Protein Transduction Domain-Mediated Delivery of Recombinant Proteins and In Vitro Transcribed mRNAs for Protein Replacement Therapy of Human Severe Genetic Mitochondrial Disorders: The Case of Sco2 Deficiency
Abstract
1. Introduction to Mitochondrial Disorders
1.1. Mitochondria: Characteristics and Function
1.2. Oxidative Phosphorylation Pathway
1.3. The Mitochondrial Machinery for Protein Import and Assembly
1.4. Mitochondrial Genetic Disorders
1.4.1. Mitochondrial Disorders Attributed to Genes Encoded by mtDNA
- Large-scale rearrangements are associated with:
- Homoplasmic point mutations are associated with:
- Leber hereditary optic neuropathy (LHON) [26]
1.4.2. Mitochondrial Disorders Attributed to Genes Encoded by nDNA
1.5. Cytochrome c Oxidase (COX) Deficiency
1.6. SCO2
2. Protein Replacement Therapy for Monogenetic Disorders
2.1. Viral-Mediated Gene Transfer and Expression as a Therapeutic Approach for the Monogenic Disorders
2.2. Non-Viral Systems for Delivery Gene Sequences
3. Protein Transduction Domains (PTDs) or Cell-Penetrating Peptides (CPPs)
4. Deliverable Recombinant Protein Delivery Mediated by PTD Technology
PTD-Mediated PRT for Mitochondrial Disorders: Transduction of the Human Recombinant TAT-Sco2 Fusion Mitochondrial Protein
5. IVT-mRNA Delivery Mediated by PTD Technology
5.1. Delivery of IVT-mRNA for Producing Proteins of Given Interest
5.2. IVT-mRNA Synthesis and Optimization Strategies
5.3. Non-Viral IVT-mRNA System for Mediated Transfer and Expression
5.3.1. Physical Approaches for the Delivery of IVT-mRNA
5.3.2. Nanocarriers for the Delivery of IVT-mRNA
5.4. Peptides for IVT-mRNA Delivery and Expression
PTD-Mediated PRT: Transduction of the IVT-mRNA of Sco2 Mitochondrial Protein
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIP | Acute intermittent porphyria |
| AAV | Adeno-associated virus |
| ATP | Adenosine trisphosphate |
| ARE | Adenylate-uridylate-rich element |
| AD | Alzheimer’s disease |
| aa | Amino acid |
| R | Arginine |
| bp | Base pair |
| β-GAL | Beta-galactosidase |
| BBB | Blood–brain barrier |
| CPP | Cell-penetrating peptide |
| CAMP | Cell-penetrating artificial mitochondria-targeting peptide |
| CNS | Central nervous system |
| CDS | Coding sequence |
| C | Cytidine |
| COX | Cytochrome c oxidase |
| DC | Dendritic cell |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| FXN | Frataxin |
| FRDA | Friedreich’s ataxia |
| HSCs | Hematopoietic stem cells |
| HSPCs | Hematopoietic stem and progenitor cells |
| HIV-1 | Human immunodeficiency virus type 1 |
| hMT1A | Human metalloprotein 1A |
| IVT-mRNA | In vitro transcribed mRNA |
| iPSCs | Induced pluripotent stem cells |
| IMM | Inner mitochondrial membrane |
| kb | Kilobase |
| L | Leader peptide |
| LHON | Leber hereditary optic neuropathy |
| LS | Leigh syndrome |
| LAD | Lipoamide dehydrogenase deficiency |
| LP | Lipoplex |
| K | Lysine |
| LSDs | Lysosomal storage disorders |
| MMA | Methylmalonic acidemia |
| mtDNA | Mitochondrial DNA |
| MPP | Mitochondrial processing peptidase |
| MTS | Mitochondrial targeting signal |
| DOTMA | N-[1-(2,3-Dioleyloxy)propyl]-N,N,N--trimethylammonium |
| NADH | Nicotinamide dinucleotide |
| nDNA | Nuclear DNA |
| NA | Nucleic acid |
| ORF | Open reading frame |
| OTC | Ornithine transcarbamylase |
| OMM | Outer mitochondrial membrane |
| OXPHOS | Oxidative phosphorylation |
| PD | Parkinson’s disease |
| PNA | Peptide nucleic acid |
| PBN | Peptide-based nanoparticle |
| pDNA | Plasmid DNA |
| PBAE | Poly(β-amino esters) |
| poly-(A) | Poly-adenylate |
| PEG | Polyethylene glycol |
| PeI | Polyethyleneimine |
| PRT | Protein replacement therapy |
| PTD | Protein transduction domain |
| Ψ | Pseudo-uridine |
| ROS | Reactive oxygen species |
| SCO2 | Synthesis of cytochrome c oxidase 2 |
| 99mTc | Technetium 99 |
| TLR | Toll-like receptor |
| TAT | Trans-activator of transcription |
| TIM | Translocase of the inner membrane |
| TOM | Translocase of the outer membrane |
| TCA | Tricarboxylic acid cycle |
| UTR | Untranslated region |
| w/t | Wild-type |
| fusion L-Sco2 | 10xHis-XaSITE-TAT-L-Sco2-HA |
References
- Ernster, L.; Schatz, G. Mitochondria: A historical review. J. Cell Biol. 1981, 91 Pt 2, 227s–255s. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Palade, G.E. An electron microscope study of the mitochondrial structure. J. Histochem. Cytochem. 1953, 1, 188–211. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Saraste, M. Oxidative phosphorylation at the fin de siecle. Science 1999, 283, 1488–1493. [Google Scholar] [CrossRef]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis*. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706. [Google Scholar] [CrossRef] [PubMed]
- Modesti, L.; Danese, A.; Angela Maria Vitto, V.; Ramaccini, D.; Aguiari, G.; Gafa, R.; Lanza, G.; Giorgi, C.; Pinton, P. Mitochondrial Ca(2+) Signaling in Health, Disease and Therapy. Cells 2021, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Rouault, T.A. Human iron-sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry 2010, 49, 4945–4956. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Wang, X. Therapeutic targets during mitochondrial lipid metabolism. Cell Biol. Toxicol. 2020, 36, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Mitochondria as signaling organelles. BMC Biol. 2014, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Protasoni, M.; Zeviani, M. Mitochondrial Structure and Bioenergetics in Normal and Disease Conditions. Int. J. Mol. Sci. 2021, 22, 586. [Google Scholar] [CrossRef] [PubMed]
- Sagan, L. On the origin of mitosing cells. J. Theor. Biol. 1967, 14, 255–274. [Google Scholar] [CrossRef]
- Singh, L.N.; Kao, S.H.; Wallace, D.C. Unlocking the Complexity of Mitochondrial DNA: A Key to Understanding Neurodegenerative Disease Caused by Injury. Cells 2021, 10, 3460. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.A. Transcription and replication of animal mitochondrial DNAs. Int. Rev. Cytol. 1992, 141, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.A. Nuclear gadgets in mitochondrial DNA replication and transcription. Trends Biochem. Sci. 1991, 16, 107–111. [Google Scholar] [CrossRef]
- Taanman, J.W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta 1999, 1410, 103–123. [Google Scholar] [CrossRef]
- Giles, R.E.; Blanc, H.; Cann, H.M.; Wallace, D.C. Maternal inheritance of human mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1980, 77, 6715–6719. [Google Scholar] [CrossRef]
- Lee, C.M.; Sedman, J.; Neupert, W.; Stuart, R.A. The DNA helicase, Hmi1p, is transported into mitochondria by a C-terminal cleavable targeting signal. J. Biol. Chem. 1999, 274, 20937–20942. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Annu. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef] [PubMed]
- Hawlitschek, G.; Schneider, H.; Schmidt, B.; Tropschug, M.; Hartl, F.U.; Neupert, W. Mitochondrial protein import: Identification of processing peptidase and of PEP, a processing enhancing protein. Cell 1988, 53, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Lunetti, P.; Curcio, R.; Lasorsa, F.M.; Capobianco, L.; Porcelli, V.; Dolce, V.; Fiermonte, G.; Scarcia, P. An Overview of Mitochondrial Protein Defects in Neuromuscular Diseases. Biomolecules 2021, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C.; Singh, G.; Lott, M.T.; Hodge, J.A.; Schurr, T.G.; Lezza, A.M.; Elsas, L.J., 2nd; Nikoskelainen, E.K. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 1988, 242, 1427–1430. [Google Scholar] [CrossRef]
- Luft, R.; Ikkos, D.; Palmieri, G.; Ernster, L.; Afzelius, B. A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: A correlated clinical, biochemical, and morphological study. J. Clin. Investig. 1962, 41, 1776–1804. [Google Scholar] [CrossRef]
- Holt, I.J.; Harding, A.E.; Morgan-Hughes, J.A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 1988, 331, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.K.; Priya, V.; Setia, A.; Mehata, A.K.; Mohan, S.; Albratty, M.; Najmi, A.; Meraya, A.M.; Makeen, H.A.; Tambuwala, M.M.; et al. Mitochondrial targeting theranostic nanomedicine and molecular biomarkers for efficient cancer diagnosis and therapy. Biomed. Pharmacother. 2022, 153, 113451. [Google Scholar] [CrossRef]
- Richter, C.; Park, J.W.; Ames, B.N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl. Acad. Sci. USA 1988, 85, 6465–6467. [Google Scholar] [CrossRef]
- Mecocci, P.; MacGarvey, U.; Kaufman, A.E.; Koontz, D.; Shoffner, J.M.; Wallace, D.C.; Beal, M.F. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann. Neurol. 1993, 34, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.M.; George, M., Jr.; Wilson, A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Lightowlers, R.N.; Chinnery, P.F.; Turnbull, D.M.; Howell, N. Mammalian mitochondrial genetics: Heredity, heteroplasmy and disease. Trends Genet. 1997, 13, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Schon, E.A.; Bonilla, E.; DiMauro, S. Mitochondrial DNA mutations and pathogenesis. J. Bioenerg. Biomembr. 1997, 29, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, C.; Lach, B.; Shoubridge, E.A. Variable distribution of mutant mitochondrial DNAs (tRNA(Leu[3243])) in tissues of symptomatic relatives with MELAS: The role of mitotic segregation. Neurology 1993, 43, 1586–1590. [Google Scholar] [CrossRef]
- Moraes, C.T.; DiMauro, S.; Zeviani, M.; Lombes, A.; Shanske, S.; Miranda, A.F.; Nakase, H.; Bonilla, E.; Werneck, L.C.; Servidei, S.; et al. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N. Engl. J. Med. 1989, 320, 1293–1299. [Google Scholar] [CrossRef]
- Rotig, A.; Colonna, M.; Bonnefont, J.P.; Blanche, S.; Fischer, A.; Saudubray, J.M.; Munnich, A. Mitochondrial DNA deletion in Pearson’s marrow/pancreas syndrome. Lancet 1989, 1, 902–903. [Google Scholar] [CrossRef]
- Leigh, D. Subacute necrotizing encephalomyelopathy in an infant. J. Neurol. Neurosurg. Psychiatry 1951, 14, 216–221. [Google Scholar] [CrossRef]
- Rahman, S.; Thorburn, D. Nuclear Gene-Encoded Leigh Syndrome Spectrum Overview. In GeneReviews((R)); Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Goto, Y.; Nonaka, I.; Horai, S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 1990, 348, 651–653. [Google Scholar] [CrossRef]
- Shoffner, J.M.; Lott, M.T.; Lezza, A.M.; Seibel, P.; Ballinger, S.W.; Wallace, D.C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell 1990, 61, 931–937. [Google Scholar] [CrossRef]
- Holt, I.J.; Harding, A.E.; Petty, R.K.; Morgan-Hughes, J.A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am. J. Hum. Genet. 1990, 46, 428–433. [Google Scholar]
- Structural Nuclear Genes for Mitochondrial Disorders. Available online: https://www.mitomap.org/foswiki/bin/view/MITOMAP/NuclearGenesStructural (accessed on 30 November 2022).
- Non-Structural Nuclear Genes for Mitochondrial Disorders. Available online: https://www.mitomap.org/bin/view.pl/MITOMAP/NuclearGenesNonStructural (accessed on 30 November 2022).
