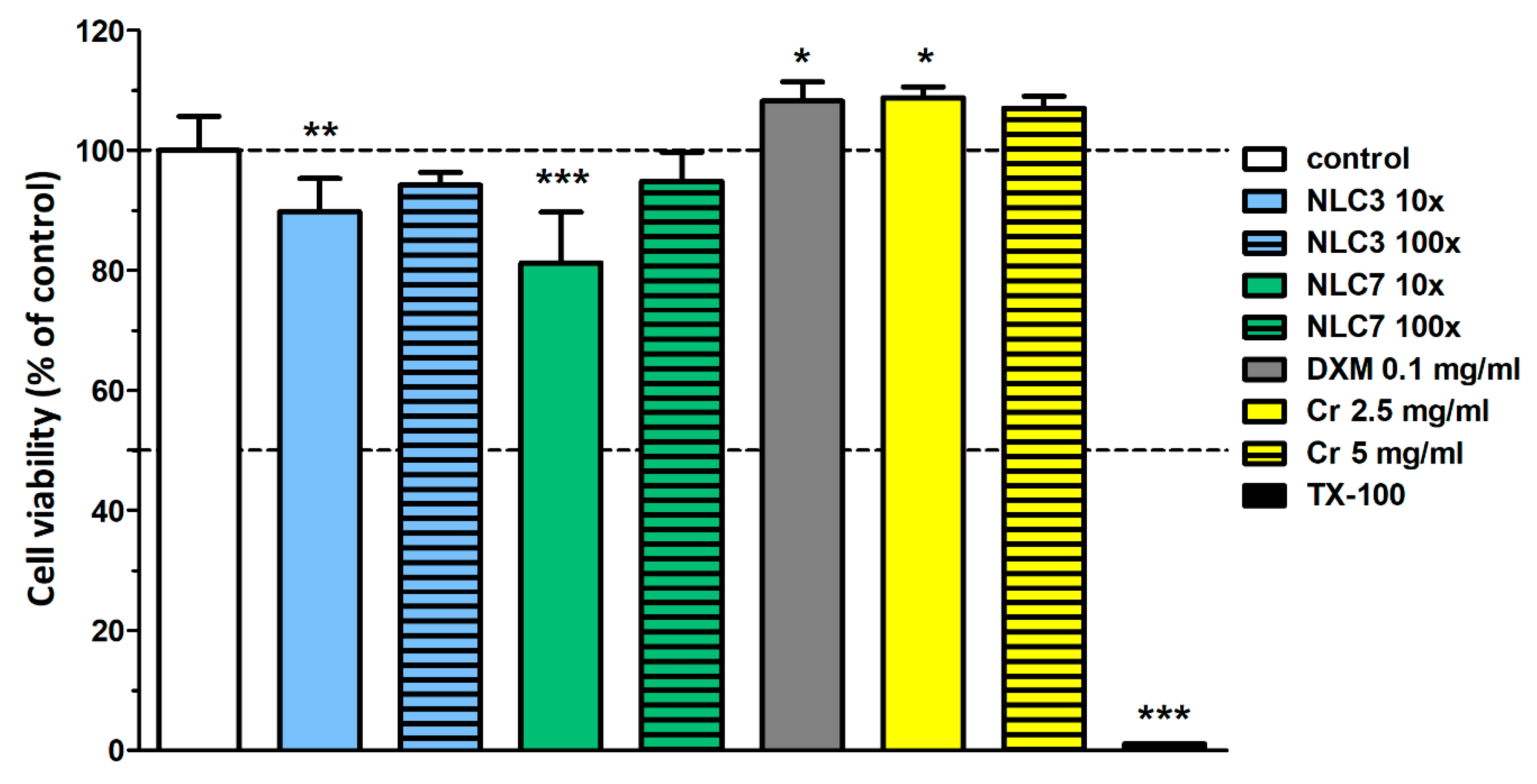
3.1. Lipid Screening
NLCs contain solid lipid, liquid lipid, and surfactant in an aqueous medium. The EE% of lipid nanoparticles can be improved by reducing the crystallinity of solid lipids. The liquid lipid decreases the crystallinity index of solid lipids in blends, and thus the crystallinity of lipid nanoparticles. The application of surfactants can also decrease the crystallinity index of lipid particles and help to form a nano emulsion system during the formulation, and their presence at the water–lipid interface can ensure the stability of nanoparticles. It is important to note that the surfactant can cause irritation and toxicity, especially on the eye. Therefore, in the case of NLC systems, a lower surfactant concentration may be sufficient. In our work, the selection criteria of the components were a low irritation potential, eye tolerability, and suitability for NLC formulation based on the literature background. Tayel et al. (2013) examined a Cremophor EL-containing formulation on in vivo rabbit eyes and it provoked no histopathological alteration in the investigated tissues, including the cornea, iris, retina, and sclera [
17].
