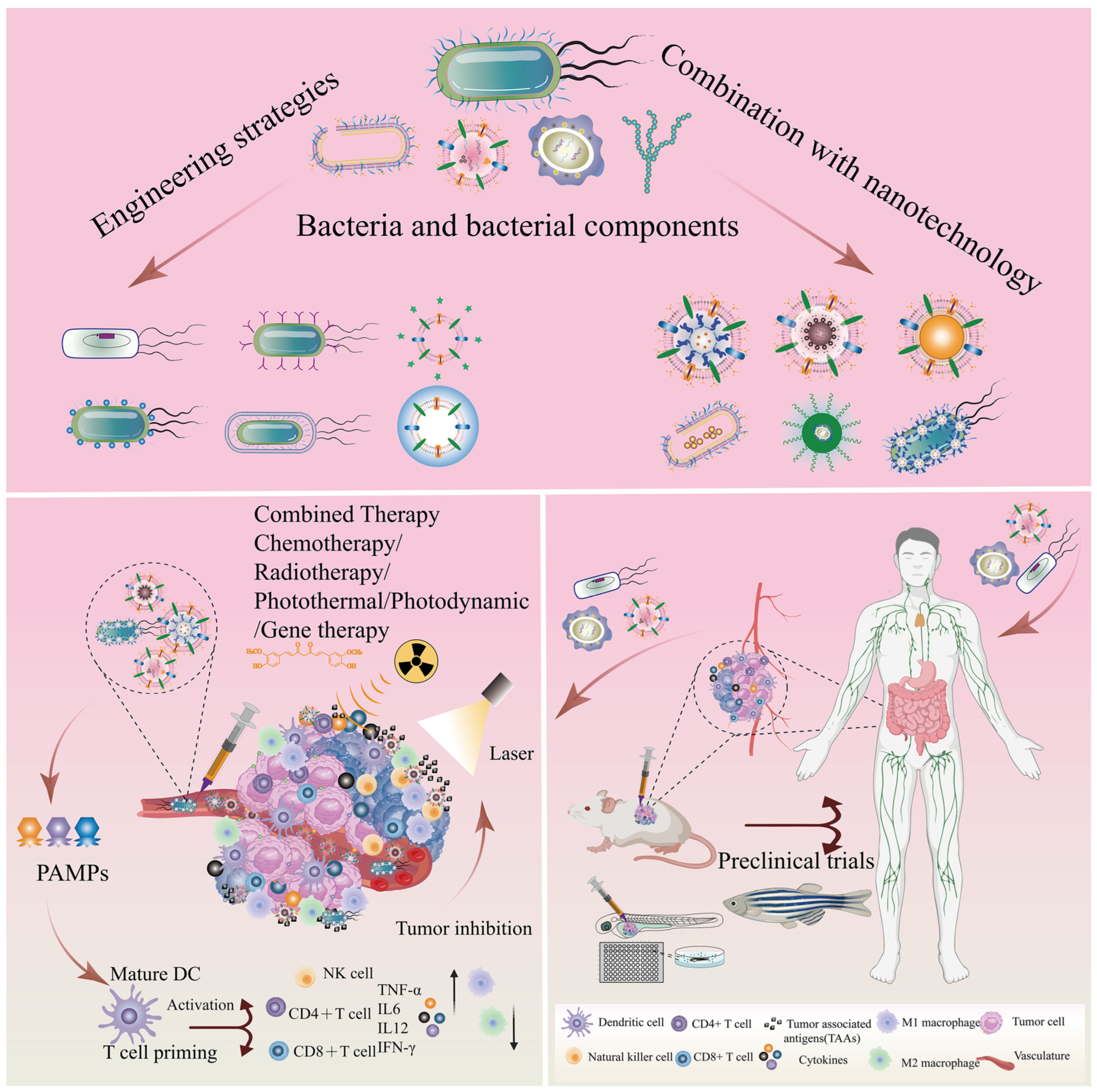
Bacteria and Bacterial Components as Natural Bio-Nanocarriers for Drug and Gene Delivery Systems in Cancer Therapy
Abstract
:1. Introduction
2. Bacteria and Bacterial Components in Cancer Therapy
2.1. Bacteria in Cancer Therapy
2.2. Bacterial Components in Cancer Therapy
2.2.1. Bacterial Outer Membrane Vesicles (OMVs)
2.2.2. Bacterial Ghosts (BGs)
2.2.3. Bacterial Spores (BSPs)
2.2.4. Other Bacterial Components
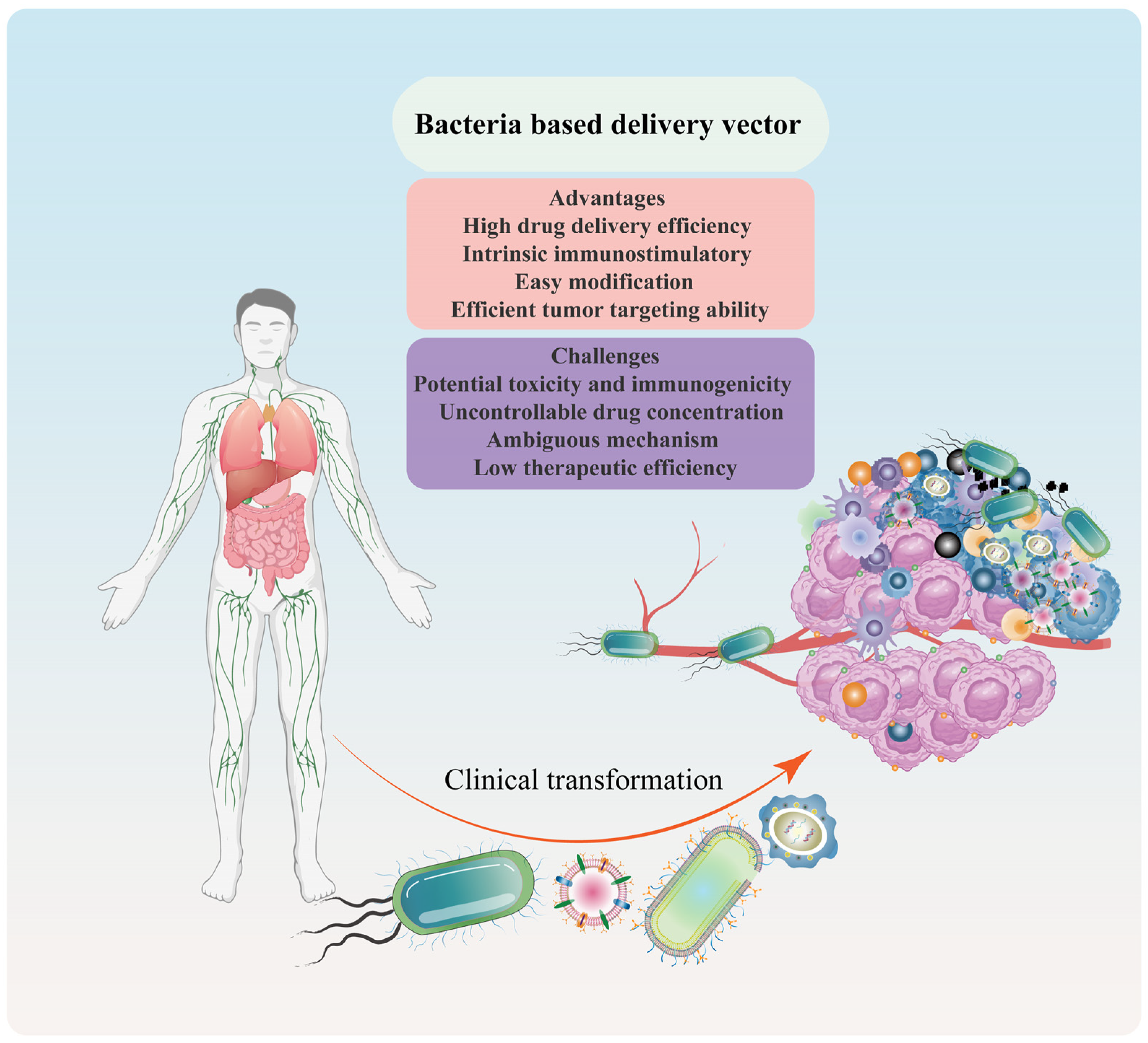
2.3. Advantages and Challenges of Bacteria-/Bacterial Component-Based Delivery Vector
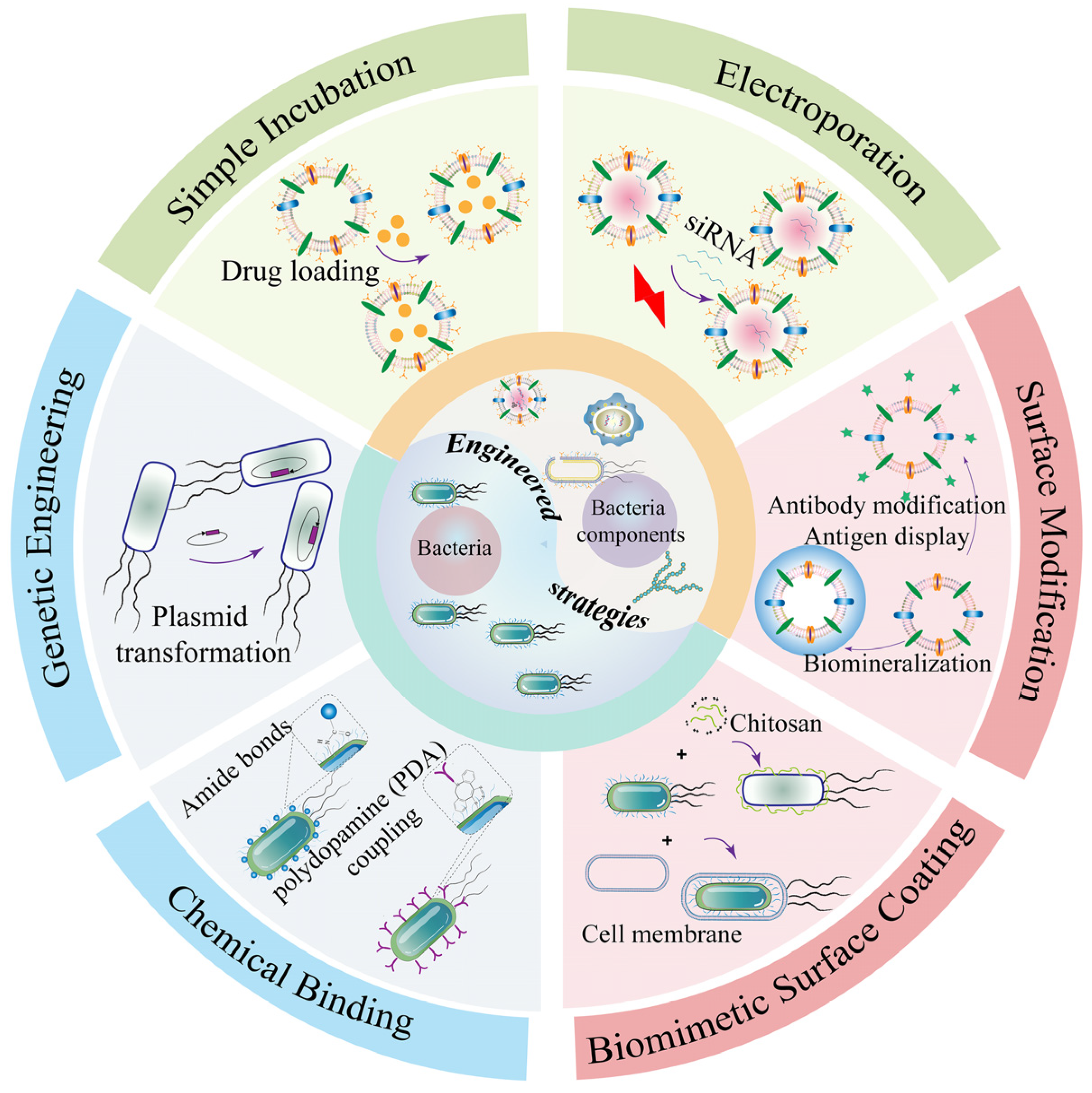
3. Engineering Strategy-Based Bacteria and Bacterial Components for Cancer Therapy
3.1. Engineering Bacteria for Drug Delivery in Cancer Therapy
3.1.1. Chemical Binding
3.1.2. Genetic Engineering
3.1.3. Biomimetic Cell-Surface Coating
3.2. Engineering Bacterial Components as Drug Carriers for Cancer Therapy
3.3. Bacteria and Bacterial Components as Nanocarriers for Gene Delivery in Cancer Therapy
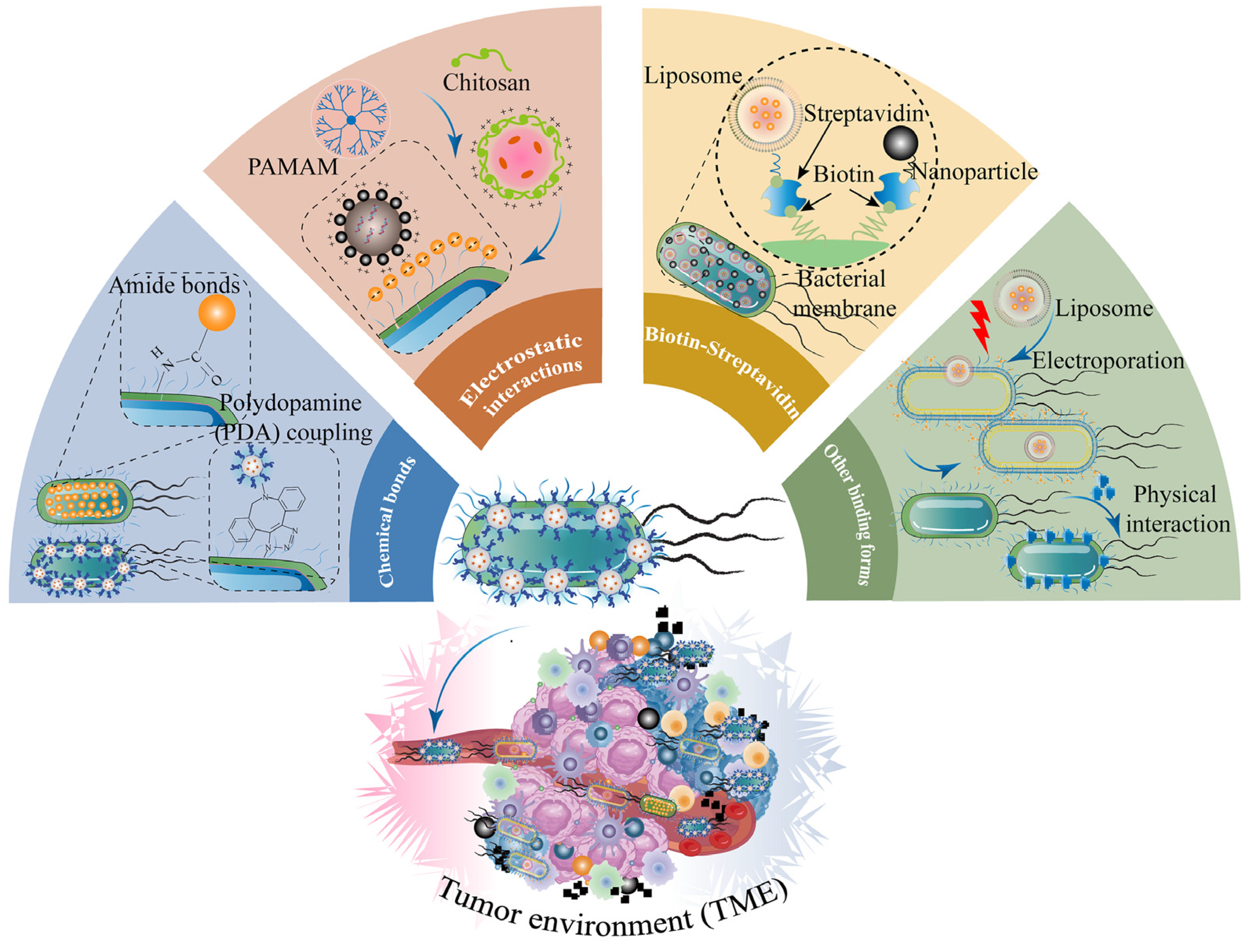
4. Engineering Strategies for Combination of Nanotechnology and Bacteria-Based Drug Systems for Cancer Treatment
4.1. Chemical Bonds
4.2. Electrostatic Interactions
4.3. Biotin–Streptavidin
4.4. Other Binding Forms
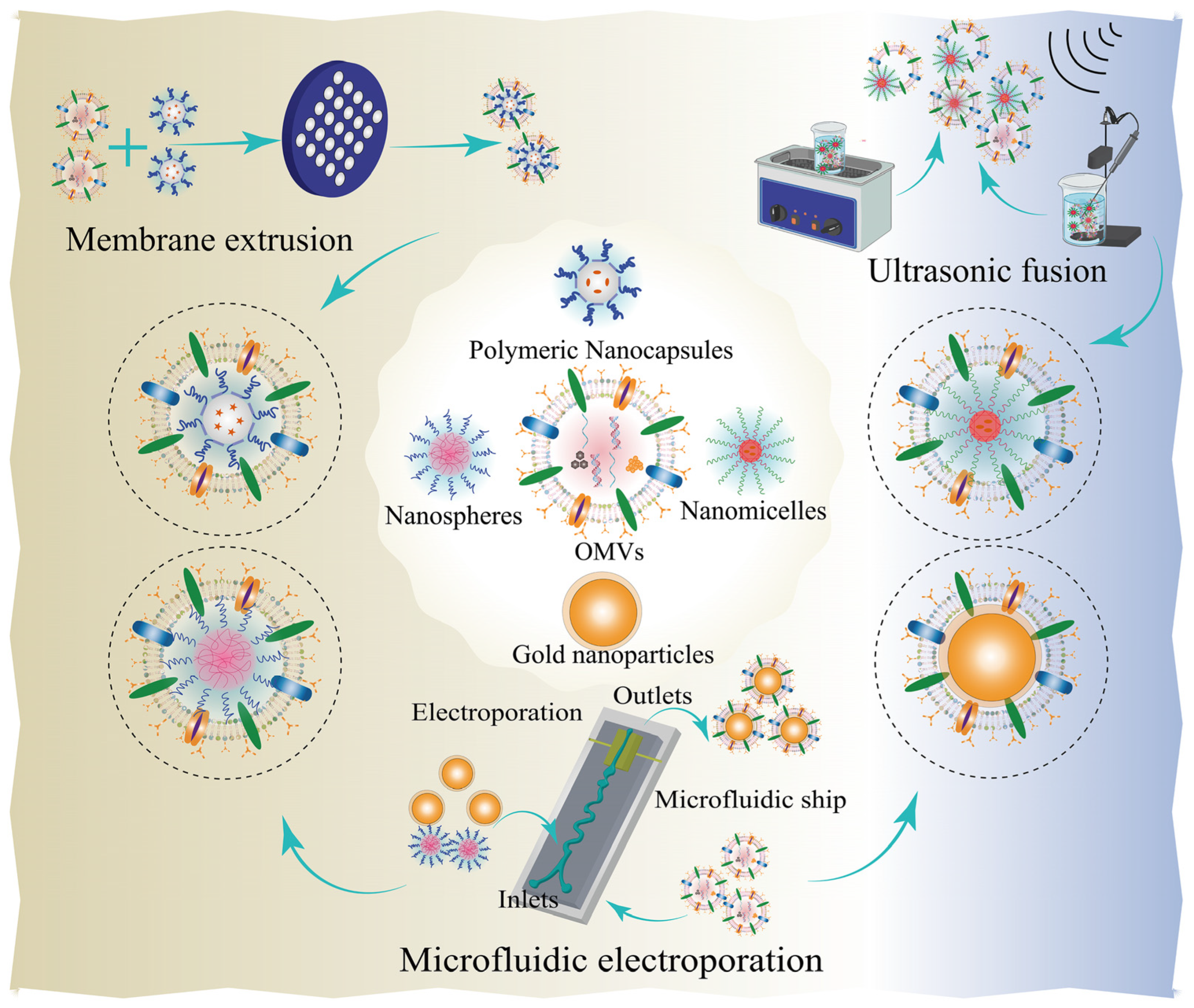
5. Combination of Nanotechnology and Bacterial Component-Based Drug Delivery Systems
5.1. Bacterial OMV Nanoparticle-Based Nanoplatforms
5.1.1. Membrane Extrusion
5.1.2. Ultrasonic Fusion
5.1.3. Other Bind Forms
5.2. Bacterial Ghost (BG) Nanoparticle-Based Nanoplatforms
5.3. Bacterial Spore–Nanoparticle-Based Nanoplatforms
5.4. Other Bacterial Component-Based Nanoplatforms
6. Future and Clinical Trials of Bacteria- and Bacterial Component-Based Nanoplatforms in Cancer Therapy
7. Safety Issues of Bacteria and Bacterial Components
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPs | nanoparticles |
| EPR | enhanced permeability and retention effect |
| PEG | polyethylene glycol |
| TME | tumor microenvironment |
| OMVs | outer membrane vesicles |
| DMVs | bilayer membrane vesicles |
| BMVs | bacterial membrane vesicles |
| BGs | bacterial ghosts |
| BSPs | bacterial spores |
| E. coli | Escherichia coli |
| EcN | E. coli Nissle 1917 |
| S. boulardii | Saccharomyces boulardii |
| L. reuteri | Lactobacillus reuteri |
| L. casei | Lactobacillus casei |
| L. rhamnosus | Lactobacillus rhamnosus |
| L. m | Listeria monocytogenes |
| LA | Lactobacillus acidophilus |
| B. infantis | Bifidobacterium infantis |
| B. breve | Bifidobacterium breve |
| BB | Bifidobacterium bifidum |
| LPS | lipopolysaccharide |
| TNF-α | tumor necrosis factor-α |
| IL-6 | interleukin-6 |
| IL-β | interleukin-β |
| EOMVs | explosive outer membrane vesicles |
| OIMVs | outer inner membrane vesicles |
| CMVs | cytoplasmic membrane vesicles |
| PAMPs | pathogen-associated molecular patterns |
| DC | dendritic |
| αPD-1 | Anti-programmed death-1 |
| PD-1 | programmed death-1 |
| PTT | photothermal therapy |
| ClyA | cytolysin |
| RBC | red blood cell |
| TLR4 | toll-like receptor 4 |
| MDR | multidrug resistance |
| DOX | doxorubicin |
| RGD | Arg-Gly-Asp |
| COVID-19 | coronavirus disease-19 |
| PDA | polydopamine |
| OVA | ovalbumin antigen |
| HSA | human serum albumin |
| LDH | layered-double-hydroxide |
| NIR | near-infrared |
| PLGA | Poly (lactic-co-glycolic acid) |
| 5-FU | 5-fluorouracil |
| BBB | blood–brain barrier |
| BCG | Bacillus Calmette-Guerin |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Etter, E.L.; Mei, K.C.; Nguyen, J. Delivering more for less: Nanosized, minimal-carrier and pharmacoactive drug delivery systems. Adv. Drug Deliv. Rev. 2021, 179, 113994. [Google Scholar] [CrossRef]
- Gao, M.; Long, X.; Du, J.; Teng, M.; Zhang, W.; Wang, Y.; Wang, X.; Wang, Z.; Zhang, P.; Li, J. Enhanced curcumin solubility and antibacterial activity by encapsulation in PLGA oily core nanocapsules. Food Funct. 2020, 11, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Duan, Y.; Huang, Y. Infection microenvironment-related antibacterial nanotherapeutic strategies. Biomaterials 2022, 280, 121249. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shi, K.; Liu, J.; Yang, P.; Han, R.; Pan, M.; Yuan, L.; Fang, C.; Yu, Y.; Qian, Z. Sustained co-delivery of 5-fluorouracil and cis-platinum via biodegradable thermo-sensitive hydrogel for intraoperative synergistic combination chemotherapy of gastric cancer. Bioact. Mater. 2023, 23, 1–15. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Feng, X.; Mai, B.; Li, X.; Wang, F.; Liu, J.; Liu, X.; Zhang, K.; Wang, X. Bacterial-based cancer therapy: An emerging toolbox for targeted drug/gene delivery. Biomaterials 2021, 277, 121124. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; He, X.; Han, R.; Yang, C.; Liu, T.; Yang, Y.; Liu, Q.; Qian, Z. RGD peptide modified platinum nanozyme Co-loaded glutathione-responsive prodrug nanoparticles for enhanced chemo-photodynamic bladder cancer therapy. Biomaterials 2023, 293, 121975. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, L.; Liu, N.; Li, S.; Hao, Y.; Zhang, X. EGF-coated nano-dendriplexes for tumor-targeted nucleic acid delivery in vivo. Drug Deliv. 2016, 23, 1718–1725. [Google Scholar] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Yang, C.; Wu, Y.; Ru, G.; He, X.; Tong, X.; Wang, S. Nanocarriers surface engineered with cell membranes for cancer targeted chemotherapy. J. Nanobiotechnol. 2022, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- McSweeney, M.; Wessler, T.; Price, L.; Ciociola, E.; Herity, L.; Piscitelli, J.; Zamboni, W.; Forest, M.; Cao, Y.; Lai, S. A minimal physiologically based pharmacokinetic model that predicts anti-PEG IgG-mediated clearance of PEGylated drugs in human and mouse. J. Control. Release 2018, 284, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, L.; Li, H.; Miao, F.; Zhang, Z.; Hu, C.; Yu, W.; Tang, Q.; Shao, G. Application of lipid nanovesicle drug delivery system in cancer immunotherapy. J. Nanobiotechnol. 2022, 20, 214. [Google Scholar] [CrossRef]
- Cullin, N.; Azevedo Antunes, C.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and cancer. Cancer Cell 2021, 39, 1317–1341. [Google Scholar] [CrossRef]
- Mayakrishnan, V.; Kannappan, P.; Tharmalingam, N.; Bose, R.J.C.; Madheswaran, T.; Ramasamy, M. Bacterial cancer therapy: A turning point for new paradigms. Drug Discov. Today 2022, 27, 2043–2050. [Google Scholar] [CrossRef]
- Li, S.; Yue, H.; Wang, S.; Li, X.; Wang, X.; Guo, P.; Ma, G.; Wei, W. Advances of bacteria-based delivery systems for modulating tumor microenvironment. Adv. Drug Deliv. Rev. 2022, 188, 114444. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Q.; Liu, W.; Wen, Z.; Li, Y. Precision strategies for cancer treatment by modifying the tumor-related bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 6183–6197. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, S.; Dong, X.; Wang, Z. RGD-expressed bacterial membrane-derived nanovesicles enhance cancer therapy via multiple tumorous targeting. Theranostics 2021, 11, 3301–3316. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Ma, X.; Cheng, K.; Liu, G.; Li, Y.; Yue, Y.; Liang, J.; Zhang, L.; Zhang, T.; Wang, X.; et al. Engineered Bacterial Outer Membrane Vesicles as Controllable Two-Way Adaptors to Activate Macrophage Phagocytosis for Improved Tumor Immunotherapy. Adv. Mater. 2022, 34, e2206200. [Google Scholar] [CrossRef] [PubMed]
