Antimicrobial and Antibiofilm Effects of Combinatorial Treatment Formulations of Anti-Inflammatory Drugs—Common Antibiotics against Pathogenic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Antibacterial Susceptibility of Isolates
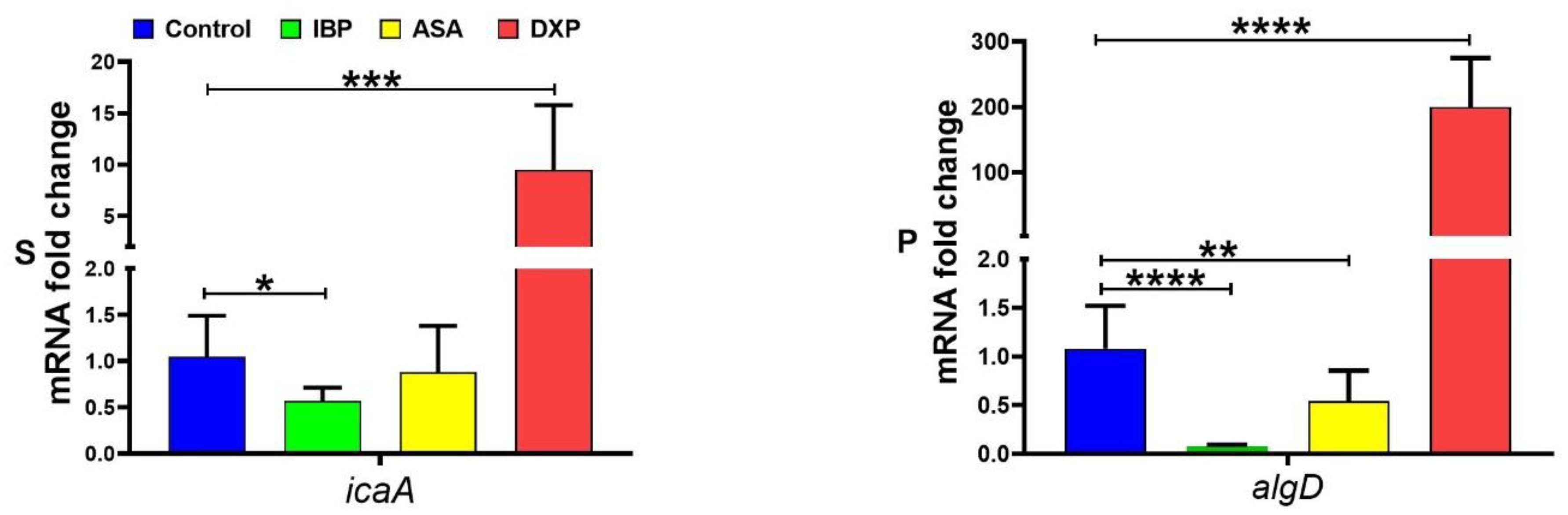
2.3. DNA Extraction and Detection of mecA and icaADBC Operon in S. aureus Isolates and algD in P. aeruginosa
2.4. Biofilm Production Assay
2.5. Anti-Inflammatory Drugs Interference Experiments
2.5.1. MICs of Isolates to Antibiotics Agents with/without ASA, IBP, and DXP
2.5.2. MBICs of Antibiotics Agents with/without ASA, IBP, and DXP for Isolates
2.5.3. MBEC of Isolates to Antibiotics Agents with/without ASA, IBP, and DXP
2.6. Gene Expression Experiment
| Gene Target | Sequence of Primer (5′-3′) | Product Size (bp) | Annealing (°C) | PCR and qPCR Conditions | Use | Reference |
|---|---|---|---|---|---|---|
| mecA | F-TCCAGATTACAACTTCACCAGG R-CCACTTCATATCTTGTAACG | 162 | 56 | 5 min at 95 °C (1 min at 95 °C, 1 min at annealing temperature, and 1 min at 72 °C for 30 cycles) and 10 min at 72 °C. | In PCR | [13] |
| icaA | F-TCTCTTGCAGGAGCAATCAA R-TCAGGCACTAACATCCAGCA | 188 | 60 | [17] | ||
| icaB | F-ATGGCTTAAAGCACACGACGC R-TATCGGCATCTGGTGTGACAG | 526 | 61 | |||
| icaC | R-CTCTCTTAACATCATTCCGACGCC F-ATCATCGTGACACACTTACTAACG | 1013 | 63 | |||
| icaD | F-GAACCGCTTGCCATGTGTTG R-GCTTGACCATGTTGCGTAACC | 483 | 61 | |||
| algD | F-GCGACCTGGACCTGGGCT R-TTGTGGTCCTGGCAGA | 457 | 56 | [18] | ||
| gyrB | F-AGGTCTTGGAGAAATGAATG R-CAAATGTTTGGTCCGCTT | 113 | 60 | 15 min at 95 °C (30 s at 95 °C, 30 s at annealing temperature, and 30 s at 72 °C for 40 cycles) and then performed melting curve analysis ranging from 60 to 95 °C | In qPCR for S. aureus | [23] |
| icaA | F-AGTTGTCGACGTTGGCTAC R-CCAAAGACCTCCCAATGT | 148 | ||||
| rpoD | F-GGGCGAAGAAGGAAATGGTC R-CAGGTGGCCTAGGTGGAGA | 178 | 60 | In qPCR for P. aeruginosa | [24] | |
| algD | F-CGCCGAGATGATCAAGTAC R-TGTAGTAGCGCGACAGGTT | 157 |
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, E.W.L.; Yee, Z.Y.; Raja, I.; Yap, J.K.Y. Synergistic effect of non-steroidal anti-inflammatory drugs (NSAIDs) on antibacterial activity of cefuroxime and chloramphenicol against methicillin-resistant Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2017, 10, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Gomes, A.; Marçal, P.H.F.; Dias-Souza, M.V. Dexamethasone abrogates the antimicrobial and antibiofilm activities of different drugs against clinical isolates of Staphylococcus aureus and Pseudomonas aeruginosa. J. Adv. Res. 2017, 8, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 2007, 42, 3–27. [Google Scholar] [PubMed]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.C.; Pritchard, M.F.; Ferguson, E.L.; Powell, K.A.; Patel, S.U.; Rye, P.D.; Sakellakou, S.M.; Buurma, N.J.; Brilliant, C.D.; Copping, J.M.; et al. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms. Microbiomes 2018, 4, 13. [Google Scholar] [CrossRef]