- Capaldi, R.A. Structure and function of cytochrome c oxidase. Annu. Rev. Biochem. 1990, 59, 569–596. [Google Scholar] [CrossRef] [PubMed]
- Balsa, E.; Marco, R.; Perales-Clemente, E.; Szklarczyk, R.; Calvo, E.; Landazuri, M.O.; Enriquez, J.A. NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab. 2012, 16, 378–386. [Google Scholar] [CrossRef]
- Tsukihara, T.; Aoyama, H.; Yamashita, E.; Tomizaki, T.; Yamaguchi, H.; Shinzawa-Itoh, K.; Nakashima, R.; Yaono, R.; Yoshikawa, S. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science 1995, 269, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, I.; Zatsepin, N.A.; Hikita, M.; Conrad, C.E.; Nelson, G.; Coe, J.D.; Basu, S.; Grant, T.D.; Seaberg, M.H.; Sierra, R.G.; et al. Crystal structure of CO-bound cytochrome c oxidase determined by serial femtosecond X-ray crystallography at room temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 8011–8016. [Google Scholar] [CrossRef]
- Pitceathly, R.D.S.; Taanman, J.W. NDUFA4 (Renamed COXFA4) Is a Cytochrome-c Oxidase Subunit. Trends Endocrinol. Metab. 2018, 29, 452–454. [Google Scholar] [CrossRef]
- Sinkler, C.A.; Kalpage, H.; Shay, J.; Lee, I.; Malek, M.H.; Grossman, L.I.; Huttemann, M. Tissue- and Condition-Specific Isoforms of Mammalian Cytochrome c Oxidase Subunits: From Function to Human Disease. Oxid. Med. Cell. Longev. 2017, 2017, 1534056. [Google Scholar] [CrossRef] [PubMed]
- Tsukihara, T.; Aoyama, H.; Yamashita, E.; Tomizaki, T.; Yamaguchi, H.; Shinzawa-Itoh, K.; Nakashima, R.; Yaono, R.; Yoshikawa, S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science 1996, 272, 1136–1144. [Google Scholar] [CrossRef]
- Brown, M.D.; Voljavec, A.S.; Lott, M.T.; MacDonald, I.; Wallace, D.C. Leber’s hereditary optic neuropathy: A model for mitochondrial neurodegenerative diseases. FASEB J. 1992, 6, 2791–2799. [Google Scholar] [CrossRef]
- Clark, K.M.; Taylor, R.W.; Johnson, M.A.; Chinnery, P.F.; Chrzanowska-Lightowlers, Z.M.; Andrews, R.M.; Nelson, I.P.; Wood, N.W.; Lamont, P.J.; Hanna, M.G.; et al. An mtDNA mutation in the initiation codon of the cytochrome C oxidase subunit II gene results in lower levels of the protein and a mitochondrial encephalomyopathy. Am. J. Hum. Genet. 1999, 64, 1330–1339. [Google Scholar] [CrossRef][Green Version]
- Johns, D.R.; Neufeld, M.J. Cytochrome c oxidase mutations in Leber hereditary optic neuropathy. Biochem. Biophys. Res. Commun. 1993, 196, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Abu-Libdeh, B.; Douiev, L.; Amro, S.; Shahrour, M.; Ta-Shma, A.; Miller, C.; Elpeleg, O.; Saada, A. Mutation in the COX4I1 gene is associated with short stature, poor weight gain and increased chromosomal breaks, simulating Fanconi anemia. Eur. J. Hum. Genet. 2017, 25, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Vondrackova, A.; Vesela, K.; Hansikova, H.; Docekalova, D.Z.; Rozsypalova, E.; Zeman, J.; Tesarova, M. High-resolution melting analysis of 15 genes in 60 patients with cytochrome-c oxidase deficiency. J. Hum. Genet. 2012, 57, 442–448. [Google Scholar] [CrossRef]
- Baertling, F.; Al-Murshedi, F.; Sanchez-Caballero, L.; Al-Senaidi, K.; Joshi, N.P.; Venselaar, H.; van den Brand, M.A.; Nijtmans, L.G.; Rodenburg, R.J. Mutation in mitochondrial complex IV subunit COX5A causes pulmonary arterial hypertension, lactic acidemia, and failure to thrive. Hum. Mutat. 2017, 38, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, G.; Makino, S.; Hayashi, M.; Abe, A.; Numakura, C.; Ueki, M.; Tanaka, A.; Ito, C.; Toshimori, K.; Ogawa, N.; et al. A mutation of COX6A1 causes a recessive axonal or mixed form of Charcot-Marie-Tooth disease. Am. J. Hum. Genet. 2014, 95, 294–300. [Google Scholar] [CrossRef]
- Inoue, M.; Uchino, S.; Iida, A.; Noguchi, S.; Hayashi, S.; Takahashi, T.; Fujii, K.; Komaki, H.; Takeshita, E.; Nonaka, I.; et al. COX6A2 variants cause a muscle-specific cytochrome c oxidase deficiency. Ann. Neurol. 2019, 86, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Massa, V.; Fernandez-Vizarra, E.; Alshahwan, S.; Bakhsh, E.; Goffrini, P.; Ferrero, I.; Mereghetti, P.; D’Adamo, P.; Gasparini, P.; Zeviani, M. Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am. J. Hum. Genet. 2008, 82, 1281–1289. [Google Scholar] [CrossRef]
- Indrieri, A.; van Rahden, V.A.; Tiranti, V.; Morleo, M.; Iaconis, D.; Tammaro, R.; D’Amato, I.; Conte, I.; Maystadt, I.; Demuth, S.; et al. Mutations in COX7B cause microphthalmia with linear skin lesions, an unconventional mitochondrial disease. Am. J. Hum. Genet. 2012, 91, 942–949. [Google Scholar] [CrossRef]
- Hallmann, K.; Kudin, A.P.; Zsurka, G.; Kornblum, C.; Reimann, J.; Stuve, B.; Waltz, S.; Hattingen, E.; Thiele, H.; Nurnberg, P.; et al. Loss of the smallest subunit of cytochrome c oxidase, COX8A, causes Leigh-like syndrome and epilepsy. Brain 2016, 139 Pt 2, 338–345. [Google Scholar] [CrossRef]
- Pitceathly, R.D.; Rahman, S.; Wedatilake, Y.; Polke, J.M.; Cirak, S.; Foley, A.R.; Sailer, A.; Hurles, M.E.; Stalker, J.; Hargreaves, I.; et al. NDUFA4 mutations underlie dysfunction of a cytochrome c oxidase subunit linked to human neurological disease. Cell Rep. 2013, 3, 1795–1805. [Google Scholar] [CrossRef]
- Tiranti, V.; Hoertnagel, K.; Carrozzo, R.; Galimberti, C.; Munaro, M.; Granatiero, M.; Zelante, L.; Gasparini, P.; Marzella, R.; Rocchi, M.; et al. Mutations of SURF-1 in Leigh disease associated with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 1998, 63, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yao, J.; Johns, T.; Fu, K.; De Bie, I.; Macmillan, C.; Cuthbert, A.P.; Newbold, R.F.; Wang, J.; Chevrette, M.; et al. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat. Genet. 1998, 20, 337–343. [Google Scholar] [CrossRef]
- Papadopoulou, L.C.; Sue, C.M.; Davidson, M.M.; Tanji, K.; Nishino, I.; Sadlock, J.E.; Krishna, S.; Walker, W.; Selby, J.; Glerum, D.M.; et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat. Genet. 1999, 23, 333–337. [Google Scholar] [CrossRef]
- Valnot, I.; Osmond, S.; Gigarel, N.; Mehaye, B.; Amiel, J.; Cormier-Daire, V.; Munnich, A.; Bonnefont, J.P.; Rustin, P.; Rotig, A. Mutations of the SCO1 gene in mitochondrial cytochrome c oxidase deficiency with neonatal-onset hepatic failure and encephalopathy. Am. J. Hum. Genet. 2000, 67, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Antonicka, H.; Leary, S.C.; Guercin, G.H.; Agar, J.N.; Horvath, R.; Kennaway, N.G.; Harding, C.O.; Jaksch, M.; Shoubridge, E.A. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum. Mol. Genet. 2003, 12, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Antonicka, H.; Mattman, A.; Carlson, C.G.; Glerum, D.M.; Hoffbuhr, K.C.; Leary, S.C.; Kennaway, N.G.; Shoubridge, E.A. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am. J. Hum. Genet. 2003, 72, 101–114. [Google Scholar] [CrossRef]
- Bugiani, M.; Tiranti, V.; Farina, L.; Uziel, G.; Zeviani, M. Novel mutations in COX15 in a long surviving Leigh syndrome patient with cytochrome c oxidase deficiency. J. Med. Genet. 2005, 42, e28. [Google Scholar] [CrossRef]
- Weraarpachai, W.; Sasarman, F.; Nishimura, T.; Antonicka, H.; Aure, K.; Rotig, A.; Lombes, A.; Shoubridge, E.A. Mutations in C12orf62, a factor that couples COX I synthesis with cytochrome c oxidase assembly, cause fatal neonatal lactic acidosis. Am. J. Hum. Genet. 2012, 90, 142–151. [Google Scholar] [CrossRef]
- Li, P.; Guo, D.; Zhang, X.; Ji, K.; Lv, H.; Zhang, Y.; Chen, Z.; Ma, J.; Fang, Y.; Liu, Y. Compound Heterozygous COX20 Variants Impair the Function of Mitochondrial Complex IV to Cause a Syndrome Involving Ophthalmoplegia and Visual Failure. Front. Neurol. 2022, 13, 873943. [Google Scholar] [CrossRef]
- Ostergaard, E.; Weraarpachai, W.; Ravn, K.; Born, A.P.; Jonson, L.; Duno, M.; Wibrand, F.; Shoubridge, E.A.; Vissing, J. Mutations in COA3 cause isolated complex IV deficiency associated with neuropathy, exercise intolerance, obesity, and short stature. J. Med. Genet. 2015, 52, 203–207. [Google Scholar] [CrossRef]
- Lim, S.C.; Smith, K.R.; Stroud, D.A.; Compton, A.G.; Tucker, E.J.; Dasvarma, A.; Gandolfo, L.C.; Marum, J.E.; McKenzie, M.; Peters, H.L.; et al. A founder mutation in PET100 causes isolated complex IV deficiency in Lebanese individuals with Leigh syndrome. Am. J. Hum. Genet. 2014, 94, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Renkema, G.H.; Visser, G.; Baertling, F.; Wintjes, L.T.; Wolters, V.M.; van Montfrans, J.; de Kort, G.A.P.; Nikkels, P.G.J.; van Hasselt, P.M.; van der Crabben, S.N.; et al. Mutated PET117 causes complex IV deficiency and is associated with neurodevelopmental regression and medulla oblongata lesions. Hum. Genet. 2017, 136, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Huigsloot, M.; Nijtmans, L.G.; Szklarczyk, R.; Baars, M.J.; van den Brand, M.A.; Hendriksfranssen, M.G.; van den Heuvel, L.P.; Smeitink, J.A.; Huynen, M.A.; Rodenburg, R.J. A mutation in C2orf64 causes impaired cytochrome c oxidase assembly and mitochondrial cardiomyopathy. Am. J. Hum. Genet. 2011, 88, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Baertling, F.; M, A.M.v.d.B.; Hertecant, J.L.; Al-Shamsi, A.; L, P.v.d.H.; Distelmaier, F.; Mayatepek, E.; Smeitink, J.A.; Nijtmans, L.G.; Rodenburg, R.J. Mutations in COA6 cause cytochrome c oxidase deficiency and neonatal hypertrophic cardiomyopathy. Hum. Mutat. 2015, 36, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Martinez Lyons, A.; Ardissone, A.; Reyes, A.; Robinson, A.J.; Moroni, I.; Ghezzi, D.; Fernandez-Vizarra, E.; Zeviani, M. COA7 (C1orf163/RESA1) mutations associated with mitochondrial leukoencephalopathy and cytochrome c oxidase deficiency. J. Med. Genet. 2016, 53, 846–849. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, P.; Fernandez-Vizarra, E.; Zeviani, M.; Van der Knaap, M.S.; Saran, R.K. Cavitating Leukoencephalopathy With Posterior Predominance Caused by a Deletion in the APOPT1 Gene in an Indian Boy. J. Child Neurol. 2018, 33, 428–431. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lepage, P.; Miller, K.; Bunkenborg, J.; Reich, M.; Hjerrild, M.; Delmonte, T.; Villeneuve, A.; Sladek, R.; Xu, F.; et al. Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics. Proc. Natl. Acad. Sci. USA 2003, 100, 605–610. [Google Scholar] [CrossRef]
- Yoo, D.H.; Choi, Y.C.; Nam, D.E.; Choi, S.S.; Kim, J.W.; Choi, B.O.; Chung, K.W. Identification of FASTKD2 compound heterozygous mutations as the underlying cause of autosomal recessive MELAS-like syndrome. Mitochondrion 2017, 35, 54–58. [Google Scholar] [CrossRef]
