23–32. [Google Scholar] [CrossRef]
- Campisi, A.; Sposito, G.; Grasso, R.; Bisicchia, J.; Spatuzza, M.; Raciti, G.; Scordino, A.; Pellitteri, R. Effect of Astaxanthin on Tissue Transglutaminase and Cytoskeletal Protein Expression in Amyloid-Beta Stressed Olfactory Ensheathing Cells: Molecular and Delayed Luminescence Studies. Antioxidants 2023, 12, 750. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Pons, R.; Carbone, C.; Fernandez-Busquets, X.; Cardia, M.C.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Physico-chemical characterization of succinyl chitosan-stabilized liposomes for the oralco-delivery of quercetin and resveratrol. Carbohydr. Polym. 2017, 157, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, S.; Zheng, Q.; Feng, X.; Tan, W.; Feng, K.; Liu, Y.; Hu, W. Preparation of Solid Lipid Nanoparticles of Cinnamaldehyde and Determination of Sustained Release Capacity. Nanomaterials 2022, 12, 4460. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Kaffka, S.; Mitchell, A.E. A long-term comparison of the influence of organic and conventional crop management practices on the content of the glycoalkaloid α-tomatine in tomatoes. J. Sci. Food Agric. 2013, 93, 1537–1542. [Google Scholar] [CrossRef]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.C.; Srivastava, P.; Pandey, P.; Khan, W.; Panda, B.P. Nose to brain delivery of astaxanthin loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016, 6, 10001–10010. [Google Scholar] [CrossRef]
- Chacko, A.; Newton, A.M.J. Synthesis and Characterization of Valacyclovir HCl Hybrid Solid Lipid Nanoparticles by Using Natural Oils. Recent Pat. Drug Deliv. Formul. 2019, 13, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chen, H.H. Entrapment and release of saquinavir using novel cationic solid lipid nanoparticles. Int. J. Pharm. 2009, 365, 206–213. [Google Scholar] [CrossRef]
- Bunjes, H.; Unruh, T.S. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef]
- Mazidi, M.; Ferns, G.A.; Banach, M. A high consumption of tomato and lycopene is associated with a lower risk of cancer mortality: Results from a multi-ethnic cohort. Public Health Nutr. 2020, 23, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Levin, C.E.; Lee, S.U.; Kim, H.J.; Lee, I.S.; Byun, J.O.; Kozukue, N. Tomatine-containing green tomato extracts inhibit growth of human breast, colon, liver, and stomach cancer cells. J. Agric. Food Chem. 2009, 57, 5727–5733. [Google Scholar] [CrossRef] [PubMed]
