Emerging Antimicrobial and Immunomodulatory Fiber-Based Scaffolding Systems for Treating Diabetic Foot Ulcers
Abstract
:1. Introduction
2. Diabetic Ulcers: Healing Impairments and Classifications
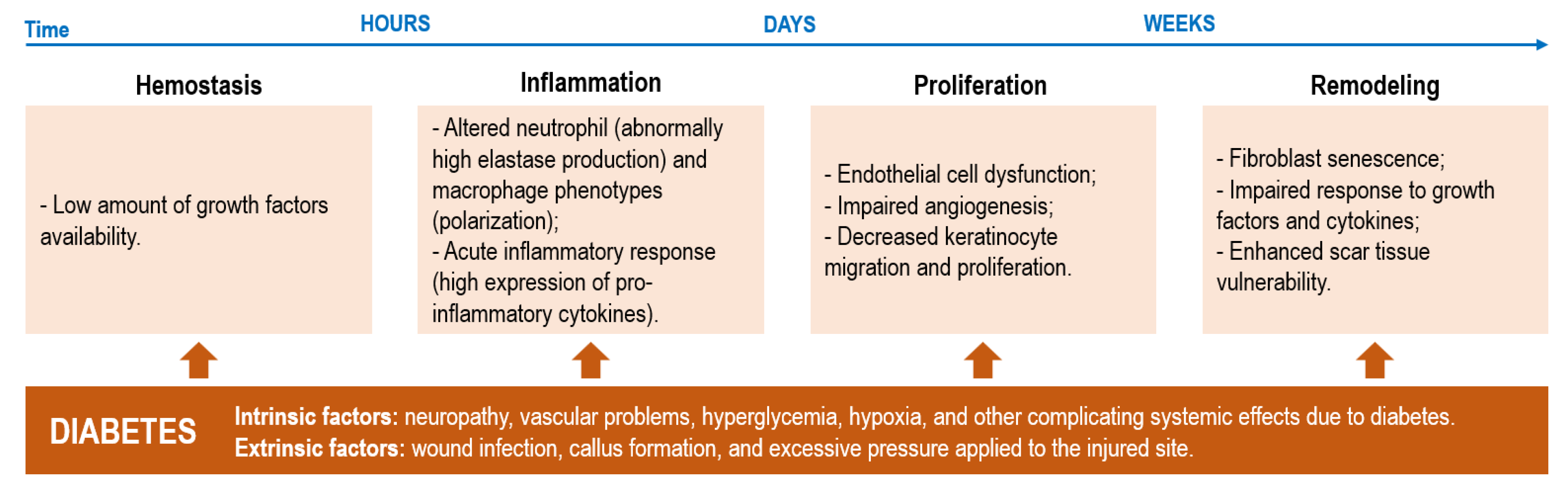
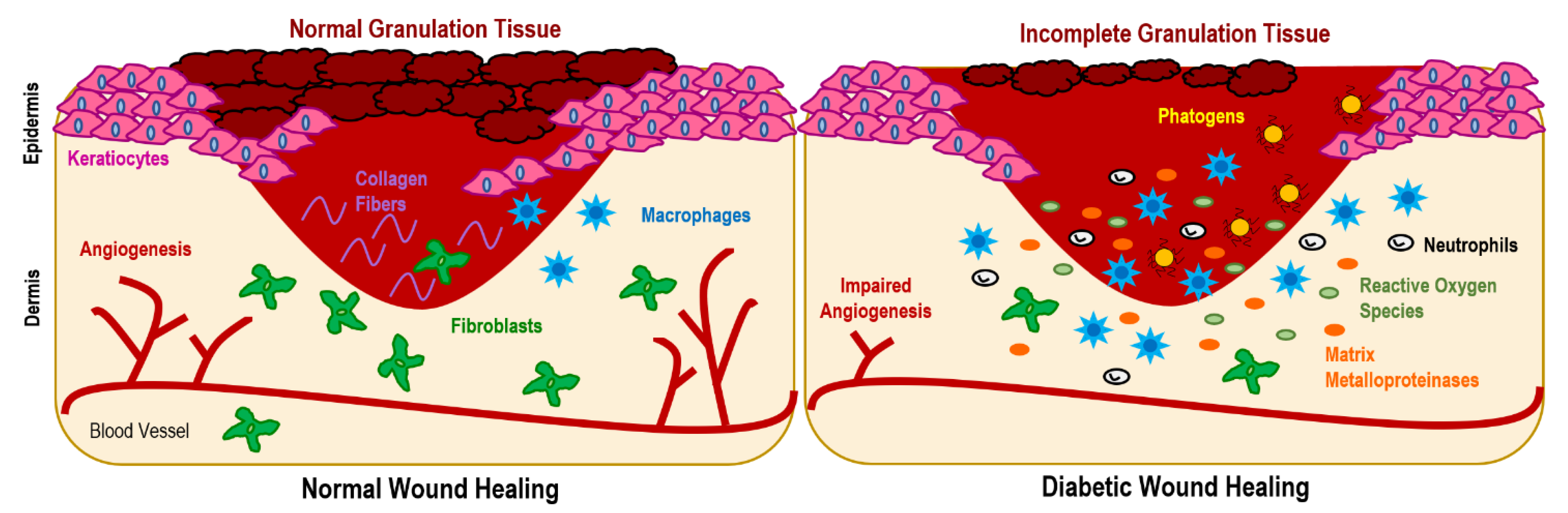
2.1. Impairments
2.2. Classification
- -
- Grade 1. Partial thickness involving only dermis and epidermis
- -
- Grade 2. Full thickness and subcutaneous tissues
- -
- Grade 3. Grade 2 plus exposed tendons, ligament, and/or joint
- -
- Grade 4. Grade 3 plus abscess and/or osteomyelitis
- -
- Grade 5. Grade 3 plus necrotic tissue in wound
- -
- Grade 6. Grade 3 plus gangrene in the wound and surrounding tissue
3. Antimicrobial Agents
3.1. Antibiotics
3.2. Natural Extracts
3.3. Inorganic Nanoparticles
3.4. Polymers: Chitosan
4. Immunomodulatory Agents
4.1. Growth Factors
4.2. Blood Components: Platelet-Rich Plasma
4.3. Natural Extracts
4.4. Proteins: Collagen, Silk Fibroin and Sericin, and Keratin
4.5. Neuropeptides
4.6. Stem Cells
4.7. Polymers: Alginate and Hyaluronic Acid
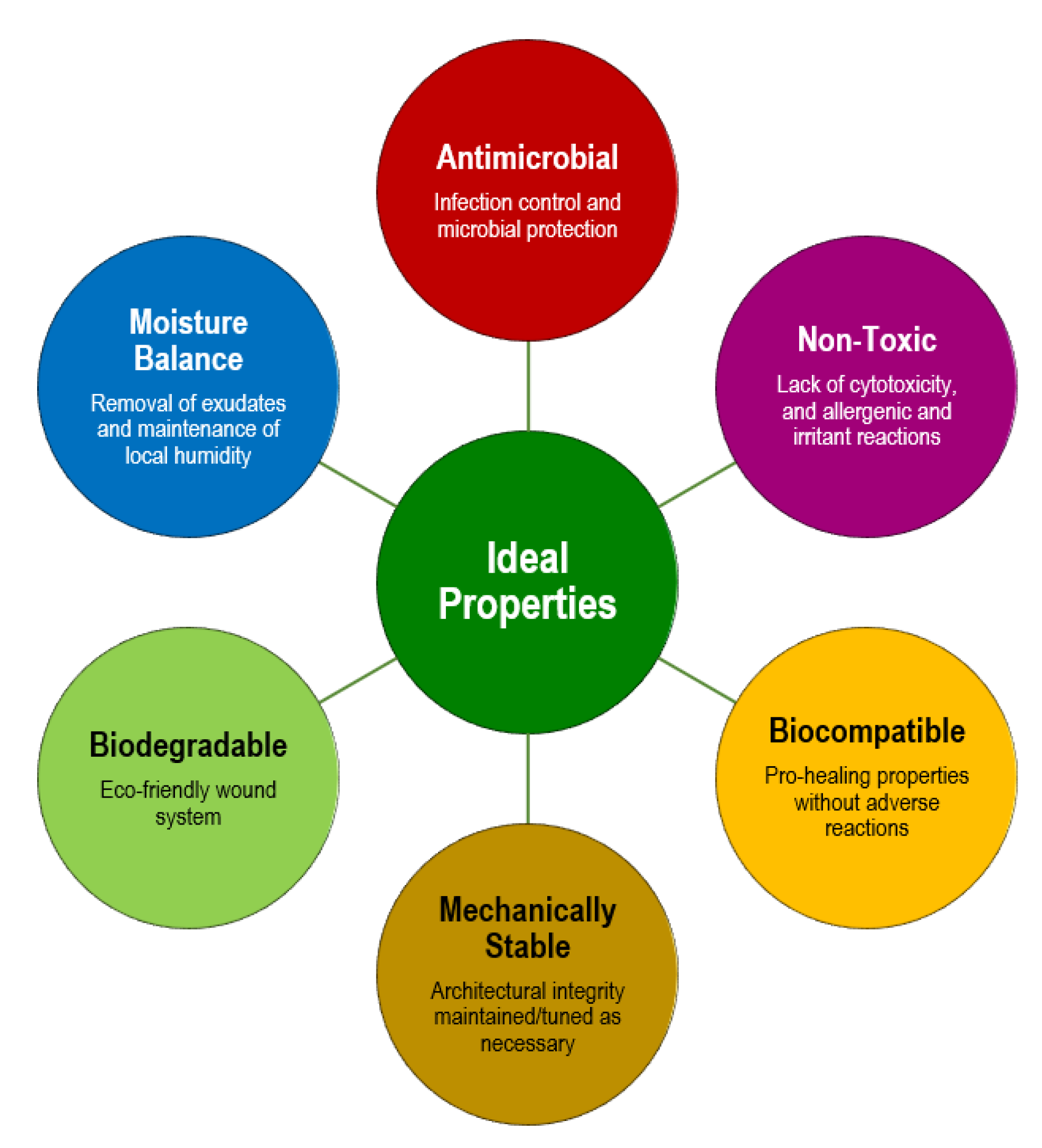
5. Advanced Fibrous Scaffolds
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medeiros, S.; Rodrigues, A.; Costa, R. Physiotherapeutic interventions in the treatment of patients with diabetic foot ulcers: A systematic literature review. Physiotherapy, 2022; in press. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Wang, Z.; Bancks, M.P.; Carnethon, M.R.; Greenland, P.; Feng, Y.-Q.; Wang, H.; Zhong, V.W. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999–2018. JAMA 2021, 326, 704–716. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers and their recurrence. New Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Peter-Riesch, B. The diabetic foot: The never-ending challenge. Nov. Diabetes 2016, 31, 108–134. [Google Scholar]
- Mbela Lusendi, F.; Matricali, G.A.; Vanherwegen, A.-S.; Doggen, K.; Nobels, F. Bottom-up approach to build a ‘precision’ risk factor classification for diabetic foot ulcer healing. Proof-of-concept. Diabetes Res. Clin. Pract. 2022, 191, 110028. [Google Scholar] [CrossRef] [PubMed]
- Glover, K.; Stratakos, A.C.; Varadi, A.; Lamprou, D.A. 3D scaffolds in the treatment of diabetic foot ulcers: New trends vs. conventional approaches. Int. J. Pharm. 2021, 599, 120423. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf. B Biointerfaces 2017, 156, 133–148. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, N.; Martínez-Jiménez, I.; García-Ojalvo, A.; Mendoza-Mari, Y.; Guillén-Nieto, G.; Armstrong, D.G.; Berlanga-Acosta, J. Wound chronicity, impaired immunity and infection in diabetic patients. MEDICC Rev. 2022, 24, 44–58. [Google Scholar] [CrossRef]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential applications of nanomaterials and technology for diabetic wound healing. Int. J. Nanomed. 2020, 15, 9717. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.-B.; Zheng, X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Hagde, P.; Pingle, P.; Mourya, A.; Katta, C.B.; Srivastava, S.; Sharma, R.; Singh, K.K.; Sodhi, R.K.; Madan, J. Therapeutic potential of quercetin in diabetic foot ulcer: Mechanistic insight, challenges, nanotechnology driven strategies and future prospects. J. Drug Deliv. Sci. Technol. 2022, 74, 103575. [Google Scholar] [CrossRef]
- Song, J.; Liu, A.; Liu, B.; Huang, W.; Jiang, Z.; Bai, X.; Hu, L.; Zheng, S.; Guo, S.; Wu, J. Natural Biologics Accelerate Healing of Diabetic Foot Ulcers by Regulating Oxidative Stress. Front. Biosci.-Landmark 2022, 27, 285. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Park, J.-U.; Oh, B.; Lee, J.P.; Choi, M.-H.; Lee, M.-J.; Kim, B.-S. Influence of Microbiota on Diabetic Foot Wound in Comparison with Adjacent Normal Skin Based on the Clinical Features. BioMed Res. Int. 2019, 2019, 7459236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, J.L.; Sotto, A.; Lavigne, J.P. New insights in diabetic foot infection. World J. Diabetes 2011, 2, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Soares, M.; Boyko, E.J.; Jeffcoate, W.; Mills, J.L.; Russell, D.; Morbach, S.; Game, F. Diabetic foot ulcer classifications: A critical review. Diabetes/Metab. Res. Rev. 2020, 36, e3272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmonds, M.; Foster, A. The use of antibiotics in the diabetic foot. Am. J. Surg. 2004, 187, S25–S28. [Google Scholar] [CrossRef]
- Felgueiras, H.P. An insight into biomolecules for the treatment of skin infectious diseases. Pharmaceutics 2021, 13, 1012. [Google Scholar] [CrossRef]
- Baquero, F.; Levin, B.R. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat. Rev. Microbiol. 2021, 19, 123–132. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater. Sci. Eng. C 2019, 104, 109745. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Liu, K.-S.; Cheng, C.-W.; Chan, E.-C.; Hung, K.-C.; Hsieh, M.-J.; Chang, S.-H.; Fu, X.; Juang, J.-H.; Hsieh, I.C.; et al. Codelivery of Sustainable Antimicrobial Agents and Platelet-Derived Growth Factor via Biodegradable Nanofibers for Repair of Diabetic Infectious Wounds. ACS Infect. Dis. 2020, 6, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Davani, F.; Alishahi, M.; Sabzi, M.; Khorram, M.; Arastehfar, A.; Zomorodian, K. Dual drug delivery of vancomycin and imipenem/cilastatin by coaxial nanofibers for treatment of diabetic foot ulcer infections. Mater. Sci. Eng. C 2021, 123, 111975. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, F.; Matsumoto, Y.; Ishii, M.; Tatsuno, K.; Okazaki, M.; Sato, T.; Moriya, K.; Sekimizu, K. Synergistic effects of vancomycin and β-lactams against vancomycin highly resistant Staphylococcus aureus. J. Antibiot. 2017, 70, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Igor Otavio, M.; Cristine Vanz, B.; Maria Izabela, F.; Hector Alonzo Gomez, G.; Chung-Yen Oliver, C.; Giuseppina Pace Pereira, L. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds; Marcos, S.-H., Mariana, P.-T., Maria del Rosario, G.-M., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- El-Ghoul, Y.; Alminderej, F.M. Bioactive and superabsorbent cellulosic dressing grafted alginate and Carthamus tinctorius polysaccharide extract for the treatment of chronic wounds. Text. Res. J. 2021, 91, 235–248. [Google Scholar] [CrossRef]