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions. J. Extracell. Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Khazaei, M.; Jalili, S.; Hasanian, S.M.; Avan, A.; Soleimanpour, S.; Cho, W.C. Bacteria as a double-action sword in cancer. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2020, 1874, 188388. [Google Scholar] [CrossRef]
- Koyama, A. Re: History of bacillus Calmette-Guerin and bladder cancer: An immunotherapy success story. J. Urol. 2008, 179, 53–56. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, J.; Rawding, P.; Bu, J.; Hong, S.; Hu, Q. Chemically and Biologically Engineered Bacteria-Based Delivery Systems for Emerging Diagnosis and Advanced Therapy. Adv. Mater. 2021, 33, e2102580. [Google Scholar] [CrossRef]
- Badie, F.; Ghandali, M.; Tabatabaei, S.A.; Safari, M.; Khorshidi, A.; Shayestehpour, M.; Mahjoubin-Tehran, M.; Morshedi, K.; Jalili, A.; Tajiknia, V. Use of Salmonella bacteria in cancer therapy: Direct, drug delivery and combination approaches. Front. Oncol. 2021, 11, 624759. [Google Scholar] [CrossRef]
- Ye, Z.; Liang, L.; Lu, H.; Shen, Y.; Zhou, W.; Li, Y. Nanotechnology-Employed Bacteria-Based Delivery Strategy for Enhanced Anticancer Therapy. Int. J. Nanomed. 2021, 16, 8069–8086. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Wang, S.; Weng, W.; Jing, Y.; Su, J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioact. Mater. 2021, 14, 169–181. [Google Scholar] [CrossRef]
- Cao, Z.; Lin, S.; Liu, J. Bacteria-Based Microdevices for the Oral Delivery of Macromolecules. Pharmaceutics 2021, 13, 1610. [Google Scholar] [CrossRef]
- Yin, T.; Diao, Z.; Blum, N.T.; Qiu, L.; Ma, A.; Huang, P. Engineering Bacteria and Bionic Bacterial Derivatives with Nanoparticles for Cancer Therapy. Small 2022, 18, e2104643. [Google Scholar] [CrossRef]
- Karbach, J.; Neumann, A.; Brand, K.; Wahle, C.; Siegel, E.; Maeurer, M.; Ritter, E.; Tsuji, T.; Gnjatic, S.; Old, L.J.; et al. Phase I clinical trial of mixed bacterial vaccine (Coley’s toxins) in patients with NY-ESO-1 expressing cancers: Immunological effects and clinical activity. Clin. Cancer Res. 2012, 18, 5449–5459. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Eidinger, D.; Bruce, A. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Toso, J.F.; Gill, V.J.; Hwu, P.; Marincola, F.M.; Restifo, N.P.; Schwartzentruber, D.J.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Stock, F. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 2002, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Zhang, Y.; Zhang, C.; Liu, X.; Shi, C. Intestinal microbiota: A potential target for enhancing the antitumor efficacy and reducing the toxicity of immune checkpoint inhibitors. Cancer Lett. 2021, 509, 53–62. [Google Scholar] [CrossRef]
- Aggarwal, N.; Breedon, A.M.E.; Davis, C.M.; Hwang, I.Y.; Chang, M.W. Engineering probiotics for therapeutic applications: Recent examples and translational outlook. Curr. Opin. Biotechnol. 2020, 65, 171–179. [Google Scholar] [CrossRef]
- Geng, Z.; Cao, Z.; Liu, R.; Liu, K.; Liu, J.; Tan, W. Aptamer-assisted tumor localization of bacteria for enhanced biotherapy. Nat. Commun. 2021, 12, 6584. [Google Scholar] [CrossRef]
- Kim, O.Y.; Park, H.T.; Dinh, N.T.H.; Choi, S.J.; Lee, J.; Kim, J.H.; Lee, S.W.; Gho, Y.S. Bacterial outer membrane vesicles suppress tumor by interferon-gamma-mediated antitumor response. Nat. Commun. 2017, 8, 626. [Google Scholar] [CrossRef]
- Park, K.S.; Svennerholm, K.; Crescitelli, R.; Lasser, C.; Gribonika, I.; Lotvall, J. Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J. Extracell. Vesicles 2021, 10, e12120. [Google Scholar] [CrossRef]
- Janku, F.; Zhang, H.H.; Pezeshki, A.; Goel, S.; Murthy, R.; Wang-Gillam, A.; Shepard, D.R.; Helgason, T.; Masters, T.; Hong, D.S.; et al. Intratumoral Injection of Clostridium novyi-NT Spores in Patients with Treatment-refractory Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 96–106. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, J. Bacteria and bacterial derivatives as drug carriers for cancer therapy. J. Control. Release 2020, 326, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Xu, J.; Deng, D.; Chao, T.; Li, J.; Zhang, R.; Peng, R.; Liu, Z. Bacteria-derived membrane vesicles to advance targeted photothermal tumor ablation. Biomaterials 2021, 268, 120550. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Moghoofei, M.; Mirkiani, S.; Ghasemi, A.; Rabiee, N.; Hadifar, S.; Beyzavi, A.; Karimi, M.; Hamblin, M.R. Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: Set the bugs to work? Biotechnol. Adv. 2018, 36, 968–985. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Marzhoseyni, Z.; Shojaie, L.; Tabatabaei, S.A.; Movahedpour, A.; Safari, M.; Esmaeili, D.; Mahjoubin-Tehran, M.; Jalili, A.; Morshedi, K.; Khan, H. Streptococcal bacterial components in cancer therapy. Cancer Gene Ther. 2022, 29, 141–155. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Nahui Palomino, R.A.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota-host communications: Bacterial extracellular vesicles as a common language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Renelli, M.; Matias, V.; Lo, R.Y.; Beveridge, T.J. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 2004, 150, 2161–2169. [Google Scholar] [CrossRef]
- Long, Q.; Zheng, P.; Zheng, X.; Li, W.; Hua, L.; Yang, Z.; Huang, W.; Ma, Y. Engineered bacterial membrane vesicles are promising carriers for vaccine design and tumor immunotherapy. Adv. Drug Deliv. Rev. 2022, 186, 114321. [Google Scholar] [CrossRef] [PubMed]
- Orench-Rivera, N.; Kuehn, M.J. Environmentally controlled bacterial vesicle-mediated export. Cell. Microbiol. 2016, 18, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Gao, X.; Feng, Q.; Wang, J.; Zhao, X. Bacterial outer membrane vesicle-based cancer nanovaccines. Cancer Biol. Med. 2022, 19, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Li, X.; Wei, Z.; Gan, L.; Yang, X. Extracellular vesicles-based drug delivery systems for cancer immunotherapy. J. Control. Release 2020, 328, 562–574. [Google Scholar] [CrossRef]
- Schetters, S.T.T.; Jong, W.S.P.; Horrevorts, S.K.; Kruijssen, L.J.W.; Engels, S.; Stolk, D.; Daleke-Schermerhorn, M.H.; Garcia-Vallejo, J.; Houben, D.; Unger, W.W.J.; et al. Outer membrane vesicles engineered to express membrane-bound antigen program dendritic cells for cross-presentation to CD8(+) T cells. Acta Biomater. 2019, 91, 248–257. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Yue, Y.; Zhang, K.; Cheng, K.; Feng, Q.; Ma, N.; Liang, J.; Zhang, T.; Zhang, L.; et al. Rapid Surface Display of mRNA Antigens by Bacteria-Derived Outer Membrane Vesicles for a Personalized Tumor Vaccine. Adv. Mater. 2022, 34, e2109984. [Google Scholar] [CrossRef]
- Liang, J.; Cheng, K.; Li, Y.; Xu, J.; Chen, Y.; Ma, N.; Feng, Q.; Zhu, F.; Ma, X.; Zhang, T.; et al. Personalized cancer vaccines from bacteria-derived outer membrane vesicles with antibody-mediated persistent uptake by dendritic cells. Fundam. Res. 2022, 2, 23–36. [Google Scholar] [CrossRef]