- Franssen, E.H.; de Bree, F.M.; Verhaagen, J. Olfactory ensheathing glia: Their contribution to primary olfactory nervous system regeneration and their regenerative potential following transplantation into the injured spinal cord. Brain Res. Rev. 2007, 56, 236–258. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal Delivery to the Central Nervous System: Mechanisms and Experimental Considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Taveira, M.; Sousa, C.; Valentão, P.; Ferreres, F.; Teixeira, J.P.; Andrade, P.B. Neuroprotective effect of steroidal alkaloids on glutamate-induced toxicity by preserving mitochondrial membrane potential and reducing oxidative stress. J. Steroid Biochem. Mol. Biol. 2014, 140, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Nepal, B.; Bhattarai, J.K.; Dhami, K.B.; Nichols, M.R.; Stine, K.J. Effect of mesoporous silica nanoparticles loaded with α-tomatine on HepG2 cancer cells studied in vitro. J. Drug Deliv. Sci. Technol. 2023, 79, 104033. [Google Scholar] [CrossRef]
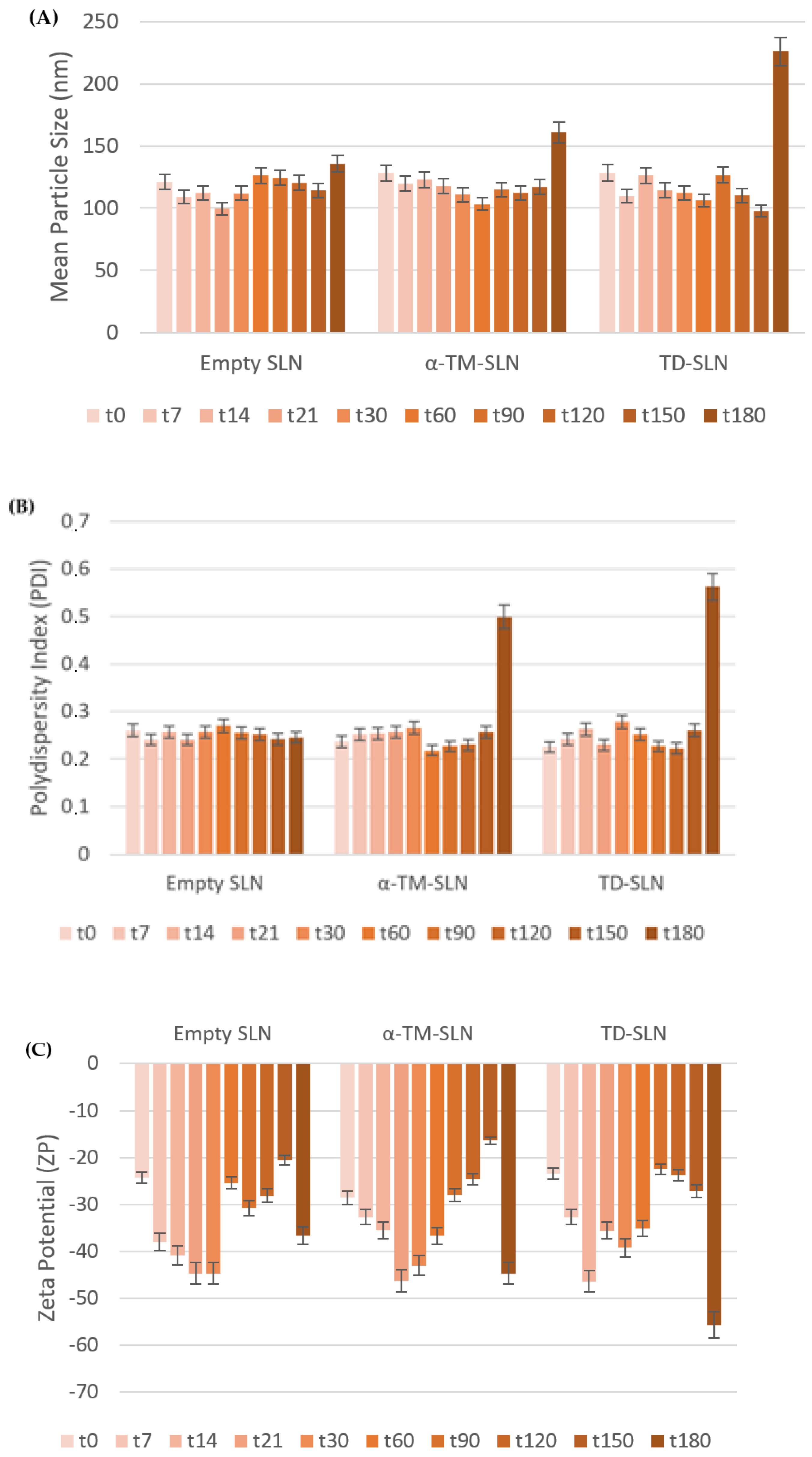
| Sample | Z-Average (nm) | PDI | ZP (mV) |
|---|---|---|---|
| Empty SLN | 121.2 ± 0.31 | 0.261 ± 0.02 | −24.3 ± 0.4 |
| α-TM-SLN | 128.2 ± 0.26 | 0.237 ± 0.12 | −28.6 ± 0.9 |
| TD-SLN | 128.4 ± 0.7 | 0.225 ± 0.44 | −23.5 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santonocito, D.; Campisi, A.; Pellitteri, R.; Sposito, G.; Basilicata, M.G.; Aquino, G.; Pepe, G.; Sarpietro, M.G.; Pittalà, M.G.G.; Schoubben, A.; et al. Lipid Nanoparticles Loading Steroidal Alkaloids of Tomatoes Affect Neuroblastoma Cell Viability in an In Vitro Model. Pharmaceutics 2023, 15, 2573. https://doi.org/10.3390/pharmaceutics15112573
Santonocito D, Campisi A, Pellitteri R, Sposito G, Basilicata MG, Aquino G, Pepe G, Sarpietro MG, Pittalà MGG, Schoubben A, et al. Lipid Nanoparticles Loading Steroidal Alkaloids of Tomatoes Affect Neuroblastoma Cell Viability in an In Vitro Model. Pharmaceutics. 2023; 15(11):2573. https://doi.org/10.3390/pharmaceutics15112573
Chicago/Turabian StyleSantonocito, Debora, Agatina Campisi, Rosalia Pellitteri, Giovanni Sposito, Manuela Giovanna Basilicata, Giovanna Aquino, Giacomo Pepe, Maria Grazia Sarpietro, Maria Gaetana Giovanna Pittalà, Aurelie Schoubben, and et al. 2023. "Lipid Nanoparticles Loading Steroidal Alkaloids of Tomatoes Affect Neuroblastoma Cell Viability in an In Vitro Model" Pharmaceutics 15, no. 11: 2573. https://doi.org/10.3390/pharmaceutics15112573
APA StyleSantonocito, D., Campisi, A., Pellitteri, R., Sposito, G., Basilicata, M. G., Aquino, G., Pepe, G., Sarpietro, M. G., Pittalà, M. G. G., Schoubben, A., Pignatello, R., & Puglia, C. (2023). Lipid Nanoparticles Loading Steroidal Alkaloids of Tomatoes Affect Neuroblastoma Cell Viability in an In Vitro Model. Pharmaceutics, 15(11), 2573. https://doi.org/10.3390/pharmaceutics15112573










