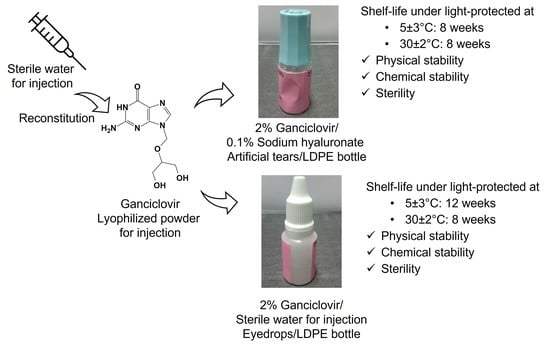
Extemporaneous Preparation of 20 mg/mL Ganciclovir in Artificial Tears in Comparison with Sterile Water for Ophthalmic Administration: Formulation and Stability Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Analysis and Validation
2.3. Compatibility Test between Ganciclovir and Artificial Tears
2.4. Extemporaneous Preparation of 20 mg/mL Ganciclovir Eye Drops (EDs)
2.4.1. Ganciclovir/HYA0.1 ED
2.4.2. Ganciclovir/HYA0.3 ED
2.4.3. Ganciclovir/SWI ED
2.5. Physicochemical Evaluation of Extemporaneous 20 mg/mL Ganciclovir EDs
2.5.1. Physical Properties
2.5.2. Drug Content Assay
2.6. Stability Testing of Extemporaneous 20 mg/mL Ganciclovir EDs
2.6.1. Physical Stability Evaluation
2.6.2. Chemical Stability Evaluation
2.6.3. Microbiological Stability Evaluation
2.7. Data Analysis
3. Results and Discussion
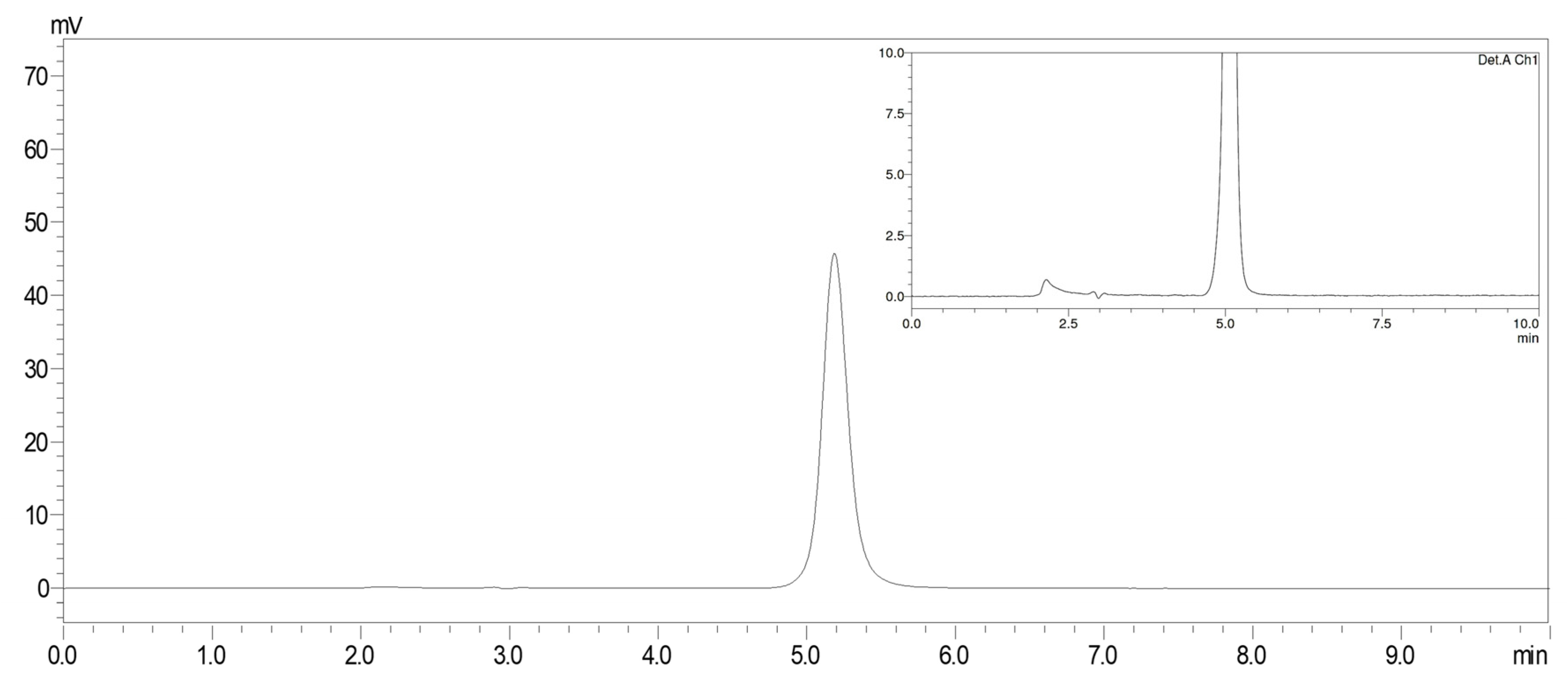
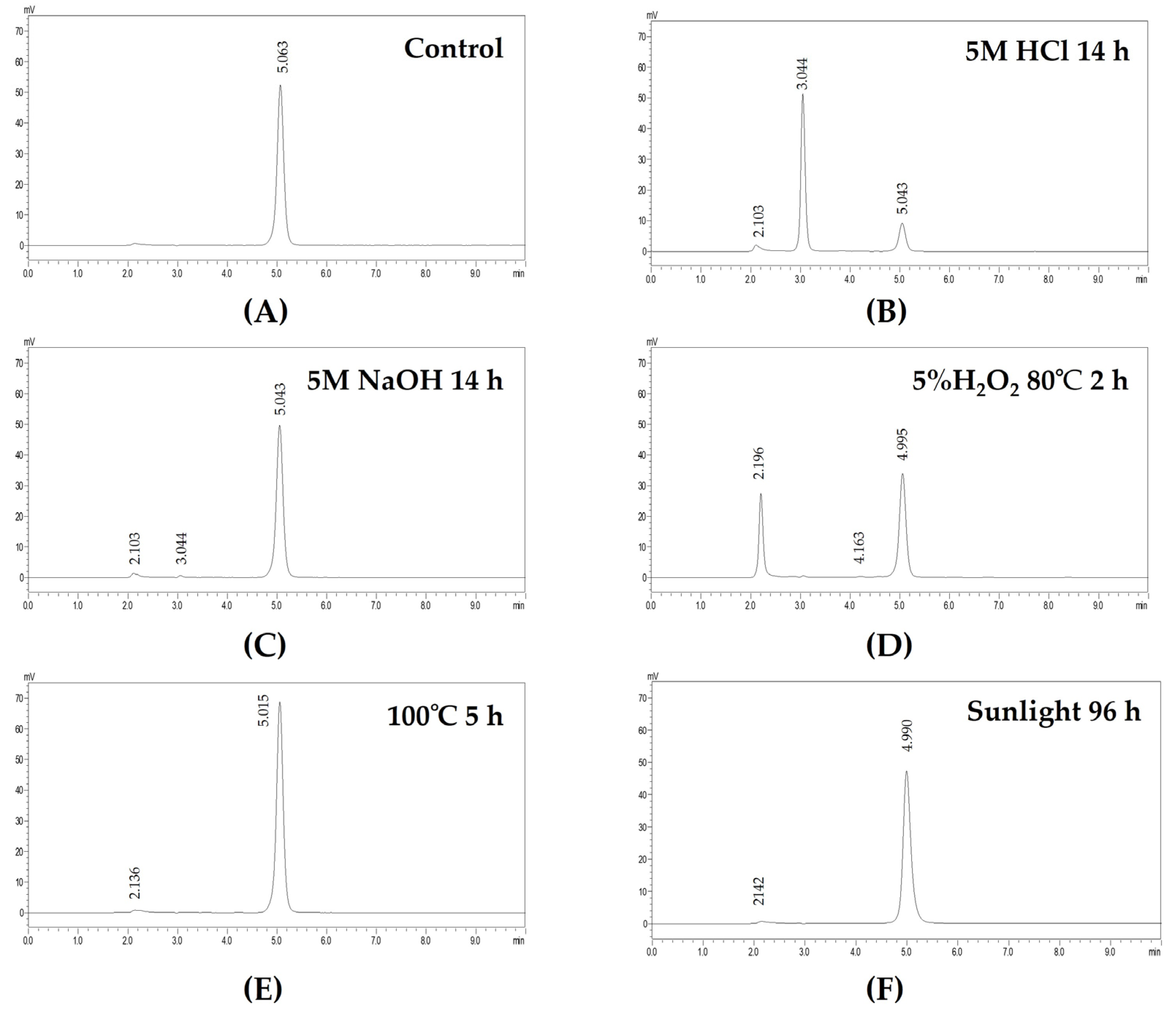
3.1. HPLC Analysis and Validation
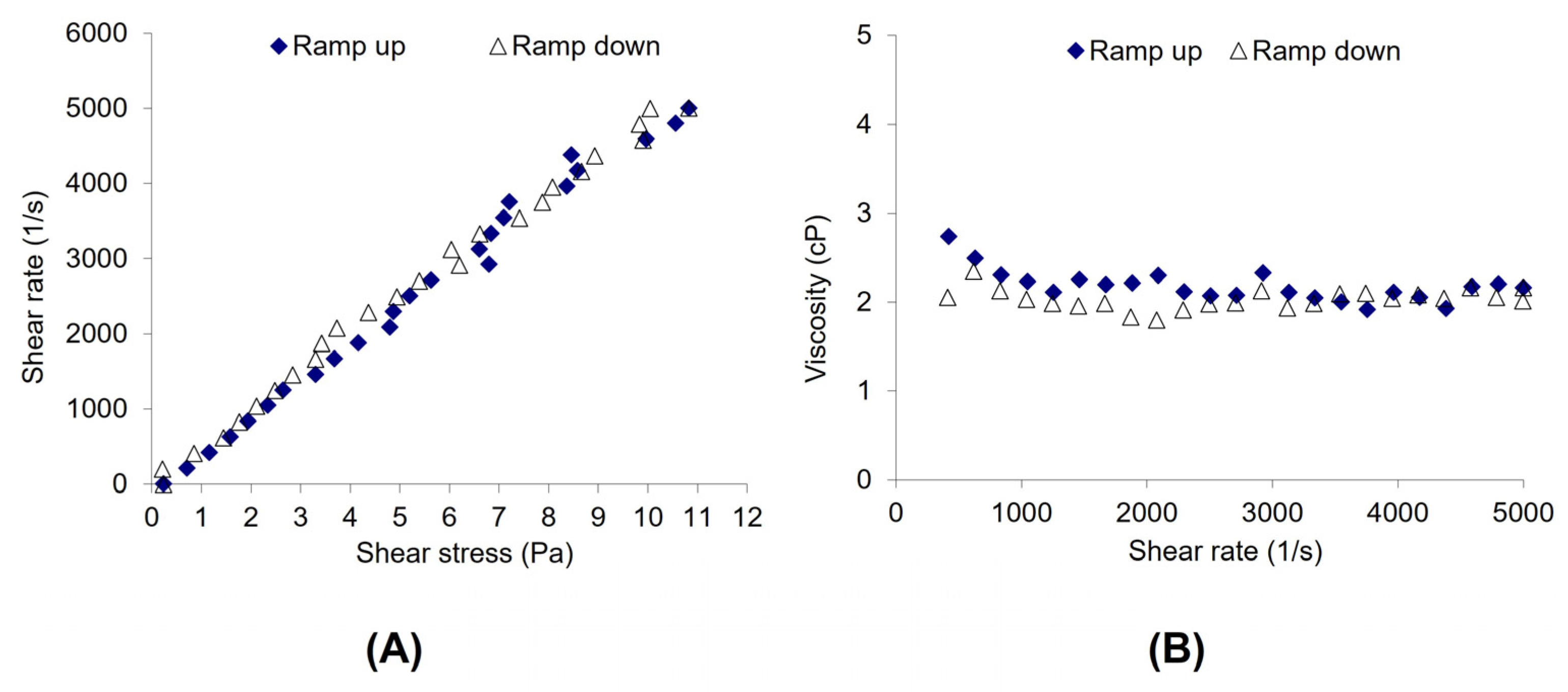
3.2. Compatibility of Ganciclovir and Artificial Tears
3.3. Extemporaneous Preparation of 20 mg/mL Ganciclovir/HYA0.1 and SWI EDs
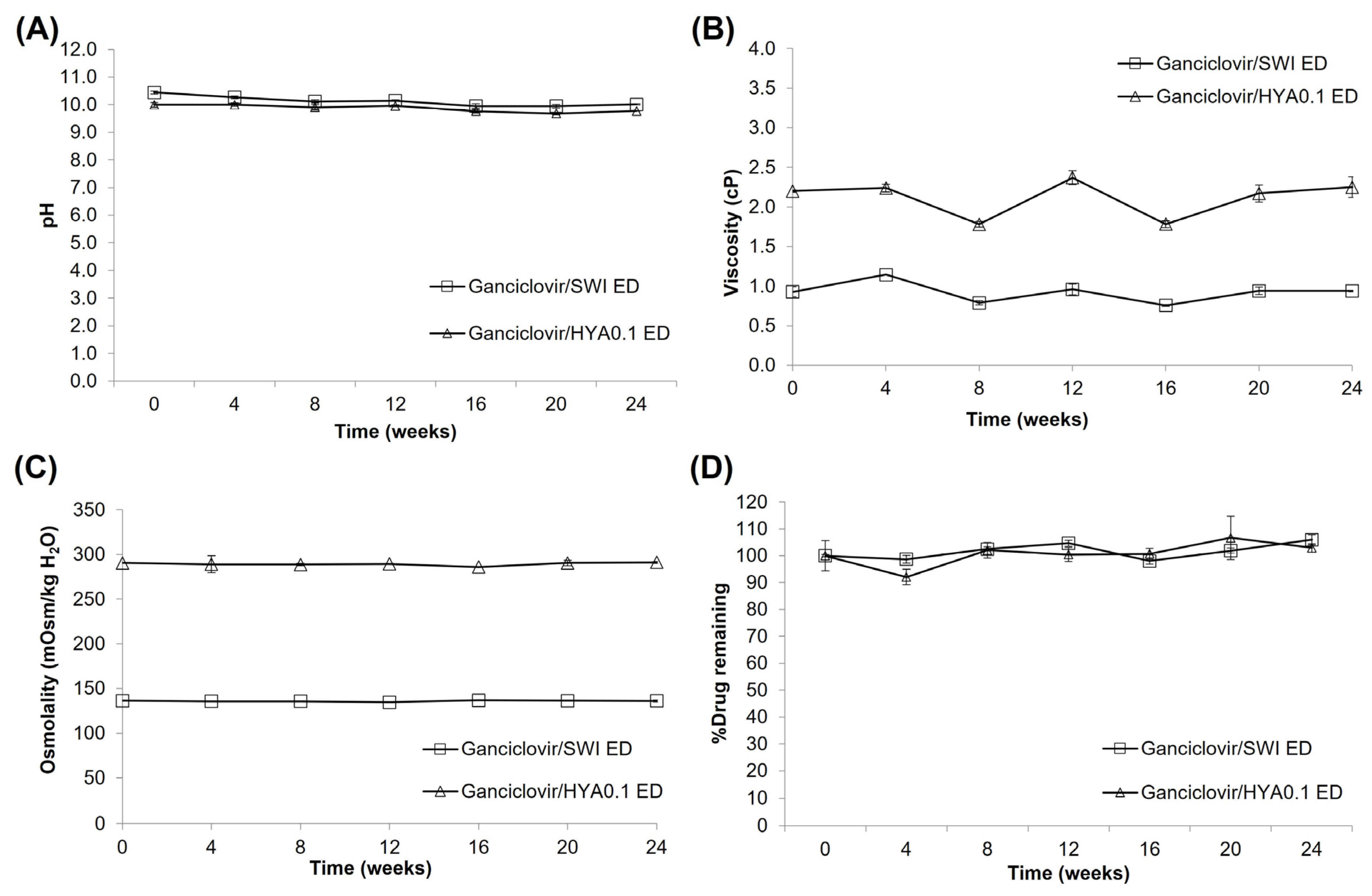
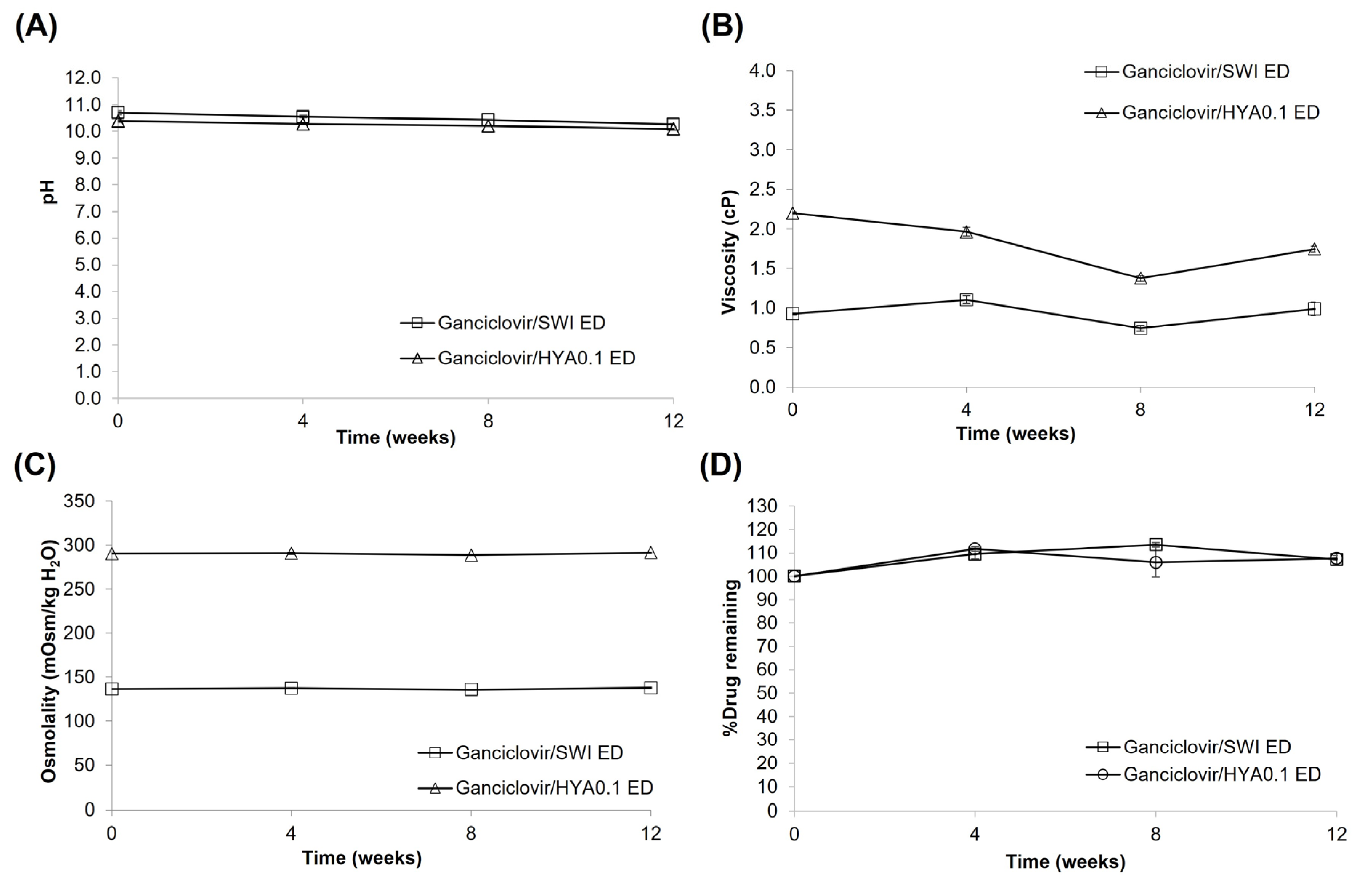
3.4. Stability Study
3.5. Preparation and Stability Study of Ganciclovir/HYA0.3 ED
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miyazaki, D.; Shimizu, D.; Shimizu, Y.; Inoue, Y.; Inoue, T.; Higaki, S.; Ueta, M.; Sugita, S.; Miyazaki, D.; Shimizu, D.; et al. Diagnostic efficacy of real-time PCR for ocular cytomegalovirus infections. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 2413–2420. [Google Scholar] [CrossRef]