The lipid screening focused on three potential critical factors that may have an effect on the performance of NLCs. The first was to find lipid compositions that are able to solve the applied dose of DXM. In the first part of the solubility study, a cost-effective method of visual observation was used to detect insoluble DXM crystals in the different combinations of lipid melts. In the second part of this study, the solubility of DXM in the solidified lipids was analyzed with XRD measurements. The second critical factor is the crystallinity of the applied lipids because their crystallinity index (CI%) may have an effect on the drug loading capacity of NLCs. This factor was investigated with DSC measurements. The third critical factor is the ability of the lipid–surfactant blends to form an NLC formulation, therefore blank (drug-free) test NLCs (T-NLCs) were formulated and analyzed by laser diffraction.
3.1.1. Visual Observations of DXM Solubility
In this part of the solubility study, a fast screening method of the visual observation of different combinations of lipid melts, including solid lipids, liquid lipids alone, and their mixtures with and without surfactant (
Table 4), was applied. When insoluble particles were observed in the thin layer of the melted lipid, it was considered as not solved.
The active pharmaceutical ingredient (API) could not be dissolved in the chosen lipids with the exception Labrasol, which was able to dissolve DXM in both concentrations. In the Compritol and Kolliphor RH60 mixtures, the API in a low concentration (0.3 w/w%) could be dissolved completely while the dissolution of a high concentration (0.7 w/w%) of DXM was partial. The same tendency was seen in the case of the Kolliphor EL and Compritol 888 ATO mixture. In the Apifil and surfactant mixtures, the API was dissolved in both concentrations. In this solubility study, there was no difference between the two solid lipids (Compritol 888 ATO, Apifil). Compared to Miglyol, Labrasol was much more effective at dissolving API. In the case of solid lipid (Compritol 888 ATO), oils (Miglyol, Labrasol), and surfactant (Cremophor RH60, Kolliphor EL)-containing mixtures, DXM was dissolved in almost all cases, except for Sol-21 and Sol-22, where the mixtures were made with Apifil, Miglyol, and Cremophor RH60(Sol-21)/Kolliphor EL(Sol-22).
3.1.2. XRD Analysis of Lipid Mixtures
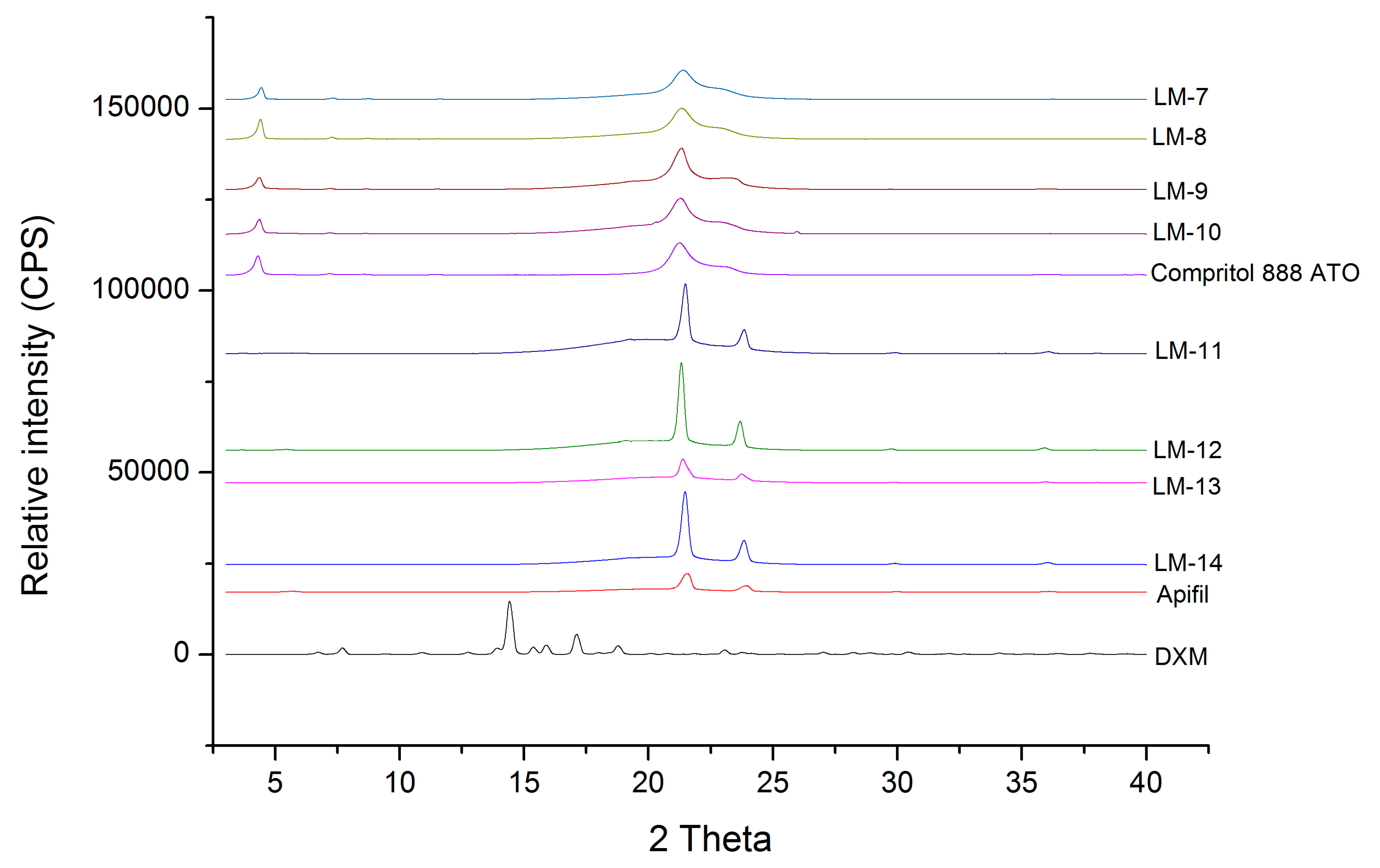