- McMillan, H.M.; Zebell, S.G.; Ristaino, J.B.; Dong, X.; Kuehn, M.J. Protective plant immune responses are elicited by bacterial outer membrane vesicles. Cell Rep. 2021, 34, 108645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, J.; Li, J.; Xia, Y.; Cao, J. Outer membrane vesicles derived from hypervirulent Klebsiella pneumoniae stimulate the inflammatory response. Microb. Pathog. 2021, 154, 104841. [Google Scholar] [CrossRef]
- Coelho, C.; Brown, L.; Maryam, M.; Vij, R.; Smith, D.F.Q.; Burnet, M.C.; Kyle, J.E.; Heyman, H.M.; Ramirez, J.; Prados-Rosales, R.; et al. Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles. J. Biol. Chem. 2019, 294, 1202–1217. [Google Scholar] [CrossRef] [PubMed]
- Watkins, H.C.; Pagan, C.L.; Childs, H.R.; Posada, S.; Chau, A.; Rios, J.; Guarino, C.; DeLisa, M.P.; Whittaker, G.R.; Putnam, D. A single dose and long lasting vaccine against pandemic influenza through the controlled release of a heterospecies tandem M2 sequence embedded within detoxified bacterial outer membrane vesicles. Vaccine 2017, 35, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Watkins, H.C.; Rappazzo, C.G.; Higgins, J.S.; Sun, X.; Brock, N.; Chau, A.; Misra, A.; Cannizzo, J.P.B.; King, M.R.; Maines, T.R.; et al. Safe Recombinant Outer Membrane Vesicles that Display M2e Elicit Heterologous Influenza Protection. Mol. Ther. 2017, 25, 989–1002. [Google Scholar] [CrossRef]
- Schulz, E.; Goes, A.; Garcia, R.; Panter, F.; Koch, M.; Muller, R.; Fuhrmann, K.; Fuhrmann, G. Biocompatible bacteria-derived vesicles show inherent antimicrobial activity. J. Control. Release 2018, 290, 46–55. [Google Scholar] [CrossRef]
- Shi, Y.; Meng, L.; Zhang, C.; Zhang, F.; Fang, Y. Extracellular vesicles of Lacticaseibacillus paracasei PC-H1 induce colorectal cancer cells apoptosis via PDK1/AKT/Bcl-2 signaling pathway. Microbiol. Res. 2021, 255, 126921. [Google Scholar] [CrossRef]
- Huang, W.; Shu, C.; Hua, L.; Zhao, Y.; Xie, H.; Qi, J.; Gao, F.; Gao, R.; Chen, Y.; Zhang, Q.; et al. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater. 2020, 108, 300–312. [Google Scholar] [CrossRef]
- Klimentova, J.; Stulik, J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol. Res. 2015, 170, 1–9. [Google Scholar] [CrossRef]
- Deo, P.; Chow, S.H.; Han, M.L.; Speir, M.; Huang, C.; Schittenhelm, R.B.; Dhital, S.; Emery, J.; Li, J.; Kile, B.T.; et al. Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat. Microbiol. 2020, 5, 1418–1427. [Google Scholar] [CrossRef]
- Jain, S.; Pillai, J. Bacterial membrane vesicles as novel nanosystems for drug delivery. Int. J. Nanomed. 2017, 12, 6329–6341. [Google Scholar] [CrossRef]
- Gnopo, Y.M.D.; Putnam, D. A lipid mixing assay to accurately quantify the fusion of outer membrane vesicles. Methods 2020, 177, 74–79. [Google Scholar] [CrossRef]
- Liu, J.H.; Chen, C.Y.; Liu, Z.Z.; Luo, Z.W.; Rao, S.S.; Jin, L.; Wan, T.F.; Yue, T.; Tan, Y.J.; Yin, H.; et al. Extracellular Vesicles from Child Gut Microbiota Enter into Bone to Preserve Bone Mass and Strength. Adv. Sci. 2021, 8, 2004831. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Mei, J.; Jiang, S.; Zhang, J.; Wang, L.; Yuan, J.; Yi, S.; Ma, Y.; Liu, Y. Lung cancer shapes commensal bacteria via exosome-like nanoparticles. Nano Today 2022, 44, 101451. [Google Scholar] [CrossRef]
- Wei, S.; Jiao, D.; Xing, W. A rapid method for isolation of bacterial extracellular vesicles from culture media using epsilon-poly-L-lysine that enables immunological function research. Front. Immunol. 2022, 13, 930510. [Google Scholar] [CrossRef]
- Bottero, D.; Zurita, M.E.; Gaillard, M.E.; Bartel, E.; Vercellini, C.; Hozbor, D. Membrane vesicles derived from Bordetella bronchiseptica: Active constituent of a new vaccine against infections caused by this pathogen. Appl. Environ. Microbiol. 2018, 84, e01877-17. [Google Scholar] [CrossRef]
- Patel, R.B.; Ye, M.; Carlson, P.M.; Jaquish, A.; Zangl, L.; Ma, B.; Wang, Y.; Arthur, I.; Xie, R.; Brown, R.J.; et al. Development of an In Situ Cancer Vaccine via Combinational Radiation and Bacterial-Membrane-Coated Nanoparticles. Adv. Mater. 2019, 31, e1902626. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.Z.; Li, Z.H.; Bai, X.F.; Liu, C.J.; Zhang, X.Z. Hybrid Vesicles Based on Autologous Tumor Cell Membrane and Bacterial Outer Membrane To Enhance Innate Immune Response and Personalized Tumor Immunotherapy. Nano Lett. 2021, 21, 8609–8618. [Google Scholar] [CrossRef]
- Ahmed, A.A.Q.; Qi, F.; Zheng, R.; Xiao, L.; Abdalla, A.M.E.; Mao, L.; Bakadia, B.M.; Liu, L.; Atta, O.M.; Li, X.; et al. The impact of ExHp-CD (outer membrane vesicles) released from Helicobacter pylori SS1 on macrophage RAW 264.7 cells and their immunogenic potential. Life Sci. 2021, 279, 119644. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.P.; Sabu, C.; Nivitha, K.P.; Sankar, R.; Ameena Shirin, V.K.; Henna, T.K.; Raphey, V.R.; Gangadharappa, H.V.; Kotta, S.; Pramod, K. Bioinspired and biomimetic micro- and nanostructures in biomedicine. J. Control. Release 2022, 343, 724–754. [Google Scholar] [CrossRef]
- Chen, H.; Ji, H.; Kong, X.; Lei, P.; Yang, Q.; Wu, W.; Jin, L.; Sun, D. Bacterial Ghosts-Based Vaccine and Drug Delivery Systems. Pharmaceutics 2021, 13, 1892. [Google Scholar] [CrossRef] [PubMed]
- Salem-Bekhit, M.M.; Youssof, A.M.E.; Alanazi, F.K.; Aleanizy, F.S.; Abdulaziz, A.; Taha, E.I.; Amara, A. Bacteria from Infectious Particles to Cell Based Anticancer Targeted Drug Delivery Systems. Pharmaceutics 2021, 13, 1984. [Google Scholar] [CrossRef]
- Xie, S.; Li, S.; Zhang, Z.; Chen, M.; Ran, P.; Li, X. Bacterial ghosts for targeting delivery and subsequent responsive release of ciprofloxacin to destruct intracellular bacteria. Chem. Eng. J. 2020, 399, 125700. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, P.; Zhang, Z.; Liu, Y.; Chen, M.; Li, S.; Li, X. Bacterial navigation for tumor targeting and photothermally-triggered bacterial ghost transformation for spatiotemporal drug release. Acta Biomater. 2021, 131, 172–184. [Google Scholar] [CrossRef]
- Rabea, S.; Salem-Bekhit, M.M.; Alanazi, F.K.; Yassin, A.S.; Moneib, N.A.; Hashem, A.E.M. A novel protocol for bacterial ghosts’ preparation using tween 80. Saudi Pharm. J. 2018, 26, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Moon, E.S.; Noh, H.B.; Park, H.J.; Kim, S.; Oh, S.; Vinod, N.; Choi, C.W.; Kwak, K. Protective Immunity against Listeria monocytogenes in Rats, Provided by HCl- and NaOH-Induced Listeria monocytogenes Bacterial Ghosts (LMGs) as Vaccine Candidates. Int. J. Mol. Sci. 2022, 23, 1946. [Google Scholar] [CrossRef]
- Nie, X.; Wang, Y.; Dong, F.; Cheng, W.; Lu, X.; Ding, C.; Lin, Q.; Liu, M.; Wang, J.; Zhuan, H.; et al. Surface interaction and biomineralization of uranium induced by the living and dead bacterial ghosts of Kocuria sp. J. Environ. Chem. Eng. 2022, 10, 107295. [Google Scholar] [CrossRef]
- Amara, A.A.; Salem-Bekhit, M.M.; Alanazi, F.K. Sponge-like: A new protocol for preparing bacterial ghosts. Sci. World J. 2013, 2013, 545741. [Google Scholar] [CrossRef]
- Song, Q.; Zheng, C.; Jia, J.; Zhao, H.; Feng, Q.; Zhang, H.; Wang, L.; Zhang, Z.; Zhang, Y. A Probiotic Spore-Based Oral Autonomous Nanoparticles Generator for Cancer Therapy. Adv. Mater. 2019, 31, e1903793. [Google Scholar] [CrossRef]