- Ramalingam, R.; Dhand, C.; Leung, C.M.; Ong, S.T.; Annamalai, S.K.; Kamruddin, M.; Verma, N.K.; Ramakrishna, S.; Lakshminarayanan, R.; Arunachalam, K.D. Antimicrobial properties and biocompatibility of electrospun poly-ε-caprolactone fibrous mats containing Gymnema sylvestre leaf extract. Mater. Sci. Eng. C 2019, 98, 503–514. [Google Scholar] [CrossRef]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Ardekani, M.R.S.; Sharifzadeh, M.; Khanavi, M. Polyurethane/carboxymethylcellulose nanofibers containing Malva sylvestris extract for healing diabetic wounds: Preparation, characterization, in vitro and in vivo studies. Mater. Sci. Eng. C 2020, 114, 111039. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Wang, X.; Chang, J.; Wu, C. Bioactive inorganic/organic nanocomposites for wound healing. Appl. Mater. Today 2018, 11, 308–319. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Noorunnisa Khanam, P.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ghiyasi, Y.; Salahi, E.; Esfahani, H. Synergy effect of Urtica dioica and ZnO NPs on microstructure, antibacterial activity and cytotoxicity of electrospun PCL scaffold for wound dressing application. Mater. Today Commun. 2021, 26, 102163. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffy, K.; Shanthi, G.; Maroky, A.S.; Selvakumar, S. Enhanced antibacterial effects of green synthesized ZnO NPs using Aristolochia indica against Multi-drug resistant bacterial pathogens from Diabetic Foot Ulcer. J. Infect. Public Health 2018, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Jeckson, T.A.; Neo, Y.P.; Sisinthy, S.P.; Foo, J.B.; Choudhury, H.; Gorain, B. Formulation and characterisation of deferoxamine nanofiber as potential wound dressing for the treatment of diabetic foot ulcer. J. Drug Deliv. Sci. Technol. 2021, 66, 102751. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Liu, Y.; Li, Y.; Zhang, C.; Qi, W.; Yeung, K.W.K.; Wong, T.M.; Zhao, X.; Pan, H. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater. Sci. Eng. C 2019, 105, 110083. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, X.; Jia, Y.; Zhang, H.; Tang, T. Hydroxypropyltrimethyl Ammonium Chloride Chitosan Functionalized-PLGA Electrospun Fibrous Membranes as Antibacterial Wound Dressing: In Vitro and In Vivo Evaluation. Polymers 2017, 9, 697. [Google Scholar] [CrossRef]
- Bennett, S.; Griffiths, G.; Schor, A.; Leese, G.; Schor, S. Growth factors in the treatment of diabetic foot ulcers. J. Br. Surg. 2003, 90, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Sondak, T.; Sivashanmugam, K.; Kim, K.-S. A Review of Immunomodulatory Reprogramming by Probiotics in Combating Chronic and Acute Diabetic Foot Ulcers (DFUs). Pharmaceutics 2022, 14, 2436. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Dalvi, Y.B.; Rehman, S.R.U.; Varghese, R.; Unni, R.N.; Yalcin, H.C.; Alfkey, R.; Thomas, S.; Al Moustafa, A.-E. Growth factor loaded in situ photocrosslinkable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater. Sci. Eng. C 2021, 118, 111519. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Nardini, M.; Collina, M.C.; Di Campli, C.; Filaci, G.; Cancedda, R.; Odorisio, T. Innovative Cell and Platelet Rich Plasma Therapies for Diabetic Foot Ulcer Treatment: The Allogeneic Approach. Front. Bioeng. Biotechnol. 2022, 10, 869408. [Google Scholar] [CrossRef]
- Meamar, R.; Ghasemi-Mobarakeh, L.; Norouzi, M.-R.; Siavash, M.; Hamblin, M.R.; Fesharaki, M. Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: A randomized clinical trial. Int. Immunopharmacol. 2021, 101, 108282. [Google Scholar] [CrossRef]
- Weibrich, G.; Kleis, W.K.; Hafner, G.; Hitzler, W.E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2002, 30, 97–102. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing—Know-how. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef]
- Pinzón-García, A.D.; Cassini-Vieira, P.; Ribeiro, C.C.; de Matos Jensen, C.E.; Barcelos, L.S.; Cortes, M.E.; Sinisterra, R.D. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J. Biomed. Mater. Research. Part B Appl. Biomater. 2017, 105, 1938–1949. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Maya Nandkumar, A.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA—Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, M.A.; Nazeri, N.; Khoshnevisan, K.; Heshmat, R.; Omidfar, K. Three-layered PCL-collagen nanofibers containing Melilotus officinalis extract for diabetic ulcer healing in a rat model. J. Diabetes Metab. Disord. 2022, 21, 313–321. [Google Scholar] [CrossRef]
