Abstract
Diabetic foot ulcers (DFUs) are one of the main complications of diabetes and are characterized by their complexity and severity, which are frequently aggravated by overexpressed inflammatory factors and polymicrobial infections. Most dressing systems offer a passive action in the treatment of DFUs, being frequently combined with antibiotic or immunomodulatory therapies. However, in many instances due to these combined therapies’ inability to properly fight microbial presence, and provide a suitable, breathable and moist environment that is also capable of protecting the site from secondary microbial invasions or further harm, aggravation of the wound state is unavoidable and lower limb amputations are necessary. Considering these limitations and knowing of the urgent demand for new and more effective therapeutic systems for DFU care that will guarantee the quality of life for patients, research in this field has boomed in the last few years. In this review, the emerging innovations in DFU dressing systems via fiber-based scaffolds modified with bioactive compounds have been compiled; data focused on the innovations introduced in the last five years (2017–2022). A generalized overview of the classifications and constraints associated with DFUs healing and the bioactive agents, both antimicrobial and immunomodulatory, that can contribute actively to surpass such issues, has also been provided.
1. Introduction
Diabetes Mellitus (DM) was one of the first illnesses to be identified, in which the recognition of symptoms by humankind has been generally understood. Because of its high prevalence, and high morbidity and mortality rates (among the top ten causes of death worldwide), DM has been deemed a global epidemic [1]. Among USA adults prevalence has been increasing overtime, above global rates, from 5.3% in 1976–1980 to 11.5% in 2011–2014, with economic costs reaching 24.0% of the total health care annual budget [2]. Diabetic foot ulcers (DFUs) are one of the main complications of DM, with important health, physical, mental, social, and economic impacts. The average global prevalence of DFUs is ≈6.3%, with North America rating at the top with 13.0% prevalence, and Oceania at the bottom with 3.0% [3]. In DM patients, the development of DFUs is a lifetime risk estimated at 25%, with wound recurrence being a very likely possibility [4]. DFUs are categorized as chronic wounds in which the loss of the epidermis and dermis is frequently followed by subcutaneous and underlying tissue exposure. Additionally, because of their location, they are highly susceptible to infections, which can spread very quickly, compromising the neighboring healthy tissues and in advanced cases or scenarios of ineffective/deficient care, leading to systemic infections and even limb loss (amputation). In fact, it is estimated that >70% of DFU patients may require lower limb amputation due to health complications, poor wound management, and treatment ineffectiveness [5]. The multifactorial pathophysiology that characterizes DFUs, namely diabetic neuropathy (DN), peripheral arterial disease and immunosuppression with an exacerbated inflammatory response, makes management and treatment selection very complex [6].
As in most diseases, early diagnoses of DFUs and treatment selection are key. Standard therapies include glycemic control, debridement, infection management and ulcer off-loading. However, as the wound progresses to more complex and difficult-to-treat stages, advanced treatment options are required [7]. It is in this category that many antimicrobial and immunomodulatory formulations based on fibrous constructs are making their mark. This review explores this further, by exposing the emerging innovations in DFU care via fiber-based scaffolding systems modified with bioactive compounds. The work provides a generalized overview of the classifications and constraints associated with DFU healing, identifies effective bioactive agents used to fight microbial infections or regulate immunomodulatory pathways and introduces emerging fiber-based formulations that are promising for DFU care (focusing on the last five years).
2. Diabetic Ulcers: Healing Impairments and Classifications
2.1. Impairments
DFUs are complex wounds that affect the lower extremities of diabetic patients. Even though DFUs are initially characterized as acute wounds, their inability to progress through the healing stages, frequently taking 12 or more weeks to properly heal (generally, acute wound healing is completed within 4 to 6 weeks), converts them into chronic wounds [4,5,8]. Ulcerations at the lower extremities are frequently associated with neuropathic pain and peripheral arterial disease with the healing cascade being largely affected by overexpressed inflammatory factors (prolonged inflammation), hyperglycemia, hypoxia, deregulated enzyme activity and alterations in various signaling pathways, including the activity of neuropeptides. Additionally, healing progression is also hindered by external influences, particularly the presence of pathogens (Figure 1) [9].
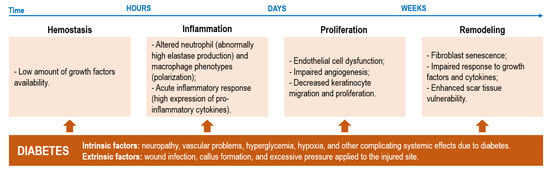
Figure 1.
Possible disruptive events occurring within each phase of healing and the intrinsic and extrinsic diabetic factors that contribute to such disturbances in the normal healing cascade of wounds (adapted with CC BY 4.0 permission from [9]).
It is general knowledge that diabetes is associated with high glucose levels in the blood. In DFUs, these abnormally high amounts of glucose aside from lowering growth factor availability and hindering leucocyte recruitment for fighting infection can also lead to vasoconstriction and microvascular dysfunctions that limit tissue oxygenation. By restricting oxygen supply (also instigated by neuropathy damage in local nerves), hypoxia scenarios may be triggered. Hypoxia increases the number of free radicals which, in combination with enhanced pro-inflammatory cytokine expression, further delays healing [10,11]. Additionally, a dysregulated M1 and M2 macrophage ratio due to the inability of macrophages to polarize from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype following inflammation has been shown to stall DFUs healing at this phase. At this stage, the expression of anti-inflammatory markers, such as interleukin-12 (IL-12), IL-6, IL-1 or tumor necrosis factor-α, is enhanced, while the activity of anti-inflammatory cues, such as IL-10 and IL-4, is reduced [7,12]. This leads to an imbalance between angiogenic factors (promoters of new blood vessel formation) and angiostatic factors, impairing angiogenesis and leading to endothelial progenitor cell dysfunction aside from hindering the proliferation and migration of fibroblasts and keratinocytes [10,13]. This is exacerbated by the limited neuropeptide activity at the site, since in hyperglycemia conditions, neuropeptides such as Substance P, neuropeptide Y or neurotensin endowed with regenerative functions, have their activity reduced. Additionally, matrix metalloproteinases (MMPs), which are responsible for promoting autolytic debridement and cell migration in acute wound scenarios, entering in remission after inflammation, become overly expressed in DFUs degrading various matrix proteins, growth factors and blocking cell proliferation [7,14]. All these events (Figure 2), exacerbate wound chronicity, conditioning granulation tissue formation.
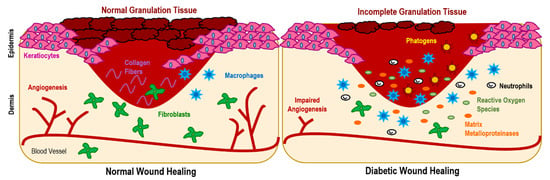
Figure 2.
Diabetic wounds are characterized by impaired angiogenesis, excessive inflammation (increase in macrophage recruitment), augmented production of matrix metalloproteinases, hypoxia and hyperglycemia, which is associated with a raise of the reactive oxygen species expression that prevents the formation of healthy tissue. All these factors condition granulation tissue formation, maintaining the wound in an inflammatory state, which is also exacerbated by installation of pathogenic microbes.
