Cydonia oblonga-Seed-Mucilage-Based pH-Sensitive Graft Copolymer for Controlled Drug Delivery—In Vitro and In Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Cydonia oblonga Mucilage
2.3. Preparation of Cydonia oblonga-Mucilage-Based Hydrogels
2.4. Physicochemical Properties of Hydrogels
2.4.1. Swelling Studies
2.4.2. Drug Loading
2.4.3. Fourier-Transform Infrared (FTIR) Analysis
2.4.4. Scanning Electron Microscopy (SEM)
2.4.5. In Vitro Drug Release Measurement
2.4.6. Percentage Drug Release
2.4.7. Evaluation of Release Kinetics
2.4.8. In Vivo Evaluation
Study Design for In Vivo Evaluation
Standard Preparation
HPLC Analysis
In Vivo Pharmacokinetic Evaluation
3. Results and Discussion
3.1. Percentage Yield and Chemical Reaction Scheme
3.2. Swelling Studies
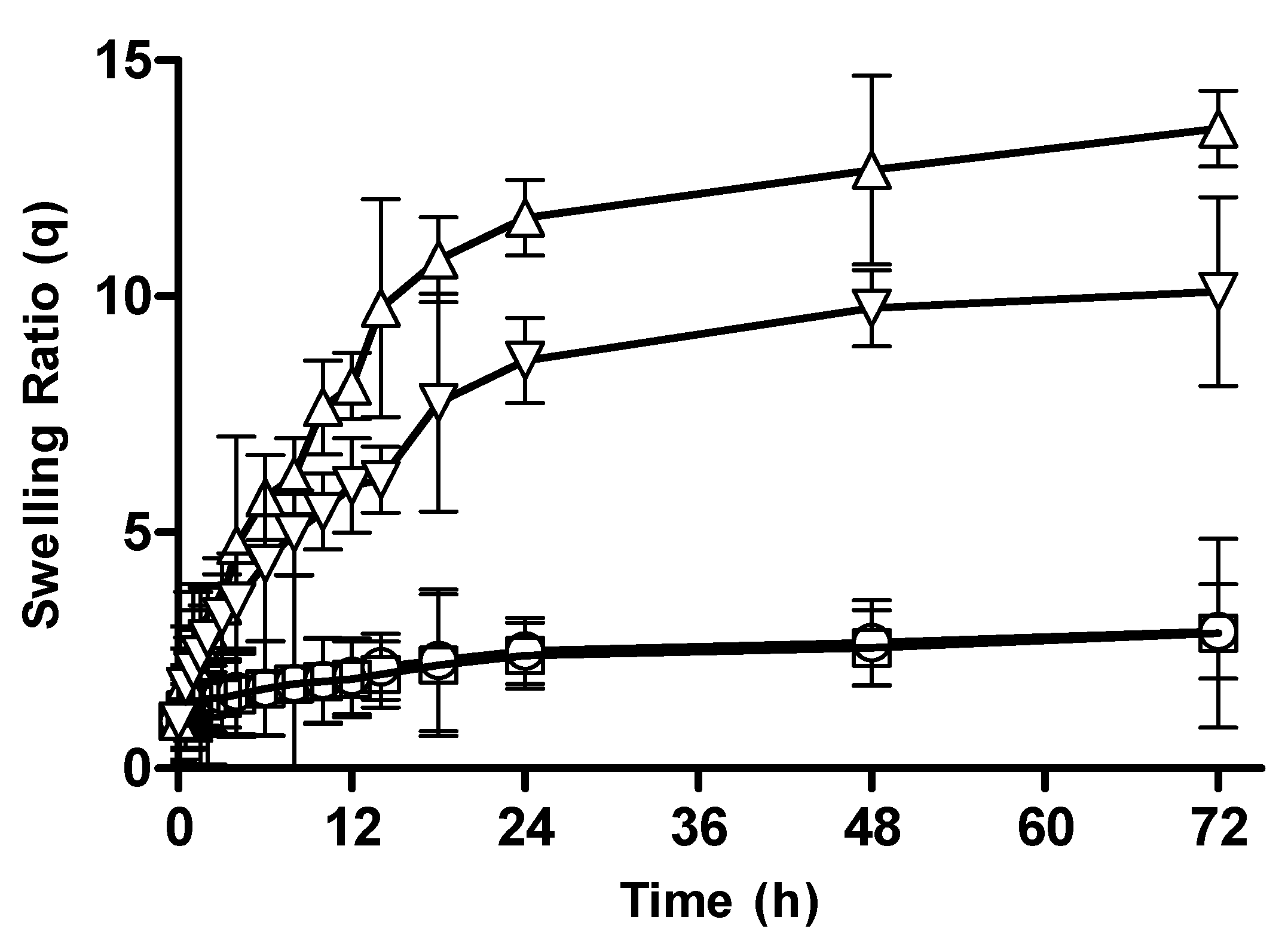
3.2.1. Hydrogel Swelling at Different pHs
3.2.2. Effect of Polymer Concentration on Swelling Behavior of CM-co-AA and CM-co-MAA Hydrogels
3.2.3. Effect of Monomer Concentration on Swelling Behavior of CM-co-AA Hydrogels
3.2.4. Effect of Monomer Concentration on Swelling Behavior of CM-co-MAA Hydrogels
3.2.5. Effect of Crosslinker Concentration on Swelling Behavior of CM-co-AA and CM-co-MAA Hydrogels
3.2.6. Drug Loading
3.2.7. FTIR Characterization of Hydrogels
3.2.8. Scanning Electron Microscopy (SEM) of CM-co-AA and CM-co-MAA
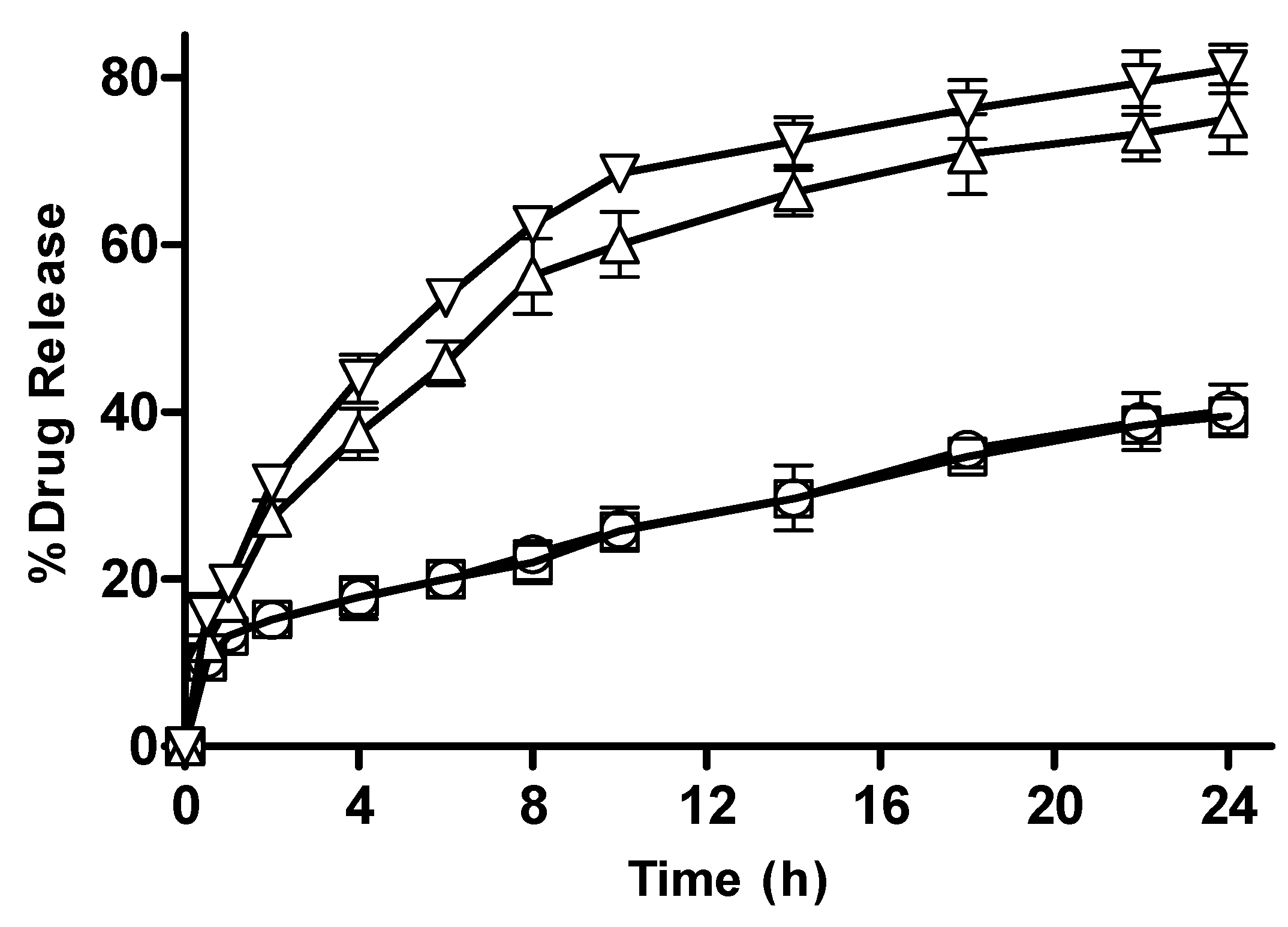
3.2.9. In Vitro Drug Release from CM-co-AA and CM-co-MAA Hydrogels
Effect of pH
3.2.10. In Vitro Drug Release from CM-co-AA and CM-co-MAA Hydrogels with Different Polymer Concentration
3.2.11. In Vitro Drug Release from CM-co-AA, and CM-co-MAA Hydrogels with Varying Crosslinker (MBA) Concentration
3.2.12. In Vitro Drug Release from CM-co-AA Hydrogels with Varying Concentration of Acrylic Acid (AA)
3.2.13. In Vitro Drug Release from CM-co-MAA Hydrogels with Varying Concentration of MAA
3.2.14. Drug Release Kinetics