- Copland, A.; Diogo, G.R.; Hart, P.; Harris, S.; Tran, A.C.; Paul, M.J.; Singh, M.; Cutting, S.M.; Reljic, R. Mucosal Delivery of Fusion Proteins with Bacillus subtilis Spores Enhances Protection against Tuberculosis by Bacillus Calmette-Guerin. Front. Immunol. 2018, 9, 346. [Google Scholar] [CrossRef]
- Park, W.; Cho, S.; Huang, X.; Larson, A.C.; Kim, D.H. Branched Gold Nanoparticle Coating of Clostridium novyi-NT Spores for CT-Guided Intratumoral Injection. Small 2017, 13, 1602722. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A., Jr.; Cheong, I.; Foss, C.A.; Zhang, X.; Peters, B.A.; Agrawal, N.; Bettegowda, C.; Karim, B.; Liu, G.; Khan, K.; et al. Pharmacologic and toxicologic evaluation of C. novyi-NT spores. Toxicol. Sci. 2005, 88, 562–575. [Google Scholar] [CrossRef]
- Zheng, D.W.; Li, R.Q.; An, J.X.; Xie, T.Q.; Han, Z.Y.; Xu, R.; Fang, Y.; Zhang, X.Z. Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer. Adv. Mater. 2020, 32, e2004529. [Google Scholar] [CrossRef]
- Lienard, D.; Avril, M.-F.; Le Gal, F.-A.; Baumgaertner, P.; Vermeulen, W.; Blom, A.; Geldhof, C.; Rimoldi, D.; Pagliusi, S.; Romero, P. Vaccination of melanoma patients with Melan-A/Mart-1 peptide and Klebsiella outer membrane protein p40 as an adjuvant. J. Immunother. 2009, 32, 875–883. [Google Scholar] [CrossRef]
- Valdes-Zayas, A.; Gonzalez, Z.; Mulens, V.; Vega, A.M.; Perez, K.; Lorenzo-Luaces, P.; Rubio, M.C.; Estevez, A.; Curbelo, I.; Fernandez, L.E. Immunologic response elicited in breast cancer patients receiving a NeuGcGM3-based vaccine as adjuvant therapy. J. Immunother. 2017, 40, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Du, M.; Chen, Z.; Yuan, Z. Recent Advances in Bacteria-Based Cancer Treatment. Cancers 2022, 14, 4945. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Z.; Zhang, M.; Lin, S.; Liu, J. Spatiotemporally Controllable Distribution of Combination Therapeutics in Solid Tumors by Dually Modified Bacteria. Adv. Mater. 2022, 34, e2106669. [Google Scholar] [CrossRef]
- Ji, P.; An, B.; Jie, Z.; Wang, L.; Qiu, S.; Ge, C.; Wu, Q.; Shi, J.; Huo, M. Genetically engineered probiotics as catalytic glucose depriver for tumor starvation therapy. Mater. Today Bio. 2023, 18, 100515. [Google Scholar] [CrossRef]
- Harimoto, T.; Hahn, J.; Chen, Y.Y.; Im, J.; Zhang, J.; Hou, N.; Li, F.; Coker, C.; Gray, K.; Harr, N.; et al. A programmable encapsulation system improves delivery of therapeutic bacteria in mice. Nat. Biotechnol. 2022, 40, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cao, Z.; Wang, L.; Wang, X.; Lin, S.; Wu, F.; Pang, Y.; Liu, J. Multimodal oncolytic bacteria by coating with tumor cell derived nanoshells. Nano Today 2022, 45, 101537. [Google Scholar] [CrossRef]
- Aly, R.G.; El-Enbaawy, M.I.; Abd El-Rahman, S.S.; Ata, N.S. Antineoplastic activity of Salmonella Typhimurium outer membrane nanovesicles. Exp. Cell Res. 2021, 399, 112423. [Google Scholar] [CrossRef] [PubMed]
- Kuerban, K.; Gao, X.; Zhang, H.; Liu, J.; Dong, M.; Wu, L.; Ye, R.; Feng, M.; Ye, L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm. Sin. B 2020, 10, 1534–1548. [Google Scholar] [CrossRef] [PubMed]
- Michalek, J.; Hezova, R.; Turanek-Knötigova, P.; Gabkova, J.; Strioga, M.; Lubitz, W.; Kudela, P. Oncolysate-loaded Escherichia coli bacterial ghosts enhance the stimulatory capacity of human dendritic cells. Cancer Immunol. Immunother. 2016, 66, 149–159. [Google Scholar] [CrossRef]
- Groza, D.; Gehrig, S.; Kudela, P.; Holcmann, M.; Pirker, C.; Dinhof, C.; Schueffl, H.H.; Sramko, M.; Hoebart, J.; Alioglu, F.; et al. Bacterial ghosts as adjuvant to oxaliplatin chemotherapy in colorectal carcinomatosis. Oncoimmunology 2018, 7, e1424676. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, V.; Prakash, J.; Malekzadeh-Najafabadi, J.; Stiel, A.; Klemm, U.; Mettenleiter, G.; Aichler, M.; Walch, A.; Ntziachristos, V. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat. Commun. 2019, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, X.; Zhou, W.; Chu, Y.; Chen, Q.; Zhang, Y.; Li, C.; Chen, H.; Liu, P.; Zhao, Z.; et al. Sequentially Triggered Bacterial Outer Membrane Vesicles for Macrophage Metabolism Modulation and Tumor Metastasis Suppression. ACS Nano 2021, 15, 13826–13838. [Google Scholar] [CrossRef]
- Gujrati, V.; Kim, S.; Kim, S.-H.; Min, J.J.; Choy, H.E.; Kim, S.C.; Jon, S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 2014, 8, 1525–1537. [Google Scholar] [CrossRef]
- Din, S.R.U.; Saeed, S.; Khan, S.U.; Arbi, F.M.; Xuefang, G.; Zhong, M. Bacteria-driven cancer therapy: Exploring advancements and challenges. Crit. Rev. Oncol./Hematol. 2023, 191, 104141. [Google Scholar] [CrossRef]
- Duong, M.T.-Q.; Qin, Y.; You, S.-H.; Min, J.-J. Bacteria-cancer interactions: Bacteria-based cancer therapy. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 2018, 18, 727–743. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Y.; Dong, Z.; Hao, Y.; Wang, C.; Li, Q.; Wu, Y.; Feng, L.; Liu, Z. Engineering bioluminescent bacteria to boost photodynamic therapy and systemic anti-tumor immunity for synergistic cancer treatment. Biomaterials 2022, 281, 121332. [Google Scholar] [CrossRef]
- Deng, X.; Yang, W.; Shao, Z.; Zhao, Y. Genetically modified bacteria for targeted phototherapy of tumor. Biomaterials 2021, 272, 120809. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Duong, M.T.; Zuo, C.; Qin, Y.; Zhang, Y.; Guo, Y.; Hong, Y.; Zheng, J.H.; Min, J.J. Targeting of pancreatic cancer cells and stromal cells using engineered oncolytic Salmonella typhimurium. Mol. Ther. 2022, 30, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Afkhami-Poostchi, A.; Mashreghi, M.; Iranshahi, M.; Matin, M.M. Use of a genetically engineered E. coli overexpressing beta-glucuronidase accompanied by glycyrrhizic acid, a natural and anti-inflammatory agent, for directed treatment of colon carcinoma in a mouse model. Int. J. Pharm. 2020, 579, 119159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, N.; Hou, W.; Qin, H. Coating bacteria for anti-tumor therapy. Front. Bioeng. Biotechnol. 2022, 10, 1020020. [Google Scholar] [CrossRef]
- Cao, Z.; Cheng, S.; Wang, X.; Pang, Y.; Liu, J. Camouflaging bacteria by wrapping with cell membranes. Nat. Commun. 2019, 10, 3452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Y.; Ning, B.; Su, X.; Yang, B.; Dong, H.; Yin, B.; Pang, Z.; Shen, S. Intravenous Delivery of Living Listeria monocytogenes Elicits Gasdmermin-Dependent Tumor Pyroptosis and Motivates Anti-Tumor Immune Response. ACS Nano 2022, 16, 4102–4115. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Pang, G.; Lang, H.; Shen, Y.; Sun, T.; Zhang, Y.; Liu, J.; Chang, J.; Kang, J.; et al. Precise Thermal Regulation of Engineered Bacteria Secretion for Breast Cancer Treatment In Vivo. ACS Synth. Biol. 2022, 11, 1167–1177. [Google Scholar] [CrossRef]
- Liu, Q.; Gai, Y.; Chen, Y.; Lan, X.; Jiang, D. Escherichiacoli Nissle 1917 as a Novel Microrobot for Tumor-Targeted Imaging and Therapy. Pharmaceutics 2021, 13, 1226. [Google Scholar] [CrossRef]
- Zhai, Y.; Ma, Y.; Pang, B.; Zhang, J.; Li, Y.; Rui, Y.; Xu, T.; Zhao, Y.; Qian, Z.; Gu, Y.; et al. A cascade targeting strategy based on modified bacterial vesicles for enhancing cancer immunotherapy. J. Nanobiotechnol. 2021, 19, 434. [Google Scholar] [CrossRef]