Aside from the previous phenomena, the increased incidence of infections in DFUs contributes significantly to impaired healing. In fact, DFUs are more prone to develop environments conducive to microbial installation than other types of chronic wounds due to the inherent stress and compressive forces that characterize the foot area, which has been known to promote the overgrowth of bacteria [14]. Additionally, due to DFUs-associated neuropathy, infection progression and inherent symptoms are more difficult to detect, delaying a proper treatment response that may allow microbes to settle and colonize the injured site. Further, even in a normal scenario, the foot microflora are very proliferative and varied. Thus, skin commensal bacteria can also colonize the wound generating a self-produced protective extracellular biofilm that may become pathogenic and immune to all antimicrobial events triggered by the healing cascade [15]. Among the many microorganisms colonizing DFUs, the most prevalent are Staphylococcus aureus, β-hemolytic streptococci, Enterobacteriaceae and various anaerobes [16].
2.2. Classification
Various classification systems have been proposed to identify the severity of DFUs. The most employed and reliable is the Wagner Ulcer Classification System established in 1981, which assesses ulcer depth and the presence of osteomyelitis or gangrene [17]:
- -
- Grade 1. Partial thickness involving only dermis and epidermis
- -
- Grade 2. Full thickness and subcutaneous tissues
- -
- Grade 3. Grade 2 plus exposed tendons, ligament, and/or joint
- -
- Grade 4. Grade 3 plus abscess and/or osteomyelitis
- -
- Grade 5. Grade 3 plus necrotic tissue in wound
- -
- Grade 6. Grade 3 plus gangrene in the wound and surrounding tissue
Another option is by following the classification introduced by the University of Texas, which categorizes DFUS based on the occurrence of ischemia or infection and ulcer depth. Additional classification systems have been proposed over the years, although the first remains the most generally accepted [7].
3. Antimicrobial Agents
Most DFUs are infected with pathogenic microorganisms. Infected DFUs result from the entrance, growth, metabolic activities, and propagation of microorganisms at the injured site and surrounding tissues, which trigger pathophysiological effects. Here, microorganisms can either colonize the wound, multiply on the surface of the wound, or invade the underlying tissues, actively penetrating the soft tissues around the ulcer. Yet, the presence of microorganisms in the DFUs is not as important as the rate of pathogen growth. Many reports have shown that an infected wound is only labeled as such if the number of microorganisms per g of tissue surpasses 100,000 [18]. To prevent that from occurring and/or to combat infection once this boundary is surpassed, many antimicrobial agents have been examined and included within antimicrobial-fighting strategies for DFUs care: antibiotics, natural extracts, antimicrobial peptides, organic and inorganic nanoparticles, bioactive polymers, etc.
3.1. Antibiotics
Antibiotics can be chemically synthesized or extracted from microbial substances. Their effectiveness is defined based on their ability to target elements within the bacterial cell wall and intracellular space and induce a detectable effect without losing their effectiveness throughout the process [19]. Studies have reported the ability of bacteriostatic (prevent bacterial growth) and bactericidal (kill bacteria) antibiotics to assist with wound closing by effectively acting against infection-producing microbes. They accomplish such deeds by targeting the bacteria cell wall biosynthesis, hindering protein synthesis or inhibiting nucleic acid replication [20]. Indeed, in DFU infection treatment strategies, doxycycline, a broad-spectrum tetracycline antibiotic and matrix metalloproteinases inhibitor, has been shown to hinder Escherichia coli bacterium activity by means of a polylactide nanofiber-based delivery system [21]. Vancomycin (glycopeptide antibiotic) and gentamicin (aminoglycoside antibiotic) have also contributed significantly to control infection and instigate regeneration when loaded onto co-axial sheath-core nanofibrous poly(lactide-co-glycolide) scaffolds modified with platelet-derived growth factors [22]. Additionally, core-shell nanofibers composed of polyethylene oxide, chitosan and vancomycin at the shell and polyvinylpyrrolidone, gelatin and imipenem/cilastatin (β-lactams) at the core have demonstrated these antibiotics effectiveness in eliminating Escherichia coli, Pseudomonas aeruginosa and multi-resistant Staphylococcus aureus bacteria colonizing DFUs [23]. Most importantly synergisms between antibiotics have been revealed, so that the amount loaded within the fibrous scaffolds required for effective action could be reduced and potential side effects against viable tissues mitigated [24]. Even though antibiotics continue to be used in DFU care, their repeated and/or improper usage has been known to increase bacterial resistance [19]. In fact, ≈ 70% of bacteria responsible for wound infections are resistant to at least one type of antibiotic, with many infectious strains revealing resistance against many [25].
3.2. Natural Extracts
Natural extracts are obtained from plants, particularly from their secondary metabolites, which have been identified as most effective in fighting pathogen-derived infections. Phenolic compounds are produced by plants in response to bacterial and fungal attacks and possess a phenol moiety in their structure. They are made of one (phenolic acids) or more (polyphenols) aromatic rings with attached hydroxyl groups. They act against microbial cells by generating non-specific interactions with proteins or by inhibiting the action of their enzymes [19,26]. Terpenes include one or more five-carbon isoprene units in their structure (the largest class of secondary metabolites found in essential oils), acting against pathogens by lipophilic membrane disruption [27]. Alkaloids are heterocyclic nitrogen complexes that are capable of inhibiting nucleic acid synthesis, via the inhibition of the enzyme dihydrofolate reductase [28]. In DFU treatments, natural extracts have been employed both in their free forms, in ointments or lotions, and loaded onto dressing systems. El-Ghoul et al. engineered a bioactive and superabsorbent cellulosic dressing grafted with alginate and Carthamus tinctorius extract and verified the ability of the polysaccharide extract to inhibit four different pathogenic bacteria, revealing particular effectiveness against Gram-positive bacteria [29]. Polycaprolactone electrospun meshes loaded with Gymnema sylvestre leaf extract also revealed great antibacterial performance via contact-mediated inhibition of Gram-positive and Gram-negative bacteria [30]. Additionally, polyurethane and carboxymethyl cellulose nanofibers containing Malva sylvestris extract were seen to effectively eradicate Staphylococcus aureus and Escherichia coli bacteria, without inducing any cytotoxic effect both in vitro and in vivo using diabetic mice [31].
3.3. Inorganic Nanoparticles
Particles of 1 to 100 nm in size are frequently denominated as nanoparticles, even though their morphology or shape can encompass spheres, capsules, liposomes, dendrimers and even micelles. They are characterized by possessing a large surface area to volume ratio and for that reason have been most sought out for biomedical purposes. Inorganic nanoparticles are subdivided into magnetic and metallic (including alloys and oxides) [32,33]. In DFU care, and particularly in fiber-based scaffolds emerging in the last five years, focus has been given to zinc oxide nanoparticles. Ahmed et al. electrospun a chitosan, poly(vinyl alcohol) and zinc oxide nanofibrous mesh and determined that antimicrobial activity was highly instigated in the presence of the inorganic particles. They also verified that by combating infection more effectively, these scaffolds were capable of accelerating healing and exhibiting a superior antioxidant profile compared to the nanoparticles-unloaded scaffolds [34]. In another study, synergisms between zinc oxide nanoparticles and the natural extract Urtica dioica were examined putting again in evidence the improved antibacterial potential of the engineered fibrous scaffolds. They were also found to support cell viability along with cell adhesion [35].