3.2.15. In Vivo Pharmacokinetic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colter, J.; Wirostko, B.; Coats, B. Finite element design optimization of a hyaluronic acid-based hydrogel drug delivery device for improved retention. Ann. Biomed. Eng. 2017, 46, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Deen, G.R.; Loh, X.J. Stimuli-responsive cationic hydrogels in drug delivery applications. Gels 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Allafchian, A.; Jalali, S.A.H.; Mousavi, S.E.; Hosseini, S.S. Preparation of cell culture scaffolds using polycaprolactone/quince seed mucilage. Int. J. Biol. Macromol. 2019, 155, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Mondal, S.; Das, P.; Bhawal, P.; Maity, P.P.; Ghosh, S.; Dhara, S.; Das, N.C. Design of psyllium-g-poly (acrylic acid-co-sodium acrylate)/cloisite 10A semi-IPN nanocomposite hydrogel and its mechanical, rheological and controlled drug release behaviour. Int. J. Biol. Macromol. 2018, 111, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Ramburrun, P.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Design and characterization of neurodurable gellan-xanthan pH-responsive hydrogels for controlled drug delivery. Expert Opin. Drug Deliv. 2017, 14, 291–306. [Google Scholar] [CrossRef] [PubMed]
- DE Santo, M.; Giovinazzo, A.; Fava, M.R.; Mazzotta, E.; De Napoli, I.E.; Greco, M.; Comandè, A.; Nigro, A.; Argurio, P.; Perrotta, I.; et al. Engineered mesoporous silica-based nanoparticles as smart chemotherapy nanodevice for bortezomib administration. Mater. Chem. Front. 2022, 7, 216–229. [Google Scholar] [CrossRef]
- Kuskov, A.; Nikitovic, D.; Berdiaki, A.; Shtilman, M.; Tsatsakis, A. Amphiphilic poly-N-vinylpyrrolidone nanoparticles as carriers for nonsteroidal, anti-inflammatory drugs: Pharmacokinetic, anti-inflammatory, and ulcerogenic activity study. Pharmaceutics 2022, 14, 925. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Wang, J.-L.; Zhang, H.