- McMillan, H.M.; Kuehn, M.J. The extracellular vesicle generation paradox: A bacterial point of view. EMBO J. 2021, 40, e108174. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J. Control. Release 2020, 323, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, M.; Zeng, Y.; Miao, T.; Luo, H.; Tong, Y.; Zhao, M.; Mu, R.; Gu, J.; Yang, S.; et al. Biomimetic Lipopolysaccharide-Free Bacterial Outer Membrane-Functionalized Nanoparticles for Brain-Targeted Drug Delivery. Adv. Sci. 2022, 9, e2105854. [Google Scholar] [CrossRef]
- Qing, S.; Lyu, C.; Zhu, L.; Pan, C.; Wang, S.; Li, F.; Wang, J.; Yue, H.; Gao, X.; Jia, R.; et al. Biomineralized Bacterial Outer Membrane Vesicles Potentiate Safe and Efficient Tumor Microenvironment Reprogramming for Anticancer Therapy. Adv. Mater. 2020, 32, e2002085. [Google Scholar] [CrossRef]
- Sagnella, S.M.; Yang, L.; Stubbs, G.E.; Boslem, E.; Martino-Echarri, E.; Smolarczyk, K.; Pattison, S.L.; Vanegas, N.; St Clair, E.; Clarke, S.; et al. Cyto-Immuno-Therapy for Cancer: A Pathway Elicited by Tumor-Targeted, Cytotoxic Drug-Packaged Bacterially Derived Nanocells. Cancer Cell 2020, 37, 354–370.e357. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, J.; Qiu, X.; Dong, S.; He, J.; Liu, J.; Xu, W.; Huang, S.; Hu, X.; Xiang, D.X. Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioact. Mater. 2023, 20, 548–560. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wen, Z.J.; Li, Y.M.; Sun, W.R.; Hu, X.Q.; Zhu, J.Z.; Li, X.Y.; Wang, P.Y.; Pedraz, J.L.; Lee, J.H.; et al. Bioengineered Bacterial Membrane Vesicles with Multifunctional Nanoparticles as a Versatile Platform for Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2023, 15, 3744–3759. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Q.; Li, J.; Kwong, C.H.; Wei, J.; Xie, B.; Lu, S.; Lee, S.M.; Wang, R. In vivo hitchhiking of immune cells by intracellular self-assembly of bacteria-mimetic nanomedicine for targeted therapy of melanoma. Sci. Adv. 2022, 8, eabn1805. [Google Scholar] [CrossRef]
- Mi, Z.; Yao, Q.; Qi, Y.; Zheng, J.; Liu, J.; Liu, Z.; Tan, H.; Ma, X.; Zhou, W.; Rong, P. Salmonella-mediated blood—brain barrier penetration, tumor homing and tumor microenvironment regulation for enhanced chemo/bacterial glioma therapy. Acta Pharm. Sin. B 2022, 13, 819–833. [Google Scholar] [CrossRef]
- Song, W.; Tiruthani, K.; Wang, Y.; Shen, L.; Hu, M.; Dorosheva, O.; Qiu, K.; Kinghorn, K.A.; Liu, R.; Huang, L. Trapping of Lipopolysaccharide to Promote Immunotherapy against Colorectal Cancer and Attenuate Liver Metastasis. Adv. Mater. 2018, 30, e1805007. [Google Scholar] [CrossRef] [PubMed]
- Youssof, A.M.E.; Alanazi, F.K.; Salem-Bekhit, M.M.; Shakeel, F.; Haq, N. Bacterial Ghosts Carrying 5-Fluorouracil: A Novel Biological Carrier for Targeting Colorectal Cancer. AAPS PharmSciTech 2019, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskiene, N.; Pasukoniene, V.; Darinskas, A.; Krasko, J.A.; Zilionyte, K.; Mlynska, A.; Gudleviciene, Z.; Miseikyte-Kaubriene, E.; Schijns, V.; Lubitz, W.; et al. Tumor lysate-loaded Bacterial Ghosts as a tool for optimized production of therapeutic dendritic cell-based cancer vaccines. Vaccine 2018, 36, 4171–4180. [Google Scholar] [CrossRef]
- Shi, Y.J.; Zheng, H.X.; Hong, Z.P.; Wang, H.B.; Wang, Y.; Li, M.Y.; Li, Z.H. Antitumor effects of different Ganoderma lucidum spore powder in cell- and zebrafish-based bioassays. J. Integr. Med. 2021, 19, 177–184. [Google Scholar] [CrossRef]
- Staedtke, V.; Roberts, N.J.; Bai, R.Y.; Zhou, S. Clostridium novyi-NT in cancer therapy. Genes Dis. 2016, 3, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Liu, N.; Guo, N.; Gao, C.; Hao, Y.; Chen, L.; Zhang, X. In vitro studies of phospholipid-modified PAMAM-siMDR1 complexes for the reversal of multidrug resistance in human breast cancer cells. Int. J. Pharm. 2017, 530, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Guo, N.; Zhang, X. Reversal of multidrug resistance in breast cancer MCF-7/ADR cells by h-R3-siMDR1-PAMAM complexes. Int. J. Pharm. 2016, 511, 436–445. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Mat Rani, N.N.I.; Alzubaidi, Z.M.; Butt, A.M.; Mohammad Faizal, N.D.F.; Sekar, M.; Azhari, H.; Mohd Amin, M.C.I. Outer membrane vesicles as biomimetic vaccine carriers against infections and cancers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1784. [Google Scholar] [CrossRef]
- Xie, J.; Li, Q.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R.E. The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. 2022, 40, 1173–1194. [Google Scholar] [CrossRef]
- Pilkington, E.H.; Suys, E.J.A.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Prakash, G.; Shokr, A.; Willemen, N.; Bashir, S.M.; Shin, S.R.; Hassan, S. Microfluidic fabrication of lipid nanoparticles for the delivery of nucleic acids. Adv. Drug Deliv. Rev. 2022, 184, 114197. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yuen, D.; Mintern, J.D.; Johnston, A.P.R. Opportunities for innovation: Building on the success of lipid nanoparticle vaccines. Curr. Opin. Colloid Interface Sci. 2021, 55, 101468. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, Y.; Mao, C.; Li, J.; Zhao, Y.; Chen, Y.; Liu, Z.; Lin, Y. Improved Tumor Infiltration and Immunomodulation for Tumor Therapy: A Pathway Based on Tetrahedral Framework Nucleic Acids Coupled Bacterial Nanocells. Nano Lett. 2023, 23, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, X.; Shao, B.; Xu, F.; Huang, X.; Guo, X.; Zhou, S. Self-Blockade of PD-L1 with Bacteria-Derived Outer-Membrane Vesicle for Enhanced Cancer Immunotherapy. Adv. Mater. 2022, 34, e2106307. [Google Scholar] [CrossRef]
- MacDiarmid, J.A.; Amaro-Mugridge, N.B.; Madrid-Weiss, J.; Sedliarou, I.; Wetzel, S.; Kochar, K.; Brahmbhatt, V.N.; Phillips, L.; Pattison, S.T.; Petti, C.; et al. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat. Biotechnol. 2009, 27, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Anselmo, A.C.; Huang, L. Nanotechnology intervention of the microbiome for cancer therapy. Nat. Nanotechnol. 2019, 14, 1093–1103. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Wang, Y.; Ning, P.; Shen, Y.; Wei, X.; Feng, Q.; Liu, Y.; Li, Z.; Xu, C.; et al. An Engineered Bacteria-Hybrid Microrobot with the Magnetothermal Bioswitch for Remotely Collective Perception and Imaging-Guided Cancer Treatment. ACS Nano 2022, 16, 6118–6133. [Google Scholar] [CrossRef]
- Akolpoglu, M.B.; Alapan, Y.; Dogan, N.O.; Baltaci, S.F.; Yasa, O.; Aybar Tural, G.; Sitti, M. Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery. Sci. Adv. 2022, 8, eabo6163. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Ji, Y.; Ding, X.; Xu, Z.; Hou, J.; Sun, J.; Xu, J.; Yu, S.; Sun, H.; Wang, T. Trojan nanobacteria hybridized with prodrug nanocapsules for efficient combined tumor therapy. Nano Res. 2023, 16, 9651–9662. [Google Scholar] [CrossRef]
- Wang, W.; Xu, H.; Ye, Q.; Tao, F.; Wheeldon, I.; Yuan, A.; Hu, Y.; Wu, J. Systemic immune responses to irradiated tumours via the transport of antigens to the tumour periphery by injected flagellate bacteria. Nat. Biomed. Eng. 2022, 6, 44–53. [Google Scholar] [CrossRef]
- Ektate, K.; Munteanu, M.C.; Ashar, H.; Malayer, J.; Ranjan, A. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci. Rep. 2018, 8, 13062. [Google Scholar] [CrossRef]