- Blanco-Cabra, N.; Paetzold, B.; Ferrar, T.; Mazzolini, R.; Torrents, E.; Serrano, L.; LLuch-Senar, M. Characterization of different alginate lyases for dissolving Pseudomonas aeruginosa biofilms. Sci. Rep. 2020, 10, 9390. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Kunz Coyne, A.J.; El Ghali, A.; Holger, D.; Rebold, N.; Rybak, M.J. Therapeutic Strategies for Emerging Multidrug-Resistant Pseudomonas aeruginosa. Infect. Dis. Ther. 2022, 12, 1–22. [Google Scholar] [CrossRef]
- Taylor, T.A.; Unakal, C.G. Staphylococcus aureus; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cue, D.; Lei, M.G.; Lee, C.Y. Genetic regulation of the intercellular adhesion locus in staphylococci. Front. Cell. Infect. Microbiol. 2012, 2, 1–13. [Google Scholar] [CrossRef]
- Paes Leme, R.C.; da Silva, R.B. Antimicrobial Activity of Non-steroidal Anti-inflammatory Drugs on Biofilm: Current Evidence and Potential for Drug Repurposing. Front. Microbiol. 2021, 12, 707629. [Google Scholar] [CrossRef]
- Pahlavanzadeh, F.; Kalantar-Neyestanaki, D.; Motamedifar, M.; Mansouri, S. In vitro reducing effect of cloxacillin on minimum inhibitory concentrations to imipenem, meropenem, ceftazidime, and cefepime in carbapenem-resistant Pseudomonas aeruginosa isolates. Yale. J. Biol. Med. 2020, 93, 29. [Google Scholar]
- Ziasistani, M.; Dabiri, S.; Soofi Abadi, M.F.; Afshari, S.A.K.; Ghaioumy, R.; Morones-Ramírez, J.R.; Tabatabaeifar, F.; Kalantar-Neyestanaki, D. Determination of antibiotic resistance genes, immune evasion cluster and agr types among Staphylococcus aureus strains isolated from children with adenoiditis. Gene Rep. 2020, 21, 100875. [Google Scholar] [CrossRef]
- Hashemizadeh, Z.; Mansouri, S.; Pahlavanzadeh, F.; Morones-Ramírez, J.R.; Tabatabaeifar, F.; Motamedifar, M.; Gholizadeh, A.; Kalantar-Neyestanaki, D. Evaluation of chromosomally and acquired mechanisms of resistance to carbapenem antibiotics among clinical isolates of Pseudomonas aeruginosa in Kerman, Iran. Gene Rep. 2020, 21, 100918. [Google Scholar] [CrossRef]
- CLSI Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020.
- Qi, C.; Pilla, V.; Jessica, H.Y.; Reed, K. Changing prevalence of Escherichia coli with CTX-M–type extended-spectrum β-lactamases in outpatient urinary E. coli between 2003 and 2008. Diag. Microbiol. Infect. Dis. 2010, 67, 87–91. [Google Scholar] [CrossRef]