-B.; Khan, M.I.; Du, X.-J.; Wang, J. Incorporation of a rhodamine B conjugated polymer for nanoparticle trafficking both in vitro and in vivo. Biomater. Sci. 2019, 7, 1933–1939. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Rahimzadeh-Sani, Z.; Feng, J.; Hosseini, H.; Jafari, S.M. The impact of essential oils on the qualitative properties, release profile, and stimuli-responsiveness of active food packaging nanocomposites. Crit. Rev. Food Sci. Nutr. 2021, 63, 1822–1845. [Google Scholar] [CrossRef]
- Kamel, R.; Afifi, S.M.; Kassem, I.A.; Elkasabgy, N.A.; Farag, M.A. Arabinoxylan and rhamnogalacturonan mucilage: Outgoing and potential trends of pharmaceutical, environmental, and medicinal merits. Int. J. Biol. Macromol. 2020, 165, 2550–2564. [Google Scholar] [CrossRef]
- Soleimani, K.; Derakhshankhah, H.; Jaymand, M.; Samadian, H. Stimuli-responsive natural gums-based drug delivery systems for cancer treatment. Carbohydr. Polym. 2021, 254, 117422. [Google Scholar] [CrossRef] [PubMed]
- Kulsoom, R.; Sarfraz, M.; Afzal, A.; Farooq, M.; Adnan, S.; Ashraf, M.U.; Khan, S.A. Synthesis of calcium carbonate-quince bio-composite for programmed and on-demand drug release of paracetamol at target site: A green chemistry approach. Polym. Bull. 2022, 80, 6965–6988. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Muhammad, G.; Haseeb, M.T.; Tahir, M.N. Quince seed mucilage: A stimuli-responsive/smart biopolymer. In Functional Biopolymers; Springer: Cham, Switzerland, 2019; pp. 127–148. [Google Scholar] [CrossRef]
- Garg, S.; Bal, T.; Panpalia, S.G.; Rajora, A.D.; Ghosh, B.D. Preparation and characterization of microwave irradiated pH-sensitive polyacrylamide grafted flax seed mucilage graft copolymeric hydrogel (PFLSM-g-PAM-cl-MBA) and its evaluation as effective polymeric scaffold. Sustain. Chem. Pharm. 2021, 22, 100479. [Google Scholar] [CrossRef]