- Koizumi, N.; Miyazaki, D.; Inoue, T.; Ohtani, F.; Kandori-Inoue, M.; Inatomi, T.; Sotozono, C.; Nakagawa, H.; Horikiri, T.; Ueta, M.; et al. The effect of topical application of 0.15% ganciclovir gel on cytomegalovirus corneal endotheliitis. Br. J. Ophthalmol. 2017, 101, 114–119. [Google Scholar] [CrossRef]
- Koizumi, N.; Inatomi, T.; Suzuki, T.; Shiraishi, A.; Ohashi, Y.; Kandori, M.; Miyazaki, D.; Inoue, Y.; Soma, T.; Nishida, K.; et al. Clinical features and management of cytomegalovirus corneal endotheliitis: Analysis of 106 cases from the Japan corneal endotheliitis study. Br. J. Ophthalmol. 2015, 99, 54–58. [Google Scholar] [CrossRef]
- Chee, S.-P.; Bacsal, K.; Jap, A.; Se-Thoe, S.-Y.; Cheng, C.L.; Tan, B.H. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am. J. Ophthalmol. 2008, 145, 834–840. [Google Scholar] [CrossRef]
- Koizumi, N.; Suzuki, T.; Uno, T.; Chihara, H.; Shiraishi, A.; Hara, Y.; Inatomi, T.; Sotozono, C.; Kawasaki, S.; Yamasaki, K.; et al. Cytomegalovirus as an etiologic factor in corneal endotheliitis. Ophthalmology 2008, 115, 292–297.e293. [Google Scholar] [CrossRef]
- Faith, S.C.; Durrani, A.F.; Jhanji, V. Cytomegalovirus keratitis. Curr. Opin. Ophthalmol. 2018, 29, 373–377. [Google Scholar] [CrossRef]