- Ziasistani, M.; Shakibaie, M.R.; Kalantar-Neyestanaki, D. Genetic characterization of two vancomycin-resistant Staphylococcus aureus isolates in Kerman, Iran. Infect. Drug. Resist. 2019, 4, 1869–1875. [Google Scholar] [CrossRef]
- Eladawy, M.; El-Mowafy, M.; El-Sokkary, M.M.A.; Barwa, R. Antimicrobial resistance and virulence characteristics in ERIC-PCR typed biofilm forming isolates of P. aeruginosa. Microb. Pathog. 2021, 158, 105042. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Shah, P.N.; Marshall-Batty, K.R.; Smolen, J.A.; Tagaev, J.A.; Chen, Q.; Rodesney, C.A.; Greenberg, D.E.; Cannon, C.L. Antimicrobial Activity of Ibuprofen against Cystic Fibrosis-Associated Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2018, 62, e01574-17. [Google Scholar] [CrossRef]
- Cruz, C.D.; Shah, S.; Tammela, P. Defining conditions for biofilm inhibition and eradication assays for Gram-positive clinical reference strains. BMC Microbiol. 2018, 18, 173. [Google Scholar] [CrossRef]
- Thieme, L.; Hartung, A.; Tramm, K.; Klinger-Strobel, M.; Jandt, K.D.; Makarewicz, O.; Pletz, M.W. MBEC Versus MBIC: The Lack of Differentiation between Biofilm Reducing and Inhibitory Effects as a Current Problem in Biofilm Methodology. Biol. Proced. 2019, 21, 18. [Google Scholar] [CrossRef]
- Kato, F.; Yabuno, Y.; Yamaguchi, Y.; Sugai, M.; Inouye, M. Deletion of mazF increases Staphylococcus aureus biofilm formation in an ica-dependent manner. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, J.L.; Neill, D.R.; Loman, N.; Winstanley, C.; Kadioglu, A. Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs. Nat. Commun. 2014, 5, 4780. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: Trends and Lessons Learned. Infect. Dis. Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Fteita, D.; Könönen, E.; Söderling, E.; Gürsoy, U.K. Effect of estradiol on planktonic growth, coaggregation, and biofilm formation of the Prevotella intermedia group bacteria. Anaerobe 2014, 27, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Patt, M.W.; Conte, L.; Blaha, M.; Plotkin, B.J. Steroid hormones as interkingdom signaling molecules: Innate immune function and microbial colonization modulation. AIMS Mol. Sci. 2018, 5, 117–130. [Google Scholar] [CrossRef]
- Sakiniene, E.; Bremell, T.; Tarkowski, A. Addition of corticosteroids to antibiotic treatment ameliorates the course of experimental Staphylococcus aureus arthritis. Arthritis. Rheum. 1996, 39, 1596–1605. [Google Scholar] [CrossRef]