- Malipeddi, V.R.; Awasthi, R.; Ghisleni, D.D.M.; Braga, M.d.S.; Kikuchi, I.S.; Pinto, T.d.J.A.; Dua, K. Preparation and characterization of metoprolol tartrate containing matrix type transdermal drug delivery system. Drug Deliv. Transl. Res. 2016, 7, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Mammadova, S.; Tapdigov, S.Z.; Humbatova, S.; Aliyeva, S.; Zeynalov, N.; Soltanov, C.A.; Cavadzadeh, A. Synthesis, structure and swelling properties of hydrogels based on polyacrylic acid. Asian J. Chem. 2017, 29, 576–580. [Google Scholar] [CrossRef]
- Ijaz, H.; Tulain, U.R.; Qureshi, J. Formulation and in vitro evaluation of pH-sensitive cross-linked xanthan gum-grafted acrylic acid copolymer for controlled delivery of perindopril erbumine (PE). Polym.-Plast. Technol. Eng. 2018, 57, 459–470. [Google Scholar] [CrossRef]
- Ashraf, M.U.; Hussain, M.A.; Haseeb, M.T.; Erum, A.; Mushtaq, M.N. Acute toxicity studies of glucuronoxylan polysaccharides from seeds of quince (cydonia oblonga). Cellul. Chem. Technol. 2019, 53, 721–729. [Google Scholar] [CrossRef]
- Sohail, M.; Ahmad, M.; Minhas, M.U.; Ali, L.; Khalid, I.; Rashid, H. Controlled delivery of valsartan by cross-linked polymeric matrices: Synthesis, in vitro and in vivo evaluation. Int. J. Pharm. 2015, 487, 110–119. [Google Scholar] [CrossRef]
- Nawaz, S.; Khan, S.; Farooq, U.; Haider, M.S.; Ranjha, N.M.; Rasul, A.; Nawaz, A.; Arshad, N.; Hameed, R. Biocompatible hydrogels for the controlled delivery of anti-hypertensive agent: Development, characterization and in vitro evaluation. Des. Monomers Polym. 2018, 21, 18–32. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.M.; Mahmoud, A.A.; Shahin, H.I. Design and in vitro/in vivo evaluation of ultra-thin mucoadhesive buccal film containing fluticasone propionate. Aaps Pharmscitech 2016, 18, 93–103. [Google Scholar] [CrossRef]