- Hwang, Y.-S.; Lin, K.-K.; Lee, J.-S.; Chang, S.H.L.; Chen, K.-J.; Lai, C.-C.; Huang, J.C.-C.; Kuo, Y.-H.; Hsiao, C.-H. Intravitreal loading injection of ganciclovir with or without adjunctive oral valganciclovir for cytomegalovirus anterior uveitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 263–269. [Google Scholar] [CrossRef]
- Villarreal, E.C. Current and potential therapies for the treatment of herpesvirus infections. In Progress in Drug Research; Wu, H., Lien, E.J., Lien, L.L., Schultz, R.M., Ram, V.J., Domingo, E., Spence, P., Gupta, S.P., Bhat, S.P., Villarreal, E.C., et al., Eds.; Birkhäuser Basel Verlag GmbH: Basel, Switzerland, 2003; pp. 263–307. [Google Scholar]
- de Schryver, I.; Rozenberg, F.; Cassoux, N.; Michelson, S.; Kestelyn, P.; LeHoang, P.; Davis, J.L.; Bodaghi, B. Diagnosis and treatment of cytomegalovirus iridocyclitis without retinal necrosis. Br. J. Ophthalmol. 2006, 90, 852–855. [Google Scholar] [CrossRef]
- Crumpacker, C.S. Ganciclovir. N. Engl. J. Med. 1996, 335, 721–729. [Google Scholar] [CrossRef]
- Choi, W.S.; Cho, J.H.; Kim, H.K.; Kim, H.S.; Shin, Y.J. A case of CMV endotheliitis treated with intravitreal ganciclovir injection. Korean J. Ophthalmol. 2013, 27, 130–132. [Google Scholar] [CrossRef]
- Wong, J.X.H.; Agrawal, R.; Wong, E.P.Y.; Teoh, S.C. Efficacy and safety of topical ganciclovir in the management of cytomegalovirus (CMV)-related anterior uveitis. J. Ophthalmic Inflamm. Infect. 2016, 6, 10. [Google Scholar] [CrossRef]
- Fan, N.-W.; Chung, Y.-C.; Liu, Y.-C.; Liu, C.J.-L.; Kuo, Y.-S.; Lin, P.-Y. Long-term topical ganciclovir and corticosteroids preserve corneal endothelial function in cytomegalovirus corneal endotheliitis. Cornea 2016, 35, 596–601. [Google Scholar] [CrossRef]