- Lortholary, O.; Nicolas, M.; Soreda, S.; Improvisi, L.; Ronin, O.; Petitjean, O.; Dupont, B.; Tod, M.; Dromer, F. Fluconazole, with or without dexamethasone for experimental cryptococcosis: Impact of treatment timing. J. Antimicrob. Chemother. 1999, 43, 817–824. [Google Scholar] [CrossRef][Green Version]
- Gong, J.Q.; Lin, L.; Lin, T.; Hao, F.; Zeng, F.Q.; Bi, Z.G.; Yi, D.; Zhao, B. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: A double-blind multicentre randomized controlled trial. Br. J. Dermatol. 2006, 155, 680–687. [Google Scholar] [CrossRef]
- Nagy, B.; Gaspar, I.; Papp, A.; Bene, Z.; Nagy, B.; Voko, Z.; Balla, G. Efficacy of methylprednisolone in children with severe community acquired pneumonia. Pediatr. Pulmonol. 2013, 48, 168–175. [Google Scholar] [CrossRef]
- Cabellos, C.; Martínez-Lacasa, J.; Tubau, F.; Fernández, A.; Viladrich, P.F.; Liñares, J.; Gudiol, F. Evaluation of combined ceftriaxone and dexamethasone therapy in experimental cephalosporin-resistant pneumococcal meningitis. J. Antimicrob. Chemother. 2000, 45, 315–320. [Google Scholar] [CrossRef]
- Martínez-Lacasa, J.; Cabellos, C.; Martos, A.; Fernández, A.; Tubau, F.; Viladrich, P.F.; Liñares, J.; Gudiol, F. Experimental study of the efficacy of vancomycin, rifampicin and dexamethasone in the therapy of pneumococcal meningitis. J. Antimicrob. Chemother. 2002, 49, 507–513. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; Sun, H.; Gong, L.; Guo, L.; Shi, Y.; Cai, C.; Gu, H.; Song, Z.; Yang, L.; et al. A novel nitro-dexamethasone inhibits agr system activity and improves therapeutic effects in MRSA sepsis models without antibiotics. Sci. Rep. 2016, 6, 20307. [Google Scholar] [CrossRef]
- Goggin, R.; Jardeleza, C.; Wormald, P.J.; Vreugde, S. Corticosteroids directly reduce Staphylococcus aureus biofilm growth: An in vitro study. Laryngoscope 2014, 124, 602–607. [Google Scholar] [CrossRef]
- Pozzi, C.; Waters, E.M.; Rudkin, J.K.; Schaeffer, C.R.; Lohan, A.J.; Tong, P.; Loftus, B.J.; Pier, G.B.; Fey, P.D.; Massey, R.C.; et al. resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 2012, 8, e1002626. [Google Scholar] [CrossRef]
- Lamret, F.; Colin, M.; Mongaret, C.; Gangloff, S.C.; Reffuveille, F. Antibiotic Tolerance of Staphylococcus aureus Biofilm in Periprosthetic Joint Infections and Antibiofilm Strategies. Antibiotics 2020, 9, 547. [Google Scholar] [CrossRef]
- Vanhommerig, E.; Moons, P.; Pirici, D.; Lammens, C.; Hernalsteens, J.P.; De Greve, H.; Kumar-Singh, S.; Goossens, H.; Malhotra-Kumar, S. Comparison of Biofilm Formation between Major Clonal Lineages of Methicillin Resistant Staphylococcus aureus. PLoS ONE 2014, 9, e104561. [Google Scholar] [CrossRef]