The inorganic nanoparticle activity against bacteria has been extensively verified, with their mechanisms of action being already detailed. In fact, inorganic nanoparticles have been shown to act against microbial cells by disrupting the cell wall and causing membrane damage, and by infiltrating through the cell membrane and inducing protein denaturation, enzyme inactivation, oxidative stress, and DNA sequence breakage [36,37]. Their small size allied to their surface charge or potential linked agents at the surface or incorporated at the core have been deemed the effectors of such successful activity.
3.4. Polymers: Chitosan
One of the most impactful polymers in DFUs’ infection control is chitosan. This polysaccharide made of D-glucosamine and N-acetyl-D-glucosamine units linked through β-(1-4) glycosidic linkages is obtained from chitin partial deacetylation and is characterized by its biocompatibility, biodegradability, and hemostasis capacity. Most importantly, it displays broad antimicrobial action against both bacteria and fungi. After dissolution, the chitosan amino groups present at the glucosamine units become protonated (particularly at acidic pH), which has been highlighted as the most significant effector of its antimicrobial potency. Indeed, it is via the electrostatic interactions established with the negatively charged microbial cells, which damage, increase the membrane permeability and lead to the leakage of intracellular components, that chitosan acts to inhibit the activity of the pathogens infecting DFUs [38,39]. Jeckson et al. demonstrated the ability of chitosan to protect the wound site from the effects of the most prevalent bacteria in DFUs, the Gram-positive Staphylococcus aureus and Gram-negative Pseudomonas aeruginosa, via a double-layer scaffolding system [40]. In a triple-layer nanofibrous scaffold, it was seen that without chitosan the antibacterial activity of the structure was inexistent. In fact, it was proven that the sub-layer with the highest chitosan content displayed the best bacteria inhibitory efficiency [41]. Similarly, Yang et al. immobilized hydroxypropyltrimethyl ammonium chloride chitosan, a chitosan derivative, onto the surface of poly(lactic-co-glycolic acid) nanofiber meshes and corroborated this polymer’s antibacterial abilities against the same bacteria. These scaffolds were also seen to stimulate the adhesion, spreading and proliferation of fibroblasts and keratinocytes and to generally improve wound healing [42]. Indeed, derivatives of chitosan with known antimicrobial features include hydroxypropyl chitosan, thioglycolic chitosan, carboxymethyl chitosan, N,N,N-trimethyl chitosan, N-(2-hydroxyl)propyl-3-trimethyl ammonium chitosan, and a variety of chitosan conjugates [38]. Even though chitosan has intervention in many immunomodulatory functions in DFUs, most applications explore its antimicrobial profile.
4. Immunomodulatory Agents
In DFUs, healing progression can be delayed by means of a variety of factors, most of which impact significantly the skin tissues’ ability to effectively induce an appropriate immune response [5,16]. As explained earlier (Section 2), there are many impairments to the successful healing of DFUs, some of which can be stimulated using bioactive, immunomodulatory agents within dressing systems, including fiber-based scaffolds.
4.1. Growth Factors
Growth factors are polypeptides capable of stimulating a variety of target cells to grow, differentiate and even alter their metabolism. They can act by paracrine (targeting nearby cells) and autocrine (targeting itself) mechanisms, inducing a complex cascade of signaling pathways. All growth factors can influence various aspects of cell performance at once [43]. In DFUs, they have been shown to affect the migration of fibroblasts and keratinocytes and collagen expression and deposition. They have also been associated with the inhibition of pro-inflammatory cytokines and the stimulation of angiogenesis and granulation tissue formation. Growth factors also prevent apoptosis pathways frequently associated with the abnormalities that occur along the stages of wound healing in DFUs [44,45]. In nanofibrous constructs, the recombinant human epidermal growth factor was found to improve fibroblast proliferation in vitro, aside from significantly instigating wound closure and re-epithelization in an in vivo full-thickness wound model [46]. Additionally, epidermal growth factor-loaded patches were deemed effective in promoting cell migration, angiogenesis, and rapid diabetic wound healing [47].
4.2. Blood Components: Platelet-Rich Plasma
Activated platelets are known to release growth factors and other cues responsible for inducing proliferation, angiogenesis, tissue remodeling and even modulating the inflammatory response in chronic wounds. Platelet-rich plasma, also known as autologous conditioned plasma, is a concentrated substance made of the patient’s own platelets. Because of its high content of growth factors, sometimes five to 10 times greater than the usual blood composition, it can reactivate latent endogenous regeneration mechanisms [48]. Platelet-rich plasma has been most sought after in DFUs because of its clot-inducing abilities and anti-inflammatory profile. Most importantly, platelet-rich plasma has been shown to reduce the cytotoxicity of implantable scaffolds. Indeed, Meamar et al. demonstrated that gelatin nanofibrous scaffolds modified with mesenchymal stem cells and platelet-rich plasma significantly promote wound healing. Even though the enriched plasma had little effect in instigating the different biological pathways associated with each wound healing stage, it was seen to effectively improve the biocompatibility of the scaffold [49]. It should be, however, be pointed out that platelet-rich plasma still requires further research as it has been associated with negative impacts in wound healing, which have been explained by the lack of standardizing methods for preparation and the variability between donors (conditioned by the patient’s medical history) [50].
4.3. Natural Extracts
From an immunomodulatory perspective, plant extracts have been widely used in traditional medicinal formulations, as they possess many bioactive compounds and metabolites: alkaloids, carotenoids, glycosides, terpenes, and flavonoids. These elements have been linked to important phenomena associated with DFUs healing, including a decrease in glucose absorption, an increase in insulin production, stimulation of fibroblasts and keratinocytes migration, proliferation, and differentiation, enhanced collagen deposition and expression, and stimulation of anti-inflammatory and antioxidant events [19,51]. Moreover, bixin, an apocarotenoid found in the seeds of the achiote tree (Bixa orellana), loaded onto polycaprolactone nanofibers was also found to accelerate wound healing and reduce the scar tissue area [52]. In another study, curcumin incorporated within poly(lactic acid) and hyperbranched polyglycerol nanofibers demonstrated great ability in promoting cell viability, adhesion and proliferation, while also instigating cell migration [53]. Melilotus officinalis extract has also been explored in a triple-layer nanofibrous scaffold made of polycaprolactone and collagen and was shown to successfully regenerate tissues of the skin in diabetic ulcers by stimulating the angiogenesis, collagen production and deposition, and re-epithelialization [54].