- Naomi, R.; Fauzi, M.B. Cellulose/Collagen Dressings for Diabetic Foot Ulcer: A Review. Pharmaceutics 2020, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Jin, W.; Li, Y.; Wan, L.; Wang, C.; Lin, C.; Chen, X.; Lei, B.; Mao, C. A highly bioactive bone extracellular matrix-biomimetic nanofibrous system with rapid angiogenesis promotes diabetic wound healing. J. Mater. Chem. B 2017, 5, 7285–7296. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Yin, H.; Yu, X.; Wang, L.; Fan, L.; Xin, J.H.; Yu, H. 3D PCL/collagen nanofibrous medical dressing for one-time treatment of diabetic foot ulcers. Colloids Surf. B Biointerfaces 2022, 214, 112480. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, S.K.; Nazir, L.S.; Thangam, R.; Perumal, R.K.; Manjubala, I.; Madhan, B.; Seetharaman, S. Type I collagen peptides and nitric oxide releasing electrospun silk fibroin scaffold: A multifunctional approach for the treatment of ischemic chronic wounds. Colloids Surf. B Biointerfaces 2019, 175, 636–643. [Google Scholar] [CrossRef]
- Tariq, M.; Tahir, H.M.; Butt, S.A.; Ali, S.; Ahmad, A.B.; Raza, C.; Summer, M.; Hassan, A.; Nadeem, J. Silk derived formulations for accelerated wound healing in diabetic mice. PeerJ 2021, 9, e10232. [Google Scholar] [CrossRef]
- Liu, J.; Yan, L.; Yang, W.; Lan, Y.; Zhu, Q.; Xu, H.; Zheng, C.; Guo, R. Controlled-release neurotensin-loaded silk fibroin dressings improve wound healing in diabetic rat model. Bioact. Mater. 2019, 4, 151–159. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Qin, C.; Khan, A.u.R.; Zhang, W.; Mo, X. Silk fibroin/poly-(L-lactide-co-caprolactone) nanofiber scaffolds loaded with Huangbai Liniment to accelerate diabetic wound healing. Colloids Surf. B Biointerfaces 2021, 199, 111557. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Zhang, J.; You, R.; Qu, J.; Li, M. Functionalized silk fibroin dressing with topical bioactive insulin release for accelerated chronic wound healing. Mater. Sci. Eng. C 2017, 72, 394–404. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Bernardes, B.G.; Borges, S.; Rodrigues, I.; Fernandes, R.; Gomes-Guerreiro, S.; Pinto, M.T.; Pintado, M.; Soares, R.; Costa, R. Exploring Silk Sericin for Diabetic Wounds: An In Situ-Forming Hydrogel to Protect against Oxidative Stress and Improve Tissue Healing and Regeneration. Biomolecules 2022, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Gilotra, S.; Chouhan, D.; Bhardwaj, N.; Nandi, S.K.; Mandal, B.B. Potential of silk sericin based nanofibrous mats for wound dressing applications. Mater. Sci. Eng. C 2018, 90, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Verma, P.; Ray, P.; Ray, A.R. Preparation of scaffolds from human hair proteins for tissue-engineering applications. Biomed. Mater. 2008, 3, 025007. [Google Scholar] [CrossRef]
- Yao, C.-H.; Lee, C.-Y.; Huang, C.-H.; Chen, Y.-S.; Chen, K.-Y. Novel bilayer wound dressing based on electrospun gelatin/keratin nanofibrous mats for skin wound repair. Mater. Sci. Eng. C 2017, 79, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Bedir, T.; Kalkandelen, C.; Sasmazel, H.T.; Basar, A.O.; Chen, J.; Ekren, N.; Gunduz, O. A drug-eluting nanofibrous hyaluronic acid-keratin mat for diabetic wound dressing. Emergent Mater. 2022, 5, 1617–1627. [Google Scholar] [CrossRef]
- Li, C.; Kim, K. Neuropeptides. In WormBook: The Online Review of C. elegans Biology [Internet]; WormBook: Pasadenak, CA, USA, 2018. [Google Scholar]
- Zheng, Z.; Liu, Y.; Huang, W.; Mo, Y.; Lan, Y.; Guo, R.; Cheng, B. Neurotensin-loaded PLGA/CNC composite nanofiber membranes accelerate diabetic wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Lopes, L.; Setia, O.; Aurshina, A.; Liu, S.; Hu, H.; Isaji, T.; Liu, H.; Wang, T.; Ono, S.; Guo, X. Stem cell therapy for diabetic foot ulcers: A review of preclinical and clinical research. Stem Cell Res. Ther. 2018, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-Z.; Gou, M.; Da, L.-C.; Zhang, W.-Q.; Xie, H.-Q. Mesenchymal stem cells for chronic wound healing: Current status of preclinical and clinical studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- An, T.; Chen, Y.; Tu, Y.; Lin, P. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in the Treatment of Diabetic Foot Ulcers: Application and Challenges. Stem Cell Rev. Rep. 2021, 17, 369–378. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Su, Y.; John, J.V.; McCarthy, A.; Wong, S.L.; Xie, J. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater. 2020, 108, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, J.; Wang, J.; Xu, M.; Jiao, J.; Qiang, Y.; Zhang, F.; Li, Z. Rock Climbing-Inspired Electrohydrodynamic Cryoprinting of Micropatterned Porous Fiber Scaffolds with Improved MSC Therapy for Wound Healing. Adv. Fiber Mater. 2022, 1–15. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Alginates: Wound dressings. Encycl. Biomed. Polym. Polym. Biomater 2015, 2014, 134–146. [Google Scholar]
- Saisuwan, R. Cellulose-Based Biosensors of Human Neutrophil Elastase (HNE) toward Chronic Wound Point-of-Care Diagnostics. Doctoral Dissertation, University of British Columbia, Vancouver, BC, Canada, 2020. [Google Scholar]
- Tort, S.; Acartürk, F.; Beşikci, A. Evaluation of three-layered doxycycline-collagen loaded nanofiber wound dressing. Int. J. Pharm. 2017, 529, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rajinikanth, P.S.; Arya, D.K.; Pandey, P.; Gupta, R.K.; Sankhwar, R.; Chidambaram, K. Multifunctional Biomimetic Nanofibrous Scaffold Loaded with Asiaticoside for Rapid Diabetic Wound Healing. Pharmaceutics 2022, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm.-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef]