- Sanyal, A.K.; Roy, D.; Chowdhury, B.; Banerjee, A.B. Ibuprofen, a unique anti-inflammatory compound with antifungal activity against dermatophytes. Lett. Appl. Microbiol. 1993, 17, 109–111. [Google Scholar] [CrossRef]
- Elvers, K.T.; Wright, S.J. Antibacterial activity of the anti-inflammatory compound ibuprofen. Lett. Appl. Microbiol. 1995, 20, 82–84. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Sansonetty, F.; Rodrigues, A.G.; Martinez-DE-Oliveira, J.; Fonseca, A.F.; Mårdh, P.A. Antifungal activity of ibuprofen alone and in combination with fluconazole against Candida species. J. Med. Microbiol. 2000, 49, 831–840. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Yu, C.; Li, T.; Liu, J.; Sun, S. Potential Antifungal Targets against a Candida Biofilm Based on an Enzyme in the Arachidonic Acid Cascade-A Review. Front. Microbiol. 2016, 7, 1925. [Google Scholar] [CrossRef]
- Rusu, E.; Radu-Popescu, M.; Pelinescu, D.; Vassu, T. Treatment with some anti-inflammatory drugs reduces germ tube formation in Candida albicans strains. Braz. J. Microbiol. 2014, 45, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Alem, M.A.S.; Douglas, L.J. Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob. Agents Chemother. 2004, 48, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wu, T.Q.; Xiong, Y.S.; Ni, H.B.; Ding, Y.; Zhang, W.C.; Chu, S.P.; Ju, S.Q.; Yu, J. Ibuprofen-mediated potential inhibition of biofilm development and quorum sensing in Pseudomonas aeruginosa. Life Sci. 2019, 237, 116947. [Google Scholar] [CrossRef] [PubMed]
- Kahlous, N.A.; Bawarish, M.A.M.; Sarhan, M.A.; Küpper, M.; Hasaba, A.; Rajab, M. Using Chemoinformatics, Bioinformatics, and Bioassay to Predict and Explain the Antibacterial Activity of Nonantibiotic Food and Drug Administration Drugs. Assay Drug Dev. Technol. 2017, 15, 89–105. [Google Scholar] [CrossRef]
- Abbas, H.A.; Atallah, H.; El-Sayed, M.A.; El-Ganiny, A.M. Diclofenac mitigates virulence of multidrug-resistant Staphylococcus aureus. Arch. Microbiol. 2020, 202, 2751–2760. [Google Scholar] [CrossRef]
- Yarwood, J.M.; Bartels, D.J.; Volper, E.M.; Greenberg, E.P. Quorum sensing in Staphylococcus aureus biofilms. J. Bacteriol. 2004, 186, 1838–1850. [Google Scholar] [CrossRef]
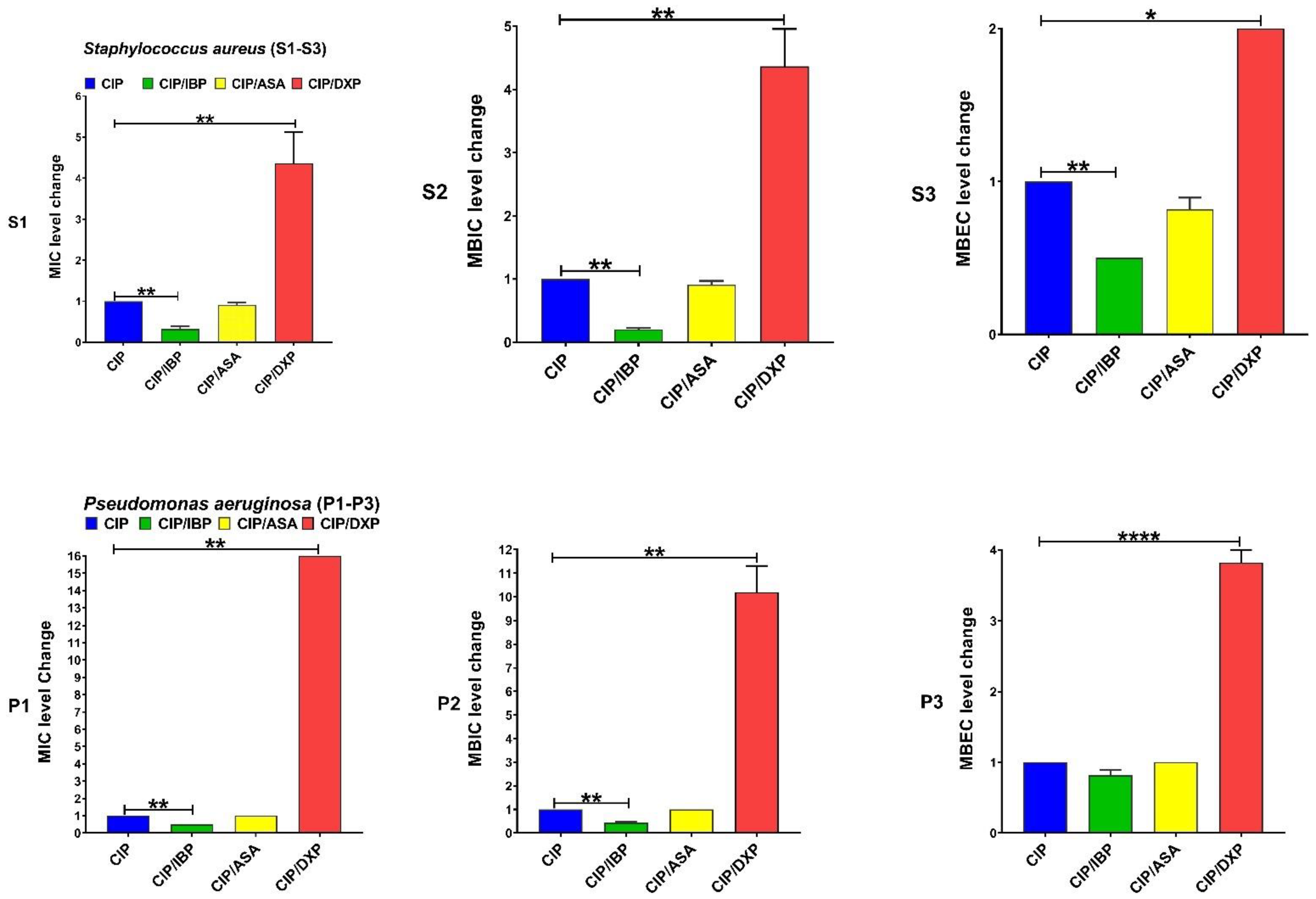
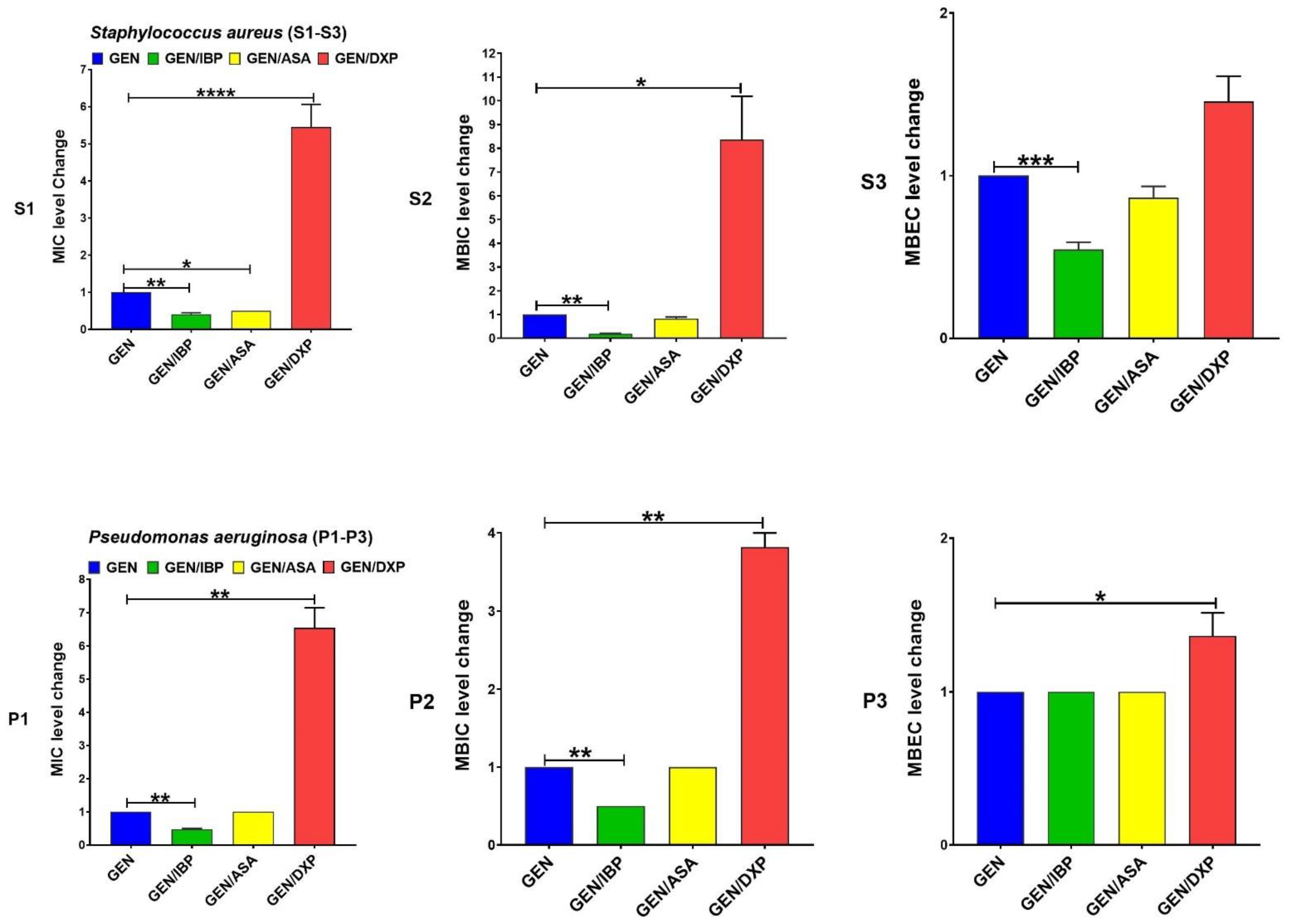
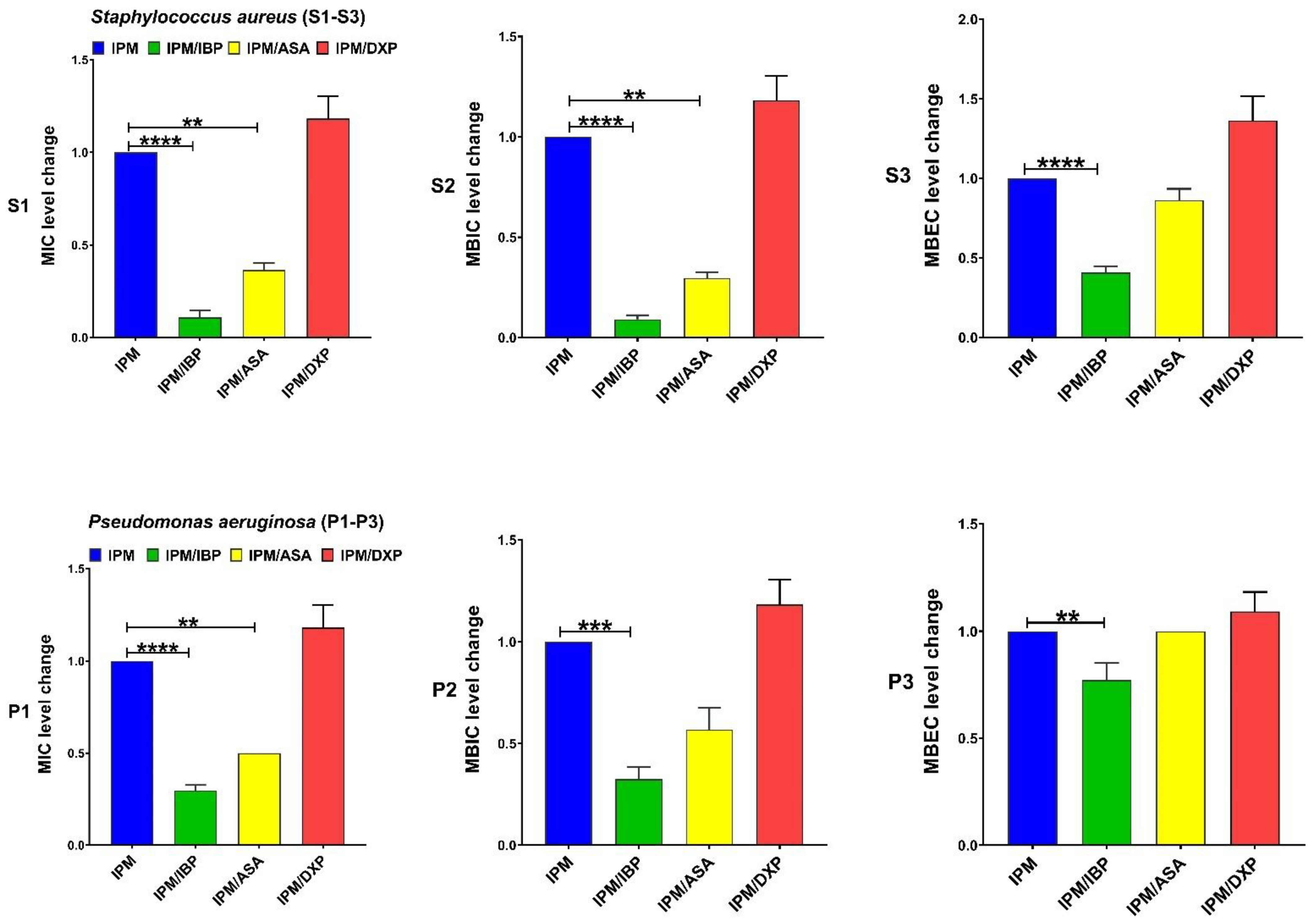
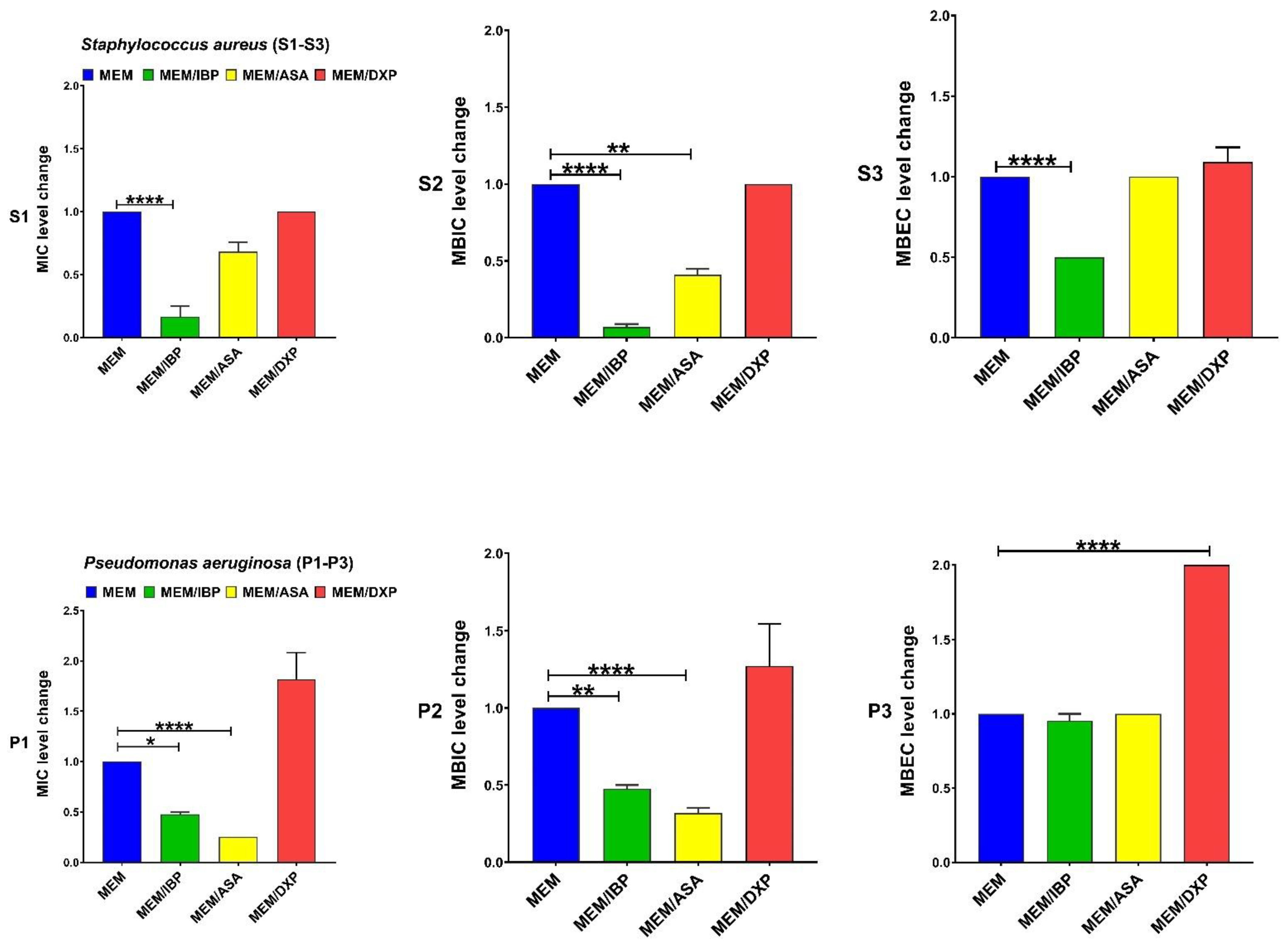
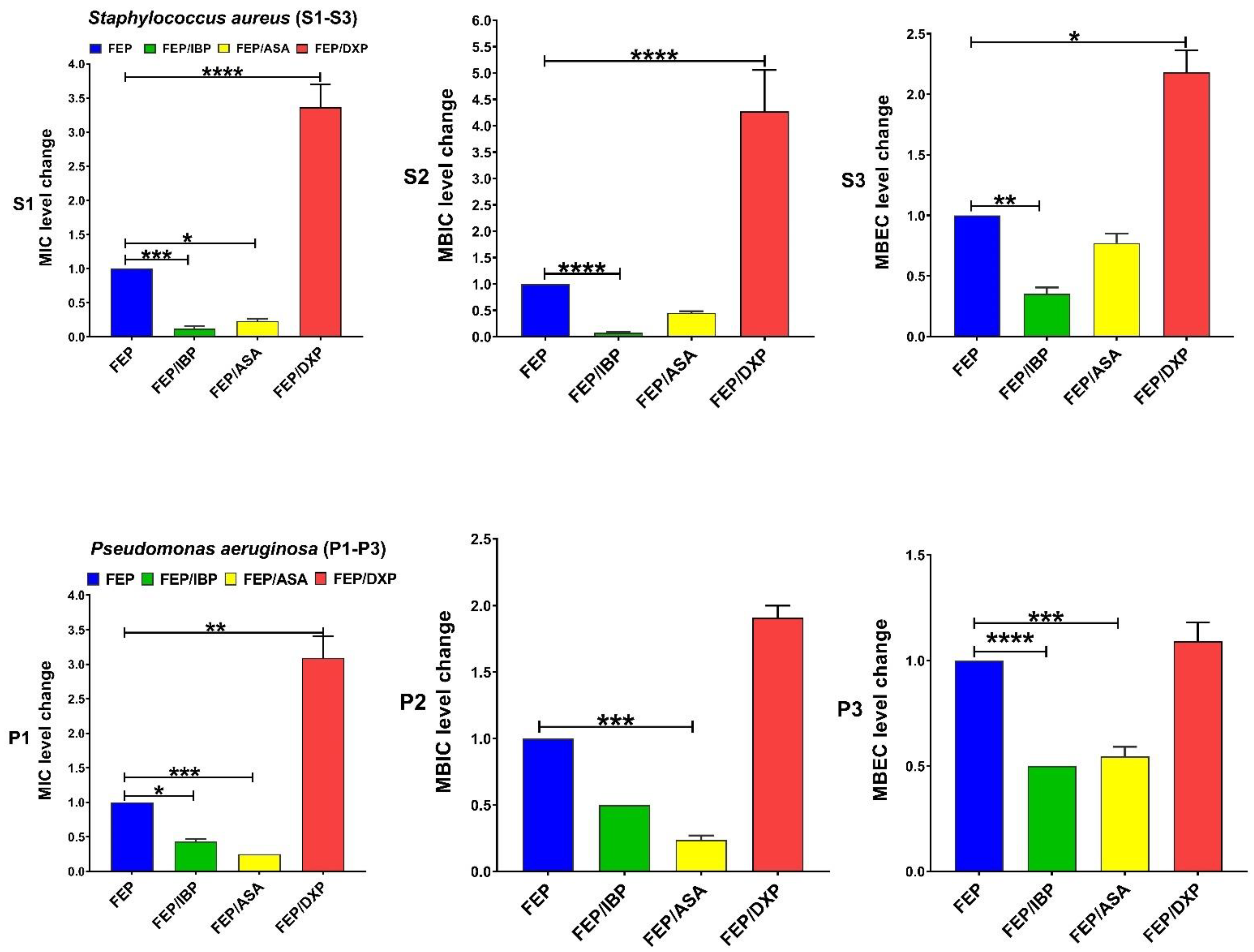