- Weraarpachai, W.; Antonicka, H.; Sasarman, F.; Seeger, J.; Schrank, B.; Kolesar, J.E.; Lochmuller, H.; Chevrette, M.; Kaufman, B.A.; Horvath, R.; et al. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat. Genet. 2009, 41, 833–837. [Google Scholar] [CrossRef]
- Brischigliaro, M.; Zeviani, M. Cytochrome c oxidase deficiency. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148335. [Google Scholar] [CrossRef]
- Foltopoulou, P.F.; Zachariadis, G.A.; Politou, A.S.; Tsiftsoglou, A.S.; Papadopoulou, L.C. Human recombinant mutated forms of the mitochondrial COX assembly Sco2 protein differ from wild-type in physical state and copper binding capacity. Mol. Genet. Metab. 2004, 81, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Pratt, A.T.; Soma, S.; Theriault, S.G.; Griffin, A.T.; Trivedi, P.P.; Gohil, V.M. Mitochondrial disease genes COA6, COX6B and SCO2 have overlapping roles in COX2 biogenesis. Hum. Mol. Genet. 2016, 25, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Jaksch, M.; Paret, C.; Stucka, R.; Horn, N.; Muller-Hocker, J.; Horvath, R.; Trepesch, N.; Stecker, G.; Freisinger, P.; Thirion, C.; et al. Cytochrome c oxidase deficiency due to mutations in SCO2, encoding a mitochondrial copper-binding protein, is rescued by copper in human myoblasts. Hum. Mol. Genet. 2001, 10, 3025–3035. [Google Scholar] [CrossRef]
- Leary, S.C.; Kaufman, B.A.; Pellecchia, G.; Guercin, G.H.; Mattman, A.; Jaksch, M.; Shoubridge, E.A. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum. Mol. Genet. 2004, 13, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Pacheu-Grau, D.; Bareth, B.; Dudek, J.; Juris, L.; Vogtle, F.N.; Wissel, M.; Leary, S.C.; Dennerlein, S.; Rehling, P.; Deckers, M. Cooperation between COA6 and SCO2 in COX2 maturation during cytochrome c oxidase assembly links two mitochondrial cardiomyopathies. Cell Metab. 2015, 21, 823–833. [Google Scholar] [CrossRef]
- Soma, S.; Morgada, M.N.; Naik, M.T.; Boulet, A.; Roesler, A.A.; Dziuba, N.; Ghosh, A.; Yu, Q.; Lindahl, P.A.; Ames, J.B.; et al. COA6 Is Structurally Tuned to Function as a Thiol-Disulfide Oxidoreductase in Copper Delivery to Mitochondrial Cytochrome c Oxidase. Cell Rep. 2019, 29, 4114–4126 e4115. [Google Scholar] [CrossRef]
- Leary, S.C.; Sasarman, F.; Nishimura, T.; Shoubridge, E.A. Human SCO2 is required for the synthesis of CO II and as a thiol-disulphide oxidoreductase for SCO1. Hum. Mol. Genet. 2009, 18, 2230–2240. [Google Scholar] [CrossRef]
- Williams, J.C.; Sue, C.; Banting, G.S.; Yang, H.; Glerum, D.M.; Hendrickson, W.A.; Schon, E.A. Crystal structure of human SCO1: Implications for redox signaling by a mitochondrial cytochrome c oxidase “assembly” protein. J. Biol. Chem. 2005, 280, 15202–15211. [Google Scholar] [CrossRef]
- Assaily, W.; Benchimol, S. Differential utilization of two ATP-generating pathways is regulated by p53. Cancer Cell 2006, 10, 4–6. [Google Scholar] [CrossRef][Green Version]
- Matoba, S.; Kang, J.G.; Patino, W.D.; Wragg, A.; Boehm, M.; Gavrilova, O.; Hurley, P.J.; Bunz, F.; Hwang, P.M. p53 regulates mitochondrial respiration. Science 2006, 312, 1650–1653. [Google Scholar] [CrossRef]
- Barcia, G.; Assouline, Z.; Pennisi, A.; Gitiaux, C.; Schiff, M.; Boddaert, N.; Munnich, A.; Bonnefont, J.P.; Rotig, A. Cytochrome c oxidase deficiency caused by biallelic SCO2 mutations in two sibs with cerebellar ataxia and progressive peripheral axonal neuropathy. Mol. Genet. Metab. Rep. 2019, 21, 100528. [Google Scholar] [CrossRef] [PubMed]
- Gangfuss, A.; Hentschel, A.; Rademacher, N.; Sickmann, A.; Stuve, B.; Horvath, R.; Gross, C.; Kohlschmidt, N.; Forster, F.; Abicht, A.; et al. Identification of a novel homozygous synthesis of cytochrome c oxidase 2 variant in siblings with early-onset axonal Charcot-Marie-Tooth disease. Hum. Mutat. 2022, 43, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Gurgel-Giannetti, J.; Oliveira, G.; Brasileiro Filho, G.; Martins, P.; Vainzof, M.; Hirano, M. Mitochondrial cardioencephalomyopathy due to a novel SCO2 mutation in a Brazilian patient: Case report and literature review. JAMA Neurol. 2013, 70, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Jaksch, M.; Ogilvie, I.; Yao, J.; Kortenhaus, G.; Bresser, H.G.; Gerbitz, K.D.; Shoubridge, E.A. Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000, 9, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Joost, K.; Rodenburg, R.; Piirsoo, A.; van den Heuvel, B.; Zordania, R.; Ounap, K. A novel mutation in the SCO2 gene in a neonate with early-onset cardioencephalomyopathy. Pediatr. Neurol. 2010, 42, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Knuf, M.; Faber, J.; Huth, R.G.; Freisinger, P.; Zepp, F.; Kampmann, C. Identification of a novel compound heterozygote SCO2 mutation in cytochrome c oxidase deficient fatal infantile cardioencephalomyopathy. Acta Paediatr. 2007, 96, 130–132. [Google Scholar] [CrossRef]
- Leary, S.C.; Mattman, A.; Wai, T.; Koehn, D.C.; Clarke, L.A.; Chan, S.; Lomax, B.; Eydoux, P.; Vallance, H.D.; Shoubridge, E.A. A hemizygous SCO2 mutation in an early onset rapidly progressive, fatal cardiomyopathy. Mol. Genet. Metab. 2006, 89, 129–133. [Google Scholar] [CrossRef]
- Mobley, B.C.; Enns, G.M.; Wong, L.J.; Vogel, H. A novel homozygous SCO2 mutation, p.G193S, causing fatal infantile cardioencephalomyopathy. Clin. Neuropathol. 2009, 28, 143–149. [Google Scholar] [CrossRef]
- Pronicka, E.; Piekutowska-Abramczuk, D.; Szymanska-Debinska, T.; Bielecka, L.; Kowalski, P.; Luczak, S.; Karkucinska-Wieckowska, A.; Migdal, M.; Kubalska, J.; Zimowski, J.; et al. The natural history of SCO2 deficiency in 36 Polish children confirmed the genotype-phenotype correlation. Mitochondrion 2013, 13, 810–816. [Google Scholar] [CrossRef]
- Pronicki, M.; Kowalski, P.; Piekutowska-Abramczuk, D.; Taybert, J.; Karkucinska-Wieckowska, A.; Szymanska-Debinska, T.; Karczmarewicz, E.; Pajdowska, M.; Migdal, M.; Milewska-Bobula, B.; et al. A homozygous mutation in the SCO2 gene causes a spinal muscular atrophy like presentation with stridor and respiratory insufficiency. Eur. J. Paediatr. Neurol. 2010, 14, 253–260. [Google Scholar] [CrossRef]
- Rebelo, A.P.; Saade, D.; Pereira, C.V.; Farooq, A.; Huff, T.C.; Abreu, L.; Moraes, C.T.; Mnatsakanova, D.; Mathews, K.; Yang, H.; et al. SCO2 mutations cause early-onset axonal Charcot-Marie-Tooth disease associated with cellular copper deficiency. Brain 2018, 141, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, S.; Salviati, L.; Sue, C.M.; Shanske, S.; Davidson, M.M.; Bonilla, E.; Naini, A.B.; De Vivo, D.C.; DiMauro, S. Mutation screening in patients with isolated cytochrome c oxidase deficiency. Pediatr. Res. 2003, 53, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Salviati, L.; Sacconi, S.; Rasalan, M.M.; Kronn, D.F.; Braun, A.; Canoll, P.; Davidson, M.; Shanske, S.; Bonilla, E.; Hays, A.P.; et al. Cytochrome c oxidase deficiency due to a novel SCO2 mutation mimics Werdnig-Hoffmann disease. Arch. Neurol. 2002, 59, 862–865. [Google Scholar] [CrossRef]
- Sambuughin, N.; Liu, X.; Bijarnia, S.; Wallace, T.; Verma, I.C.; Hamilton, S.; Muldoon, S.; Tallon, L.J.; Wang, S. Exome sequencing reveals SCO2 mutations in a family presented with fatal infantile hyperthermia. J. Hum. Genet. 2013, 58, 226–228. [Google Scholar] [CrossRef][Green Version]
- Szymanska-Debinska, T.; Karkucinska-Wieckowska, A.; Piekutowska-Abramczuk, D.; Jurkiewicz, E.; Iwanicka-Pronicka, K.; Rokicki, D.; Pronicki, M. Leigh disease due to SCO2 mutations revealed at extended autopsy. J. Clin. Pathol. 2015, 68, 397–399. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Bourgeois, J.M.; Fu, M.H.; Kataeva, G.; Shah, J.; Simon, D.K.; Mahoney, D.; Johns, D.; MacKay, N.; Robinson, B.H. Novel SCO2 mutation (G1521A) presenting as a spinal muscular atrophy type I phenotype. Am. J. Med. Genet. A 2004, 125A, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.K.; Shanske, S.; Kaplan, P.; DiMauro, S. Association of mutations in SCO2, a cytochrome c oxidase assembly gene, with early fetal lethality. Arch. Neurol 2004, 61, 950–952. [Google Scholar] [CrossRef]
- Tran-Viet, K.N.; Powell, C.; Barathi, V.A.; Klemm, T.; Maurer-Stroh, S.; Limviphuvadh, V.; Soler, V.; Ho, C.; Yanovitch, T.; Schneider, G.; et al. Mutations in SCO2 are associated with autosomal-dominant high-grade myopia. Am. J. Hum. Genet. 2013, 92, 820–826. [Google Scholar] [CrossRef]
- Verdijk, R.M.; de Krijger, R.; Schoonderwoerd, K.; Tiranti, V.; Smeets, H.; Govaerts, L.C.; de Coo, R. Phenotypic consequences of a novel SCO2 gene mutation. Am. J. Med. Genet. A 2008, 146A, 2822–2827. [Google Scholar] [CrossRef]
- Vesela, K.; Hansikova, H.; Tesarova, M.; Martasek, P.; Elleder, M.; Houstek, J.; Zeman, J. Clinical, biochemical and molecular analyses of six patients with isolated cytochrome c oxidase deficiency due to mutations in the SCO2 gene. Acta Paediatr. 2004, 93, 1312–1317. [Google Scholar] [CrossRef]
- Vesela, K.; Hulkova, H.; Hansikova, H.; Zeman, J.; Elleder, M. Structural analysis of tissues affected by cytochrome C oxidase deficiency due to mutations in the SCO2 gene. APMIS 2008, 116, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Vondrackova, A.; Vesela, K.; Kratochvilova, H.; Kucerova Vidrova, V.; Vinsova, K.; Stranecky, V.; Honzik, T.; Hansikova, H.; Zeman, J.; Tesarova, M. Large copy number variations in combination with point mutations in the TYMP and SCO2 genes found in two patients with mitochondrial disorders. Eur. J. Hum. Genet. 2014, 22, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Rucheton, B.; Ewenczyk, C.; Gaignard, P.; de Sainte Agathe, J.M.; Fauret, A.L.; Saillour, V.; Leonard-Louis, S.; Touitou, V.; Mochel, F. Adult Cerebellar Ataxia, Axonal Neuropathy, and Sensory Impairments Caused by Biallelic SCO2 Variants. Neurol. Genet. 2021, 7, e630. [Google Scholar] [CrossRef]
- Wakazono, T.; Miyake, M.; Yamashiro, K.; Yoshikawa, M.; Yoshimura, N. Association between SCO2 mutation and extreme myopia in Japanese patients. Jpn. J. Ophthalmol. 2016, 60, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.; Pronicka, E.; Karczmarewicz, E.; Pronicki, M.; Piekutowska-Abramczuk, D.; Sykut-Cegielska, J.; Mierzewska, H.; Hansikova, H.; Vesela, K.; Tesarova, M.; et al. Retrospective, multicentric study of 180 children with cytochrome C oxidase deficiency. Pediatr. Res. 2006, 59, 21–26. [Google Scholar] [CrossRef]