- Su, C.-C.; Wang, I.J.; Chen, W.-L.; Lin, C.-P.; His, B.; Hu, F.-R. Topical ganciclovir treatment in patients with cytomegalovirus endotheliitis receiving penetrating keratoplasty. Clin. Experiment. Ophthalmol. 2013, 41, 339–347. [Google Scholar] [CrossRef]
- Su, C.-C.; Hu, F.-R.; Wang, T.-H.; Huang, J.-Y.; Yeh, P.-T.; Lin, C.-P.; Wang, I.J. Clinical outcomes in cytomegalovirus-positive Posner-Schlossman syndrome patients treated with topical ganciclovir therapy. Am. J. Ophthalmol. 2014, 158, 1024–1031.e2. [Google Scholar] [CrossRef]
- Keorochana, N.; Choontanom, R. Efficacy and safety of an extemporaneous preparation of 2% ganciclovir eye drops in CMV anterior uveitis. BMJ Open Ophthalmol. 2017, 2, e000061. [Google Scholar] [CrossRef]
- Colin, J. Ganciclovir ophthalmic gel, 0.15%: A valuable tool for treating ocular herpes. Clin. Ophthalmol. 2007, 1, 441–453. [Google Scholar]
- Srisangchun, J.; Noppawinyoowong, C. Chemical stability and sterility of frozen ganciclovir injections. Srinagarind Med. J. 2013, 23, 2–6. [Google Scholar]
- Okumura, N.; Tanaka, T.; Fukui, Y.; Koizumi, N. Stability, safety, and pharmacokinetics of ganciclovir eye drops prepared from ganciclovir for intravenous infusion. Jpn. J. Ophthalmol. 2019, 63, 289–296. [Google Scholar] [CrossRef]
- Kathuria, A.; Shamloo, K.; Jhanji, V.; Sharma, A. Categorization of marketed artificial tear formulations based on their ingredients: A rational approach for their use. J. Clin. Med. 2021, 10, 1289. [Google Scholar] [CrossRef]