| Antibiotics Agents | MIC, MBIC, and MBEC (µg/mL) 50 and 90 for Clinical Isolates of S. aureus Isolates | MIC, MBIC, and MBEC (µg/mL) 50 and 90 for Clinical Isolates of P. aeruginosa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | MBIC50 | MBIC90 | MBEC50 | MBEC90 | MIC50 | MIC90 | MBIC50 | MBIC90 | MBEC50 | MBEC90 | |
| CIP | 64 | 128 | 128 | 256 | 1024 | 2048 | 8 | 16 | 32 | 64 | 512 | 512 |
| CIP/IBP | 16 | 32 | 32 | 64 | 1024 | 2048 | 4 | 8 | 16 | 16 | 256 | 512 |
| CIP/ASA | 64 | 128 | 128 | 256 | 1024 | 2048 | 8 | 16 | 32 | 64 | 512 | 512 |
| CIP/DXP | 256 | 256 | 256 | 1024 | 4096 | ≥8192 | 128 | 256 | 256 | 1024 | 2048 | 2048 |
| GEN | 64 | 64 | 256 | 256 | 1024 | 1024 | 16 | 32 | 64 | 128 | 1024 | 1024 |
| GEN/IBP | 32 | 32 | 64 | 64 | 512 | 1024 | 8 | 16 | 32 | 64 | 1024 | 1024 |
| GEN/ASA | 32 | 32 | 128 | 256 | 1024 | 1024 | 16 | 32 | 64 | 128 | 1024 | 1024 |
| GEN/DXP | 256 | 256 | 1024 | 1024 | 1024 | 2048 | 128 | 128 | 256 | 512 | 1024 | 1024 |
| IPM | 16 | 32 | 64 | 64 | 1024 | 2048 | 32 | 32 | 512 | 512 | 2048 | 2048 |
| IPM/IBP | 1 | 2 | 2 | 8 | 512 | 512 | 8 | 16 | 64 | 256 | 1024 | 2048 |
| IPM/ASA | 8 | 8 | 8 | 16 | 1024 | 2048 | 16 | 16 | 128 | 256 | 2048 | 2048 |
| IPM/DXP | 64 | 64 | 32 | 64 | 2048 | 2048 | 32 | 32 | 256 | 512 | 2048 | 2048 |
| MEM | 16 | 32 | 64 | 128 | 1024 | 2048 | 32 | 32 | 64 | 128 | 1024 | 1024 |
| MEM/IBP | 1 | 2 | 4 | 8 | 512 | 1024 | 16 | 16 | 32 | 64 | 1024 | 1024 |
| MEM/ASA | 16 | 16 | 16 | 64 | 1024 | 2048 | 8 | 8 | 16 | 32 | 1024 | 1024 |
| MEM/DXP | 16 | 32 | 64 | 128 | 1024 | 2048 | 32 | 64 | 64 | 128 | 1024 | 2048 |
| FEP | 16 | 64 | 64 | 256 | 2048 | 2048 | 32 | 64 | 512 | 512 | 4096 | 4096 |
| FEP/IBP | 2 | 4 | 4 | 8 | 512 | 512 | 16 | 32 | 256 | 256 | 2048 | 2048 |
| FEP/ASA | 8 | 8 | 32 | 64 | 1024 | 1024 | 8 | 16 | 64 | 128 | 2048 | 2048 |
| FEP/DXP | 64 | 128 | 256 | 1024 | 2048 | 4096 | 128 | 128 | 512 | 1024 | 4096 | 4096 |
| Drugs | MIC Range | MIC Fold Changes Range | Isolates n (%) | p-Value | MBIC Range | MBIC Fold Changes Range | Isolates n (%) | p-Value | MBEC Range | MBEC Fold Changes Range | Isolates n (%) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | 16–128 | C | - | - | 64–256 | C | - | - | 1024–4096 | C | - | |
| CIP + IBP | 4–32 | 4 ↓ | 10 (100) | 0.0058 | 8–64 | 4–8 ↓ | 10 (100) | 0.0051 | 512–2048 | 2 ↓ | 10 (100) | 0.0039 |
| CIP + ASA | 16–128 | 0–2 ↓ | 2 (20) | >0.9999 | 128–256 | 0–2 ↓ | 2 (20) | >0.9999 | 1024–2048 | 0–2 ↓ | 4 (40) | 0.7261 |
| CIP + DXP | 128–256 | 2–8 ↑ | 10 (100) | 0.0055 | 256–1024 | 2–8 ↑ | 10 (100) | 0.0058 | 2048–8192 | 2 ↑ | 10 (100) | 0.0141 |
| GEN | 8–64 | C | - | - | 64–256 | C | - | - | 1024–2048 | C | - | - |