4.4. Proteins: Collagen, Silk Fibroin and Sericin, and Keratin
Collagen is a natural fibrous protein commonly found in the connective tissues and in the skin extracellular matrix (ECM). It is a biocompatible, structural, biomimetic and low immunogenic protein that has been used as a biological component in many regenerative medicine scaffolding systems by instigating the formation of cell-level interactions with living tissues (fibrillogenesis) [55]. In DFUs, collagen has been shown to stimulate the attachment, proliferation, and migration of fibroblasts and to modulate granulation tissue formation [39,55]. However, because of its low mechanical and chemical stability, it is frequently combined with other polymers for producing wound dressings. Gao et al. proposed the combination of collagen with polycaprolactone and bioactive glass nanoparticles and determined that the scaffold could improve endothelial cell attachment and proliferation, and significantly enhance angiogenesis, granulation tissue formation, collagen matrix remodeling and epidermis differentiation [56]. Another collagen-containing polycaprolactone scaffold was found to improve water uptake and cell biocompatibility, reducing dressing change frequency [57]. In combination with silk fibroin and a poly(vinyl alcohol-based scaffold), collagen was also found to promote cell attachment, spreading and proliferation [58]. Silk fibroin is a fibrous protein secreted by Bombyx mori silk glands, which exhibits excellent mechanical performance, controllable biodegradability, improved hemostatic profile, great biocompatibility, and low antigenicity [59,60]. Most importantly, in DFUs, it can reduce inflammation and augment the expression of transforming growth factor-β signaling pathway and collagen during wound healing, as reported by Xu et al. [61]. Additionally, modified with insulin, silk fibroin dressings can also accelerate wound closure, collagen deposition and vascularization [62]. Sericin is another protein that can be extracted from Bombyx mori silk glands, however, at a lower amount than silk fibroin (80% versus 20% for sericin) [59]. Silk sericin is a globular protein with remarkable biodegradability, biocompatibility, antioxidant capacity, and regenerative abilities, including faster wound healing abilities, accelerated cell proliferation, and a low inflammatory profile. Silk sericin is less explored than silk fibroin; however, fiber-based scaffolding systems for DFU care have already been engineered [59,63]. Indeed, Gilotra et al. demonstrated the antioxidant potential of silk sericin without compromising cell viability and verified its ability to accelerate wound healing without triggering any inflammatory response [64].
Keratin is a fibrous protein with excellent mechanical features. It is abundant in the skin, hair, nails, claws, horns, feathers, and hooves of many animals. In humans, keratin, from the epidermal proteins family, works as an important skin barrier element due to its structural properties. Moreover, keratin contains cell adhesion sequences, such as the arginine-glycine-aspartic acid (RGD) or the leucine-aspartic acid-valine (LDV) motifs, which can support cell attachment, spreading and proliferation [65,66]. This is exactly what was determined via Yao et al.’s experiments. They concluded that by generating a double-layer keratin-loaded gelatin mesh they could accelerate cell attachment and proliferation above commercial dressing systems [67]. In addition, keratin has been shown to improve the biocompatibility of wound-healing scaffolds, while guaranteeing their structural stability for sustained drug delivery [68].
4.5. Neuropeptides
Neuropeptides consist of short-sequence amino acids that work to modulate synaptic activity or as primary neurotransmitters, activating the immune system and cell proliferation during the healing process. Neuropeptides such as calcitonin gene-related peptide, neuropeptide Y, neurotensin, and α-melanocorticotropin-releasing hormone have been identified as biomarkers in DFUs by promoting the expression of interferon-β, transforming growth factor-β, and the macrophage inflammatory protein-1α [45,69]. Zheng et al. even showed that polylactide-polyglycolide and cellulose nanocrystals fibrous meshes loaded with neurotensin are capable of inducing epidermal and dermal regeneration and increasing the ratios of the fibrotic area to the whole affected region. These neuropeptide-containing scaffolds were also seen to reduce the expression of the inflammatory cytokines interleukin-1β and interleukin-6 [70].
4.6. Stem Cells
Stem cells are the body’s raw matter, from which other cells and tissues with specialized functions can be generated. They can be subdivided into embryonic, derived from the inner cell mass of a blastocyst, and adult stem cells, which can be obtained from bone marrow, peripheral blood, and hair follicles [39,71]. In DFUs, stem cells can repopulate lost or injured areas with differentiated cells (i.e., keratinocytes, endothelial cells), aiding the self-renewal of tissues due to their multipotency, or promoting the recruitment of inflammatory cells through paracrine secretions (i.e., cytokines, chemokines, growth factors, and extracellular vesicles containing proteins, mRNA, microRNAs, and mitochondria) [72,73]. Indeed, stem cells have been shown to migrate into injured sites and actively work towards the elimination of infections and to promote tissue homeostasis, aside from enhancing angiogenesis, accelerating re-epithelialization, and instigating granulation tissue formation [73]. Stem cells have been mostly employed in full-thickness wound treatments, both as free, topically delivered agents and as part of scaffolding systems, including fiber-based constructs. Chen et al. demonstrated that by engineering a 3D scaffold consisting of radially or vertically aligned nanofibers modified with bone marrow mesenchymal stem cells. They generated a personalized platform, adaptable to all DFUs’ patient needs, that significantly enhanced angiogenesis and ECM deposition, promoted granulation tissue formation and, generally, elicited a pro-regenerative response from the wounded tissues [74]. Nanofibrous meshes containing human placenta-derived mesenchymal stem cells and platelet-rich plasma were also found to induce cell recruitment and proliferation to the injured area, and to stimulate wound closure, while reducing pain [49]. More recently, micropatterned fiber scaffolds prepared via the electrohydrodynamic cryo-printing method and bonded with adipose-derived mesenchymal stem cells were seen to improve the secretion of growth factors and chemokines, thus instigating fibroblast migration and vascular endothelial cell tube formation, along with collagen deposition and angiogenesis and reducing pro-inflammatory reactions [75].
4.7. Polymers: Alginate and Hyaluronic Acid
Alginate is an anionic polysaccharide made of b-L-guluronic acid and (1–4) related to a-D-mannuronic acid and extracted from brown seaweed. It is a biodegradable, biocompatible, non-toxic polymer known to promote oxygen permeability and hemostasis and to reduce odor and pain in wounds. Its most attractive features for DFU healing rely on its hemostat, gel-forming, highly exudate-absorbent abilities, which guarantee local moisture balance, stimulating re-epithelialization and granulation [51,76]. Additionally, alginate has been shown to decrease pro-inflammatory cytokines expression and inhibit free radicals’ formation via elastase binding, an enzyme that becomes highly expressed in chronic wound scenarios [8,77]. In DFUs, a three-layered nanofiber wound dressing has been shown to inhibit matrix metalloproteinase-2 effect via doxycycline, improve remodeling via collagen, and guarantee proper wettability, absorption capacity and bio-adhesion due to the combinatory effects of chitosan and alginate [78]. A similar effect was demonstrated by Anand et al.’s scaffolds made of polyvinyl alcohol (PVA), sodium alginate and silk fibroin, in which alginate was found to guarantee the wound site moisture control, with critical water retention for effective drug delivery and wound healing [79].