The visual observation revealed that the lipid mixtures containing solid, liquid lipids, and surfactant were able to solve the required DXM amounts. XRD is a technique used to investigate the solid state of a drug, giving information about its amorphous or crystalline nature. The disappearance of the crystalline peaks mainly indicates the amorphous form of the drug within the lipid matrix and/or its molecularly dispersion in the lipid. In this section, 7:3 and 9:1 ratios of the solid and liquid lipids were evaluated.
During the XRD measurements, the presence of the diffraction peaks of crystalline DXM was investigated. The X-ray diffraction pattern of dexamethasone showed major peaks at 2θ = 6.1, 9.1, 10.9, 12.86, 14.66, 15.4, and 17.02 in our measurements, similar to the investigation by Ali et al. (2013) [
18]. The fact that the characteristic peaks of crystalline DXM are not detectable can prove its amorphous form and/or molecular dispersion in all the lipid matrices and it can be concluded that the ratio of the solid and liquid lipids did not influence it (
Figure 1).
On the other hand, the characteristic peaks of the solid lipids (the characteristics peaks of Apifil are 2θ = 21.51, 39.32, 36.06, 5.67 and the main peaks of Compritol 888 ATO are 2θ = 4.27, 21.25, 22.97) can be found in the lipid mixtures, indicating the presence of some crystalline solid lipid in all cases. The amount of crystalline solid lipid is detailed with the DSC measurements in
Section 3.1.3.
3.1.3. Investigation of Lipid Crystallinity with DSC Measurements
Lipid crystallization plays a very important role in the performance of NLC carriers because it has a great influence on the drug loading capacity of lipid particles and the drug release from them.
It must be considered that the application of liquid lipids/oily components can decrease the melting point of the lipid nanoparticle, which can result in easy diffusion of the API from the nanocarriers. From this point of view, the melting behavior of the lipid mixture can be an important factor because it can predict the permanence of the amount of the incorporated drug in the nanocarriers during storage and can have an influence on the evaluation of the drug effect.
As it was mentioned, a strategy to decrease crystallinity is the application of liquid lipids. In our work, two oil and solid lipid ratios (7:3 and 9:1) were evaluated. On the other hand, emulsifiers can also have an effect on the crystallinity, so two emulsifier types (Cremophor RH60 and Kolliphor EL) in the same concentration were also investigated in this section.
Based on the results, it can be seen that Cremophor RH60 decreased the melting point of the lipid mixture in the case of the 7:3 solid lipid:oil ratio (samples LM-3 and LM-7). In the case of Kolliphor EL (LM-4,5) and the 9:1 solid lipid:oil ratio, this decrease in the melting point was not experienced.