- Chen, H.; Peng, Y.; Wu, S.; Tan, L.P. Electrospun 3D Fibrous Scaffolds for Chronic Wound Repair. Materials 2016, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, H.P. Electrospun Nanocomposites Containing Cellulose and Its Derivatives Modified with Specialized Biomolecules for an Enhanced Wound Healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Potential applications of natural origin polymer-based systems in soft tissue regeneration. Crit. Rev. Biotechnol. 2010, 30, 200–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, M.; Govindharajan, T. Study of hydrocellular functional material as microbicidal wound dressing for diabetic wound healing. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211054930. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Kumar, P.; Choonara, Y.E.; Du Toit, L.C.; Pillay, V. Artificial, Triple-Layered, Nanomembranous Wound Patch for Potential Diabetic Foot Ulcer Intervention. Materials 2018, 11, 2128. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-H.; Hung, K.-C.; Hsieh, M.-J.; Chang, S.-H.; Juang, J.-H.; Hsieh, I.C.; Wen, M.-S.; Liu, S.-J. Core-shell insulin-loaded nanofibrous scaffolds for repairing diabetic wounds. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102123. [Google Scholar] [CrossRef]
- Lv, F.; Wang, J.; Xu, P.; Han, Y.; Ma, H.; Xu, H.; Chen, S.; Chang, J.; Ke, Q.; Liu, M.; et al. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater. 2017, 60, 128–143. [Google Scholar] [CrossRef]
- Grip, J.; Engstad, R.E.; Skjæveland, I.; Škalko-Basnet, N.; Isaksson, J.; Basnet, P.; Holsæter, A.M. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur. J. Pharm. Sci. 2018, 121, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Han, Y.; Wang, J.; Jiang, Y.; Yi, Z.; Xu, H.; Ke, Q. An aligned porous electrospun fibrous membrane with controlled drug delivery—An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018, 70, 140–153. [Google Scholar] [CrossRef]
- Aduba, D.C.; An, S.-S.; Selders, G.S.; Yeudall, W.A.; Bowlin, G.L.; Kitten, T.; Yang, H. Electrospun gelatin–arabinoxylan ferulate composite fibers for diabetic chronic wound dressing application. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 660–668. [Google Scholar] [CrossRef]
- Cam, M.E.; Crabbe-Mann, M.; Alenezi, H.; Hazar-Yavuz, A.N.; Ertas, B.; Ekentok, C.; Ozcan, G.S.; Topal, F.; Guler, E.; Yazir, Y.; et al. The comparision of glybenclamide and metformin-loaded bacterial cellulose/gelatin nanofibres produced by a portable electrohydrodynamic gun for diabetic wound healing. Eur. Polym. J. 2020, 134, 109844. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, X.; Pang, L.; Liu, Y.; Lin, Y.; Xiang, T.; Li, J.; Liao, S.; Jiang, Y. Astragalus Polysaccharides/PVA Nanofiber Membranes Containing Astragaloside IV-Loaded Liposomes and Their Potential Use for Wound Healing. Evid.-Based Complement. Altern. Med. 2022, 2022, 9716271. [Google Scholar] [CrossRef] [PubMed]
- Losi, P.; Al Kayal, T.; Buscemi, M.; Foffa, I.; Cavallo, A.; Soldani, G. Bilayered Fibrin-Based Electrospun-Sprayed Scaffold Loaded with Platelet Lysate Enhances Wound Healing in a Diabetic Mouse Model. Nanomaterials 2020, 10, 2128. [Google Scholar] [CrossRef]
- Ilomuanya, M.O.; Okafor, P.S.; Amajuoyi, J.N.; Onyejekwe, J.C.; Okubanjo, O.O.; Adeosun, S.O.; Silva, B.O. Polylactic acid-based electrospun fiber and hyaluronic acid-valsartan hydrogel scaffold for chronic wound healing. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 31. [Google Scholar] [CrossRef]
- Ghalei, S.; Nourmohammadi, J.; Solouk, A.; Mirzadeh, H. Enhanced cellular response elicited by addition of amniotic fluid to alginate hydrogel-electrospun silk fibroin fibers for potential wound dressing application. Colloids Surf. B: Biointerfaces 2018, 172, 82–89. [Google Scholar] [CrossRef]
- Blanchette, V.; Belosinschi, D.; Lai, T.T.; Cloutier, L.; Barnabé, S. New Antibacterial Paper Made of Silver Phosphate Cellulose Fibers: A Preliminary Study on the Elimination of Staphylococcus aureus Involved in Diabetic Foot Ulceration. BioMed Res. Int. 2020, 2020, 1304016. [Google Scholar] [CrossRef] [Green Version]
- Cam, M.E.; Yildiz, S.; Alenezi, H.; Cesur, S.; Ozcan, G.S.; Erdemir, G.; Edirisinghe, U.; Akakin, D.; Kuruca, D.S.; Kabasakal, L.; et al. Evaluation of burst release and sustained release of pioglitazone-loaded fibrous mats on diabetic wound healing: An in vitro and in vivo comparison study. J. R. Soc. Interface 2020, 17, 20190712. [Google Scholar] [CrossRef] [Green Version]
- Miranda, C.S.; Silva, A.F.G.; Pereira-Lima, S.M.; Costa, S.P.; Homem, N.C.; Felgueiras, H.P. Tunable spun fiber constructs in biomedicine: Influence of processing parameters in the fibers’ architecture. Pharmaceutics 2022, 14, 164. [Google Scholar] [CrossRef]
| Technique | Final System | Components | Main Bioactive Agents Categories | DFUs Care Potential | Ref. |
|---|---|---|---|---|---|
| Electrospinning | Triple Layer Mesh | Outer and middle layers: sodium alginate (SA) and chitosan (CS); Inner layer: co-axial nanofibers with core made of polycaprolactone and collagen, and shell of doxycycline and polyethylene oxide | CS (polymer), collagen (protein) and doxycycline (antibiotic); each layer contained one main inducer of biological activity | - Porous, flexible, mechanically resilient and wettable aligned fiber meshes were produced; - Absence of cytotoxic effects against keratinocyte cells even with quick release of drug; - Guaranteed prolonged protection of the payload (12 months); - Expression of matrix metalloproteinase-2 enzyme was inhibited, and ECM remodeling was promoted. | [78] |