Hyaluronic acid is a hygroscopic glycosaminoglycan made of repeating polymeric disaccharides of D-glucuronic acid and N-acetyl-D-glucosamine linked through a glucuronidic β (1–3) bond. It is characterized by its biocompatibility, biodegradability, hydration and lubrication capacities, anti-adhesive and bioresorption features, and its viscoelasticity [51]. It is mostly found in the connective tissues of the ECM. Considering its abundance in the skin, accounting for 50% of the total body presence of hyaluronic acid, it is frequently employed in dressing systems to treat DFUs. Aside from its high-water uptake capacity which prevents wound dryness, hyaluronic acid is known to increase collagen secretion via fibroblast migration and proliferation and to activate inflammatory cells towards an enhanced immune response. It has also been shown to contribute to angiogenesis [80,81]. In a core-shell nanofiber scaffold made of polyethylene oxide, polycaprolactone, keratin and hyaluronic acid, this biopolymer was seen to guarantee great swelling capacity, fast degradation and increased cumulative drug release, aside from enhancing the scaffold biocompatibility in vitro [68]. Similar observations were made with core-shell nanofibrous structures made of polyurethane, starch and hyaluronic acid. This biopolymer wound healing impact was also confirmed in vivo, by contributing to accelerated cell repair [81].
5. Advanced Fibrous Scaffolds
Limitations in the ECM (cell defects, protein degradation, dysregulated enzyme activity, etc.), accumulation of devitalized/necrotic tissues and appearance of infections caused by polymicrobial wound bed invasion and colonization are the main characteristics of non-healing DFUs [8,82]. Advanced fibrous scaffolding systems may aid in restoring functions and replacing missing ECM and fighting pathogens. Ideally, these constructs should entail properties that closely mimic the naturally occurring elements that they are trying to resemble or pass for, provide physical and mechanical stability for quick integration, restoration of functions and protection from additional harm, and release chemical cues or bioactive agents that will actively work to protect the region against invaders and/or trigger events that will promote cell recruitment and the progression of the healing cascade (Figure 3) [83].
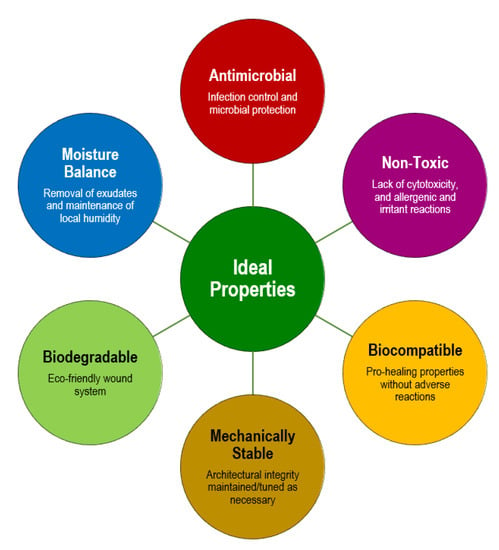
Figure 3.
Requirements for the formulation of a scaffolding system for applications in DFUs therapies.
Three-dimensional fibrous scaffolds, with a porous structure and small fiber diameters (mostly in the nanoscale) are gaining immense popularity and applicability in DFU treatments. Their similarity to the ECM structure, particularly those scaffolding systems produced with a random fiber arrangement, can closely mimic the fibrous entangled organization of the ECM proteins, offering many advantages for cell recruitment, attachment, and proliferation. Among the many techniques used to produce fiber-based scaffolds for DFU therapies, the electrospinning approach is the most employed. This technique allows the control of several parameters during mesh production, namely porosity, pore size, fiber diameter, alignment, etc., and is cost-effective, versatile, and easy to process. Most importantly, it allows for the production of fibers on the nanometer scale, whose arrangement guarantees effective gas permeation (oxygen transfer), and displays a large surface area for effective binding with bioactive agents or cells (depending on the intended functions) or for the sustained liberation of agents pre-loaded into the polymeric solution prior to nanofiber mesh production [8,84]. As seen in Table 1, electrospun nanofiber scaffolds of single and multiple layers have an impactful place in therapeutic formulations for DFU care. They can be modified with a variety of bioactive agents for inducing antimicrobial and/or immunomodulatory effects. Many reports have exposed formulations with natural-origin polymers (i.e., chitosan, gelatin, sodium alginate, cellulose, etc.), synthetic polymers (i.e., polycaprolactone, poly(vinyl alcohol), polylactic-co-glycolic acid, poly(lactic acid), etc.), or combinations of both. Extensive revision work has been conducted on the properties, characteristics, and benefits of using each of these polymers [85,86,87,88]. Additionally, other techniques such as aqueous phase fiber reassembly [57] and cryoprinting [75] may also be used in the production of 3D fibrous scaffolding systems with great biological performance. Textile knitting is another very common technique for bandage production applied to DFUs care [29,89], either as a main (Table 1 only reports studies in which knitted fiber constructs are the main therapy) or a co-adjuvant, protective therapy.

Table 1.
Examples of fiber-based systems containing bioactive components of different origins with potential for applications in DFU care and detailed analyses of their biological contributions (antimicrobial and immunomodulatory). Data report work published only within the last five years (2017–2022).
6. Conclusions
DFUs are very complex chronic wounds, demanding active strategies to manage and treat the affected regions. Polymers processed in the form of fibers and modified with bioactive agents have been proven to have great efficiency in generating environments conducive to wound healing, with exceptional outcomes both in vitro and in vivo. Among the many technical approaches available for fiber-based scaffolding systems production for DFUs care, electrospinning has taken the lead, gaining much interest for its ability in generating scaffolds with improved porosity and air and water-vapor permeabilities essential for cell growth, attachment and proliferation; high water intake and hydration capacity which guarantees local moisture balance; good mechanical stability and tunable flexibility for appropriate adaptation to the injured site, offering protection, and tunable fiber size diameters (micro to nanoscale), alignment (random, aligned) and chemical composition so resemblances can be drawn to the skin ECM and, hence, facilitate recognition and integration of the scaffolding system. Most importantly, these structures allow the integration of bioactive compounds or chemical cues that actively intervene in different stages of the healing of these diabetic wounds, accelerating injury recovery by promoting vascularization, collagen deposition, and the restoration of physiological functions. Both antimicrobial and immunomodulatory agents have been shown to play decisive roles in DFU care, actively contributing to the healing cascade. In fact, many of the formulations that combine these bioactive agents and fiber constructs described in this work have already been tested against commercial products demonstrating improved efficacy. Considering the Nanospider™ technology is the only available option for electrospinning these nanofibrous systems at an industrial scale, investments should be conducted to optimize these approaches, either by approximating laboratory-scale testing to the available technology or by improving on the pre-existent technology, augmenting the features available for processing. Nowadays, much investment is also being conducted in seamless technology for bandage production, 3D printing for fiber patterned constructs, or even on wet-spun systems [103]. This continued research for new, faster healing and more target-directed dressings is essential for efficient treatment strategies, adaptable for each situation and patient, and plays major roles in the physical and psychological states of patients dealing with DFUs.