The two emulsifiers (Cremophor RH60 and Kolliphor EL) reduced the crystallinity index, but there was no remarkable difference between them (
Table 5). The higher liquid lipid concentration resulted in the most relevant CI% depression. The presence of the API (samples LM 7–10) can also change the CI%, but just in the case of the 7:3 solid lipid:oil ratio. In this latter case, the slight increase in CI% (LM-7: 23.26 vs. LM-3: 20.95, and LM-8: 23.11 vs. LM-4: 18.33) could be observed.
Thanks to the favorable CI% depression effect of the 7:3 solid lipid:oil ratio (with minimal melting point depression), this ratio was applied for further optimization.
3.1.4. Investigation of NLC Formulations
In order to clarify the ability of the chosen lipid mixtures to form a nanosystem, drug-free test NLCs (T-NLCs) were made. To characterize the NLC systems, the following factors were measured using the laser diffraction method: d(0.1), d(0.5), d(0.9), and span values.
On the basis of Equation (2), the lower span value (at about 1 or less) indicates a more monodispersed distribution, thus better stability for NLC systems. The span value was high for T-NLCs 1, 5, 6, and 11. The T-NLCs made with Apifil (T-NLCs 1, 2, 5, 6, and 11) had higher span values than Compritol 888 ATO-containing NLCs (
Table 6). The mixtures containing Labrasol as liquid lipid did not form NLC systems (T-NLCs 10–16); the only exception was T-NLC 11, where nanosized particles could be detected but the span value was very high (2.776) (
Table 6). As a consequence, Labrasol as an oil component was excluded from further measurements.
The lowest span value was found for T-NLC 3, which was made with Compritol 888 ATO, Miglyol, and Cremophor RH60. So, components of T-NLC 3, such as Compritol 888 ATO, Miglyol 812N, and Cremophor RH60, were chosen for further measurements and formulation optimization.
3.2. Optimization of DXM-Loaded NLCs with Factorial Experimental Design
During the lipid screening, we could conclude that the optimal components for NLC formulation are Compritol 888 ATO, Miglyol 812N, and Cremophor RH60 with a 7:3 solid lipid:liquid lipid ratio. To evaluate the effect of the component concentration and find the optimal ratio, a factorial experimental design was used. With the application of a 23 full factorial design, over the optimal ratio, information about the interactions of factors can also be provided. Three formulation parameters of DXM-NLC were chosen as independent factors (the concentration of the emulsifier, the drug, and the total lipid). As for the optimization parameters (dependent factors), the particle size (Zave), zeta potential (ZP), polydispersity index (PDI), and entrapment efficacy (EE%) of the NLC systems were chosen.
As it was mentioned above, the emulsifier is essential for the formulation of NLC systems, but it can be the main irritating and toxic component of the formulations. For this reason, an effort should be made to minimize the amount of surfactant. In the factorial design, the two levels of emulsifier concentration were 5% and 2.5%. As the high lipid concentration can make it possible to solve more API, 10% and 15% total lipid contents were evaluated with 0.05% and 0.1% total DXM concentrations during our experiments.
EE% provides information about the amount of DXM, which can be incorporated into the lipid matrix of the NLC.
ZP is a key indicator of the stability of colloid dispersions. The magnitude of ZP indicates the degree of electrostatic repulsion between adjacent, similarly charged particles in the dispersion. For molecules and particles that are small enough, the high absolute value of ZP provides stability, and these dispersions have moderate aggregation. If the absolute value of ZP is low, the attractive forces may exceed this barrier and the dispersion may break and flocculate. Thus, colloids with a high ZP (negative or positive) are electrically stabilized while colloids with low ZP tend to coagulate or flocculate [
19,
20]. In the case of an electrostatic and steric stabilized system, about ZP ± 20 mV is advised [
7].
PDI is used to estimate the average uniformity of a dispersed solution, and larger PDI values correspond to a larger size distribution in the dispersed sample. PDI can also indicate nanoparticle aggregation and the stability of the dispersed system. A sample is considered monodispersed when the PDI value is less than 0.1. The low PDI (0.1–0.25) shows a narrow size distribution [
7] while PDI over 0.5 indicates a wide size distribution, resulting in more polydispersity [
21,
22].