- Sabbagh, N.; Akbari, A.; Arsalani, N.; Eftekhari-Sis, B.; Hamishekar, H. Halloysite-based hybrid bionanocomposite hydrogels as potential drug delivery systems. Appl. Clay Sci. 2017, 148, 48–55. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K. Synthesis and characterization of karaya gum-g-poly (acrylic acid) hydrogels and in vitro release of hydrophobic quercetin. Polymer 2018, 147, 108–120. [Google Scholar] [CrossRef]
- Ijaz, H.; Qureshi, J.; Danish, Z.; Zaman, M.; Abdel-Daim, M.; Hanif, M.; Waheed, I.; Mohammad, I.S. Formulation and in-vitro evaluation of floating bilayer tablet of lisinopril maleate and metoprolol tartrate. Pak. J. Pharm. Sci. 2015, 28, 2019–2025. [Google Scholar] [PubMed]
- Ghosh, A.; Nayak, U.K.; Rout, P.; Nag, T.; Roy, P. Preparation, Evaluation and in vitro-in vivo Correlation (IVIVC) study of Lamivudine Loaded Microspheres. Res. J. Pharm. Technol. 2008, 1, 353–356. [Google Scholar]
- Seeli, D.S.; Prabaharan, M. Guar gum oleate-graft-poly (methacrylic acid) hydrogel as a colon-specific controlled drug delivery carrier. Carbohydr. Polym. 2017, 158, 51–57. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis and characterization of superabsorbent hydrogels based on hydroxyethylcellulose and acrylic acid. Carbohydr. Polym. 2017, 166, 300–308. [Google Scholar] [CrossRef]
- Mamman, I.S.; Teo, Y.Y.; Misran, M. Synthesis, characterization and rheological study of Arabic gum-grafted-poly (methacrylic acid) hydrogels. Polym. Bull. 2020, 78, 3399–3423. [Google Scholar] [CrossRef]
- Thakur, S.; Arotiba, O.A. Synthesis, swelling and adsorption studies of a pH-responsive sodium alginate–poly (acrylic acid) superabsorbent hydrogel. Polym. Bull. 2018, 75, 4587–4606. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, G.; Li, H. A pH sensitive graphene oxide/poly (N-methylolacrylamide-methyl acrylate) composite hydrogel. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Changsha, China, 2017; Volume 274, p. 012114. [Google Scholar]