Funding
This research was funded by the Portuguese Foundation for Science and Technology (FCT) via grant UIDP/00264/2020 of 2C2T Strategic Project 2020–2023. H.P.F. also acknowledges FCT for contract 2021.02720.CEECIND.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Medeiros, S.; Rodrigues, A.; Costa, R. Physiotherapeutic interventions in the treatment of patients with diabetic foot ulcers: A systematic literature review. Physiotherapy, 2022; in press. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Wang, Z.; Bancks, M.P.; Carnethon, M.R.; Greenland, P.; Feng, Y.-Q.; Wang, H.; Zhong, V.W. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999–2018. JAMA 2021, 326, 704–716. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers and their recurrence. New Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Peter-Riesch, B. The diabetic foot: The never-ending challenge. Nov. Diabetes 2016, 31, 108–134. [Google Scholar]
- Mbela Lusendi, F.; Matricali, G.A.; Vanherwegen, A.-S.; Doggen, K.; Nobels, F. Bottom-up approach to build a ‘precision’ risk factor classification for diabetic foot ulcer healing. Proof-of-concept. Diabetes Res. Clin. Pract. 2022, 191, 110028. [Google Scholar] [CrossRef] [PubMed]
- Glover, K.; Stratakos, A.C.; Varadi, A.; Lamprou, D.A. 3D scaffolds in the treatment of diabetic foot ulcers: New trends vs. conventional approaches. Int. J. Pharm. 2021, 599, 120423. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf. B Biointerfaces 2017, 156, 133–148. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, N.; Martínez-Jiménez, I.; García-Ojalvo, A.; Mendoza-Mari, Y.; Guillén-Nieto, G.; Armstrong, D.G.; Berlanga-Acosta, J. Wound chronicity, impaired immunity and infection in diabetic patients. MEDICC Rev. 2022, 24, 44–58. [Google Scholar] [CrossRef]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential applications of nanomaterials and technology for diabetic wound healing. Int. J. Nanomed. 2020, 15, 9717. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.-B.; Zheng, X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Hagde, P.; Pingle, P.; Mourya, A.; Katta, C.B.; Srivastava, S.; Sharma, R.; Singh, K.K.; Sodhi, R.K.; Madan, J. Therapeutic potential of quercetin in diabetic foot ulcer: Mechanistic insight, challenges, nanotechnology driven strategies and future prospects. J. Drug Deliv. Sci. Technol. 2022, 74, 103575. [Google Scholar] [CrossRef]
- Song, J.; Liu, A.; Liu, B.; Huang, W.; Jiang, Z.; Bai, X.; Hu, L.; Zheng, S.; Guo, S.; Wu, J. Natural Biologics Accelerate Healing of Diabetic Foot Ulcers by Regulating Oxidative Stress. Front. Biosci.-Landmark 2022, 27, 285. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Park, J.-U.; Oh, B.; Lee, J.P.; Choi, M.-H.; Lee, M.-J.; Kim, B.-S. Influence of Microbiota on Diabetic Foot Wound in Comparison with Adjacent Normal Skin Based on the Clinical Features. BioMed Res. Int. 2019, 2019, 7459236. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.L.; Sotto, A.; Lavigne, J.P. New insights in diabetic foot infection. World J. Diabetes 2011, 2, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Soares, M.; Boyko, E.J.; Jeffcoate, W.; Mills, J.L.; Russell, D.; Morbach, S.; Game, F. Diabetic foot ulcer classifications: A critical review. Diabetes/Metab. Res. Rev. 2020, 36, e3272. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M.; Foster, A. The use of antibiotics in the diabetic foot. Am. J. Surg. 2004, 187, S25–S28. [Google Scholar] [CrossRef]
- Felgueiras, H.P. An insight into biomolecules for the treatment of skin infectious diseases. Pharmaceutics 2021, 13, 1012. [Google Scholar] [CrossRef]
- Baquero, F.; Levin, B.R. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat. Rev. Microbiol. 2021, 19, 123–132. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater. Sci. Eng. C 2019, 104, 109745. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Liu, K.-S.; Cheng, C.-W.; Chan, E.-C.; Hung, K.-C.; Hsieh, M.-J.; Chang, S.-H.; Fu, X.; Juang, J.-H.; Hsieh, I.C.; et al. Codelivery of Sustainable Antimicrobial Agents and Platelet-Derived Growth Factor via Biodegradable Nanofibers for Repair of Diabetic Infectious Wounds. ACS Infect. Dis. 2020, 6, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Davani, F.; Alishahi, M.; Sabzi, M.; Khorram, M.; Arastehfar, A.; Zomorodian, K. Dual drug delivery of vancomycin and imipenem/cilastatin by coaxial nanofibers for treatment of diabetic foot ulcer infections. Mater. Sci. Eng. C 2021, 123, 111975. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, F.; Matsumoto, Y.; Ishii, M.; Tatsuno, K.; Okazaki, M.; Sato, T.; Moriya, K.; Sekimizu, K. Synergistic effects of vancomycin and β-lactams against vancomycin highly resistant Staphylococcus aureus. J. Antibiot. 2017, 70, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Igor Otavio, M.; Cristine Vanz, B.; Maria Izabela, F.; Hector Alonzo Gomez, G.; Chung-Yen Oliver, C.; Giuseppina Pace Pereira, L. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds; Marcos, S.-H., Mariana, P.-T., Maria del Rosario, G.-M., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- El-Ghoul, Y.; Alminderej, F.M. Bioactive and superabsorbent cellulosic dressing grafted alginate and Carthamus tinctorius polysaccharide extract for the treatment of chronic wounds. Text. Res. J. 2021, 91, 235–248. [Google Scholar] [CrossRef]
- Ramalingam, R.; Dhand, C.; Leung, C.M.; Ong, S.T.; Annamalai, S.K.; Kamruddin, M.; Verma, N.K.; Ramakrishna, S.; Lakshminarayanan, R.; Arunachalam, K.D. Antimicrobial properties and biocompatibility of electrospun poly-ε-caprolactone fibrous mats containing Gymnema sylvestre leaf extract. Mater. Sci. Eng. C 2019, 98, 503–514. [Google Scholar] [CrossRef]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Ardekani, M.R.S.; Sharifzadeh, M.; Khanavi, M. Polyurethane/carboxymethylcellulose nanofibers containing Malva sylvestris extract for healing diabetic wounds: Preparation, characterization, in vitro and in vivo studies. Mater. Sci. Eng. C 2020, 114, 111039. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Wang, X.; Chang, J.; Wu, C. Bioactive inorganic/organic nanocomposites for wound healing. Appl. Mater. Today 2018, 11, 308–319. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Noorunnisa Khanam, P.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ghiyasi, Y.; Salahi, E.; Esfahani, H. Synergy effect of Urtica dioica and ZnO NPs on microstructure, antibacterial activity and cytotoxicity of electrospun PCL scaffold for wound dressing application. Mater. Today Commun. 2021, 26, 102163. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [PubMed]