| Outer layer: polycaprolactone (PCL) Middle layer: PCL and type I collagen Inner layer: type I collagen and Melilotus officinalis extract | Collagen (protein) and Melilotus officinalis extract (plant extract) | - Smooth and defect-free three-layer nanofiber structures were obtained; - In vitro cell studies verified the fibroblast cells viability; - 18-day in vivo studies demonstrated the scaffold to induce proper re-epithelialization in diabetic wounds and to instigate collagen production and deposition in the newly formed skin. | [54] | ||
| Outer layer: PCL Middle layer: polyvinyl pyrrolidone (PVP) and ciprofloxacin Inner layer: poly(acrylic acid) (PAA) | Ciprofloxacin (antibiotic) | - The scaffolding system displayed great mechanical resilience, with the PCL outer layer exhibiting lower wettability, adherence, and moisture uptake than the remainder layers; - Drug release was guaranteed in a sustained manner and was influenced by loading amount on the scaffold. | [90] | ||
| Outer layer: poly(vinyl alcohol (PVA) and nanobioglass (nBG) Middle layer: CS and PVA Inner layer: CS | nBG (inorganic particles) and CS (polymer) | - The multilayer mesh exhibited excellent biocompatibility, antibacterial activity and regenerative effects; - Each layer allowed for a microenvironment to be generated so optimal performances of each component could be attained; - In vivo wound model revealed the mesh to significantly accelerate and enhance healing, in terms of complete re-epithelialization, improved collagen alignment and formation of skin appendages. | [41] | ||
| Double Layer Mesh | Inner layer: gelatin (GN) with keratin Outer layer: polyurethane (PU) | Keratin (protein) | - Nanofibers presented a uniform morphology and bead-free structure; - Fibroblast-like cells attachment was improved (increased cell spreading) and proliferation was accelerated; - Engineered meshes promoted faster vascularization, facilitating wound repair and growth of thicker epidermis. | [67] | |
| Inner layer: CS, PVA and deferoxamine Outer Layer: SA and PVA | CS (polymer) and deferoxamine (immunomodulatory drug) | - The double layer dressing displayed high swelling degree, sufficient water-vapor permeation, and high drug entrapment efficiency with a sustained release up to 48 h; - Meshes antibacterial effectiveness was confirmed; - The dressing was also deemed cytocompatible, with in vitro scratch testing revealing wound healing potential. | [40] | ||
| Core-Shell Nanofibers | Shell: polyethylene oxide (PEO) and PCL Core: hyaluronic acid (HA), keratin and metformin hydrochloride | HA (biopolymer), keratin (protein) and metformin hydrochloride (biguanide antihyperglycemic agent) | - Shell structure guaranteed a prolonged released of the core-entrapped bioactive agents; - In vitro cell cultures demonstrated the enhanced biocompatibility of the engineered core-shell fibers and attested to their efficacy as drug delivery platforms. | [68] | |
| Shell: PEO, CS and vancomycin Core: PVP, GN and imipenem/cilastatin | CS (polymer) and vancomycin and imipenem/cilastatin (antibiotics) | - Core-sell nanofibers displayed a smooth morphology with no cytotoxic evidence against fibroblastic cells; - Shell structure protected antibiotic at the core, guaranteeing a prolonged liberation, while the antibiotic at the shell experienced a faster release; - Nanofibers exhibit significant antibacterial profile against bacteria prevalent in DFUs. | [23] | ||
| Shell: PU Core: starch and HA | HA (biopolymer) | - Porous core-shell structures were obtained; - In vitro cell morphology, viability and attachment were enhanced in the presence of the HA-loaded scaffold; - In vivo studies demonstrated the scaffolds to accelerate wound healing. | [81] | ||
| Shell: poly-D-L-lactide-glycolide (PLGA) Core: insulin | Insulin (peptide hormone) | - Core-shell fibers sustained the release of insulin for 4 weeks, guaranteeing a balanced moist environment as well compared to single layer meshes; - Nanofibers reduced the amount of type I collagen in vitro but increased the transforming growth factor-beta (TGF-β) content in vivo and promoted diabetic wound repair. | [91] | ||
| Shell: PLGA, vancomycin and gentamycin Core: recombinant human platelet-derived growth factor-BB (rhPDGF-BB) | Vancomycin and gentamycin (antibiotics) and rhPDGF-BB (growth factor) | - Scaffolds guaranteed a sustained release of growth factor and antibiotics for 3 weeks; - They also decreased phosphatase and tensin homolog content, enhanced angiogenesis marker presence (CD31), and accelerated healing in early-stage infected diabetic wounds. | [22] | ||
| Single Layer Mesh | PLGA modified with recombinant human epidermal growth factor (rhEGF) and Aloe vera extract | rhEGF (growth factor) and Aloe vera (plant extract) | - Uniform, bead free meshes of improved porosity were obtained; - Presence of rhEGF and the extracts improved fibroblast proliferation and accelerated significantly wound closure and reepithelization in an in vivo full thickness wound mice model. | [46] | |
| GN modified with human placenta-derived mesenchymal stem cells (hPDMSCs) and platelet-rich plasma (PRP) | hPDMSCs (stem cells) and PRP (blood components) | - Clinical testing was conducted on 28 patients with DFUs, from which 18 were treated with the smooth, homogenous nanofibers mats with and without PRP; - Cell proliferation and wound closure were significantly enhanced by hPDMSCs, however, PRP had little impact on the outcomes; - Pain was significantly reduced in the presence of the engineered nanofibers as compared to conventional standard care therapies (control group). | [49] | ||