- Tatarchuk, T.; Liaskovska, M.; Kotsyubynsky, V.; Bououdina, M. Green synthesis of cobalt ferrite nanoparticles using Cydonia oblonga extract: Structural and mössbauer studies. Mol. Cryst. Liq. Cryst. 2018, 672, 54–66. [Google Scholar] [CrossRef]
- Ciciliati, M.A.; Cavalheiro, E.T. Studies of thermal behavior of metoprolol tartrate. J. Therm. Anal. Calorim. 2019, 138, 3653–3663. [Google Scholar] [CrossRef]
- Junior, C.R.F.; de Moura, M.R.; Aouada, F.A. Synthesis and characterization of intercalated nanocomposites based on poly (methacrylic acid) hydrogel and nanoclay cloisite-Na+ for possible application in agriculture. J. Nanosci. Nanotechnol. 2017, 17, 5878–5883. [Google Scholar] [CrossRef]
- Tulain, U.R.; Ahmad, M.; Rashid, A.; Iqbal, F.M. development and characterization of smart drug delivery System. Acta Pol. Pharm.-Drug Res. 2016, 73, 1009–1022. [Google Scholar]
- Ijaz, H.; Tulain, U.R. Development of interpenetrating polymeric network for controlled drug delivery and its evaluation. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 1099–1107. [Google Scholar] [CrossRef]
- Shujaat, J.; Erum, A.; Tulain, U.; Noreen, S.; Ismail, A. Formulation, optimization and characterization of microspheres using biomaterial arabinoxylan. J. Polym Mater 2017, 34, 809–821. [Google Scholar]
- Natarajan, J.V.; Nugraha, C.; Ng, X.W.; Venkatraman, S. Sustained-release from nanocarriers: A review. J. Control. Release 2014, 193, 122–138. [Google Scholar] [CrossRef]
- Issa, M.G.; de Souza, N.V.; Jou, B.W.C.; Duque, M.D.; Ferraz, H.G. Development of Extended-Release Mini-Tablets Containing Metoprolol Supported by Design of Experiments and Physiologically Based Biopharmaceutics Modeling. Pharmaceutics 2022, 14, 892. [Google Scholar] [CrossRef]
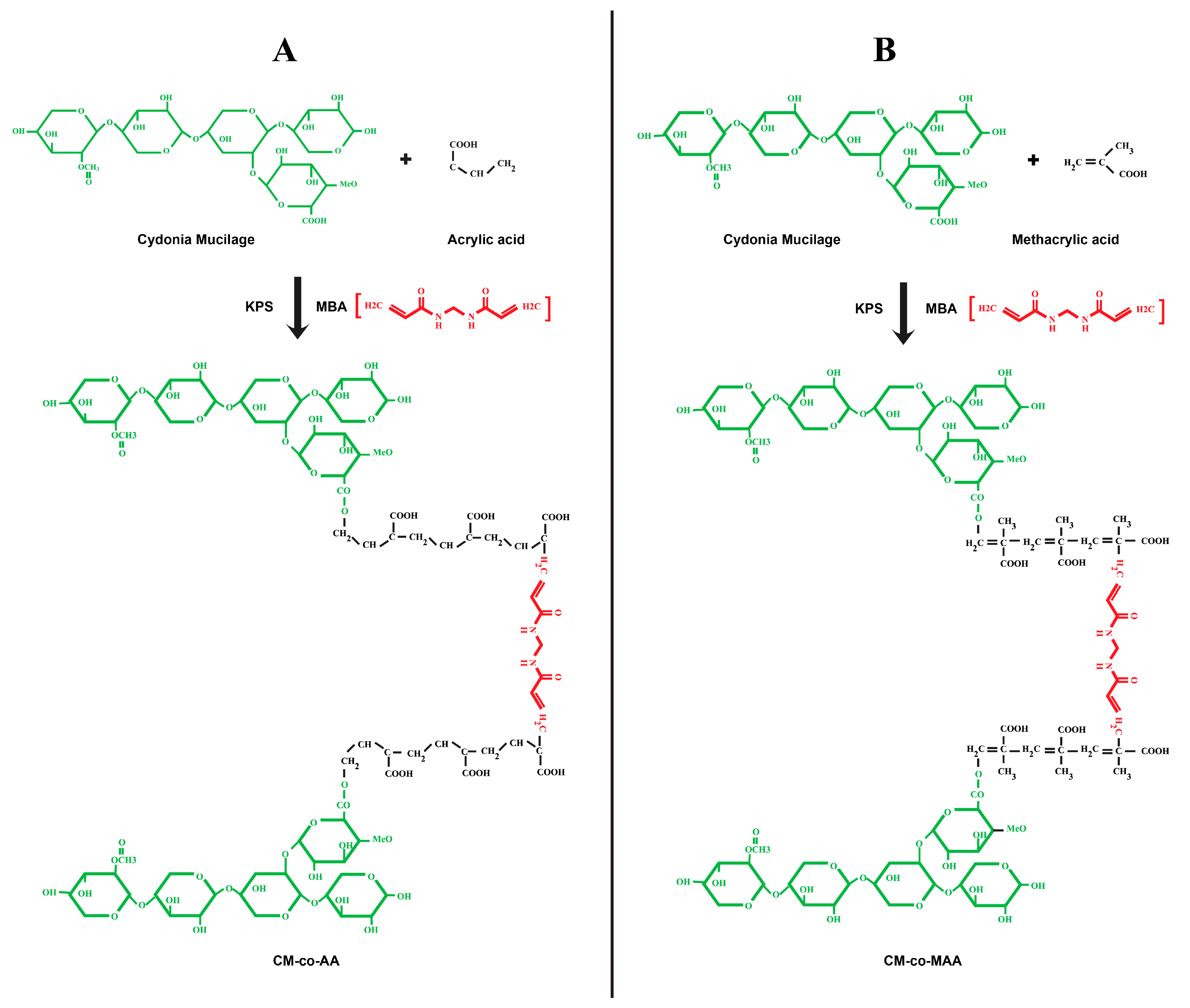














| Formulation Code | Polymer Cydonia oblonga Mucilage (g) | Crosslinker MBA (% Mole Ratio of Monomer) | Initiator KPS (% Mole Ratio of Monomer) | Acrylic Acid (g) |
|---|---|---|---|---|
| M-1 | 1 | 0.02 | 0.02 | 12.5 |
| M-2 | 1 | 0.02 | 0.02 | 17.5 |