- Brossard, D.; Chedru-Legros, V.; Crauste-Manciet, S.; Fleury-Souverain, S.; Lagarce, F.; Odou, P.; Roy, S.; Sadeghipour, F.; Sautou, V. Methodological Guidelines for Stability Studies of Hospital Pharmaceutical Preparations Part 1: Liquid Preparations, 1st ed.; Printconseil: Romagnat, France, 2013; pp. 1–75. [Google Scholar]
- ICH Topic Q 1 A (R2) Stability Testing of New Drug Substances and Products. 2003, pp. 1–20. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf (accessed on 1 August 2022).
- United States Pharmacopeia 45-the National Formulary 40; United States Pharmacopeial Convention Inc.: Rockville, MD, USA, 2022.
- ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2(R1). 2005, pp. 1–17. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 1 August 2022).
- Ramesh, P.J.; Basavaiah, K.; Vinay, K.B.; Xavier, C.M. Development and validation of RP-HPLC method for the determination of ganciclovir in bulk drug and in formulations. ISRN Chromatogr. 2012, 2012, 894965. [Google Scholar] [CrossRef]
- Nuchtavorn, N.; Leanpolchareanchai, J.; Chanton, D.; Supapsophon, P.; Chongruchiroj, S.; Chatmapanrangsee, J.; Suksiriworapong, J. A rapid stability indicating HPLC method for determination of quetiapine fumarate in tablets and extemporaneous formulations. Pharm. Chem. J. 2021, 55, 845–854. [Google Scholar] [CrossRef]