- Jiang, D.; Li, J.; Xiao, X.; Li, S.; Jia, X.; Sun, W.; Guo, X.; Zhang, Q. Detection of mutations in LRPAP1, CTSH, LEPREL1, ZNF644, SLC39A5, and SCO2 in 298 families with early-onset high myopia by exome sequencing. Investig. Ophthalmol. Vis. Sci. 2014, 56, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.B.; Zheng, Y.H.; Chen, D.F.; Zhou, F.Y.; Xia, L.Q.; Wen, X.R.; Yuan, Y.M.; Han, F.; Piao, S.Y.; Zhuang, W.; et al. Expanding the Phenotypic and Genotypic Landscape of Nonsyndromic High Myopia: A Cross-Sectional Study in 731 Chinese Patients. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4052–4062. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Cai, X.B.; Xia, L.Q.; Zhou, F.Y.; Wen, X.R.; Chen, D.F.; Han, F.; Zhou, K.J.; Jin, Z.B.; Zhuang, W.J.; et al. Mutational screening of AGRN, SLC39A5, SCO2, P4HA2, BSG, ZNF644, and CPSF1 in a Chinese cohort of 103 patients with nonsyndromic high myopia. Mol. Vis. 2021, 27, 706–717. [Google Scholar]
- Jaksch, M.; Horvath, R.; Horn, N.; Auer, D.P.; Macmillan, C.; Peters, J.; Gerbitz, K.D.; Kraegeloh-Mann, I.; Muntau, A.; Karcagi, V.; et al. Homozygosity (E140K) in SCO2 causes delayed infantile onset of cardiomyopathy and neuropathy. Neurology 2001, 57, 1440–1446. [Google Scholar] [CrossRef]
- Yang, H.; Brosel, S.; Acin-Perez, R.; Slavkovich, V.; Nishino, I.; Khan, R.; Goldberg, I.J.; Graziano, J.; Manfredi, G.; Schon, E.A. Analysis of mouse models of cytochrome c oxidase deficiency owing to mutations in Sco2. Hum. Mol. Genet. 2010, 19, 170–180. [Google Scholar] [CrossRef]
- Hill, S.; Deepa, S.S.; Sataranatarajan, K.; Premkumar, P.; Pulliam, D.; Liu, Y.; Soto, V.Y.; Fischer, K.E.; Van Remmen, H. Sco2 deficient mice develop increased adiposity and insulin resistance. Mol. Cell. Endocrinol. 2017, 455, 103–114. [Google Scholar] [CrossRef]
- Antonarakis, S.E.; Beckmann, J.S. Mendelian disorders deserve more attention. Nat. Rev. Genet. 2006, 7, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.K. Monogenic hereditary diseases. Ugeskr. Laeger 2003, 165, 805–809. [Google Scholar] [PubMed]
- Sauer, V.; Roy-Chowdhury, N.; Roy-Chowdhury, J. Monogenic Liver Diseases. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1857–1865. [Google Scholar] [CrossRef]
- ESHRE PGD Consortium. Available online: https://www.eshre.eu/Data-collection-and-research/Consortia/PGD-Consortium/ (accessed on 29 May 2020).
- Chen, C.K.; Yu, H.T.; Soong, Y.K.; Lee, C.L. New perspectives on preimplantation genetic diagnosis and preimplantation genetic screening. Taiwan. J. Obstet. Gynecol. 2014, 53, 146–150. [Google Scholar] [CrossRef]
- Tambuyzer, E.; Vandendriessche, B.; Austin, C.P.; Brooks, P.J.; Larsson, K.; Miller Needleman, K.I.; Valentine, J.; Davies, K.; Groft, S.C.; Preti, R.; et al. Therapies for rare diseases: Therapeutic modalities, progress and challenges ahead. Nat. Rev. Drug Discov. 2020, 19, 93–111. [Google Scholar] [CrossRef] [PubMed]
- List of Rare Diseases Tops 10,000—and Is Growing—New Analysis Finds. Available online: https://fabrydiseasenews.com/news/list-rare-diseases-tops-10000-growing-analysis/ (accessed on 14 July 2022).
- Gorzelany, J.A.; de Souza, M.P. Protein replacement therapies for rare diseases: A breeze for regulatory approval? Sci. Transl. Med. 2013, 5, 178fs110. [Google Scholar] [CrossRef][Green Version]
- Bentia, D.; Saceleanu, M.V.; Marinescu, A.A.; Ciurea, A.V. Centenary of Insulin Discovery (1921–2021): Nicolae Paulescu’s Original Contributions. Acta Endocrinol. 2021, 17, 406–411. [Google Scholar] [CrossRef]
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A.A. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can. Med. Assoc. J. 1922, 12, 141–146. [Google Scholar]
- Jurecka, A.; Malinova, V.; Tylki-Szymanska, A. Effect of rapid cessation of enzyme replacement therapy: A report of 5 more cases. Mol. Genet. Metab. 2014, 111, 212–213. [Google Scholar] [CrossRef]
- Desnick, R.J.; Schuchman, E.H. Enzyme replacement therapy for lysosomal diseases: Lessons from 20 years of experience and remaining challenges. Annu. Rev. Genom. Hum. Genet. 2012, 13, 307–335. [Google Scholar] [CrossRef]
- Peters, R.; Harris, T. Advances and innovations in haemophilia treatment. Nat. Rev. Drug Discov. 2018, 17, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Beck, M. Treatment strategies for lysosomal storage disorders. Dev. Med. Child Neurol. 2018, 60, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Ravina, B.M.; Bankiewicz, K.S.; Paul, S.M.; Sah, D.W.Y. Gene therapy for neurological disorders: Progress and prospects. Nat. Rev. Drug Discov. 2018, 17, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Nance, M.E.; Duan, D. Perspective on Adeno-Associated Virus Capsid Modification for Duchenne Muscular Dystrophy Gene Therapy. Hum. Gene Ther. 2015, 26, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.B. Historical Perspective on the Current Renaissance for Hematopoietic Stem Cell Gene Therapy. Hematol. Oncol. Clin. N. Am. 2017, 31, 721–735. [Google Scholar] [CrossRef]
- Wilson, J.M. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol. Genet. Metab. 2009, 96, 151–157. [Google Scholar] [CrossRef]
- Yla-Herttuala, S. Endgame: Glybera finally recommended for approval as the first gene therapy drug in the European union. Mol. Ther. 2012, 20, 1831–1832. [Google Scholar] [CrossRef]
- Gaziev, J.; Lucarelli, G. Allogeneic cellular gene therapy for hemoglobinopathies. Hematol. Oncol. Clin. N. Am. 2010, 24, 1145–1163. [Google Scholar] [CrossRef]
- Ali, G.; Tariq, M.A.; Shahid, K.; Ahmad, F.J.; Akram, J. Advances in genome editing: The technology of choice for precise and efficient beta-thalassemia treatment. Gene Ther. 2021, 28, 6–15. [Google Scholar] [CrossRef]
- Xie, S.Y.; Ren, Z.R.; Zhang, J.Z.; Guo, X.B.; Wang, Q.X.; Wang, S.; Lin, D.; Gong, X.L.; Li, W.; Huang, S.Z.; et al. Restoration of the balanced alpha/beta-globin gene expression in beta654-thalassemia mice using combined RNAi and antisense RNA approach. Hum. Mol. Genet. 2007, 16, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Voon, H.P.; Vadolas, J. Controlling alpha-globin: A review of alpha-globin expression and its impact on beta-thalassemia. Haematologica 2008, 93, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.E.; Kamran, S.C.; Lessard, S.; Xu, J.; Fujiwara, Y.; Lin, C.; Shao, Z.; Canver, M.C.; Smith, E.C.; Pinello, L.; et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science 2013, 342, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.C.; Fedosyuk, H.; Neades, R.; de Los Rios, J.B.; Barbas, C.F., 3rd; Peterson, K.R. Induction of Fetal Hemoglobin In Vivo Mediated by a Synthetic gamma-Globin Zinc Finger Activator. Anemia 2012, 2012, 507894. [Google Scholar] [CrossRef] [PubMed]
- Guda, S.; Brendel, C.; Renella, R.; Du, P.; Bauer, D.E.; Canver, M.C.; Grenier, J.K.; Grimson, A.W.; Kamran, S.C.; Thornton, J.; et al. miRNA-embedded shRNAs for Lineage-specific BCL11A Knockdown and Hemoglobin F Induction. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Karponi, G.; Zogas, N. Gene Therapy For Beta-Thalassemia: Updated Perspectives. Appl. Clin. Genet. 2019, 12, 167–180. [Google Scholar] [CrossRef]
- Almannai, M.; El-Hattab, A.W.; Alic, M.; Soler-Alfonsoc, C.; Scaglia, F. Clinical trials in mitochondrial disorders, an update. Mol. Genet. Metab. 2020, 131, 1–13. [Google Scholar] [CrossRef]
- Almannai, M.; El-Hattab, A.W.; Scaglia, F. Mitochondrial DNA replication: Clinical syndromes. Essays Biochem. 2018, 62, 297–308. [Google Scholar] [CrossRef]
- DiMauro, S.; Schon, E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 2003, 348, 2656–2668. [Google Scholar] [CrossRef]
- Meyerson, C.; Van Stavern, G.; McClelland, C. Leber hereditary optic neuropathy: Current perspectives. Clin. Ophthalmol. 2015, 9, 1165–1176. [Google Scholar] [CrossRef]
- Koilkonda, R.D.; Yu, H.; Chou, T.H.; Feuer, W.J.; Ruggeri, M.; Porciatti, V.; Tse, D.; Hauswirth, W.W.; Chiodo, V.; Boye, S.L.; et al. Safety and effects of the vector for the Leber hereditary optic neuropathy gene therapy clinical trial. JAMA Ophthalmol. 2014, 132, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Hanaford, A.R.; Cho, Y.J.; Nakai, H. AAV-vector based gene therapy for mitochondrial disease: Progress and future perspectives. Orphanet J. Rare Dis. 2022, 17, 217. [Google Scholar] [CrossRef]
- Suerth, J.D.; Schambach, A.; Baum, C. Genetic modification of lymphocytes by retrovirus-based vectors. Curr. Opin. Immunol. 2012, 24, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Slivac, I.; Guay, D.; Mangion, M.; Champeil, J.; Gaillet, B. Non-viral nucleic acid delivery methods. Expert Opin. Biol. Ther. 2017, 17, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Kim, M.; Seo, Y.; Moon, Y.S.; Lee, H.J.; Lee, K.; Lee, H. Emergence of synthetic mRNA: In vitro synthesis of mRNA and its applications in regenerative medicine. Biomaterials 2018, 156, 172–193. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- Magadum, A.; Kaur, K.; Zangi, L. mRNA-Based Protein Replacement Therapy for the Heart. Mol. Ther. 2019, 27, 785–793. [Google Scholar] [CrossRef]
- Sahu, I.; Haque, A.; Weidensee, B.; Weinmann, P.; Kormann, M.S.D. Recent Developments in mRNA-Based Protein Supplementation Therapy to Target Lung Diseases. Mol. Ther. 2019, 27, 803–823. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tran, D.M.; Cavedon, A.; Cai, X.; Rajendran, R.; Lyle, M.J.; Martini, P.G.V.; Miao, C.H. Treatment of Hemophilia A Using Factor VIII Messenger RNA Lipid Nanoparticles. Mol. Ther. Nucleic Acids 2020, 20, 534–544. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, X.; Yu, H.; Yang, L.; Lin, J.; Yang, W.; Liu, J.; Fan, X.; Xu, Y. Chemically modified in-vitro-transcribed mRNA encoding thrombopoietin stimulates thrombopoiesis in mice. Mol. Ther. Nucleic Acids 2022, 29, 657–671. [Google Scholar] [CrossRef]
- Plews, J.R.; Li, J.; Jones, M.; Moore, H.D.; Mason, C.; Andrews, P.W.; Na, J. Activation of pluripotency genes in human fibroblast cells by a novel mRNA based approach. PLoS ONE 2010, 5, e14397. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Kariko, K.; Tureci, O. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Milletti, F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.W.; Liu, B.R.; Wu, C.Y.; Lu, S.W.; Lee, H.J. Protein transport in human cells mediated by covalently and noncovalently conjugated arginine-rich intracellular delivery peptides. Peptides 2009, 30, 1669–1678. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, C.P.; Chan, M.H.; Chang, M.; Hou, Y.W.; Chen, H.H.; Hsu, H.R.; Liu, K.; Lee, H.J. Arginine-rich intracellular delivery peptides noncovalently transport protein into living cells. Biochem. Biophys. Res. Commun. 2006, 346, 758–767. [Google Scholar] [CrossRef] [PubMed]
- CPPsite 2.0 Database of Cell-Penetrating Peptides. Available online: https://webs.iiitd.edu.in/raghava/cppsite/ (accessed on 7 March 2012).