As indicators of the stability of NLCs, the optimization parameters were measured after preparation (after 24 h), and also after 1 month (except for EE%) (
Table 7). On the basis of the results, the particle size of the NLC systems was less than 300 nm and the PDIs were less than 0.2, which means a narrow size distribution in all cases. The smallest particles and the narrowest size distributions were detected in the case of NLCs 5 and 7, where the total lipid concentration was low (10
w/
w%) and the surfactant concentration was high (5
w/
w%). The ZP values changed between 8 and 15, which corresponds to the literature data, where the ZP of NLC prepared with the Cremophor-type emulsifier was about −12 mV [
23]. These values, the low particle size, and the narrow size distribution can help to retain the stability of the nano systems. The EE% of the nano lipids was very high, over 87%, indicating the good drug loading capacity of the nano lipid carriers. EE% was better in the NLCs containing lower surfactant concentrations (NLCs 1–4), which may be explained by the appearance of the redundant surfactant in the aqueous phase, which can dissolve DXM in the aqueous phase in a higher amount.
When the samples were measured after 1 month, in two compositions (NLCs 4 and 6), the aggregation of the nano lipid particles resulted in the formation of a lipid cake on the top of the container, therefore none of the tested parameters could be measured in these cases. In the case of the homogenous samples, it could be observed that the PDI values remained within the narrow size. The absolute ZP values (if it was applicable) increased with storage time (after 1 month), which means the aqueous–lipid interface changed and became more stable after 1 month.
A mathematical model was used to analyze the single and combined effect of the factors. The coefficients in the equations describe the size and direction (negative: Inversely proportional; or positive: Directly proportional) of the relationship between a term in the model and the response variable [
24]. The mathematical model is shown in the following equations (Equations (5)–(8)) of the response surfaces:
where factor A is the lipid concentration, B is the DXM concentration, and C is the surfactant concentration.
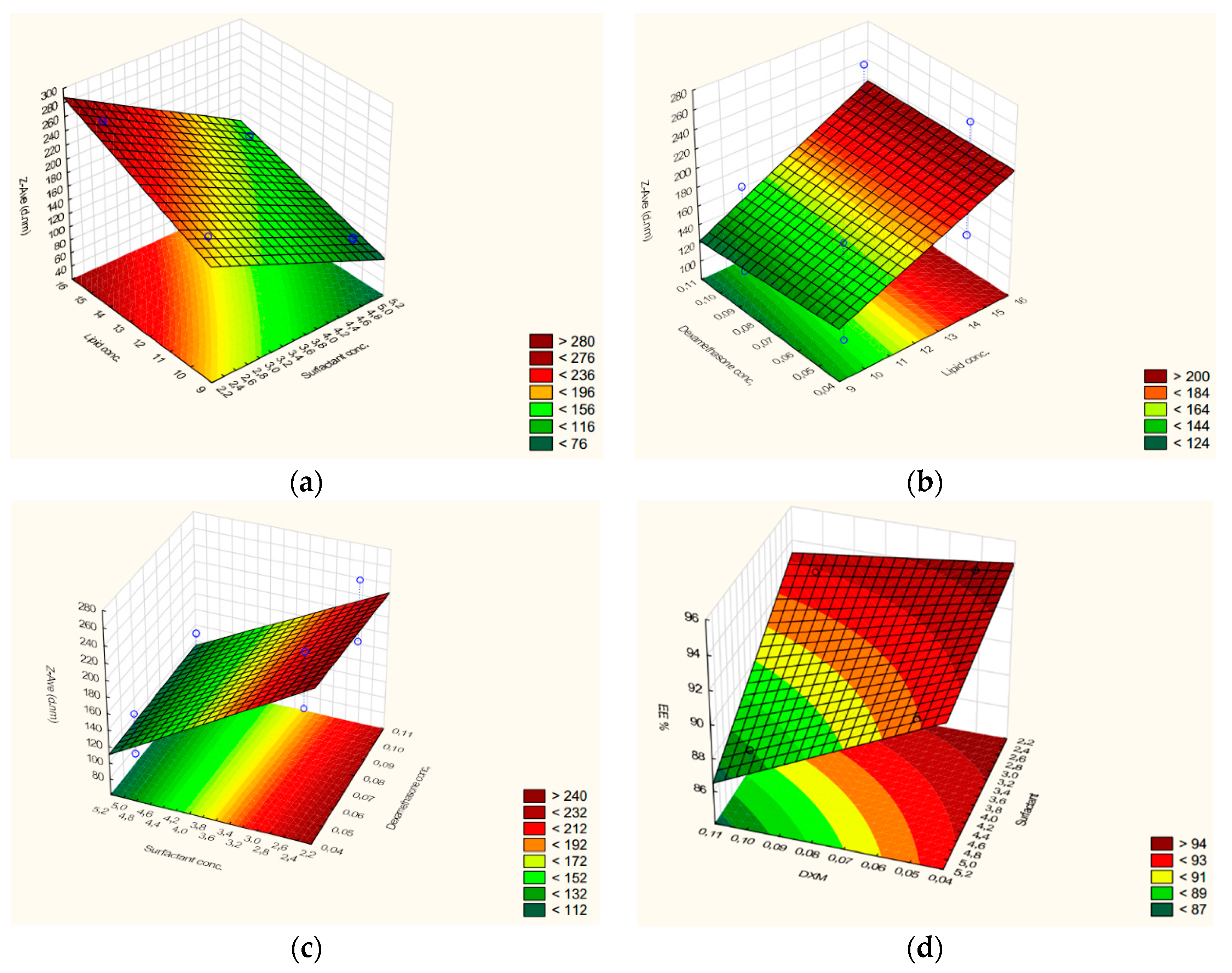
The results suggest that EE% depends on DXM and the surfactant concentration (inversely proportional) as well (
Figure 2d). The combined effect of factors is also inversely related to EE%. On the basis of the coefficient value, the changes of entrapment efficiency are significant (higher coefficient values) with the DXM concentration and surfactant concentration while the combined effect of the factors is not remarkable (lower coefficient values). The mathematical model demonstrated that the lipid content does not influence EE% (very low coefficient value), so in this total lipid concentration range (10–15%), the drug loading capacity of NLCs cannot improve by increasing this concentration.
Z
ave is also affected by lipid (directly proportional), DXM, and surfactant concentration (inversely proportional) (
Figure 2a–c), but the amount of surfactant showed the most remarkable impact. The combined effect of factors is less expressed as it was observed in the case of EE%, too. The increase of the surfactant amount can be beneficial for particle size reduction, but as we could conclude from Equation (4), it can deteriorate the drug loading capacity (lower EE% values).