| PCL and GN composite containing silicate-based bioceramic particles of nagelschmidtite (NAGEL) | NAGEL (inorganic particles) | - The scaffolding system promoted the adhesion, proliferation and migration of human umbilical vein endothelial cells (HUVECs) and human keratinocytes (HaCaTs) in vitro; - In vivo evaluations demonstrated their ability in inducing angiogenesis, collagen deposition and re-epithelialization, as well as in inhibiting inflammation. | [92] | ||
| Hydroxypropyl methylcellulose (HPMC) and PEO loaded with β-glucan | β-glucan (immunomodulatory drug) | - Electrospinning Nanospider™ technology ensure the reproducible and reliable production of nanofibers; - The scaffolds exhibited no cellular toxicity in vitro; - Wound healing assessment in a wound model confirmed the significant improvement introduced by βG-nanofibers. | [93] | ||
| Poly(L-lactic acid) (PLLA) and dimethyloxalylglycine-loaded mesoporous silica nanoparticles (MSi NPs) | Dimethyloxalylglycine (immunomodulatory drug) and MSi NPs (inorganic particles) | - The engineered aligned porous meshes stimulated the proliferation, migration and angiogenesis-related gene expression of endothelial cells; - In vivo study demonstrated the meshes’ ability to improve neo-vascularization, re-epithelialization and collagen formation, while inhibiting inflammatory reactions in the diabetic wound bed. | [94] | ||
| GN, arabinoxylan ferulate (AXF) and silver sulfadiazine | AXF (polysaccharide) and silver sulfadiazine (inorganic compound) | - Continuous, homogeneous fibers were attained with excellent biocompatibility and antimicrobial profiles; - Prolonged liberation of silver compound was guaranteed potentiating the scaffold testing in in vivo scenarios. | [95] | ||
| ECM-componential collagen, PCL and bioactive glass nanoparticles (BGNs) | Collagen (protein) and BGNs (inorganic particles) | - Endothelial cell attachment and proliferation were enhanced; - Angiogenesis marker CD31 expression was upregulated in vitro; - Angiogenesis was also improved in vivo, by greatly upregulating the mRNA and protein expressions of hypoxia-inducible factor 1-α (Hif-1α), vascular endothelial growth factor (VEGF), collagen I and α-smooth muscle actin (α-SMA); - Granulation tissue formation, collagen matrix remodeling and epidermis differentiation were accelerated. | [56] | ||
| Bixin-loaded PCL nanofibers | Bixin (carotenoid pigment extracted from Bixa orellana L. seeds) | - Increasing bixin concentration resulted in higher polymeric solution electrical conductivity and, consequently, in smaller fiber diameters; - Bixin release kinetics was guaranteed for 14 days (30–40% release in the first 10 h); - The bixin-loaded meshes accelerated wound healing while reducing the scar tissue area. | [52] | ||
| PCL and Gymnema sylvestre | Gymnema sylvestre (plant extract) | - Scaffolds exhibited good wettability and enhanced mechanical properties; - Contact-mediated bacterial inhibition was achieved against Gram-positive and Gram-negative bacteria; - Scaffolds were identified as cytocompatible towards fibroblasts. | [30] | ||
| PU and carboxymethyl cellulose (CMC) nanofibers containing Malva sylvestris extract | Malva sylvestris (plant extract) | - Meshes allowed great fluid absorption and sustained release of the extract; - Significant antibacterial activity was observed; - In vivo wound-healing testing indicated the scaffold accelerated healing significantly, aside from lowering acute and chronic inflammations; - Collagen deposition and neovascularization were also instigated. | [31] | ||
| Poly(lactic acid) (PLA) and hyperbranched polyglycerol (HPG) modified with curcumin | Curcumin (plant extract) | - Meshes were deemed highly hydrophilic, absorbent and with great drug uptake; - In vitro cell viability, adhesion and proliferation were instigated as well as cell migration (scratch test). | [53] | ||
| Neurotensin-loaded PLGA and cellulose nanocrystals (CNCs) composite | Neurotensin (neuropeptide) | - PLGA/CNCs nanofibers showed excellent cytocompatibility and facilitated fibroblast adhesion, spreading and proliferation; - Neurotensin could be released from the mats in a sustained manner for up 2 weeks; - In vivo data reported the composite abilities to induce faster epidermal and dermal regeneration, while decreasing the expressions of the inflammatory cytokines interleukin-1β (IL-1β) and IL-6. | [70] | ||
| GN and bacterial cellulose (BC) modified with metformin and glybenclamide | Metformin and glybenclamide (diabetic drugs) | - Nanofibers were produced using a portable electrohydrodynamic gun with great homogeneity; - Diabetic wounds treated with nanofibers loaded with glybenclamide revealed moderate to complete re-epithelialization and well-formed granulation tissue, a result superior to the metformin-modified fibers; - TNF-α levels were significantly reduced with both drugs, but again glybenclamide was more effective. | [96] | ||
| PLA and doxycycline | Doxycycline (antibiotic) | - Antibiotic homogeneous distribution along the fibers for a sustained release was achieved; - The mats’ mechanical features, water-vapor permeability and absorbency met the requirement for wound dressings; - In vitro data confirmed the mats’ cytocompatibility and antibacterial profile; - In vivo full-thickness wound healing was also stimulated. | [21] | ||