- Doomkaew, A.; Prutthiwanasan, B.; Suntornsuk, L. Stability indicating MEKC method for the determination of gliclazide and its specified impurities. J. Pharm. Biomed. Anal. 2015, 102, 119–128. [Google Scholar] [CrossRef]
- Power, L.A.; Coyne, J.W. ASHP guidelines on handling hazardous drugs. Am. J. Health Syst. Pharm. 2018, 75, 1996–2031. [Google Scholar] [CrossRef]
- ICH Topic Q6A Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances. 2000, pp. 1–32. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-6-test-procedures-acceptance-criteria-new-drug-substances-new-drug-products-chemical_en.pdf (accessed on 1 August 2022).
- Epshtein, N.A. System suitability requirements for liquid chromatography methods: Controlled parameters and their recommended values (review). Pharm. Chem. J. 2020, 54, 518–525. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research (CDER), Reviewer Guidance: Validation of Chromatographic Methods. pp. 1–33. 1994. Available online: https://www.fda.gov/media/75643/download (accessed on 1 August 2022).
- Guichard, N.; Bonnabry, P.; Rudaz, S.; Fleury-Souverain, S. Long-term stability of ganciclovir in polypropylene containers at room temperature. J. Oncol. Pharm. Pract. 2019, 25, 303–308. [Google Scholar] [CrossRef]
- Chen, X.; Ooi, C.P.; Lim, T.H. Effect of ganciclovir on the hydrolytic degradation of poly(lactide-co-glycolide) microspheres. J. Biomater. Appl. 2006, 20, 287–302. [Google Scholar] [CrossRef]
- Full Prescribing Information: Hialid 0.1 Ophthalmic Solution; Santen Pharmaceutical Co. Ltd.: Ishikawa, Japan, 2019; pp. 1–3.