Concerning PDI, very low coefficient values were calculated. In this case, the preparation technique and/or type of composition may have a more relevant influence on it, but these parameters were constant in our current experiments.
ZP is influenced by DXM, surfactant (directly related), and lipid concentration (inversely related). As it was concluded in the case of EE% and Zave, the combined effect of factors does not have a considerable effect on it. The most remarkable effect can be observed in the case of the surfactant, which means the increase in the surfactant concentration can improve the stability of NLCs (higher absolute ZP value), but higher values were found to reduce EE%.
As a conclusion, the surfactant concentration has the most remarkable effect on the stability parameter (Zave and ZP) of NLCs; an increased amount can be favorable, but we must bear in mind that its higher concentration can result in a lower drug loading capacity and potential irritation and toxicity.
3.5. Porcine Corneal Penetration Study of NLCs
Topically applied ophthalmic formulations are faced with a number of elimination mechanisms and barriers that reduce the bioavailability of the active ingredients. After instillation, tear production slightly increases in order to wash away the foreign material from the surface of the eye. Following this, through the nasolacrimal sac, the material washed out can enter into the systemic circulation, increasing the risk of potential side effects. In addition, a part of the active agent can also be eliminated via the highly vascularized conjunctiva, further reducing the possibility of its penetration into the deeper ocular tissue layers [
27]. The main absorption way of the topically applied drugs is through the cornea, which has a remarkable barrier function. The cornea consists of three main layers: The epithelium (next to the conjunctiva), the stroma (the middle layer), and the endothelium (the inner layer). The epithelium and the endothelium are lipid-rich layers that limit the absorption of hydrophilic drugs while the stroma as an aqueous layer limits the permeation of lipophilic substances. In addition, both the conjunctiva and the corneal epithelium have tight junctions that limit the absorption of substances (regardless of their physicochemical properties) by paracellular permeation, further reducing the bioavailability [
27,
28].
DXM as a lipophilic drug is considered to be able to penetrate through the epithelial layer, but the stromal layer of the cornea is a significant barrier for this molecule. When DXM is present in a suspension form, a part of it is removed from the ocular surface due to the elimination mechanisms following administration. A part of the suspended lipophilic drug might be soluble in the lipophilic layer of the tear film and can be present in higher concentrations than in the aqueous layer of the tear film. It may penetrate into the lipophilic epithelium but is supposed to poorly penetrate across the hydrophilic stromal layer. However, when NLC is used, it is probable that the better distribution and lower mechanical irritation (less foreign material sensation) of the nano-sized material can result in decreased elimination. The active ingredient will also be present in the lipophilic layer of the tear film as well as in the lipophilic epithelial layer, like in the case of the conventional suspension form. However, the nanocarrier can also pass through the hydrophilic stromal layer and can be distributed in it due to its small size and emulsifier content.