| C-1 | 1 | 0.03 | 0.02 | 15 |
| C-2 | 1 | 0.04 | 0.02 | 15 |
| P-1 | 0.5 | 0.02 | 0.02 | 15 |
| P-2 | 1.5 | 0.02 | 0.02 | 15 |
| Formulation Code | Polymer Cydonia oblonga Mucilage (g) | Crosslinker MBA (% Mole Ratio of Monomer) | Initiator KPS (% Mole Ratio of Monomer) | Methacrylic Acid(g) |
|---|---|---|---|---|
| M-3 | 1 | 0.02 | 0.02 | 30 |
| M-4 | 1 | 0.02 | 0.02 | 35 |
| C-3 | 1 | 0.03 | 0.02 | 35 |
| C-4 | 1 | 0.04 | 0.02 | 35 |
| P-3 | 0.5 | 0.02 | 0.02 | 35 |
| P-4 | 1.5 | 0.02 | 0.02 | 35 |
| Chromatography | LC-20AD Shimadzu |
| Column | C 18.5 μ, (250 mm × 4.6 mm) |
| Mobile phase | Acetonitrile:methanol:20 mM ammonium acetate buffer (25:55:20) |
| Flow rate | One milliliter per minute |
| Temperature | Ambient |
| Wavelength for detection | 274 nm |
| Dilution solvent | HPLC graded water |
| Retention time | 5.53 min |
| Formulation | Zero-Order Model | First-Order Model | Higuchi Model | Korsmeyer–Peppas Model | Hixson–Crowell Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| code | R2 | K0 | R2 | K1 | R2 | kH | R2 | kKP | n | R2 | kHC |
| M1 | 0.801 | 3.196 | 0.94 | 0.051 | 0.994 | 13.321 | 0.997 | 12.463 | 0.525 | 0.908 | 0.015 |
| M2 | 0.613 | 4.351 | 0.946 | 0.105 | 0.939 | 18.49 | 0.943 | 10.107 | 0.451 | 0.892 | 0.028 |
| M5 | 0.846 | 3.092 | 0.963 | 0.049 | 0.984 | 12.812 | 0.991 | 10.616 | 0.571 | 0.936 | 0.014 |
| M6 | 0.74 | 3.528 | 0.926 | 0.063 | 0.951 | 14.79 | 0.951 | 14.168 | 0.516 | 0.883 | 0.018 |
| C1 | 0.79 | 3.394 | 0.949 | 0.258 | 0.992 | 14.171 | 0.993 | 13.38 | 0.522 | 0.913 | 0.016 |
| C2 | 0.82 | 3.24 | 0.954 | 0.053 | 0.99 | 13.473 | 0.993 | 11.997 | 0.544 | 0.924 | 0.015 |
| C5 | 0.844 | 3.214 | 0.966 | 0.052 | 0.984 | 13.321 | 0.99 | 11.077 | 0.569 | 0.939 | 0.015 |
| C6 | 0.861 | 3.036 | 0.968 | 0.047 | 0.977 | 12.546 | 0.987 | 9.857 | 0.59 | 0.943 | 0.014 |
| P1 | 0.604 | 0.333 | 0.943 | 0.104 | 0.937 | 18.427 | 0.942 | 21.177 | 0.447 | 0.887 | 0.028 |