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Fawell, S.; Seery, J.; Daikh, Y.; Moore, C.; Chen, L.L.; Pepinsky, B.; Barsoum, J. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA 1994, 91, 664–668. [Google Scholar] [CrossRef]
- Vives, E.; Brodin, P.; Lebleu, B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef]
- Nagahara, H.; Vocero-Akbani, A.M.; Snyder, E.L.; Ho, A.; Latham, D.G.; Lissy, N.A.; Becker-Hapak, M.; Ezhevsky, S.A.; Dowdy, S.F. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 1998, 4, 1449–1452. [Google Scholar] [CrossRef]
- Joliot, A.; Pernelle, C.; Deagostini-Bazin, H.; Prochiantz, A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, S.R.; Ho, A.; Vocero-Akbani, A.; Dowdy, S.F. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science 1999, 285, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840. [Google Scholar] [CrossRef] [PubMed]
- Pooga, M.; Hallbrink, M.; Zorko, M.; Langel, U. Cell penetration by transportan. FASEB J. 1998, 12, 67–77. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Morris, M.C.; Deshayes, S.; Heitz, F.; Divita, G. Cell-penetrating peptides: From molecular mechanisms to therapeutics. Biol. Cell 2008, 100, 201–217. [Google Scholar] [CrossRef]
- Gao, C.; Mao, S.; Ditzel, H.J.; Farnaes, L.; Wirsching, P.; Lerner, R.A.; Janda, K.D. A cell-penetrating peptide from a novel pVII-pIX phage-displayed random peptide library. Bioorg. Med. Chem. 2002, 10, 4057–4065. [Google Scholar] [CrossRef]
- Stewart, K.M.; Horton, K.L.; Kelley, S.O. Cell-penetrating peptides as delivery vehicles for biology and medicine. Org. Biomol. Chem. 2008, 6, 2242–2255. [Google Scholar] [CrossRef]
- Hirose, H.; Takeuchi, T.; Osakada, H.; Pujals, S.; Katayama, S.; Nakase, I.; Kobayashi, S.; Haraguchi, T.; Futaki, S. Transient focal membrane deformation induced by arginine-rich peptides leads to their direct penetration into cells. Mol. Ther. 2012, 20, 984–993. [Google Scholar] [CrossRef]
- Gestin, M.; Dowaidar, M.; Langel, U. Uptake Mechanism of Cell-Penetrating Peptides. Adv. Exp. Med. Biol. 2017, 1030, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Porosk, L.; Gaidutsik, I.; Langel, U. Approaches for the discovery of new cell-penetrating peptides. Expert Opin. Drug Discov. 2021, 16, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Al Musaimi, O.; Lombardi, L.; Williams, D.R.; Albericio, F. Strategies for Improving Peptide Stability and Delivery. Pharmaceuticals 2022, 15, 1283. [Google Scholar] [CrossRef]
- Taylor, R.E.; Zahid, M. Cell Penetrating Peptides, Novel Vectors for Gene Therapy. Pharmaceutics 2020, 12, 225. [Google Scholar] [CrossRef]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Foltopoulou, P.F.; Tsiftsoglou, A.S.; Bonovolias, I.D.; Ingendoh, A.T.; Papadopoulou, L.C. Intracellular delivery of full length recombinant human mitochondrial L-Sco2 protein into the mitochondria of permanent cell lines and SCO2 deficient patient’s primary cells. Biochim. Biophys. Acta 2010, 1802, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Horton, K.L.; Stewart, K.M.; Fonseca, S.B.; Guo, Q.; Kelley, S.O. Mitochondria-penetrating peptides. Chem. Biol. 2008, 15, 375–382. [Google Scholar] [CrossRef]
- Kang, Y.C.; Son, M.; Kang, S.; Im, S.; Piao, Y.; Lim, K.S.; Song, M.Y.; Park, K.S.; Kim, Y.H.; Pak, Y.K. Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson’s disease models. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef]
- Parent, A.; Elduque, X.; Cornu, D.; Belot, L.; Le Caer, J.P.; Grandas, A.; Toledano, M.B.; D’Autreaux, B. Mammalian frataxin directly enhances sulfur transfer of NFS1 persulfide to both ISCU and free thiols. Nat. Commun. 2015, 6, 5686. [Google Scholar] [CrossRef]
- Vyas, P.M.; Tomamichel, W.J.; Pride, P.M.; Babbey, C.M.; Wang, Q.; Mercier, J.; Martin, E.M.; Payne, R.M. A TAT-frataxin fusion protein increases lifespan and cardiac function in a conditional Friedreich’s ataxia mouse model. Hum. Mol. Genet. 2012, 21, 1230–1247. [Google Scholar] [CrossRef]
- Britti, E.; Delaspre, F.; Feldman, A.; Osborne, M.; Greif, H.; Tamarit, J.; Ros, J. Frataxin-deficient neurons and mice models of Friedreich ataxia are improved by TAT-MTScs-FXN treatment. J. Cell. Mol. Med. 2018, 22, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Puccio, H.; Simon, D.; Cossee, M.; Criqui-Filipe, P.; Tiziano, F.; Melki, J.; Hindelang, C.; Matyas, R.; Rustin, P.; Koenig, M. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 2001, 27, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, D.W.; Jeong, H.J.; Sohn, E.J.; Shin, M.J.; Ahn, E.H.; Kwon, S.W.; Kim, Y.N.; Kim, D.S.; Park, J.; et al. Tat-Frataxin protects dopaminergic neuronal cells against MPTP-induced toxicity in a mouse model of Parkinson’s disease. Biochimie 2012, 94, 2448–2456. [Google Scholar] [CrossRef]
- Cao, G.; Pei, W.; Ge, H.; Liang, Q.; Luo, Y.; Sharp, F.R.; Lu, A.; Ran, R.; Graham, S.H.; Chen, J. In Vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis. J. Neurosci. 2002, 22, 5423–5431. [Google Scholar] [CrossRef] [PubMed]
- Parrasia, S.; Szabo, I.; Zoratti, M.; Biasutto, L. Peptides as Pharmacological Carriers to the Brain: Promises, Shortcomings and Challenges. Mol. Pharm. 2022, 19, 3700–3729. [Google Scholar] [CrossRef]
- Rapoport, M.; Saada, A.; Elpeleg, O.; Lorberboum-Galski, H. TAT-mediated delivery of LAD restores pyruvate dehydrogenase complex activity in the mitochondria of patients with LAD deficiency. Mol. Ther. 2008, 16, 691–697. [Google Scholar] [CrossRef]
- Rapoport, M.; Salman, L.; Sabag, O.; Patel, M.S.; Lorberboum-Galski, H. Successful TAT-mediated enzyme replacement therapy in a mouse model of mitochondrial E3 deficiency. J. Mol. Med. 2011, 89, 161–170. [Google Scholar] [CrossRef]
- Marcus, D.; Lichtenstein, M.; Saada, A.; Lorberboum-Galski, H. Replacement of the C6ORF66 assembly factor (NDUFAF4) restores complex I activity in patient cells. Mol. Med. 2013, 19, 124–134. [Google Scholar] [CrossRef]
- Papadopoulou, L.C.; Tsiftsoglou, A.S. Transduction of human recombinant proteins into mitochondria as a protein therapeutic approach for mitochondrial disorders. Pharm. Res. 2011, 28, 2639–2656. [Google Scholar] [CrossRef]
- Kaiafas, G.C.; Papagiannopoulou, D.; Miliotou Alpha, N.; Tsingotjidou, A.S.; Chalkidou, P.C.; Tsika, A.C.; Spyroulias, G.A.; Tsiftsoglou, A.S.; Papadopoulou, L.C. In vivo biodistribution study of TAT-L-Sco2 fusion protein, developed as protein therapeutic for mitochondrial disorders attributed to SCO2 mutations. Mol. Genet. Metab. Rep. 2020, 25, 100683. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247 Pt 1, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Boczkowski, D.; Nair, S.K.; Snyder, D.; Gilboa, E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 1996, 184, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Hoerr, I.; Obst, R.; Rammensee, H.G.; Jung, G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur. J. Immunol. 2000, 30, 1–7. [Google Scholar] [CrossRef]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- mRNA Therapeutics Market Size, Share & Trends Analysis Report By Application (Infectious Diseases, Oncology), By Type (Prophylactic Vaccines, Therapeutic Drugs), By End-use, By Region, And Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/mrna-therapeutics-market-report (accessed on 30 November 2022).