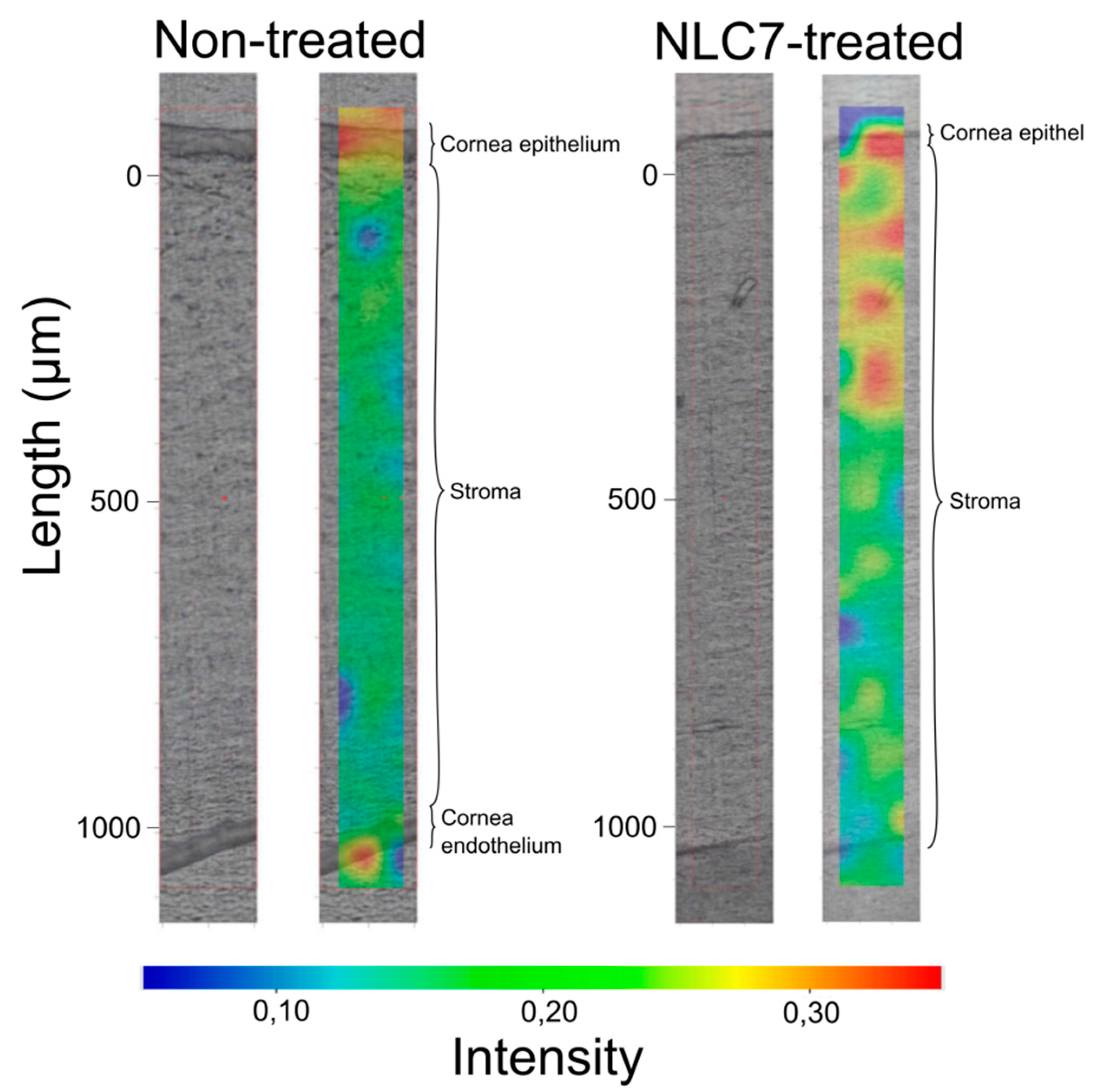
In order to follow the penetration of nanocarriers through the cornea, in our study, semi-quantitative Raman mapping was applied, where porcine cornea was treated with NLC formulation, and the penetration depth was analyzed.