- Chen, Q.; Bai, H.; Wu, W.; Huang, G.; Li, Y.; Wu, M.; Tang, G.; Ping, Y. Bioengineering Bacterial Vesicle-Coated Polymeric Nanomedicine for Enhanced Cancer Immunotherapy and Metastasis Prevention. Nano Lett. 2020, 20, 11–21. [Google Scholar] [CrossRef]
- Yin, L.; Meng, Z.; Zhang, Y.; Hu, K.; Chen, W.; Han, K.; Wu, B.Y.; You, R.; Li, C.H.; Jin, Y.; et al. Bacillus spore-based oral carriers loading curcumin for the therapy of colon cancer. J. Control. Release 2018, 271, 31–44. [Google Scholar] [CrossRef]
- Fan, J.-X.; Niu, M.-T.; Qin, Y.-T.; Sun, Y.-X.; Zhang, X.-Z. Progress of engineered bacteria for tumor therapy. Adv. Drug Deliv. Rev. 2022, 185, 114296. [Google Scholar] [CrossRef]
- Pan, P.; Dong, X.; Chen, Y.; Zeng, X.; Zhang, X.Z. Engineered Bacteria for Enhanced Radiotherapy against Breast Carcinoma. ACS Nano 2022, 16, 801–812. [Google Scholar] [CrossRef]
- Ma, X.; Liang, X.; Li, Y.; Feng, Q.; Cheng, K.; Ma, N.; Zhu, F.; Guo, X.; Yue, Y.; Liu, G.; et al. Modular-designed engineered bacteria for precision tumor immunotherapy via spatiotemporal manipulation by magnetic field. Nat. Commun. 2023, 14, 1606. [Google Scholar] [CrossRef]
- Sun, M.; Yang, S.; Huang, H.; Gao, P.; Pan, S.; Cheng, Z.; He, Z.; Wang, Z.; Sun, J.; Liu, F. Boarding Oncolytic Viruses onto Tumor-Homing Bacterium-Vessels for Augmented Cancer Immunotherapy. Nano Lett. 2022, 22, 5055–5064. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Q.; Guo, H.; Fu, Z.; Liu, Y.; Lin, S.; Liu, J. Decorating Bacteria with Triple Immune Nanoactivators Generates Tumor-Resident Living Immunotherapeutics. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202409. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Wang, X.; Yang, F.; Cao, Z.; Wang, L.; Liu, J. Dressing Bacteria With a Hybrid Immunoactive Nanosurface to Elicit Dual Anticancer and Antiviral Immunity. Adv Mater. 2023, 35, e2210949. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Pu, Y.; Yao, H.; Lin, H.; Shi, J. Microbiotic nanomedicine for tumor-specific chemotherapy-synergized innate/adaptive antitumor immunity. Nano Today 2022, 42, 101377. [Google Scholar] [CrossRef]
- Sun, R.; Liu, M.; Lu, J.; Chu, B.; Yang, Y.; Song, B.; Wang, H.; He, Y. Bacteria loaded with glucose polymer and photosensitive ICG silicon-nanoparticles for glioblastoma photothermal immunotherapy. Nat. Commun. 2022, 13, 5127. [Google Scholar] [CrossRef]
- Bhoopathy, S.; Inbakandan, D.; Rajendran, T.; Chandrasekaran, K.; Kasilingam, R.; Gopal, D. Curcumin loaded chitosan nanoparticles fortify shrimp feed pellets with enhanced antioxidant activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111737. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, Y.; Zhang, Z.; Sun, X. Advances in Salmonella Typhimurium-based drug delivery system for cancer therapy. Adv. Drug Deliv. Rev. 2022, 185, 114295. [Google Scholar] [CrossRef]
- Redenti, A.; Hahn, J.; Danino, T. Bacterial couriers as cancer vaccines. Nat. Biomed. Eng. 2022, 6, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, X.; Pang, G.; Zhang, Y.; Pan, H.; Li, L.; Cui, M.; Liu, B.; Kang, R.; Xue, X.; et al. Hydrogel microcapsules containing engineered bacteria for sustained production and release of protein drugs. Biomaterials 2022, 287, 121619. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Liu, Z.; Huang, C.; Zeng, N.; Jiang, W.; Li, Q. Novel Engineered Bacterium/Black Phosphorus Quantum Dot Hybrid System for Hypoxic Tumor Targeting and Efficient Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 10564–10573. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Chen, Q.W.; Luo, G.F.; Ji, P.; Han, Z.Y.; Song, W.F.; Chen, W.H.; Zhang, X.Z. Interference of Glucose Bioavailability of Tumor by Engineered Biohybrids for Potentiating Targeting and Uptake of Antitumor Nanodrugs. Nano Lett. 2022, 22, 8735–8743. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Du, Y.; Lin, L.; Zhang, Z.; Ou, X.; Chen, S.; Wang, Q.; Zou, J. Genetically engineered bacteria-mediated multi-functional nanoparticles for synergistic tumor-targeting therapy. Acta Biomater. 2022, 150, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, R.; Jiang, Y.; Shen, W.; Pei, H.; Wang, G.; Pei, P.; Yang, K. The role of bacteria and its derived biomaterials in cancer radiotherapy. Acta Pharm. Sin. B 2022, 13, 4149–4171. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, T.; Bian, Y.; Meng, F.; Yu, S.; Li, H.; Zhang, Q.; Gu, L.; Weng, X.; Tan, C.; et al. Coupling Probiotics with 2D CoCuMo-LDH Nanosheets as a Tumor-Microenvironment-Responsive Platform for Precise NIR-II Photodynamic Therapy. Adv. Mater. 2023, 35, e2211205. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Han, J.-W.; Choi, Y.J.; Cho, S.; Zheng, S.; Ko, S.Y.; Park, J.-O.; Park, S. Active tumor-therapeutic liposomal bacteriobot combining a drug (paclitaxel)-encapsulated liposome with targeting bacteria (Salmonella Typhimurium). Sens. Actuator B Chem. 2016, 224, 217–224. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, X.; Cheng, L.; Chen, X.; Tang, J.; Zhang, P.; Wang, C.; Liu, J. Surface programmed bacteria as photo-controlled NO generator for tumor immunological and gas therapy. J. Control. Release 2023, 353, 889–902. [Google Scholar] [CrossRef]
- Reghu, S.; Miyako, E. Nanoengineered Bifidobacterium bifidum with Optical Activity for Photothermal Cancer Immunotheranostics. Nano Lett. 2022, 22, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, M.; Du, L.; Zeng, J.; Yao, T.; Jin, Y. Paclitaxel-in-liposome-in-bacteria for inhalation treatment of primary lung cancer. Int. J. Pharm. 2020, 578, 119177. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Xiao, N.; Liu, Y.; Wang, Z.; Tóth, J.; Gyenis, J.; Thakur, V.K.; Oyane, A.; Shubhra, Q.T.H. Engineering polymer nanoparticles using cell membrane coating technology and their application in cancer treatments: Opportunities and challenges. Nano Mater. Sci. 2021, 4, 295–321. [Google Scholar] [CrossRef]
- Naskar, A.; Cho, H.; Lee, S.; Kim, K.-S. Biomimetic nanoparticles coated with bacterial outer membrane vesicles as a new-generation platform for biomedical applications. Pharmaceutics 2021, 13, 1887. [Google Scholar] [CrossRef]
- Imran, M.; Jha, L.A.; Hasan, N.; Shrestha, J.; Pangeni, R.; Parvez, N.; Mohammed, Y.; Jha, S.K.; Paudel, K.R. “Nanodecoys”—Future of drug delivery by encapsulating nanoparticles in natural cell membranes. Int. J. Pharm. 2022, 621, 121790. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, H.; Peng, H.; Zhou, H.; Li, P.Y.; Langer, R. Non-genetic engineering of cells for drug delivery and cell-based therapy. Adv. Drug Deliv. Rev. 2015, 91, 125–140. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, M.; Gao, Y.; Cheng, X.; Liu, X.; Tang, S.; Peng, Y.; Wang, N.; Hu, D.; Peng, H.; et al. Biomimetic erythrocytes engineered drug delivery for cancer therapy. Chem. Eng. J. 2022, 433, 133498. [Google Scholar] [CrossRef]
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.M.; Zhang, L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef]