- Carney, L.G.; Mauger, T.F.; Hill, R.M. Buffering in human tears: pH responses to acid and base challenge. Investig. Ophthalmol. Vis. Sci. 1989, 30, 747–754. [Google Scholar]
- Carney, L.G.; Hill, R.M. Human tear buffering capacity. Arch. Ophthalmol. 1979, 97, 951–952. [Google Scholar] [CrossRef]
- Bright, A.M.; Tighe, B.J. The composition and interfacial properties of tears, tear substitutes and tear models. J. Br. Contact Lens Assoc. 1993, 16, 57–66. [Google Scholar] [CrossRef]
- Willshire, C.; Buckley, R.J.; Bron, A.J. Estimating basal tear osmolarity in normal and dry eye subjects. Cont. Lens Anterior Eye 2018, 41, 34–46. [Google Scholar] [CrossRef]
- Terry, J.E.; Hill, R.M. Human tear osmotic pressure: Diurnal variations and the closed eye. Arch. Ophthalmol. 1978, 96, 120–122. [Google Scholar] [CrossRef]
- Motolko, M.; Breslin, C.W. The effect of pH and osmolarity on the ability of tolerate artificial tears. Am. J. Ophthalmol. 1981, 91, 781–784. [Google Scholar] [CrossRef]
- Che Arif, F.; Hilmi, M.R.; Kamal, K.; Ithnin, M. Evaluation of 18 artificial tears based on viscosity and pH. Malays. J. Ophthalmol. 2020, 2, 96–111. [Google Scholar] [CrossRef]
- Simmons, P.A.; Liu, H.; Carlisle-Wilcox, C.; Vehige, J.G. Efficacy and safety of two new formulations of artificial tears in subjects with dry eye disease: A 3-month, multicenter, active-controlled, randomized trial. Clin. Ophthalmol. 2015, 9, 665–675. [Google Scholar] [CrossRef]
- Aragona, P.; Simmons, P.A.; Wang, H.; Wang, T. Physicochemical properties of hyaluronic acid–based lubricant eye drops. Transl. Vis. Sci. Technol. 2019, 8, 2. [Google Scholar] [CrossRef]
- Arshinoff, S.A.; Hofmann, I.; Nae, H. Role of rheology in tears and artificial tears. J. Cataract Refract. Surg. 2021, 47, 655–661. [Google Scholar] [CrossRef]
- Mole, L.; Oliva, C.; O’Hanley, P. Extended stability of ganciclovir for outpatient parenteral therapy for cytomegalovirus retinitis. J. Acquir. Immune Defic. Syndr. 1992, 5, 354–358. [Google Scholar]
| Product Code | Source | Active Ingredients | Inactive Ingredients |
|---|---|---|---|
| CMCG | Allergan Australia Pty Ltd., North Sydney, NSW, Australia | 0.5% Carboxymethyl cellulose sodium and 0.9% glycerin | Boric acid, calcium chloride dihydrate, erythritol, levocarnitine, magnesium chloride hexahydrate, potassium chloride, purified water, PURITE® (stabilized oxychloro complex), sodium borate decahydrate, and sodium citrate dihydrate |
| DEXH | Alcon Laboratories, Puurs-Sint-Amands, Belgium | 0.1% Dextran 70 and 0.3% hydroxypropyl methyl cellulose | Disodium edetate, sodium chloride, potassium chloride, benzalkonium chloride, hydrochloric acid and/or sodium hydroxide (to adjust pH), and purified water |
| PEGP | Alcon Laboratories, Lake Forest, CA, USA | 0.4% Polyethylene glycol 400 and 0.3% propylene glycol | Aminomethyl propanol, boric acid, hydroxypropyl guar, POLYQUAD® (polyquarternium-1), potassium chloride, sodium chloride, sorbitol, hydrochloric acid and/or sodium hydroxide (to adjust pH), and purified water |
| HPMC0.3A | Alcon Laboratories, Fort Worth, TX, USA | 0.3% Hydroxypropyl methyl cellulose | Carbopol 980, phosphonic acid, purified water, sodium hydroxide, sodium perborate, and sorbitol |
| CMC | Allergan Australia Pty Ltd., North Sydney, NSW, Australia | 0.5% Carboxymethyl cellulose sodium | Boric acid, calcium chloride, magnesium chloride, potassium chloride, purified water, sodium borate, sodium chloride, and PURITE® (stabilized oxychloro complex) |
| HPMC0.5 | Sangthai Medical Co., Ltd., Bangkok, Thailand | 0.5% Hydroxypropyl methyl cellulose | Benzalkonium chloride (other ingredients are not available.) |
| HYA0.1 | Santen Pharmaceutical Co., Ltd., Osaka, Japan | 0.1% Sodium hyaluronate (HYA) | ε-Aminocaproic acid, disodium edetate hydrate, propylene glycol, sodium chloride, chlorhexidine gluconate solution, and pH adjuster |
| HYA0.3 | Santen Pharmaceutical Co., Ltd., Osaka, Japan | 0.3% Sodium hyaluronate (HYA) | ε-Aminocaproic acid, disodium edetate hydrate, propylene glycol, sodium chloride, chlorhexidine gluconate solution, and pH adjuster |