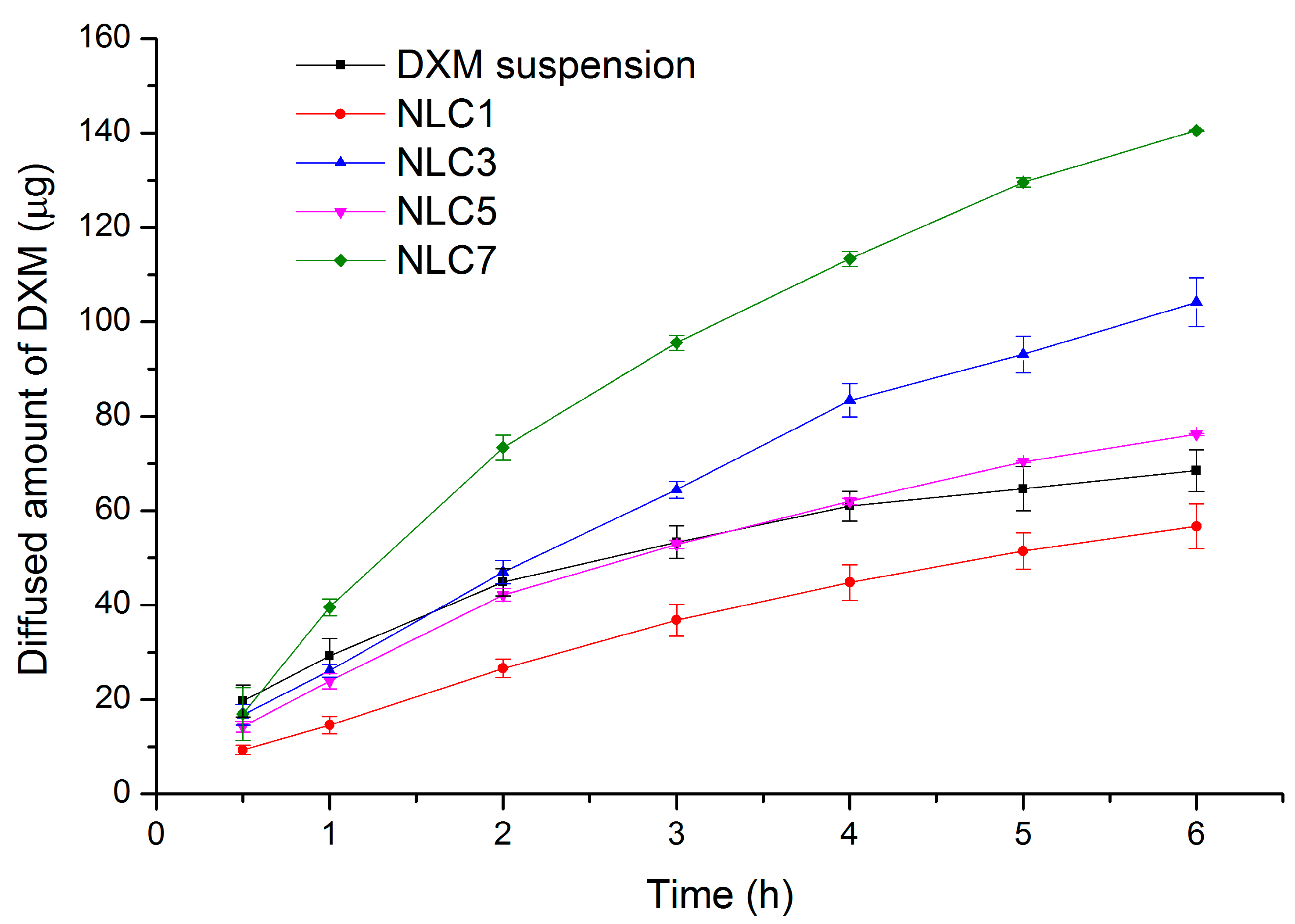
Based on the results of the in vitro drug release study, NLC7 was chosen for the penetration study.
NLC7-treated porcine cornea specimen was compared with non-treated cornea specimen using Raman correlation mapping. The spectrum of NLC7, containing DXM as well, was used for the cornea distribution correlation maps.
Figure 5 shows a high Raman intensity on the top of the cornea specimens in both cases (non-treated, treated), which can correspond to the high lipid content of the epithelial layer and the surface of the tear film. In contrast, the NLC7-treated cornea has a higher Raman intensity for the lipid components in the 200–400 µm depth of the cornea, the depth of which corresponds to the stroma layer. This observation means the nanocarriers entered and enriched in the hydrophilic part of the cornea, which is considered as a main limiting barrier for hydrophobic materials. Based on these results, the penetration of DXM through the cornea might be achieved by using nano lipid carriers.