| P2 | 0.654 | 3.964 | 0.933 | 0.082 | 0.958 | 16.784 | 0.961 | 18.531 | 0.463 | 0.876 | 0.022 |
| P5 | 0.771 | 3.517 | 0.948 | 0.062 | 0.97 | 14.709 | 0.971 | 13.743 | 0.526 | 0.909 | 0.017 |
| P6 | 0.854 | 3.186 | 0.968 | 0.051 | 0.98 | 13.183 | 0.989 | 10.649 | 0.58 | 0.942 | 0.015 |
| Parameter | Unit | Hydrogel Formulation | Marketed Product |
|---|---|---|---|
| Lambda_z | 1/h | 0.094999903 | 0.0089321 |
| Tmax | h | 4 | 2 |
| Cmax | ng/mL | 156.4879899 | 96 |
| AUC 0-t | ng/mL × h | 1321.630847 | 574.456 |
| MRT 0-inf_obs | h | 8.807584284 | 4.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarfraz, M.; Tulain, U.R.; Erum, A.; Malik, N.S.; Mahmood, A.; Sumaira; Aslam, S.; Sandhu, M.A.; Tayyab, M. Cydonia oblonga-Seed-Mucilage-Based pH-Sensitive Graft Copolymer for Controlled Drug Delivery—In Vitro and In Vivo Evaluation. Pharmaceutics 2023, 15, 2445. https://doi.org/10.3390/pharmaceutics15102445
Sarfraz M, Tulain UR, Erum A, Malik NS, Mahmood A, Sumaira, Aslam S, Sandhu MA, Tayyab M. Cydonia oblonga-Seed-Mucilage-Based pH-Sensitive Graft Copolymer for Controlled Drug Delivery—In Vitro and In Vivo Evaluation. Pharmaceutics. 2023; 15(10):2445. https://doi.org/10.3390/pharmaceutics15102445
Chicago/Turabian StyleSarfraz, Muhammad, Ume Ruqia Tulain, Alia Erum, Nadia Shamshad Malik, Arshad Mahmood, Sumaira, Sidra Aslam, Mansur Abdullah Sandhu, and Muhammad Tayyab. 2023. "Cydonia oblonga-Seed-Mucilage-Based pH-Sensitive Graft Copolymer for Controlled Drug Delivery—In Vitro and In Vivo Evaluation" Pharmaceutics 15, no. 10: 2445. https://doi.org/10.3390/pharmaceutics15102445
APA StyleSarfraz, M., Tulain, U. R., Erum, A., Malik, N. S., Mahmood, A., Sumaira, Aslam, S., Sandhu, M. A., & Tayyab, M. (2023). Cydonia oblonga-Seed-Mucilage-Based pH-Sensitive Graft Copolymer for Controlled Drug Delivery—In Vitro and In Vivo Evaluation. Pharmaceutics, 15(10), 2445. https://doi.org/10.3390/pharmaceutics15102445