- Steffy, K.; Shanthi, G.; Maroky, A.S.; Selvakumar, S. Enhanced antibacterial effects of green synthesized ZnO NPs using Aristolochia indica against Multi-drug resistant bacterial pathogens from Diabetic Foot Ulcer. J. Infect. Public Health 2018, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Jeckson, T.A.; Neo, Y.P.; Sisinthy, S.P.; Foo, J.B.; Choudhury, H.; Gorain, B. Formulation and characterisation of deferoxamine nanofiber as potential wound dressing for the treatment of diabetic foot ulcer. J. Drug Deliv. Sci. Technol. 2021, 66, 102751. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Liu, Y.; Li, Y.; Zhang, C.; Qi, W.; Yeung, K.W.K.; Wong, T.M.; Zhao, X.; Pan, H. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater. Sci. Eng. C 2019, 105, 110083. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, X.; Jia, Y.; Zhang, H.; Tang, T. Hydroxypropyltrimethyl Ammonium Chloride Chitosan Functionalized-PLGA Electrospun Fibrous Membranes as Antibacterial Wound Dressing: In Vitro and In Vivo Evaluation. Polymers 2017, 9, 697. [Google Scholar] [CrossRef]
- Bennett, S.; Griffiths, G.; Schor, A.; Leese, G.; Schor, S. Growth factors in the treatment of diabetic foot ulcers. J. Br. Surg. 2003, 90, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Sondak, T.; Sivashanmugam, K.; Kim, K.-S. A Review of Immunomodulatory Reprogramming by Probiotics in Combating Chronic and Acute Diabetic Foot Ulcers (DFUs). Pharmaceutics 2022, 14, 2436. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Dalvi, Y.B.; Rehman, S.R.U.; Varghese, R.; Unni, R.N.; Yalcin, H.C.; Alfkey, R.; Thomas, S.; Al Moustafa, A.-E. Growth factor loaded in situ photocrosslinkable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater. Sci. Eng. C 2021, 118, 111519. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Nardini, M.; Collina, M.C.; Di Campli, C.; Filaci, G.; Cancedda, R.; Odorisio, T. Innovative Cell and Platelet Rich Plasma Therapies for Diabetic Foot Ulcer Treatment: The Allogeneic Approach. Front. Bioeng. Biotechnol. 2022, 10, 869408. [Google Scholar] [CrossRef]
- Meamar, R.; Ghasemi-Mobarakeh, L.; Norouzi, M.-R.; Siavash, M.; Hamblin, M.R.; Fesharaki, M. Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: A randomized clinical trial. Int. Immunopharmacol. 2021, 101, 108282. [Google Scholar] [CrossRef]
- Weibrich, G.; Kleis, W.K.; Hafner, G.; Hitzler, W.E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2002, 30, 97–102. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing—Know-how. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef]
- Pinzón-García, A.D.; Cassini-Vieira, P.; Ribeiro, C.C.; de Matos Jensen, C.E.; Barcelos, L.S.; Cortes, M.E.; Sinisterra, R.D. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J. Biomed. Mater. Research. Part B Appl. Biomater. 2017, 105, 1938–1949. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Maya Nandkumar, A.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA—Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, M.A.; Nazeri, N.; Khoshnevisan, K.; Heshmat, R.; Omidfar, K. Three-layered PCL-collagen nanofibers containing Melilotus officinalis extract for diabetic ulcer healing in a rat model. J. Diabetes Metab. Disord. 2022, 21, 313–321. [Google Scholar] [CrossRef]
- Naomi, R.; Fauzi, M.B. Cellulose/Collagen Dressings for Diabetic Foot Ulcer: A Review. Pharmaceutics 2020, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Jin, W.; Li, Y.; Wan, L.; Wang, C.; Lin, C.; Chen, X.; Lei, B.; Mao, C. A highly bioactive bone extracellular matrix-biomimetic nanofibrous system with rapid angiogenesis promotes diabetic wound healing. J. Mater. Chem. B 2017, 5, 7285–7296. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Yin, H.; Yu, X.; Wang, L.; Fan, L.; Xin, J.H.; Yu, H. 3D PCL/collagen nanofibrous medical dressing for one-time treatment of diabetic foot ulcers. Colloids Surf. B Biointerfaces 2022, 214, 112480. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, S.K.; Nazir, L.S.; Thangam, R.; Perumal, R.K.; Manjubala, I.; Madhan, B.; Seetharaman, S. Type I collagen peptides and nitric oxide releasing electrospun silk fibroin scaffold: A multifunctional approach for the treatment of ischemic chronic wounds. Colloids Surf. B Biointerfaces 2019, 175, 636–643. [Google Scholar] [CrossRef]
- Tariq, M.; Tahir, H.M.; Butt, S.A.; Ali, S.; Ahmad, A.B.; Raza, C.; Summer, M.; Hassan, A.; Nadeem, J. Silk derived formulations for accelerated wound healing in diabetic mice. PeerJ 2021, 9, e10232. [Google Scholar] [CrossRef]
- Liu, J.; Yan, L.; Yang, W.; Lan, Y.; Zhu, Q.; Xu, H.; Zheng, C.; Guo, R. Controlled-release neurotensin-loaded silk fibroin dressings improve wound healing in diabetic rat model. Bioact. Mater. 2019, 4, 151–159. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Qin, C.; Khan, A.u.R.; Zhang, W.; Mo, X. Silk fibroin/poly-(L-lactide-co-caprolactone) nanofiber scaffolds loaded with Huangbai Liniment to accelerate diabetic wound healing. Colloids Surf. B Biointerfaces 2021, 199, 111557. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Zhang, J.; You, R.; Qu, J.; Li, M. Functionalized silk fibroin dressing with topical bioactive insulin release for accelerated chronic wound healing. Mater. Sci. Eng. C 2017, 72, 394–404. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Bernardes, B.G.; Borges, S.; Rodrigues, I.; Fernandes, R.; Gomes-Guerreiro, S.; Pinto, M.T.; Pintado, M.; Soares, R.; Costa, R. Exploring Silk Sericin for Diabetic Wounds: An In Situ-Forming Hydrogel to Protect against Oxidative Stress and Improve Tissue Healing and Regeneration. Biomolecules 2022, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Gilotra, S.; Chouhan, D.; Bhardwaj, N.; Nandi, S.K.; Mandal, B.B. Potential of silk sericin based nanofibrous mats for wound dressing applications. Mater. Sci. Eng. C 2018, 90, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Verma, V.; Verma, P.; Ray, P.; Ray, A.R. Preparation of scaffolds from human hair proteins for tissue-engineering applications. Biomed. Mater. 2008, 3, 025007. [Google Scholar] [CrossRef]
- Yao, C.-H.; Lee, C.-Y.; Huang, C.-H.; Chen, Y.-S.; Chen, K.-Y. Novel bilayer wound dressing based on electrospun gelatin/keratin nanofibrous mats for skin wound repair. Mater. Sci. Eng. C 2017, 79, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Bedir, T.; Kalkandelen, C.; Sasmazel, H.T.; Basar, A.O.; Chen, J.; Ekren, N.; Gunduz, O. A drug-eluting nanofibrous hyaluronic acid-keratin mat for diabetic wound dressing. Emergent Mater. 2022, 5, 1617–1627. [Google Scholar] [CrossRef]
- Li, C.; Kim, K. Neuropeptides. In WormBook: The Online Review of C. elegans Biology [Internet]; WormBook: Pasadenak, CA, USA, 2018. [Google Scholar]
- Zheng, Z.; Liu, Y.; Huang, W.; Mo, Y.; Lan, Y.; Guo, R.; Cheng, B. Neurotensin-loaded PLGA/CNC composite nanofiber membranes accelerate diabetic wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46, 493–501. [Google Scholar] [CrossRef]