| PVA incorporating active silk sericin | Silk sericin (protein) | - Dressings were endowed with free radical scavenging capacity, antibacterial activity, swelling capacity, and biocompatibility due to the incorporation of silk sericin; - Fibroblasts and keratinocytes spreading and proliferation were improved; - The nanofibers exhibited excellent antioxidant potential without hampering cell viability even under H2O2 driven oxidative stress; - In vivo tolerance to the engineered dressing was confirmed over 4 weeks of testing, with no inflammatory events being triggered. | [64] | ||
| CS, PVA and zinc oxide nanoparticles (ZnO NPs) | ZnO NPs (inorganic particles) | - CS/PVA/ZnO nanofibrous meshes possessed exceptional antibacterial activity against DFUs-prevalent bacteria; - They also exhibited excellent antioxidant potential; - In vivo wound healing studies showed that CS/PVA/ZnO meshes accelerated wound healing. | [34] | ||
| PCL, ZnO NPs and Urtica dioica | ZnO NPs (inorganic particles) and Urtica dioica (plant extract) | - Incorporation of Urtica dioica and ZnO NPs improved the fibers’ water uptake and controlled the release of the plant extract; - Antibacterial activity was augmented and cell cytotoxicity was diminished in the presence of the hybrid scaffold; - Cell adhesion and, consequent, scaffold integration was instigated. | [35] | ||
| PVA, astragalus and astragaloside IV liposomes. | Astragalus (polysaccharide, plant extract) and astragaloside IV (cycloartane-type triterpene obtained from Astragalus membranaceus) | - In vivo testing revealed the nanofibers to inhibit inflammation, enhance deposition of collagen and the repair of regenerated epithelium, and effectively strengthen wound healing of diabetic rats. | [97] | ||
| PVA, SA and silk fibroin (SF) fibers loaded with asiaticoside. | SF (protein) and asiaticoside (pentacyclic triterpenoid isolated from Centella asiatica plant) | - Homogeneous nanofibers with sustained asiaticoside liberation over extended periods were electrospun; - Mats exhibited low cytotoxicity, promoting improved cell migration and anti-microbial efficacy; - In vivo testing revealed wound healing efficacy and abilities to restore normal skin structure. | [79] | ||
| PVA, SF, type I collagen and S-Nitrosoglutathione | SF (protein), type I collagen (protein) and S-Nitrosoglutathione (nitric oxide donor) | - Continuous, bead free and randomly oriented nanofibers meshes were obtained with a highly porous morphology; - In vitro evaluations attested to the scaffold biocompatibility with a high level of cell attachment, expansion, inter-cellular connections and proliferation, mainly promoted by type I collagen; - Nitric oxide release, essential for effective wound healing, was guaranteed for 1 day. | [58] | ||
| Poly-(L-lactide-co-caprolactone) (PLCL) and SF loaded with Huangbai Liniment | SF (protein) and Huangbai Liniment (plant extract) | - Smooth and bead-free nanofibers allowed for a sustained release of the natural-origin drug; - Antibacterial effects were observed against Gram-positive and Gram-negative bacteria; - In vitro cell adhesion and proliferation were enhanced; - In vivo testing demonstrated the loaded nanofibers to instigate the expression of the TGF-β signaling pathway and collagen and to inhibit pro-inflammatory factors, thus effectively promoting healing. | [61] | ||
| Electrospinning and Entrapment-Graft | Single Layer Mesh | PLGA-hydroxypropyltrimethyl ammonium chloride chitosan (HACC) composite | HACC (polymer) | - Effective antibacterial activity towards both Gram-positive and Gram-negative bacteria; - Meshes were cytocompatible, significantly stimulating adhesion, spreading and proliferation of fibroblasts and keratinocytes; - PLGA-HACC exhibited excellent wound healing efficacy in vivo. | [42] |
| Electrospinning combined with a Spray Phase-Inversion Method | Double-Layer Meshes | PU combined with fibrin fibers loaded with platelet lysate | Fibrin (protein) and platelet lysate (blood component) | - The bilayer dressing allowed a sustained release of bioactive platelet-derived growth factors; - The engineered scaffold also significantly accelerated wound closure in in vivo full-thickness wounds; - Histological data demonstrated the scaffold’s effectiveness in increasing re-epithelialization and collagen deposition. | [98] |
| Electrospinning followed by Hydrogel Loading | Fiber-Hydrogel Composite | PLA nanofibers loaded with HA, valsartan, and ascorbic acid hydrogel | HA (biopolymer), valsartan (immunomodulatory drug) and ascorbic acid (vitamin C) | - Scaffolds offered a large surface area for enhanced drug solubility, oxygen permeability, and fluid uptake; - Presence of valsartan significantly impacted the re-epithelization rate, accelerating it; - Scaffolds also reduced the number of inflammatory cell infiltrates at the wound site. | [99] |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) nanofibers modified with GN-methacryloyl (GNMA) hydrogels containing epidermal growth factors (EGFs) | EGFs (growth factors) | - Patches promoted cells migration and proliferation and enhanced angiogenesis in vitro; - In vivo wound healing testing in diabetic rats showed the patches stimulated favorable cell responses, angiogenesis and rapid wound healing. | [47] | ||