| GEN + IBP | 2–32 | 2–4 ↓ | 10 (100) | 0.0012 | 4–64 | 4–16 ↓ | 10 (100) | 0.0013 | 512–1024 | 2 ↓ | 10 (100) | 0.0007 |
| GEN + ASA | 4–32 | 2 ↓ | 10 (100) | 0.0166 | 64–128 | 0–2 ↓ | 3 (100) | >0.9999 | 1024 | 2 ↓ | 3 (30) | 0.8025 |
| GEN + DXP | 64–256 | 4–8 ↑ | 10 (100) | 0.0001 | 1024 | 4–16 ↑ | 10 (100) | 0.0176 | 1024–2048 | 4 ↑ | 4 (40) | 0.4255 |
| IPM | 8–32 | C | - | - | 32–64 | C | - | - | 1024–2048 | C | - | - |
| IPM + IBP | 0.5–2 | 8–32 ↓ | 10 (100) | <0.0001 | 2–8 | 8–16 ↓ | 10 (100) | <0.0001 | 512 | 2–4 ↓ | 10 (100) | <0.0001 |
| IPM + ASA | 4–8 | 2–4 ↓ | 10 (100) | 0.0052 | 8–16 | 2–4 ↓ | 10 (100) | 0.0028 | 512–2048 | 0–2 ↓ | 20 (20) | 0.9313 |
| IPM + DXP | 16–32 | 2 ↑ | 2 (20) | >0.9999 | 32–64 | 0–2 ↑ | 2 (20) | >0.9999 | 1024–2048 | 0–2 ↑ | 4 (40) | 0.7792 |
| MEM | 8–32 | C | - | - | 32–128 | C | - | - | 1024–2048 | C | - | - |
| MEM + IBP | 1–2 | 8–16 ↓ | 10 (100) | <0.0001 | 1–8 | 16–32 ↓ | 10 (100) | <0.0001 | 512–1024 | 2 ↓ | 10 (10) | <0.0001 |
| MEM + ASA | 4–16 | 0–2 ↓ | 7 (70) | 0.0707 | 16–64 | 2–4 ↓ | 10 (100) | 0.0032 | 1024–2048 | 0 | - | >0.9999 |
| MEM + DXP | 8–32 | 0 | - | >0.9999 | 32–128 | 0 | - | >0.9999 | 1024–2048 | 0 | - | >0.9999 |
| FEP | 16–64 | C | - | - | 32–256 | C | - | - | 1024–2048 | C | - | - |
| FEP + IBP | 1–4 | 8–16 ↓ | 10 (100) | 0.0003 | 2–8 | 16–32 ↓ | 10 (100) | <0.0001 | 256–1024 | 2–8 ↓ | 10 (100) | 0.0023 |
| FEP + ASA | 2–8 | 4–8 ↓ | 10 (100) | 0.0468 | 16–64 | 2–4 ↓ | 10 (100) | 0.0934 | 1024–2048 | 2 ↓ | 5 (50) | 0.6736 |
| FEP + DXP | 64–128 | 2–4 ↑ | 10 (100) | <0.0001 | 64–1024 | 2–8 ↑ | 10 (100) | <0.0001 | 2048–4096 | 2 ↑ | 10 (100) | 0.0226 |
| Drugs | MIC Range | MIC Fold Changes Range | Isolates n (%) | p-Value | MBIC Range | MBIC Fold Changes Range | Isolates n (%) | p-Value | MBEC Range | MBEC Fold Changes Range | Isolates n (%) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | 4–16 | C | - | - | 32–64 | C | - | - | 256–512 | C | - | - |
| CIP + IBP | 2–8 | 2 ↓ | 10 (100) | 0.0031 | 16 | 2–4 ↓ | 10 (100) | 0.0035 | 256–512 | 0–2 ↓ | 4 (40) | 0.5728 |
| CIP + ASA | 4–16 | 0 | - | >0.9999 | 32–64 | 0 | 0 | >0.9999 | 256–512 | 0 | - | >0.9999 |
| CIP + DXP | 64–256 | 16 ↑ | 10 (100) | 0.0031 | 256–1024 | 8–16 ↑ | 10 (100) | 0.0035 | 1024–2048 | 4 ↑ | 10 (100) | <0.0001 |
| GEN | 16–32 | C | - | - | 64–128 | C | - | - | 512–1024 | C | - | - |
| GEN + IBP | 8–16 | 2 ↓ | 10 (100) | 0.0035 | 32–64 | 2 ↓ | 10 (100) | 0.0032 | 512–1024 | 0 | - | >0.9999 |
| GEN + ASA | 16–32 | 0 | - | >0.9999 | 64–128 | 0 | 0 | >0.9999 | 512–1024 | 0 | - | >0.9999 |
| GEN + DXP | 128 | 4–8 ↑ | 10 (100) | 0.0035 | 256–512 | 2–4 ↑ | 10 (100) | 0.0032 | 1024 | 0–2 ↑ | 3 (30) | 0.0101 |