- Lopes, L.; Setia, O.; Aurshina, A.; Liu, S.; Hu, H.; Isaji, T.; Liu, H.; Wang, T.; Ono, S.; Guo, X. Stem cell therapy for diabetic foot ulcers: A review of preclinical and clinical research. Stem Cell Res. Ther. 2018, 9, 188. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Gou, M.; Da, L.-C.; Zhang, W.-Q.; Xie, H.-Q. Mesenchymal stem cells for chronic wound healing: Current status of preclinical and clinical studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- An, T.; Chen, Y.; Tu, Y.; Lin, P. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in the Treatment of Diabetic Foot Ulcers: Application and Challenges. Stem Cell Rev. Rep. 2021, 17, 369–378. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Su, Y.; John, J.V.; McCarthy, A.; Wong, S.L.; Xie, J. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater. 2020, 108, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, J.; Wang, J.; Xu, M.; Jiao, J.; Qiang, Y.; Zhang, F.; Li, Z. Rock Climbing-Inspired Electrohydrodynamic Cryoprinting of Micropatterned Porous Fiber Scaffolds with Improved MSC Therapy for Wound Healing. Adv. Fiber Mater. 2022, 1–15. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Alginates: Wound dressings. Encycl. Biomed. Polym. Polym. Biomater 2015, 2014, 134–146. [Google Scholar]
- Saisuwan, R. Cellulose-Based Biosensors of Human Neutrophil Elastase (HNE) toward Chronic Wound Point-of-Care Diagnostics. Doctoral Dissertation, University of British Columbia, Vancouver, BC, Canada, 2020. [Google Scholar]
- Tort, S.; Acartürk, F.; Beşikci, A. Evaluation of three-layered doxycycline-collagen loaded nanofiber wound dressing. Int. J. Pharm. 2017, 529, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rajinikanth, P.S.; Arya, D.K.; Pandey, P.; Gupta, R.K.; Sankhwar, R.; Chidambaram, K. Multifunctional Biomimetic Nanofibrous Scaffold Loaded with Asiaticoside for Rapid Diabetic Wound Healing. Pharmaceutics 2022, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm.-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef]
- Chen, H.; Peng, Y.; Wu, S.; Tan, L.P. Electrospun 3D Fibrous Scaffolds for Chronic Wound Repair. Materials 2016, 9, 272. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, H.P. Electrospun Nanocomposites Containing Cellulose and Its Derivatives Modified with Specialized Biomolecules for an Enhanced Wound Healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Potential applications of natural origin polymer-based systems in soft tissue regeneration. Crit. Rev. Biotechnol. 2010, 30, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Govindharajan, T. Study of hydrocellular functional material as microbicidal wound dressing for diabetic wound healing. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211054930. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Kumar, P.; Choonara, Y.E.; Du Toit, L.C.; Pillay, V. Artificial, Triple-Layered, Nanomembranous Wound Patch for Potential Diabetic Foot Ulcer Intervention. Materials 2018, 11, 2128. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hung, K.-C.; Hsieh, M.-J.; Chang, S.-H.; Juang, J.-H.; Hsieh, I.C.; Wen, M.-S.; Liu, S.-J. Core-shell insulin-loaded nanofibrous scaffolds for repairing diabetic wounds. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102123. [Google Scholar] [CrossRef]
- Lv, F.; Wang, J.; Xu, P.; Han, Y.; Ma, H.; Xu, H.; Chen, S.; Chang, J.; Ke, Q.; Liu, M.; et al. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater. 2017, 60, 128–143. [Google Scholar] [CrossRef]
- Grip, J.; Engstad, R.E.; Skjæveland, I.; Škalko-Basnet, N.; Isaksson, J.; Basnet, P.; Holsæter, A.M. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur. J. Pharm. Sci. 2018, 121, 269–280. [Google Scholar] [CrossRef]
- Ren, X.; Han, Y.; Wang, J.; Jiang, Y.; Yi, Z.; Xu, H.; Ke, Q. An aligned porous electrospun fibrous membrane with controlled drug delivery—An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018, 70, 140–153. [Google Scholar] [CrossRef]
- Aduba, D.C.; An, S.-S.; Selders, G.S.; Yeudall, W.A.; Bowlin, G.L.; Kitten, T.; Yang, H. Electrospun gelatin–arabinoxylan ferulate composite fibers for diabetic chronic wound dressing application. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 660–668. [Google Scholar] [CrossRef]
- Cam, M.E.; Crabbe-Mann, M.; Alenezi, H.; Hazar-Yavuz, A.N.; Ertas, B.; Ekentok, C.; Ozcan, G.S.; Topal, F.; Guler, E.; Yazir, Y.; et al. The comparision of glybenclamide and metformin-loaded bacterial cellulose/gelatin nanofibres produced by a portable electrohydrodynamic gun for diabetic wound healing. Eur. Polym. J. 2020, 134, 109844. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, X.; Pang, L.; Liu, Y.; Lin, Y.; Xiang, T.; Li, J.; Liao, S.; Jiang, Y. Astragalus Polysaccharides/PVA Nanofiber Membranes Containing Astragaloside IV-Loaded Liposomes and Their Potential Use for Wound Healing. Evid.-Based Complement. Altern. Med. 2022, 2022, 9716271. [Google Scholar] [CrossRef] [PubMed]
- Losi, P.; Al Kayal, T.; Buscemi, M.; Foffa, I.; Cavallo, A.; Soldani, G. Bilayered Fibrin-Based Electrospun-Sprayed Scaffold Loaded with Platelet Lysate Enhances Wound Healing in a Diabetic Mouse Model. Nanomaterials 2020, 10, 2128. [Google Scholar] [CrossRef]
- Ilomuanya, M.O.; Okafor, P.S.; Amajuoyi, J.N.; Onyejekwe, J.C.; Okubanjo, O.O.; Adeosun, S.O.; Silva, B.O. Polylactic acid-based electrospun fiber and hyaluronic acid-valsartan hydrogel scaffold for chronic wound healing. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 31. [Google Scholar] [CrossRef]
- Ghalei, S.; Nourmohammadi, J.; Solouk, A.; Mirzadeh, H. Enhanced cellular response elicited by addition of amniotic fluid to alginate hydrogel-electrospun silk fibroin fibers for potential wound dressing application. Colloids Surf. B: Biointerfaces 2018, 172, 82–89. [Google Scholar] [CrossRef]
- Blanchette, V.; Belosinschi, D.; Lai, T.T.; Cloutier, L.; Barnabé, S. New Antibacterial Paper Made of Silver Phosphate Cellulose Fibers: A Preliminary Study on the Elimination of Staphylococcus aureus Involved in Diabetic Foot Ulceration. BioMed Res. Int. 2020, 2020, 1304016. [Google Scholar] [CrossRef]
- Cam, M.E.; Yildiz, S.; Alenezi, H.; Cesur, S.; Ozcan, G.S.; Erdemir, G.; Edirisinghe, U.; Akakin, D.; Kuruca, D.S.; Kabasakal, L.; et al. Evaluation of burst release and sustained release of pioglitazone-loaded fibrous mats on diabetic wound healing: An in vitro and in vivo comparison study. J. R. Soc. Interface 2020, 17, 20190712. [Google Scholar] [CrossRef]
- Miranda, C.S.; Silva, A.F.G.; Pereira-Lima, S.M.; Costa, S.P.; Homem, N.C.; Felgueiras, H.P. Tunable spun fiber constructs in biomedicine: Influence of processing parameters in the fibers’ architecture. Pharmaceutics 2022, 14, 164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).