| SA, SF and amniotic fluid | SF (protein) and amniotic fluid (complex substance containing a wide range of growth factors) | - SF fibers were successfully linked to the SA-based hydrogel formulation; - Sustained release of amniotic fluid was guaranteed at the optimal ratio with SA (superior amount of amnionic fluid); - Fibroblast-like cells proliferation, spreading, and secretion of collagen were enhanced with increasing concentrations of amniotic fluid. | [100] | ||
| Electrospinning followed by Cryogenic Cutting and Thermal Treatment | Cylindrical 3D Scaffolds made of Radially or Vertically Aligned Nanofibers | PCL, GN and Pluronic-F-127, modified with bone marrow mesenchymal stem cells (BMSCs) | BMSCs (stem cells) | - Scaffolds can be customized with different sizes, depths, and shapes for a variety of type 2 diabetic wounds; - They were also shape-recoverable in the atmosphere and water following compression; - Enhanced the formation of granulation tissue, promoting angiogenesis, and facilitating collagen deposition; - Inhibited the expression of pro-inflammatory cytokines IL-6 and tumor necrosis factor-α (TNF-α) and promote the expression of anti-inflammatory cytokines IL-4 and IL-10. | [74] |
| Electrospraying | Fibrous Sponge Functionalized with Co-axial Microparticles | Insulin-encapsulated SF microparticles loaded onto SF sponge | SF (protein) | - SF microparticles guaranteed the sustained release of insulin for up to 28 days; - Insulin retained its bioactivity and promoted cell migration; - The engineered dressing was seen to accelerate wound closure, collagen deposition and vascularization. | [62] |
| Weft Knitting | Three-Layer Fabric | Polyethylene terephthalate (PET) and PU yarns modified with quaternary ammonium salt (QAS) | QAS (cationic salt; organic particles) | - Effective exudates management, with optimal moisture balance, and oxygenation of the wound (porous nature); - Excellent broad-spectrum antimicrobial activity, with non-leaching of salts, thus preventing the development of mutations or microbial resistance; - Durable and cost-effective dressing. | [89] |
| Knitting followed by Grafting (adjustable curing and impregnation conditions) | Cellulosic Textile Woven | Cellulosic textile woven grafted with alginate and Carthamus tinctorius polymer extract | Alginate (polymer) and Carthamus tinctorius polymer extract (plant extract) | - Grafting was successfully conducted without compromising textile properties; - Excellent biocompatibility, with increased cell viability after grafting; - Antimicrobial testing against four of the most prevalent DFU bacteria demonstrated the engineered dressing potent antimicrobial effect. | [29] |
| Tappi T 205 sp-02 (standard compression method of pulp and paper industry) | Fibrous paper | Cellulose fibers from bleached softwood Kraft lignin modified with silver phosphate | Silver phosphate (inorganic salt) | - Efficient antimicrobial activity against Staphylococcus aureus bacterium; - Antimicrobial effectiveness equal or superior to commercial products. | [101] |
| Aqueous Phase Fiber Reassembly | 3D Fibrous Scaffold | PCL and collagen nanofibers loaded with doxycycline hyclate-modified halloysite nanotubes and cephalexin | Collagen (protein), doxycycline hyclate (tetracycline antibiotic), halloysite nanotubes (inorganic nanostructures made of alumina-silicate) and cephalexin (antibiotic) | - The scaffold exhibited high water absorption capacity and swelling capacity, potentially reducing dressing change frequency in DFUs; - It also displayed excellent antibacterial activity against Escherichia coli and Staphylococcus aureus bacteria; - Additionally, the dressing demonstrated good biocompatibility, significantly improving wound healing. | [57] |
| Electrohydrodynamic Cryoprinting | Micropatterned fiber scaffolds | PC loaded with adipose-derived MSCs (AMSCs) | AMSCs (stem cells) | - Efficient pore formation along the scaffold for increased surface roughness (facilitated cell adhesion); - In vitro enhanced secreting of growth factors and chemokines, which promoted fibroblast migration and vascular endothelial cell tube formation; - Augmented scarless collagen deposition and angiogenesis, and reduction of pro-inflammatory reactions in in vivo experimentation. | [75] |
| Pressurized Gyration | Single Layer Mesh | PVP and PCL loaded with pioglitazone hydrochloride (PHR) | PHR (immunomodulatory drug) | - PHR-loaded fibrous mats expedited diabetic wound healing in type-1 diabetic rats without triggering any cytotoxic effect on cells; - Additionally, mats improved neutrophil infiltration, edema, and reduced inflammation, aside from increasing epidermal regeneration and fibroblast proliferation. | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felgueiras, H.P. Emerging Antimicrobial and Immunomodulatory Fiber-Based Scaffolding Systems for Treating Diabetic Foot Ulcers. Pharmaceutics 2023, 15, 258. https://doi.org/10.3390/pharmaceutics15010258
Felgueiras HP. Emerging Antimicrobial and Immunomodulatory Fiber-Based Scaffolding Systems for Treating Diabetic Foot Ulcers. Pharmaceutics. 2023; 15(1):258. https://doi.org/10.3390/pharmaceutics15010258
Chicago/Turabian StyleFelgueiras, Helena P. 2023. "Emerging Antimicrobial and Immunomodulatory Fiber-Based Scaffolding Systems for Treating Diabetic Foot Ulcers" Pharmaceutics 15, no. 1: 258. https://doi.org/10.3390/pharmaceutics15010258
APA StyleFelgueiras, H. P. (2023). Emerging Antimicrobial and Immunomodulatory Fiber-Based Scaffolding Systems for Treating Diabetic Foot Ulcers. Pharmaceutics, 15(1), 258. https://doi.org/10.3390/pharmaceutics15010258