- Berraondo, P.; Martini, P.G.V.; Avila, M.A.; Fontanellas, A. Messenger RNA therapy for rare genetic metabolic diseases. Gut 2019, 68, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D. mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Thran, M.; Jordan, I. mRNA as novel technology for passive immunotherapy. Cell. Mol. Life Sci. CMLS 2019, 76, 301–328. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Roose, K. How mRNA therapeutics are entering the monoclonal antibody field. J. Transl. Med. 2019, 17, 54. [Google Scholar] [CrossRef]
- Devoldere, J.; Dewitte, H.; De Smedt, S.C.; Remaut, K. Evading innate immunity in nonviral mRNA delivery: Don’t shoot the messenger. Drug Discov. Today 2016, 21, 11–25. [Google Scholar] [CrossRef]
- Grudzien-Nogalska, E.; Stepinski, J.; Jemielity, J.; Zuberek, J.; Stolarski, R.; Rhoads, R.E.; Darzynkiewicz, E. Synthesis of anti-reverse cap analogs (ARCAs) and their applications in mRNA translation and stability. Methods Enzymol. 2007, 431, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Roers, A.; Hiller, B.; Hornung, V. Recognition of Endogenous Nucleic Acids by the Innate Immune System. Immunity 2016, 44, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Kocmik, I.; Piecyk, K.; Rudzinska, M.; Niedzwiecka, A.; Darzynkiewicz, E.; Grzela, R.; Jankowska-Anyszka, M. Modified ARCA analogs providing enhanced translational properties of capped mRNAs. Cell Cycle 2018, 17, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Jemielity, J.; Fowler, T.; Zuberek, J.; Stepinski, J.; Lewdorowicz, M.; Niedzwiecka, A.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Novel “anti-reverse” cap analogs with superior translational properties. RNA 2003, 9, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Suknuntha, K.; Tao, L.; Brok-Volchanskaya, V.; D’Souza, S.S.; Kumar, A.; Slukvin, I. Optimization of Synthetic mRNA for Highly Efficient Translation and its Application in the Generation of Endothelial and Hematopoietic Cells from Human and Primate Pluripotent Stem Cells. Stem Cell Rev. Rep. 2018, 14, 525–534. [Google Scholar] [CrossRef]
- Creusot, R.J.; Chang, P.; Healey, D.G.; Tcherepanova, I.Y.; Nicolette, C.A.; Fathman, C.G. A short pulse of IL-4 delivered by DCs electroporated with modified mRNA can both prevent and treat autoimmune diabetes in NOD mice. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 2112–2120. [Google Scholar] [CrossRef]
- Asrani, K.H.; Farelli, J.D.; Stahley, M.R.; Miller, R.L.; Cheng, C.J.; Subramanian, R.R.; Brown, J.M. Optimization of mRNA untranslated regions for improved expression of therapeutic mRNA. RNA Biol. 2018, 15, 756–762. [Google Scholar] [CrossRef]
- Kuhn, A.N.; Beibetaert, T.; Simon, P.; Vallazza, B.; Buck, J.; Davies, B.P.; Tureci, O.; Sahin, U. mRNA as a versatile tool for exogenous protein expression. Curr. Gene Ther. 2012, 12, 347–361. [Google Scholar] [CrossRef]
- Kozak, M. An analysis of 5’-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987, 15, 8125–8148. [Google Scholar] [CrossRef]
- Kozak, M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature 1984, 308, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Goss, D.J.; Kleiman, F.E. Poly(A) binding proteins: Are they all created equal? Wiley Interdiscip. Rev. RNA 2013, 4, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Avci-Adali, M.; Hann, L.; Michel, T.; Steinle, H.; Stoppelkamp, S.; Stang, K.; Narita, M.; Schlensak, C.; Wendel, H.P. In vitro test system for evaluation of immune activation potential of new single-stranded DNA-based therapeutics. Drug Test. Anal. 2015, 7, 300–308. [Google Scholar] [CrossRef]
- Kormann, M.S.; Hasenpusch, G.; Aneja, M.K.; Nica, G.; Flemmer, A.W.; Herber-Jonat, S.; Huppmann, M.; Mays, L.E.; Illenyi, M.; Schams, A.; et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011, 29, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 2015, 217, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Mir, F.F.; Jhunjhunwala, S.; Kaczmarek, J.C.; Hurtado, J.E.; Yang, J.H.; Webber, M.J.; Kowalski, P.S.; Heartlein, M.W.; DeRosa, F.; et al. Efficacy and immunogenicity of unmodified and pseudouridine-modified mRNA delivered systemically with lipid nanoparticles in vivo. Biomaterials 2016, 109, 78–87. [Google Scholar] [CrossRef]
- Gomez-Aguado, I.; Rodriguez-Castejon, J.; Vicente-Pascual, M.; Rodriguez-Gascon, A.; Solinis, M.A.; Del Pozo-Rodriguez, A. Nanomedicines to Deliver mRNA: State of the Art and Future Perspectives. Nanomaterials 2020, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liang, Y.; Huang, L. Development and Delivery Systems of mRNA Vaccines. Front. Bioeng. Biotechnol. 2021, 9, 718753. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef]
- Guan, S.; Rosenecker, J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017, 24, 133–143. [Google Scholar] [CrossRef]
- Wang, H.X.; Li, M.; Lee, C.M.; Chakraborty, S.; Kim, H.W.; Bao, G.; Leong, K.W. CRISPR/Cas9-Based Genome Editing for Disease Modeling and Therapy: Challenges and Opportunities for Nonviral Delivery. Chem. Rev. 2017, 117, 9874–9906. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Q.; Zhu, F.; Zhao, M.; Yan, Y. mRNA delivery via non-viral carriers for biomedical applications. Int. J. Pharm. 2021, 607, 121020. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.; Pilz, M.; Steinle, H.; Kochba, E.; Levin, Y.; Lunter, D.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Intradermal Delivery of Synthetic mRNA Using Hollow Microneedles for Efficient and Rapid Production of Exogenous Proteins in Skin. Mol. Ther. Nucleic Acids 2018, 11, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Moody, S.A. Microinjection of mRNAs and Oligonucleotides. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot097261. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Xu, Y.; Tao, W.; Ubellacker, J.M.; Lim, M.; Aum, D.; Lee, G.Y.; Zhou, K.; Zope, H.; Yu, M.; et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2018, 2, 850–864. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines. Curr. Top. Microbiol. Immunol. 2020, 440, 71–110. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Control. Release 2016, 240, 227–234. [Google Scholar] [CrossRef]
- Lefebvre, B.; Vandewalle, B.; Longue, J.; Moerman, E.; Lukowiak, B.; Gmyr, V.; Maedler, K.; Kerr-conte, J.; Pattou, F. Efficient gene delivery and silencing of mouse and human pancreatic islets. BMC Biotechnol. 2010, 10, 28. [Google Scholar] [CrossRef]
- Marucci, G.; Lammi, C.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Amantini, C.; Santoni, G.; Kandhavelu, M.; Abbracchio, M.P.; Lecca, D.; et al. Comparison and optimization of transient transfection methods at human astrocytoma cell line 1321N1. Anal. Biochem. 2011, 414, 300–302. [Google Scholar] [CrossRef]
- Ahlemeyer, B.; Vogt, J.F.; Michel, V.; Hahn-Kohlberger, P.; Baumgart-Vogt, E. Microporation is an efficient method for siRNA-induced knockdown of PEX5 in HepG2 cells: Evaluation of the transfection efficiency, the PEX5 mRNA and protein levels and induction of peroxisomal deficiency. Histochem. Cell Biol. 2014, 142, 577–591. [Google Scholar] [CrossRef]
- Kigasawa, K.; Kajimoto, K.; Hama, S.; Saito, A.; Kanamura, K.; Kogure, K. Noninvasive delivery of siRNA into the epidermis by iontophoresis using an atopic dermatitis-like model rat. Int. J. Pharm. 2010, 383, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.C.; Kim, D.I.; Kim, S.H.; Wang, H.-M.D.; Hwang, B.H. Synergistic Transdermal Delivery of Biomacromolecules Using Sonophoresis after Microneedle Treatment. Biotechnol. Bioprocess Eng. 2018, 23, 286–292. [Google Scholar] [CrossRef]
- Hamm, A.; Krott, N.; Breibach, I.; Blindt, R.; Bosserhoff, A.K. Efficient transfection method for primary cells. Tissue Eng. 2002, 8, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Tager, J.; Kohler, K.; Haerle, M.; Werdin, F.; Schaller, H.E.; Sinis, N. Non-viral genetic transfection of rat Schwann cells with FuGENE HD(c) lipofection and AMAXA(c) nucleofection is feasible but impairs cell viability. Neuron Glia Biol. 2010, 6, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Heiser, A.; Coleman, D.; Dannull, J.; Yancey, D.; Maurice, M.A.; Lallas, C.D.; Dahm, P.; Niedzwiecki, D.; Gilboa, E.; Vieweg, J. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J. Clin. Investig. 2002, 109, 409–417. [Google Scholar] [CrossRef]
- Miliotou, A.N.; Pappas, I.S.; Vizirianakis, I.S.; Papadopoulou, L.C. In Vitro-Transcribed mRNAs as a New Generation of Therapeutics in the Dawn of Twenty-First Century: Exploitation of Peptides as Carriers for Their Intracellular Delivery. In Messenger RNA Therapeutics. RNA Technologies; Jurga, S., Barciszewski, J., Eds.; Springer: Cham, Switzerland, 2022; Volume 13. [Google Scholar]
- Felgner, J.H.; Kumar, R.; Sridhar, C.N.; Wheeler, C.J.; Tsai, Y.J.; Border, R.; Ramsey, P.; Martin, M.; Felgner, P.L. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem. 1994, 269, 2550–2561. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.A.; Chan, M.; Shaw, C.A.; Hekele, A.; Carsillo, T.; Schaefer, M.; Archer, J.; Seubert, A.; Otten, G.R.; Beard, C.W.; et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther. 2014, 22, 2118–2129. [Google Scholar] [CrossRef]
- Landesman-Milo, D.; Peer, D. Toxicity profiling of several common RNAi-based nanomedicines: A comparative study. Drug Deliv. Transl. Res. 2014, 4, 96–103. [Google Scholar] [CrossRef]
- Lallana, E.; Rios de la Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/Hyaluronic Acid Nanoparticles: Rational Design Revisited for RNA Delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef]
- Soliman, O.Y.; Alameh, M.G.; De Cresenzo, G.; Buschmann, M.D.; Lavertu, M. Efficiency of Chitosan/Hyaluronan-Based mRNA Delivery Systems In Vitro: Influence of Composition and Structure. J. Pharm. Sci. 2020, 109, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Magini, D.; Giovani, C.; Mangiavacchi, S.; Maccari, S.; Cecchi, R.; Ulmer, J.B.; De Gregorio, E.; Geall, A.J.; Brazzoli, M.; Bertholet, S. Self-Amplifying mRNA Vaccines Expressing Multiple Conserved Influenza Antigens Confer Protection against Homologous and Heterosubtypic Viral Challenge. PLoS ONE 2016, 11, e0161193. [Google Scholar] [CrossRef] [PubMed]