- Wu, G.; Ji, H.; Guo, X.; Li, Y.; Ren, T.; Dong, H.; Liu, J.; Liu, Y.; Shi, X.; He, B. Nanoparticle reinforced bacterial outer-membrane vesicles effectively prevent fatal infection of carbapenem-resistant Klebsiella pneumoniae. Nanomedicine 2020, 24, 102148. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Jiang, W.; Yang, C.; Miao, H.; Wang, Y. Nanovaccines integrating endogenous antigens and pathogenic adjuvants elicit potent antitumor immunity. Nano Today 2020, 35, 101007. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.; You, S.; Zhang, K.; Li, M.; Cao, Y.; Wang, C.; Dong, H.; Zhang, X. Bacterial Vesicle-Cancer Cell Hybrid Membrane-Coated Nanoparticles for Tumor Specific Immune Activation and Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 41138–41147. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Lo, C.; Zhuang, J.; Angsantikul, P.; Zhang, Q.; Wei, X.; Zhou, Z.; Obonyo, M.; Fang, R.H.; et al. Inhibition of Pathogen Adhesion by Bacterial Outer Membrane-Coated Nanoparticles. Angew. Chem. Int. Ed. Engl. 2019, 58, 11404–11408. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, L.; Yang, B.; Fan, F.; Yang, L. Kill the Real with the Fake: Eliminate Intracellular Staphylococcus aureus Using Nanoparticle Coated with Its Extracellular Vesicle Membrane as Active-Targeting Drug Carrier. ACS Infect. Dis. 2019, 5, 218–227. [Google Scholar] [CrossRef]
- Qin, J.; Yang, T.; Li, J.; Zhan, G.; Li, X.; Wei, Z.; Chen, Z.; Zheng, W.; Chen, H.; Yang, X.; et al. Bacterial outer membrane vesicle-templated biomimetic nanoparticles for synergistic photothermo-immunotherapy. Nano Today 2022, 46, 101591. [Google Scholar] [CrossRef]
- Ong, S.; Chitneni, M.; Lee, K.; Ming, L.; Yuen, K. Evaluation of Extrusion Technique for Nanosizing Liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- Di Toro, R.; Betti, V.; Spampinato, S. Biocompatibility and integrin-mediated adhesion of human osteoblasts to poly(DL-lactide-co-glycolide) copolymers. Eur. J. Pharm. Sci. 2004, 21, 161–169. [Google Scholar] [CrossRef]
- Chen, L.; Qin, H.; Zhao, R.; Zhao, X.; Lin, L.; Chen, Y.; Lin, Y.; Li, Y.; Qin, Y.; Li, Y. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci. Transl. Med. 2021, 13, eabc2816. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, G.; Wu, W.; Wang, J.; Hu, J.; Mao, J.; Chu, P.K.; Bai, H.; Tang, G. A Hybrid Eukaryotic-Prokaryotic Nanoplatform with Photothermal Modality for Enhanced Antitumor Vaccination. Adv. Mater. 2020, 32, e1908185. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Dong, X.; Chen, Y.; Ye, J.J.; Sun, Y.X.; Zhang, X.Z. A heterogenic membrane-based biomimetic hybrid nanoplatform for combining radiotherapy and immunotherapy against breast cancer. Biomaterials 2022, 289, 121810. [Google Scholar] [CrossRef]
- Guo, P.; Huang, J.; Zhao, Y.; Martin, C.R.; Zare, R.N.; Moses, M.A. Nanomaterial Preparation by Extrusion through Nanoporous Membranes. Small 2018, 14, e1703493. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Song, Y.; Bai, S.; He, C.; Guo, Z.; Zhu, Y.; Zhang, Z.; Sun, X. Cloaking Mesoporous Polydopamine with Bacterial Membrane Vesicles to Amplify Local and Systemic Antitumor Immunity. ACS Nano 2023, 17, 7733–7749. [Google Scholar] [CrossRef]
- Witwer, K.W.; Wolfram, J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat. Rev. Mater. 2021, 6, 103–106. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, J.; Qiu, M.; Chen, J.; Liang, Q.; Peng, G.; Zou, Z. Bacteria-mediated metformin-loaded peptide hydrogel reprograms the tumor immune microenvironment in glioblastoma. Biomaterials 2022, 288, 121711. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Chen, Q.-W.; Fu, Z.-J.; Cheng, S.-X.; Zhang, X.-Z. Probiotic Spore-Based Oral Drug Delivery System for Enhancing Pancreatic Cancer Chemotherapy by Gut–Pancreas-Axis-Guided Delivery. Nano Lett. 2022, 22, 8608–8617. [Google Scholar] [CrossRef]
- Dong, C.Y.; Huang, Q.X.; Cheng, H.; Zheng, D.W.; Hong, S.; Yan, Y.; Niu, M.T.; Xu, J.G.; Zhang, X.Z. Neisseria meningitidis Opca Protein/MnO2 Hybrid Nanoparticles for Overcoming the Blood-Brain Barrier to Treat Glioblastoma. Adv. Mater. 2022, 34, e2109213. [Google Scholar] [CrossRef]
- Hwang, J.; An, E.K.; Kim, S.J.; Zhang, W.; Jin, J.O. Escherichia coli Mimetic Gold Nanorod-Mediated Photo- and Immunotherapy for Treating Cancer and Its Metastasis. ACS Nano 2022, 16, 8472–8483. [Google Scholar] [CrossRef]
- Tsung, K.; Norton, J.A. Lessons from Coley’s Toxin. Surg. Oncol. 2006, 15, 25–28. [Google Scholar] [CrossRef]
- Huh, W.K.; Brady, W.E.; Fracasso, P.M.; Dizon, D.S.; Powell, M.A.; Monk, B.J.; Leath, C.A., 3rd; Landrum, L.M.; Tanner, E.J.; Crane, E.K.; et al. Phase II study of axalimogene filolisbac (ADXS-HPV) for platinum-refractory cervical carcinoma: An NRG oncology/gynecologic oncology group study. Gynecol. Oncol. 2020, 158, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.; Cunningham, C.; Senzer, N.; Kuhn, J.; Cramm, J.; Litz, C.; Cavagnolo, R.; Cahill, A.; Clairmont, C.; Sznol, M. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003, 10, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Gniadek, T.J.; Augustin, L.; Schottel, J.; Leonard, A.; Saltzman, D.; Greeno, E.; Batist, G. A phase I, dose escalation, single dose trial of oral attenuated Salmonella typhimurium containing human IL-2 in patients with metastatic gastrointestinal cancers. J. Immunother. 2020, 43, 217. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Abedishirehjin, S.; Baghbadorani, M.A.; Handali, S. Bacteria and Archaea: A new era of cancer therapy. J. Control. Release 2021, 338, 1–7. [Google Scholar] [CrossRef]
- Effenberger-Neidnicht, K.; Hartmann, M. Mechanisms of Hemolysis During Sepsis. Inflammation 2018, 41, 1569–1581. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T. The ‘cytokine storm’: Molecular mechanisms and therapeutic prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef]
- Gurbatri, C.R.; Arpaia, N.; Danino, T. Engineering bacteria as interactive cancer therapies. Science 2022, 378, 858–864. [Google Scholar] [CrossRef]
- Huang, X.; Pan, J.; Xu, F.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Bacteria-Based Cancer Immunotherapy. Adv. Sci. 2021, 8, 2003572. [Google Scholar] [CrossRef] [PubMed]
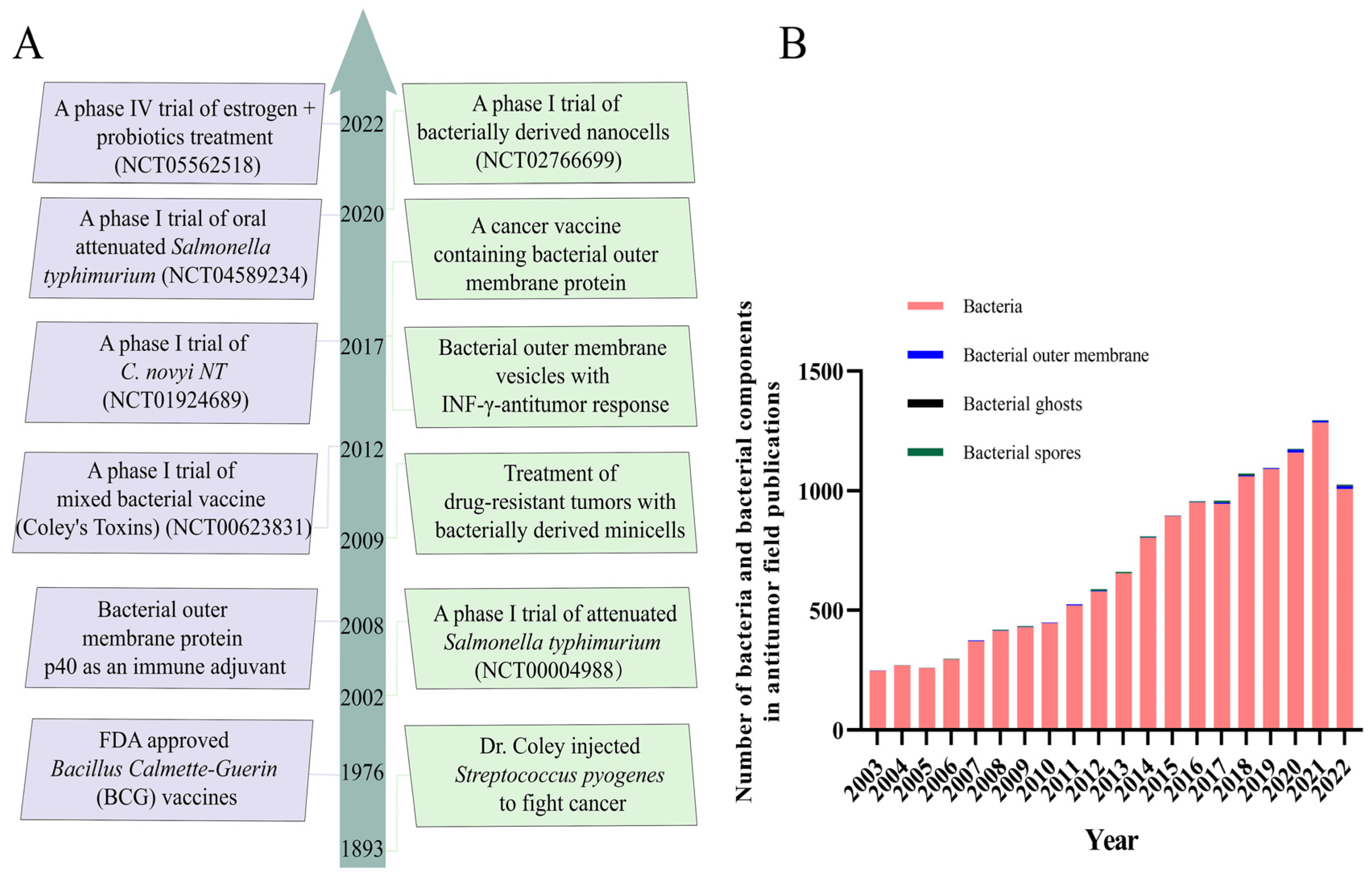
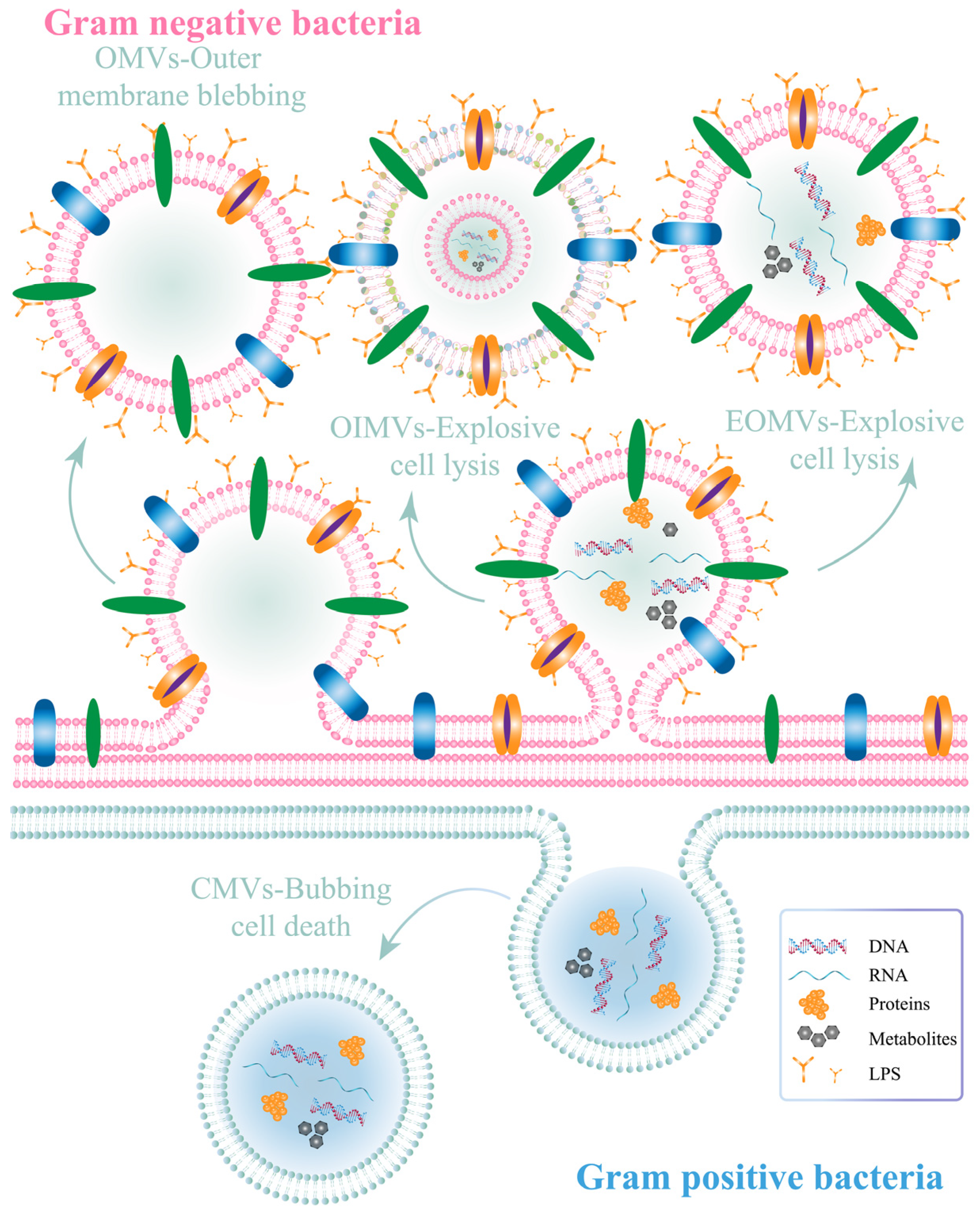
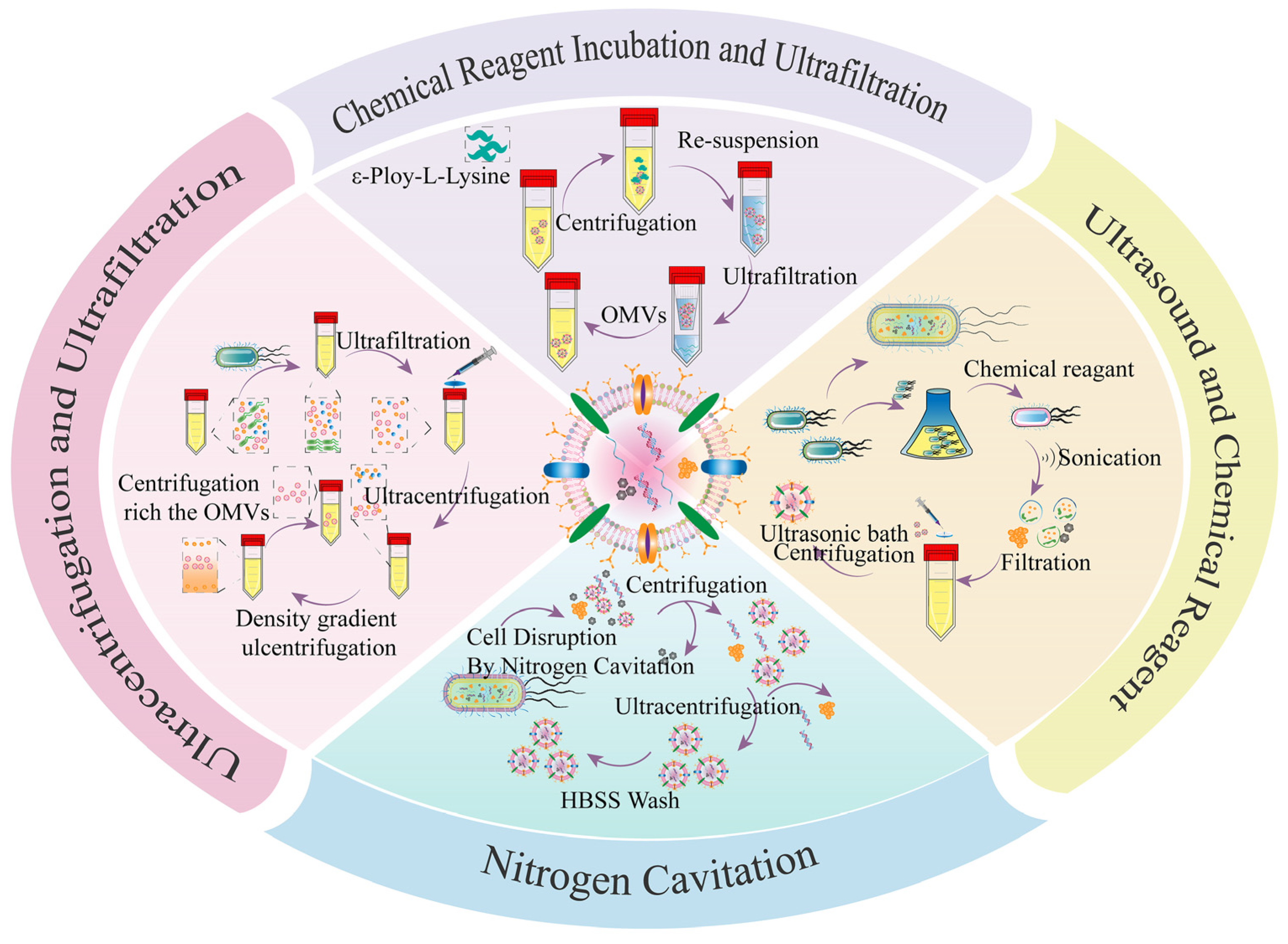
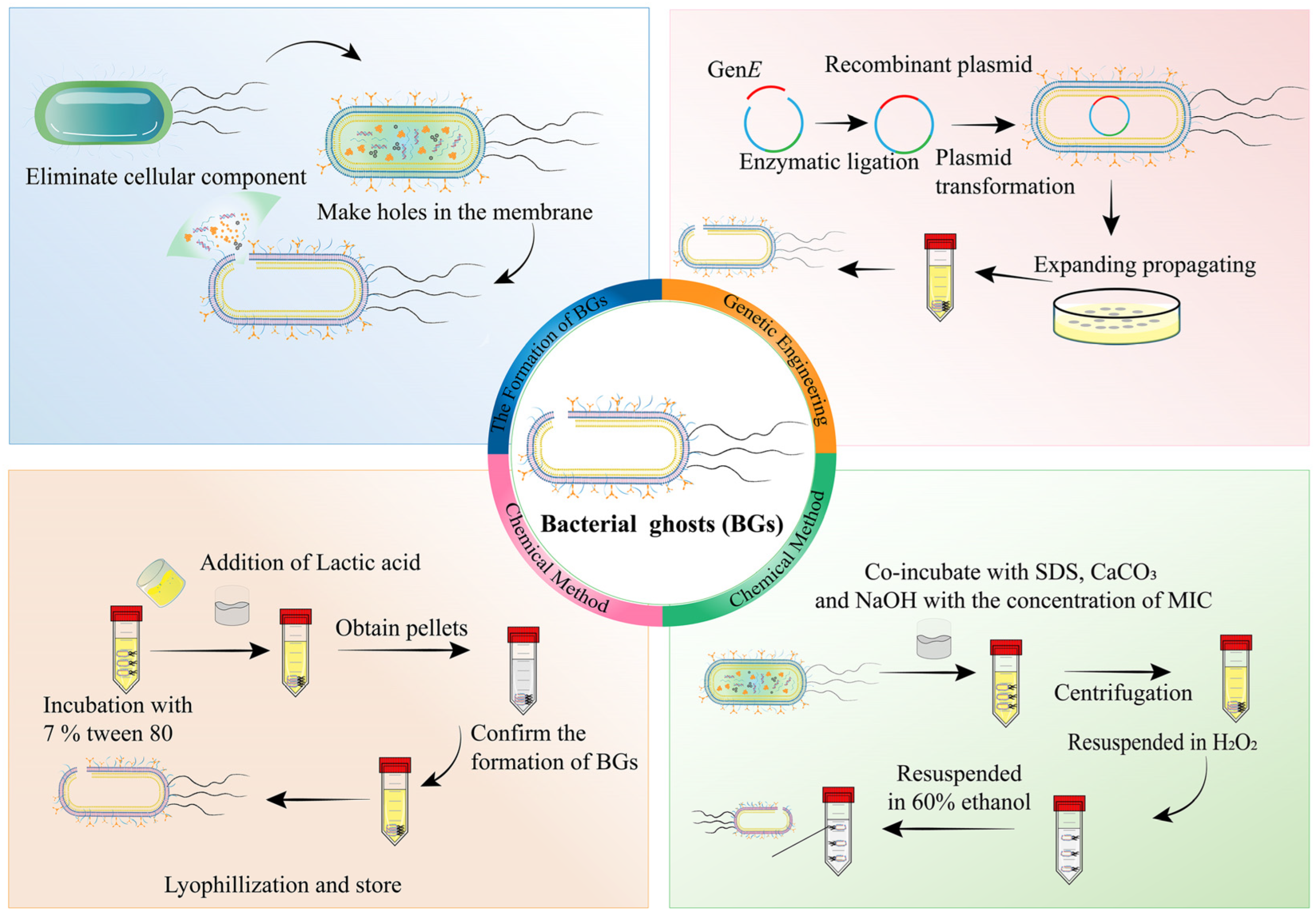
| Bacteria/ Bacterial Components | Microorganism | Method | Results | Ref. |
|---|---|---|---|---|
| Bacteria | E. coli Nissle 1917 Salmonella typhimurium VNP20009 | Chemical binding | Activated immune responses Targeted intratumoral localication | [38] |
| Bacteria | E. coli BL21(DE3) | Chemical binding | Dual ability of tumor immune activation | [97] |
| Bacteria | E. coli MG1655 | Genetic engineering | Actively targeted to solid tumor regions Induced tumor cell autophagy | [98] |
| Bacteria | E. coli Nissle 1917 | Genetic engineering Biomimetic surface coating | Improved antitumor efficacy in vivo Increased microbial translocation in distal tumors | [99] |
| Bacteria | Attenuated Salmonella typhimurium VNP20009 | Biomimetic surface coating | Synergistic and systematic antitumor immune responses Inhibited tumor progression and metastasis | [100] |
| OMVs | E. coli BL21 (DE3) | Genetic engineering Surface modification | Remodeled TME Long-time adaptive immune response | [23] |
| OMVs | E. coli DH5α | Genetic engineering | Inhibited tumor angiogenesis Promoted tumor cell apoptosis | [68] |
| OMVs | Salmonella typhimurium ATCC 14028 | Simple incubation | Enhanced autophagy and apoptosis of tumor cells | [101] |