| IMP | 8–32 | C | - | - | 16–512 | C | - | - | 1024–2048 | C | - | - |
| IMP + IBP | 2–16 | 2–4 ↓ | 10 (100) | <0.0001 | 8–256 | 2–8 ↓ | 10 (100) | 0.0001 | 1024–2048 | 0–2 ↓ | 4 (40) | 0.0082 |
| IMP + ASA | 4–16 | 2 ↓ | 10 (100) | 0.0038 | 32–256 | 0–4 ↓ | 60 (60) | 0.00191 | 1024–2048 | 0 | - | >0.9999 |
| IMP + DXP | 16–32 | 2 ↑ | 2 (20) | >0.9999 | 32–512 | 2 ↑ | 2 (20) | >0.9999 | 1024–2048 | 0 | - | >0.9999 |
| MEM | 16–32 | C | - | - | 64–128 | C | - | - | 1024 | C | - | - |
| MEM + IBP | 8–16 | 2 ↓ | 10 (100) | 0.0313 | 32–64 | 2 ↓ | 10 (100) | 0.0012 | 1024 | 0 | - | >0.9999 |
| MEM + ASA | 4–8 | 4 ↓ | 10 (100) | <0.0001 | 32 | 2–4 ↓ | 10 (100) | <0.0001 | 1024 | 0 | - | >0.9999 |
| MEM + DXP | 32–64 | 2 ↑ | 6 (60) | 0.5526 | 64–128 | 0 | - | >0.9999 | 1024–2048 | 2 ↑ | 10 (100) | <0.0001 |
| FEP | 32–64 | C | - | - | 256–512 | C | - | - | 4096 | C | - | - |
| FEP + IBP | 8–32 | 2–4 ↓ | 10 (100) | 0.0153 | 128–256 | 2 ↓ | 10 (100) | 0.0724 | 2048 | 2 ↓ | 10 (100) | <0.0001 |
| FEP + ASA | 8–16 | 4 ↓ | 10 (100) | 0.0003 | 64–128 | 2–8 ↓ | 10 (100) | 0.0001 | 2048 | 2 ↓ | 10 (100) | 0.0001 |
| FEP + DXP | 128 | 2–4 ↑ | 10 (100) | 0.0001 | 512–1024 | 2 ↑ | 10 (100) | 0.1806 | 4096 | 0 ↑ | - | >0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabatabaeifar, F.; Isaei, E.; Kalantar-Neyestanaki, D.; Morones-Ramírez, J.R. Antimicrobial and Antibiofilm Effects of Combinatorial Treatment Formulations of Anti-Inflammatory Drugs—Common Antibiotics against Pathogenic Bacteria. Pharmaceutics 2023, 15, 4. https://doi.org/10.3390/pharmaceutics15010004
Tabatabaeifar F, Isaei E, Kalantar-Neyestanaki D, Morones-Ramírez JR. Antimicrobial and Antibiofilm Effects of Combinatorial Treatment Formulations of Anti-Inflammatory Drugs—Common Antibiotics against Pathogenic Bacteria. Pharmaceutics. 2023; 15(1):4. https://doi.org/10.3390/pharmaceutics15010004
Chicago/Turabian StyleTabatabaeifar, Fatemehalsadat, Elham Isaei, Davood Kalantar-Neyestanaki, and José Rubén Morones-Ramírez. 2023. "Antimicrobial and Antibiofilm Effects of Combinatorial Treatment Formulations of Anti-Inflammatory Drugs—Common Antibiotics against Pathogenic Bacteria" Pharmaceutics 15, no. 1: 4. https://doi.org/10.3390/pharmaceutics15010004
APA StyleTabatabaeifar, F., Isaei, E., Kalantar-Neyestanaki, D., & Morones-Ramírez, J. R. (2023). Antimicrobial and Antibiofilm Effects of Combinatorial Treatment Formulations of Anti-Inflammatory Drugs—Common Antibiotics against Pathogenic Bacteria. Pharmaceutics, 15(1), 4. https://doi.org/10.3390/pharmaceutics15010004