- Geall, A.J.; Verma, A.; Otten, G.R.; Shaw, C.A.; Hekele, A.; Banerjee, K.; Cu, Y.; Beard, C.W.; Brito, L.A.; Krucker, T.; et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA 2012, 109, 14604–14609. [Google Scholar] [CrossRef] [PubMed]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, O.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Rohss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified mRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 169, 176. [Google Scholar] [CrossRef]
- Feldman, R.A.; Fuhr, R.; Smolenov, I.; Mick Ribeiro, A.; Panther, L.; Watson, M.; Senn, J.J.; Smith, M.; Almarsson; Pujar, H.S.; et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 2019, 37, 3326–3334. [Google Scholar] [CrossRef]
- Uchida, H.; Itaka, K.; Nomoto, T.; Ishii, T.; Suma, T.; Ikegami, M.; Miyata, K.; Oba, M.; Nishiyama, N.; Kataoka, K. Modulated protonation of side chain aminoethylene repeats in N-substituted polyaspartamides promotes mRNA transfection. J. Am. Chem. Soc. 2014, 136, 12396–12405. [Google Scholar] [CrossRef]
- Kaczmarek, J.C.; Kauffman, K.J.; Fenton, O.S.; Sadtler, K.; Patel, A.K.; Heartlein, M.W.; DeRosa, F.; Anderson, D.G. Optimization of a Degradable Polymer-Lipid Nanoparticle for Potent Systemic Delivery of mRNA to the Lung Endothelium and Immune Cells. Nano Lett. 2018, 18, 6449–6454. [Google Scholar] [CrossRef]
- Jiang, Y.; Gaudin, A.; Zhang, J.; Agarwal, T.; Song, E.; Kauffman, A.C.; Tietjen, G.T.; Wang, Y.; Jiang, Z.; Cheng, C.J.; et al. A “top-down” approach to actuate poly(amine-co-ester) terpolymers for potent and safe mRNA delivery. Biomaterials 2018, 176, 122–130. [Google Scholar] [CrossRef]
- Rosenkranz, A.A.; Sobolev, A.S. Polyethylenimine-based polyplex nanoparticles and features of their behavior in cells and tissues. Russ. Chem. Bull. 2015, 64, 2749–2755. [Google Scholar] [CrossRef]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Feyerabend, S.; Stevanovic, S.; Gouttefangeas, C.; Wernet, D.; Hennenlotter, J.; Bedke, J.; Dietz, K.; Pascolo, S.; Kuczyk, M.; Rammensee, H.G.; et al. Novel multi-peptide vaccination in Hla-A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate 2009, 69, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Pascolo, S.; Scheel, B.; Derhovanessian, E.; Pflugfelder, A.; Eigentler, T.K.; Pawelec, G.; Hoerr, I.; Rammensee, H.G.; Garbe, C. Direct injection of protamine-protected mRNA: Results of a phase 1/2 vaccination trial in metastatic melanoma patients. J. Immunother. 2009, 32, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Westdorp, H.; Creemers, J.H.A.; van Oort, I.M.; Schreibelt, G.; Gorris, M.A.J.; Mehra, N.; Simons, M.; de Goede, A.L.; van Rossum, M.M.; Croockewit, A.J.; et al. Blood-derived dendritic cell vaccinations induce immune responses that correlate with clinical outcome in patients with chemo-naive castration-resistant prostate cancer. J. Immunother. Cancer 2019, 7, 302. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef]
- Snyder, E.L.; Dowdy, S.F. Cell penetrating peptides in drug delivery. Pharm. Res. 2004, 21, 389–393. [Google Scholar] [CrossRef]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef]
- Turner, J.J.; Jones, S.; Fabani, M.M.; Ivanova, G.; Arzumanov, A.A.; Gait, M.J. RNA targeting with peptide conjugates of oligonucleotides, siRNA and PNA. Blood Cells Mol. Dis. 2007, 38, 1–7. [Google Scholar] [CrossRef]
- Jarver, P.; Langel, K.; El-Andaloussi, S.; Langel, U. Applications of cell-penetrating peptides in regulation of gene expression. Biochem. Soc. Trans. 2007, 35 Pt 4, 770–774. [Google Scholar] [CrossRef]
- Di Pisa, M.; Chassaing, G.; Swiecicki, J.M. When cationic cell-penetrating peptides meet hydrocarbons to enhance in-cell cargo delivery. J. Pept. Sci. 2015, 21, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Scheicher, B.; Schachner-Nedherer, A.L.; Zimmer, A. Protamine-oligonucleotide-nanoparticles: Recent advances in drug delivery and drug targeting. Eur. J. Pharm. Sci. 2015, 75, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Lehto, T.; Ezzat, K.; Wood, M.J.A.; El Andaloussi, S. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 106 Pt A, 172–182. [Google Scholar] [CrossRef]
- Boisguerin, P.; Deshayes, S.; Gait, M.J.; O’Donovan, L.; Godfrey, C.; Betts, C.A.; Wood, M.J.; Lebleu, B. Delivery of therapeutic oligonucleotides with cell penetrating peptides. Adv. Drug Deliv. Rev. 2015, 87, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Margus, H.; Padari, K.; Pooga, M. Cell-penetrating peptides as versatile vehicles for oligonucleotide delivery. Mol. Ther. J. Am. Soc. Gene Ther. 2012, 20, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Liang, J.F.; Ko, K.S.; Kim, S.W.; Yang, V.C. Low molecular weight protamine as an efficient and nontoxic gene carrier: In vitro study. J. Gene Med. 2003, 5, 700–711. [Google Scholar] [CrossRef]
- Lehto, T.; Kurrikoff, K.; Langel, U. Cell-penetrating peptides for the delivery of nucleic acids. Expert Opin. Drug Deliv. 2012, 9, 823–836. [Google Scholar] [CrossRef]
- Aldrian-Herrada, G.; Desarmenien, M.G.; Orcel, H.; Boissin-Agasse, L.; Mery, J.; Brugidou, J.; Rabie, A. A peptide nucleic acid (PNA) is more rapidly internalized in cultured neurons when coupled to a retro-inverso delivery peptide. The antisense activity depresses the target mRNA and protein in magnocellular oxytocin neurons. Nucleic Acids Res. 1998, 26, 4910–4916. [Google Scholar] [CrossRef]
- Bendifallah, N.; Rasmussen, F.W.; Zachar, V.; Ebbesen, P.; Nielsen, P.E.; Koppelhus, U. Evaluation of cell-penetrating peptides (CPPs) as vehicles for intracellular delivery of antisense peptide nucleic acid (PNA). Bioconjug. Chem. 2006, 17, 750–758. [Google Scholar] [CrossRef]
- Guo, Z.; Peng, H.; Kang, J.; Sun, D. Cell-penetrating peptides: Possible transduction mechanisms and therapeutic applications. Biomed. Rep. 2016, 4, 528–534. [Google Scholar] [CrossRef]
- Huang, Y.W.; Lee, H.J.; Tolliver, L.M.; Aronstam, R.S. Delivery of nucleic acids and nanomaterials by cell-penetrating peptides: Opportunities and challenges. BioMed Res. Int. 2015, 2015, 834079. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Nigatu, A.S.; Berkland, C.J.; Ramsey, J.D. Noncovalently associated cell-penetrating peptides for gene delivery applications. Ther. Deliv. 2013, 4, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Coolen, A.L.; Lacroix, C.; Mercier-Gouy, P.; Delaune, E.; Monge, C.; Exposito, J.Y.; Verrier, B. Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials 2019, 195, 23–37. [Google Scholar] [CrossRef] [PubMed]
- van den Brand, D.; Gorris, M.A.J.; van Asbeck, A.H.; Palmen, E.; Ebisch, I.; Dolstra, H.; Hallbrink, M.; Massuger, L.; Brock, R. Peptide-mediated delivery of therapeutic mRNA in ovarian cancer. Eur. J. Pharm. Biopharm. 2019, 141, 180–190. [Google Scholar] [CrossRef]
- Bell, G.D.; Yang, Y.; Leung, E.; Krissansen, G.W. mRNA transfection by a Xentry-protamine cell-penetrating peptide is enhanced by TLR antagonist E6446. PLoS ONE 2018, 13, e0201464. [Google Scholar] [CrossRef]
- Lockhart, J.H.; VanWye, J.; Banerjee, R.; Wickline, S.A.; Pan, H.; Totary-Jain, H. Self-assembled miRNA-switch nanoparticles target denuded regions and prevent restenosis. Mol. Ther. 2021, 29, 1744–1757. [Google Scholar] [CrossRef]
- Haenssle, H.A.; Riedl, P.; Buhl, T.; Schardt, A.; Rosenberger, A.; Schon, M.P.; Schirmbeck, R. Intracellular delivery of major histocompatibility complex class I-binding epitopes: Dendritic cells loaded and matured with cationic peptide/poly(I:C) complexes efficiently activate T cells. Exp. Dermatol. 2010, 19, 19–28. [Google Scholar] [CrossRef]
- Lee, K.; Yu, P.; Lingampalli, N.; Kim, H.J.; Tang, R.; Murthy, N. Peptide-enhanced mRNA transfection in cultured mouse cardiac fibroblasts and direct reprogramming towards cardiomyocyte-like cells. Int. J. Nanomed. 2015, 10, 1841–1854. [Google Scholar] [CrossRef]
- Konate, K.; Crombez, L.; Deshayes, S.; Decaffmeyer, M.; Thomas, A.; Brasseur, R.; Aldrian, G.; Heitz, F.; Divita, G. Insight into the cellular uptake mechanism of a secondary amphipathic cell-penetrating peptide for siRNA delivery. Biochemistry 2010, 49, 3393–3402. [Google Scholar] [CrossRef]
- Boisguerin, P.; Konate, K.; Josse, E.; Vives, E.; Deshayes, S. Peptide-Based Nanoparticles for Therapeutic Nucleic Acid Delivery. Biomedicines 2021, 9, 583. [Google Scholar] [CrossRef]
- Roberts, R.W.; Szostak, J.W. RNA-peptide fusions for the in vitro selection of peptides and proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 12297–12302. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, N.; Miyamoto-Sato, E.; Husimi, Y.; Yanagawa, H. In vitro virus: Bonding of mRNA bearing puromycin at the 3’-terminal end to the C-terminal end of its encoded protein on the ribosome in vitro. FEBS Lett. 1997, 414, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.W. Totally in vitro protein selection using mRNA-protein fusions and ribosome display. Curr. Opin. Chem. Biol. 1999, 3, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Barrick, J.E.; Szostak, J.W.; Roberts, R.W. Optimized synthesis of RNA-protein fusions for in vitro protein selection. Methods Enzymol. 2000, 318, 268–293. [Google Scholar] [CrossRef]
- Lou, B.; De Koker, S.; Lau, C.Y.J.; Hennink, W.E.; Mastrobattista, E. mRNA Polyplexes with Post-Conjugated GALA Peptides Efficiently Target, Transfect, and Activate Antigen Presenting Cells. Bioconjug. Chem. 2019, 30, 461–475. [Google Scholar] [CrossRef]
- Rhee, M.; Davis, P. Mechanism of uptake of C105Y, a novel cell-penetrating peptide. J. Biol. Chem. 2006, 281, 1233–1240. [Google Scholar] [CrossRef]