| OMVs | Attenuated K. pneumonia ACCC 60095 | Simple incubation | Recruited macrophages in TME Promoted tumor cell apoptosis | [102] |
| BGs | E. coli Nissle 1917 | Simple incubation | Promoted DC maturation Increased CD4+ and CD8+ T-cell proliferation | [103] |
| BGs | E. coli Nissle 1917 | Simple incubation | Exhibited synergistic antitumor activity Induced immunogenic cell death | [104] |
| Spores | Clostridium novyi-NT | Simple incubation | Induced a systemic immune cytokine response Enhanced the tumor cell-specific T-cell activation | [41] |
| Spores | C. butyricum ATCC 19398 | Simple incubation | Targeted and enriched in tumor sites | [93] |
| Bacteria/ Bacterial Component | Microorganism | Gene | Type of Ligand | Method | Ref. |
|---|---|---|---|---|---|
| Bacteria | Streptococcus mutans (S. m) | ssDNAs | Nucleic aptamer AS1411 | Surface modification | [143] |
| OMVs | E. coli BL21 (ΔmsbB) | Redd1 siRNA | DSPE -PEG | Electroporation Surface modification | [106] |
| OMVs | E. coli BL21 (DE3) | Box C/D mRNA | -- | Genetic engineering Surface modification | [59] |
| OMVs | Engineered K-12 W3110 E. coli | KSP siRNA | HER2 | Genetic engineering Biotin–streptavidin | [107] |
| OMVs | Engineered K-12 W3110 E. coli | PD-1 pDNA | LyP1 | Genetic engineering Plasmid transfection | [144] |
| OMVs | Attenuated Salmonella | CD38 siRNA | -- | Ultrasonic fusion | [120] |
| Minicells | Salmonella enterica serovar Typhimurium | PLK siRNA KSP siRNA MDR1 siRNA | EGFR | Simple incubation | [145] |
| Bacteria/ Bacterial Components | Microorganism | Nanoparticles | Method | Results | Ref. |
|---|---|---|---|---|---|
| Bacteria | E. coli Nissle 1917 | Magnetic nanoparticles | Chemical bonds Genetic engineering Surface modification | Triggered with magnetothermal ablation NDH-2-induced ROS damage | [147] |
| Bacteria | E. coli MG1655 | Magnetic nanoparticles Nanoliposomes | Streptavidin–biotin | Moved through the tumor spheroids autonomously under magnetic field | [148] |
| Bacteria | E. coli MG 1655 | Nanocapsules | Chemical bonds | Colonized to the tumor sites Enhanced T-cell infiltration | [149] |
| Bacteria | Salmonella typhimurium VNP20009 | Polyamidoamine dendrimer | Electrostatic interactions Biospecific binding | Enhanced antitumor immune responses | [150] |
| Bacteria | Attenuated Salmonella typhimurium (YS1646) | Liposomes | Streptavidin–biotin | Enhanced immune cell infiltration Excellent tumor-suppressive effects | [151] |
| OMVs | Attenuated Salmonella typhimurium | Nanomicelles | Membrane extrusion | Activated macrophages for the stimulation of T cells Prevented tumor metastasis | [152] |
| OMVs | E. coli K1 | PLGA nanoparticles | Membrane extrusion | Prolonged the elimination of drugs Superior brain-targeting ability | [123] |
| OMVs | E. coli ATCC 25922 | Fe3O4-MnO2 nanoparticles | Ultrasonic fusion | Targeted to solid tumor sites Induced antitumor immune responses | [127] |
| BGs | E. coli Nissle 1917 | Au nanorods | Electroporation Physical adsorption | Stimulated the immune response Synergistic tumor inhibition efficacy | [84] |
| BSPs | Bacillus coagulans | Nanomicelles | Chemical bonds | Targeted to tumor sites Resulted in tumor cell apoptosis | [153] |
| Method | Advantages | Challenges | Refs. | |
|---|---|---|---|---|
| Bacteria | Chemical bonds | Strong bond association High spatiotemporal control | Modification of limited ligands Unavoidable bacteria damage | [29,147,155,158] |
| Electrostatic interactions | Easy formation Multifunctional therapeutics | Poor stability of assemble conjugations | [29,166,167] | |
| Biotin–streptavidin | High binding affinity Better therapeutic effect | -- | [29,151,172] | |
| Electroporation | Highly efficient anticancer effects High accumulative distribution | Side effect of viability of bacteria | [175] | |
| Bacterial components | Membrane extrusion | Uniform size Better preservation of biomolecules | Time-consuming Difficult for large-scale production | [176,177] |
| Ultrasonic fusion | Safe and non-toxic Faster and easier to perform Reduced loss of material | Denaturation of membrane proteins Drug leakage Lack of uniformity | [176,177,178] | |
| Microfluidic electroporation | Accurated control of size High reproducibility | Not commercially available Need to explore the scalability | [176,178] |
| Bacteria /Bacterial Component | Clinical Trial Identifier | Cancer Types | Interventions | Status | Route |
|---|---|---|---|---|---|
| Bacteria | NCT05562518 | Breast Cancer | Probiotics | Phase IV | Local administration |
| Bacteria | NCT04874883 | Colorectal Cancer | Simbyotic | Phase IV | Oral administration |
| Bacteria | NCT03742596 | Colorectal Cancer | Probiotic formula capsule | Phase II/Phase III | Oral administration |
| Bacteria | NCT01579591 | Rectal Cancer | Probiotics | Phase III | Oral administration |
| Bacteria | NCT02002182 | Squamous Cell Carcinoma | Modified Listeria monocytogenes | Phase II | Intravenous administrations |
| Bacteria | NCT03847519 | Lung Cancer | Attenuated Listeria monocytogenes | Phase I/Phase II | Intravenous administrations |
| Bacteria | NCT01266460 | Carcinoma | Attenuated live Listeria Encoding HPV 16 E7 | Phase II | Intravenous administrations |
| Bacteria | NCT01099631 | Liver Cancer Biliary Cancer | Biological: Salmonella typhimurium | Phase I | Oral administration |
| Bacteria | NCT00004988 | Advanced or Metastatic Cancer | Salmonella typhimurium VNP20009 | Phase II | Intravenous administrations |
| Bacteria | NCT04589234 | Pancreatic Cancer | Salmonela-IL2 | Phase II | Oral administration |
| Bacteria | NCT00623831 | Malignancies | Mixed bacteria vaccine | Phase I | Subcutaneous administration |
| Bacterial Components | NCT02766699 | Glioblastoma | Bacterially derived nonviable nanocells | Phase I | Intravenous administrations |
| Bacterial Components | NCT01924689 | Solid Tumor Malignancies | Clostridium novyi-NT spores | Phase I | Intratumoral injection |
| Bacterial Components | NCT01118819 | Solid Tumor Malignancies | Clostridium novyi-NT spores | Phase I | Intratumoral injection |
| Bacterial Components | NCT00358397 | Tumors | Clostridium novyi-NT spores | Phase I | Intravenous administrations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, R.; Ruan, H.; Liu, C.; Fan, S.; Li, J. Bacteria and Bacterial Components as Natural Bio-Nanocarriers for Drug and Gene Delivery Systems in Cancer Therapy. Pharmaceutics 2023, 15, 2490. https://doi.org/10.3390/pharmaceutics15102490
Zong R, Ruan H, Liu C, Fan S, Li J. Bacteria and Bacterial Components as Natural Bio-Nanocarriers for Drug and Gene Delivery Systems in Cancer Therapy. Pharmaceutics. 2023; 15(10):2490. https://doi.org/10.3390/pharmaceutics15102490
Chicago/Turabian StyleZong, Rui, Hainan Ruan, Chanmin Liu, Shaohua Fan, and Jun Li. 2023. "Bacteria and Bacterial Components as Natural Bio-Nanocarriers for Drug and Gene Delivery Systems in Cancer Therapy" Pharmaceutics 15, no. 10: 2490. https://doi.org/10.3390/pharmaceutics15102490
APA StyleZong, R., Ruan, H., Liu, C., Fan, S., & Li, J. (2023). Bacteria and Bacterial Components as Natural Bio-Nanocarriers for Drug and Gene Delivery Systems in Cancer Therapy. Pharmaceutics, 15(10), 2490. https://doi.org/10.3390/pharmaceutics15102490