- Jones, S.; Lukanowska, M.; Suhorutsenko, J.; Oxenham, S.; Barratt, C.; Publicover, S.; Copolovici, D.M.; Langel, U.; Howl, J. Intracellular translocation and differential accumulation of cell-penetrating peptides in bovine spermatozoa: Evaluation of efficient delivery vectors that do not compromise human sperm motility. Hum. Reprod. 2013, 28, 1874–1889. [Google Scholar] [CrossRef]
- Barkalina, N.; Jones, C.; Townley, H.; Coward, K. Functionalization of mesoporous silica nanoparticles with a cell-penetrating peptide to target mammalian sperm in vitro. Nanomedicine 2015, 10, 1539–1553. [Google Scholar] [CrossRef]
- Fang, B.; Guo, H.Y.; Zhang, M.; Jiang, L.; Ren, F.Z. The six amino acid antimicrobial peptide bLFcin6 penetrates cells and delivers siRNA. FEBS J. 2013, 280, 1007–1017. [Google Scholar] [CrossRef]
- Miliotou, A.N.; Pappas, I.S.; Spyroulias, G.A.; Vlachaki, E.; Tsiftsoglou, A.S.; Vizirianakis, I.S.; Papadopoulou, L.C. Development of a novel PTD-mediated IVT-mRNA delivery platform for potential protein replacement therapy of metabolic/genetic disorders. Mol. Ther.-Nucleic Acids 2021, 26, 694–710. [Google Scholar] [CrossRef]
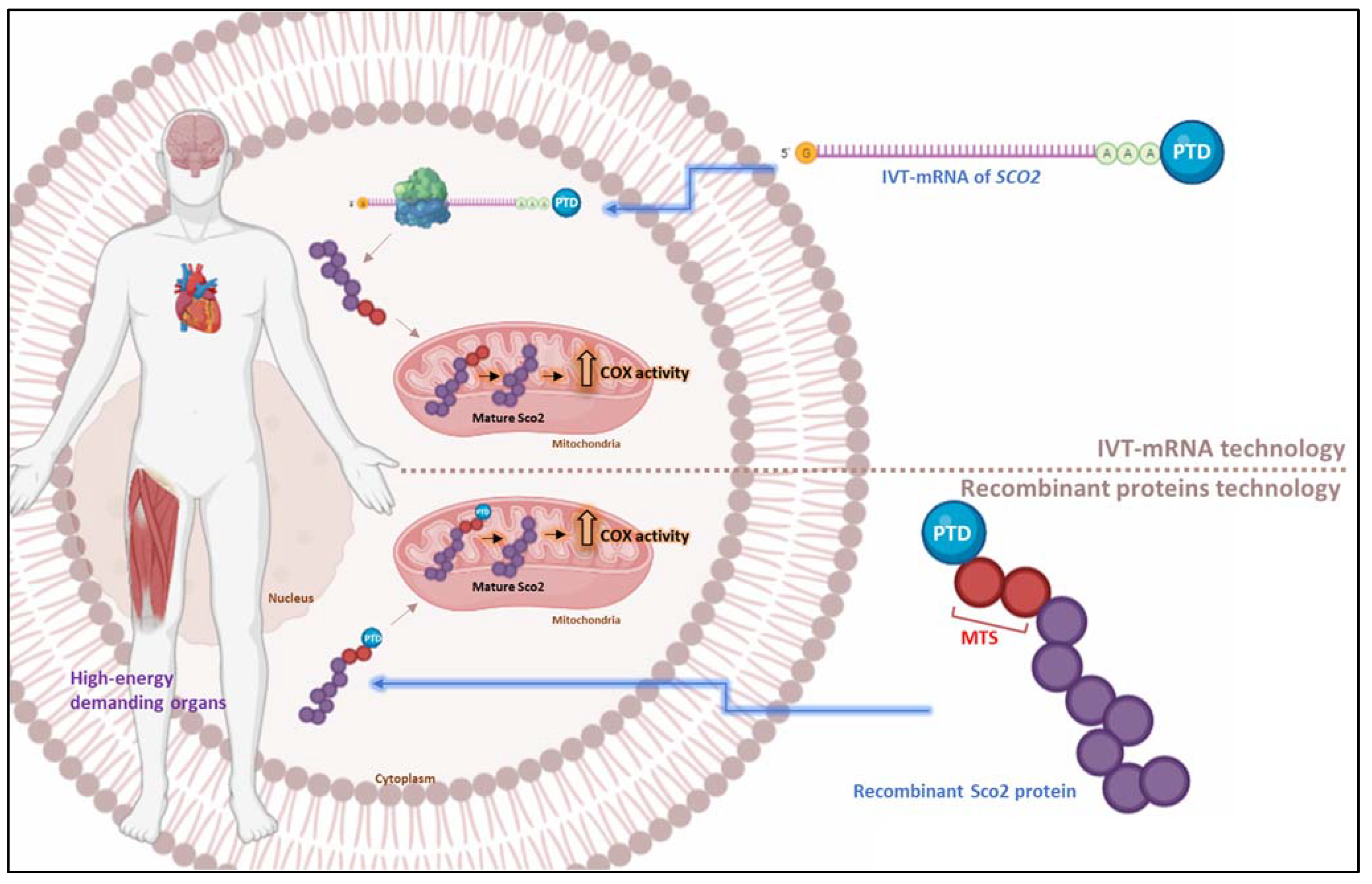
| SCO2 Genotype | Clinical Outcome | Affected Individual(s) | References * |
|---|---|---|---|
| Q53X/E140K | Fatal infantile cardioencephalomyopathy with COX deficiency, neurological symptoms with lactic/metabolic acidosis, respiratory difficulties | 6 | Papadopoulou L.C. et al., 1999 [66]; Tay S.K.H. et al., 2004 [110]; Vesela K. et al., 2004 [113]; Pronicki M. et al., 2010 [103]; Pronicka E. et al., 2013 [102] |
| E140K/S225F | Fatal infantile cardioencephalomyopathy with COX deficiency | 1 | Papadopoulou L.C. et al., 1999 [66] |
| E140K/R171W | Fatal hypertrophic cardiomyopathy (HCMP), seizures, muscle hypotonia (MH), respiratory insufficiency | 1 | Jaksch M. et al., 2000 [97] |
| R90X/E140K | Fatal hypertrophic cardiomyopathy (HCMP), seizures, muscle hypotonia (MH), respiratory insufficiency | 2 | Jaksch M. et al., 2000 [97] |
| E140K/E140K | Delayed infantile onset of cardiomyopathy and neuropathy, laryngeal inspiratory stridor, infantile SMA-like/Leigh-like picture | 39 | Jaksch M. et al., 2001 [122]; Vesela K. et al., 2004 [113]; Bohm M. et al., 2006 [118]; Pronicki M. et al., 2010 [103]; Pronicka E. et al., 2013 [102] |
| 10 bp duplication (1302–1311)/E140K | Prominent spinal cord involvement mimicking spinal muscular atrophy (Werdnig–Hoffmann disease) | 1 | Salviati L. et al., 2002 [106] |
| E140K/L151P | Hypertrophic cardiomyopathy and encephalomyopathy | 1 | Sacconi S. et al., 2003 [105] |
| C133S/E140K | Neonatal hypotonia with a spinal muscular atrophy (SMA) type 1 phenotype | 1 | Tarnopolsky M.A. et al., 2004 [109] |
| 1518delA/E140K | Cytochrome c oxidase deficiency and a Werdnig–Hoffmann disease phenotype | 2 | Bohm M. et al., 2006 [118]; Vesela K. et al., 2008 [114]; |
| Hemizygosity 16 bp deletion within the intron_E140K | Early onset rapidly progressive, fatal cardiomyopathy | 1 | Leary S.C. et al., 2006 [100] |
| E140K/V160G | Cytochrome c oxidase deficiency, fatal infantile cardioencephalomyopathy | 1 | Knuf M. et al., 2007 [99] |
| W36X/E140K | Fatal infantile cardioencephalomyopathy | 2 | Verdijk R.M. et al., 2008 [112] |
| G193S/G193S | Fatal infantile cardioencephalomyopathy | 1 | Mobley B.C. et al., 2009 [101] |
| E140K/M177T | Classical SMA or SMA-like picture, laryngeal inspiratory stridor, milder encephalopathic | 4 | Pronicki M. et al., 2010 [103]; Pronicka E. et al., 2013 [102] |
| E140K/w/t | Respiratory failure, artificial ventilation, hypotony, high-grade myopia | 4 | Pronicki M. et al. 2010 [103]; Tran-Viet K.N. et al. 2013 [111] |
| 19 bp insertion at position 17 (17INS19bp)/ E140K | Failure to thrive, muscular hypotonia, hypertrophic cardiomyopathy, and lactic acidemia, totally absent of COX activity | 1 | Joost K. et al., 2010 [98] |
| 12 bp deletion (c.1519_1530del)/ E140K | Cardioencephalomyopathy, stridor, neuropathy | 1 | Gurgel-Giannetti J. et al., 2013 [96] |
| Q53X/w/t | High-grade myopia | 7 | Tran-Viet K.N. et al., 2013 [111] |
| R114H/w/t | High-grade myopia | 1 | Tran-Viet K.N. et al. 2013 [111] |
| p82delK/E140K | Progressive encephalopathy, cardiomegaly, spinal muscular atrophy, COX deficiency | 1 | Pronicka E. et al., 2013 [102] |
| W75R/E140K | Neonatal cardiomyopathy, muscle weakness | 1 | Pronicka E. et al., 2013 [102] |
| E140K/T241X | Neonatal cardiomyopathy, muscle weakness, Leigh disease | 1 | Pronicka E. et al., 2013 [102] |
| V160A/P233T | Fatal hyperthermia and metabolic acidosis | 1 | Sambuughin N. et al., 2013 [107] |
| A259V/w/t | High-grade myopia | 1 | Tran-Viet K.N. et al., 2013 [111] |
| D223N/87 kb deletion on chr.22 | Severe hypotonic syndrome, failure to thrive, divergent strabismus and ataxia, regression of psychomotor development | 1 | Vondrackova A. et al., 2014 [115] |
| R112W/w/t | Early-onset high myopia | 1 | Jiang D. et al., 2014 [119] |
| R120W/w/t | Early-onset high myopia | 1 | Jiang D. et al., 2014 [119] |
| A97V/w/t | Extreme myopia | 1 | Wakazono T. et al., 2016 [117] |
| D135G/R171Q | Early-onset axonal Charcot–Marie–Tooth disease associated with cellular copper deficiency | 1 | Rebelo A.P. et al., 2018 [104] |
| E140K/P169T | Early-onset axonal Charcot–Marie–Tooth disease associated with cellular copper deficiency | 1 | Rebelo A.P. et al., 2018 [104] |
| D173V/w/t | Non-syndromic high myopia | 1 | Cai X.B. et al., 2019 [120] |
| A201P/w/t | Non-syndromic high myopia | 1 | Cai X.B. et al., 2019 [120] |
| I221V/w/t | Non-syndromic high myopia | 1 | Cai X.B. et al., 2019 [120] |
| R255W/R255W | Cerebellar ataxia, progressive peripheral axonal neuropathy and long survival | 2 | Barcia G. et al., 2019 [94] |
| p82delK/w/t | Non-syndromic high myopia | 1 | Zheng Y.H et al., 2021 [121] |
| R60Q/G193S | Adult cerebellar ataxia, axonal neuropathy, and sensory impairments | 1 | Rucheton B. et al., 2021 [116] |
| G121R/G121R | Early-onset axonal Charcot–Marie–Tooth disease | 2 | Gangfuß A. et al., 2022 [95] |
| Condition/ Disease | Title | Interventions | Phase | Clinical Trial |
|---|---|---|---|---|
| Leber Hereditary Optic Neuropathy (LHON) disease | Gene Therapy Clinical Trial for the Treatment Of Leber’s Hereditary Optic Neuropathy (GOLD) | Drug: NR082 injection Device: sham injection | Phase 2 Phase 3 | NCT04912843 |
| Safety Study of an Adeno-associated Virus Vector for Gene Therapy of Leber’s Hereditary Optic Neuropathy | Drug: injection of scAAV2-P1ND4v2 (low–higher) | Phase 1 | NCT02161380 | |
| Efficacy & Safety Study of Bilateral IVT Injection of GS010 in LHON Subjects Due to the ND4 Mutation for up to 1 Year | Genetic: GS010 Drug: placebo | Phase 3 | NCT03293524 | |
| A Single Intravitreal Injection of rAAV2-ND4 for the Treatment of Leber’s Hereditary Optic Neuropathy | Drug: rAAV2-ND4 | Phase 2 Phase 3 | NCT03153293 | |
| RESCUE and REVERSE Long-term Follow-up | Genetic: GS010 Other: sham | Completed (2022) | NCT03406104 | |
| Safety Evaluation of Gene Therapy in Leber Hereditary Optic Neuropathy (LHON) Patients | Genetic: GS010 | Completed (2020) | NCT02064569 | |
| REALITY LHON Registry | Other: patient-reported outcomes (PROs) | Completed (2020) | NCT03295071 | |
| Efficacy Study of GS010 for the Treatment of Vision Loss up to 6 Months From Onset in LHON Due to the ND4 Mutation | Biological: GS010 Device: sham intravitreal injection | Completed (2020) | NCT02652767 | |
| Safety and Efficacy Study of rAAV2-ND4 Treatment of Leber Hereditary Optic Neuropathy (LHON) | Drug: rAAV2-ND4 | Completed (2016) | NCT01267422 | |
| Friedreich Ataxia | Gene Therapy for Cardiomyopathy Associated With Friedreich’s Ataxia | Genetic: low–high dose LX2006 | Phase 1 Phase 2 | NCT05445323 |
| Phase IA Study of AAVrh.10hFXN Gene Therapy for the Cardiomyopathy of Friedreich’s Ataxia | Biological: AAVrh.10hFXN, serotype rh.10 adeno-associated virus (AAV) gene transfer vector expressing the cDNA coding for human FXN Drug: Prednisone | Phase 1 | NCT05302271 | |
| Multiple Ascending Dose Study of CTI-1601 Versus Placebo in Subjects With Friedreich’s Ataxia | Biological: CTI-1601 (a recombinant fusion protein intended to deliver human frataxin into the mitochondria of patients with Friedreich’s ataxia) | Phase 1 | NCT04519567 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miliotou, A.N.; Foltopoulou, P.F.; Ingendoh-Tsakmakidis, A.; Tsiftsoglou, A.S.; Vizirianakis, I.S.; Pappas, I.S.; Papadopoulou, L.C. Protein Transduction Domain-Mediated Delivery of Recombinant Proteins and In Vitro Transcribed mRNAs for Protein Replacement Therapy of Human Severe Genetic Mitochondrial Disorders: The Case of Sco2 Deficiency. Pharmaceutics 2023, 15, 286. https://doi.org/10.3390/pharmaceutics15010286
Miliotou AN, Foltopoulou PF, Ingendoh-Tsakmakidis A, Tsiftsoglou AS, Vizirianakis IS, Pappas IS, Papadopoulou LC. Protein Transduction Domain-Mediated Delivery of Recombinant Proteins and In Vitro Transcribed mRNAs for Protein Replacement Therapy of Human Severe Genetic Mitochondrial Disorders: The Case of Sco2 Deficiency. Pharmaceutics. 2023; 15(1):286. https://doi.org/10.3390/pharmaceutics15010286
Chicago/Turabian StyleMiliotou, Androulla N., Parthena F. Foltopoulou, Alexandra Ingendoh-Tsakmakidis, Asterios S. Tsiftsoglou, Ioannis S. Vizirianakis, Ioannis S. Pappas, and Lefkothea C. Papadopoulou. 2023. "Protein Transduction Domain-Mediated Delivery of Recombinant Proteins and In Vitro Transcribed mRNAs for Protein Replacement Therapy of Human Severe Genetic Mitochondrial Disorders: The Case of Sco2 Deficiency" Pharmaceutics 15, no. 1: 286. https://doi.org/10.3390/pharmaceutics15010286
APA StyleMiliotou, A. N., Foltopoulou, P. F., Ingendoh-Tsakmakidis, A., Tsiftsoglou, A. S., Vizirianakis, I. S., Pappas, I. S., & Papadopoulou, L. C. (2023). Protein Transduction Domain-Mediated Delivery of Recombinant Proteins and In Vitro Transcribed mRNAs for Protein Replacement Therapy of Human Severe Genetic Mitochondrial Disorders: The Case of Sco2 Deficiency. Pharmaceutics, 15(1), 286. https://doi.org/10.3390/pharmaceutics15010286