| HPMC0.3S | Silom Medical Co., Ltd., Bangkok, Thailand | 0.3% Hydroxypropyl methyl cellulose | Sodium perborate (other ingredients are not available.) |
| Parameters | Obtained Value a | Criteria b [23,30,31] |
|---|---|---|
| Linearity | ||
| Range | 1–20 µg/mL | N/A |
| Slope | 70,482 | N/A |
| Y-intercept | 2202 | N/A |
| r | 0.9998 | ≥0.9990 |
| Precision | ||
| Repeatability: %RSD | 1.2 | ≤2.0 |
| Intermediate: %RSD | 2.0 | ≤2.0 |
| Accuracy: %Recovery | ||
| Solution | 99.5 ± 1.2 c (1.2) | 98–102% |
| Exemplified samples | 99.1 ± 2.5 c (2.6) | 95–105% |
| LOD | 0.03 | N/A |
| LOQ | 0.10 (1.8) | N/A |
| Vehicles | pH before Drug Mixing | Concentration of Ganciclovir (mg/mL) | |||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | |||||
| pH | Appearance a | pH | Appearance a | pH | Appearance a | ||
| SWI | 7.1 | 10.5 | C | 10.5 | C | 10.4 | C |
| CMCG | 7.3 | 7.9 | C | 8.4 | P | 9.2 | P |
| DEXH | 7.5 | 8.8 | P* | 9.2 | P* | 9.7 | P* |
| PEGP | 7.9 | 8.4 | C | 8.8 | P | 9.5 | P |
| HPMC0.3A | 6.7 | 8.3 | C | 8.8 | C | 9.4 | P |
| CMC | 7.2 | 8.3 | C | 8.7 | P | 9.4 | P |
| HPMC0.5 | 7.0 | 9.3 | P* | 9.8 | P* | 10.2 | P* |
| HYA0.1 | 6.3 | 9.9 | C | 10.1 | C | 10.3 | C |
| HPMC0.3S | 7.0 | 8.0 | C | 8.4 | P | 9.1 | P |
| Tests | Ganciclovir/HYA0.1 ED | Ganciclovir/SWI ED |
|---|---|---|
| Appearance | ||
| Color | Colorless | Colorless |
| Clarity | Clear | Clear |
| Precipitation | No | No |
| Gas bubble | No | No |
| pH | 9.99 ± 0.08 a | 10.44 ± 0.06 a |
| Osmolality (mOsm/kg H2O) | 290 ± 1 a | 136 ± 1 a |
| Viscosity (cP) | 2.20 ± 0.01 a | 0.93 ± 0.06 a |
| % Labeled amount | 105.6 ± 6.6 a | 99.7 ± 0.7 a |
| Sterility test | pass | pass |
| Test | Time (Weeks) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ganciclovir/HYA0.1 ED | Ganciclovir/SWI ED | |||||||||||
| 4 | 8 | 12 | 16 | 20 | 24 | 4 | 8 | 12 | 16 | 20 | 24 | |
| Packaging | ||||||||||||
| Integrity a | C | C | C | C | C | C | C | C | C | C | C | C |
| Leakage b | − | − | − | − | − | − | − | − | − | − | − | − |
| Solution | ||||||||||||
| Color c | C | C | C | C | C | C | C | C | C | C | C | C |
| Clarity b | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Precipitation b | − | − | + d | + d | + d | + d | − | − | − | − | − | − |
| Gas bubble b | − | − | − | − | − | − | − | − | − | − | +++ | +++ |
| Sterility | Pass | Pass | Pass | Fail | ND e | ND e | Pass | Pass | Pass | Fail | ND e | ND e |
| Test | Time (Weeks) | |||||
|---|---|---|---|---|---|---|
| Ganciclovir/HYA0.1 ED | Ganciclovir/SWI ED | |||||
| 4 | 8 | 12 | 4 | 8 | 12 | |
| Packaging | ||||||
| Integrity a | C | C | C | C | C | C |
| Leakage b | − | − | − | − | − | − |
| Solution | ||||||
| Color c | C | C | C | C | C | C |
| Clarity b | +++ | +++ | ++ | +++ | +++ | ++ |
| Precipitation b | − | − | − | − | − | − |
| Gas bubble b | − | − | − | − | − | − |
| Sterility | Pass | Pass | Pass | Pass | Pass | Pass |
| Test | Time (Weeks) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 ± 3 °C | 30 ± 2 °C | ||||||
| 4 | 8 | 12 | 16 | 20 | 24 | 4 | ||
| Packaging | ||||||||
| Integrity a | C | C | C | C | C | C | C | C |
| Leakage b | − | − | − | − | − | − | − | − |
| Solution | ||||||||
| Color c | C | C | C | C | C | C | C | C |
| Clarity b | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Precipitation b | − | − | − | + d | + d | + d | + d | + |
| Gas bubble b | − | − | − | − | − | − | − | − |
| pH e | 10.42 (0.02) | 10.71 (0.03) | 10.78 (0.07) | 9.73 (0.00) | 10.59 (0.00) | 10.61 (0.06) | 10.53 (0.06) | 10.07 (0.03) |
| Viscosity e (cP) | 6.69 (0.13) | 6.59 (0.03) | 6.68 (0.14) | 6.41 (0.11) | 6.51 (0.05) | 6.28 (0.42) | 6.52 (0.20) | 6.12 (0.07) |
| Osmolality e (mOsm/kg H2O) | 290 (2) | 292 (1) | 293 (1) | 290 (0) | 288 (2) | 292 (1) | 289 (1) | 278 (1) |
| %Drug remaining e | 100.0 (3.4) | 98.0 (3.9) | 95.9 (4.8) | 93.2 (7.7) | 97.7 (4.3) | 90.8 (9.9) | 88.6 (2.0) | 85.5 (3.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leanpolchareanchai, J.; Tangteerakoon, P.; Supapsophon, P.; Sukavatcharin, S.; Simaroj, P.; Suksiriworapong, J. Extemporaneous Preparation of 20 mg/mL Ganciclovir in Artificial Tears in Comparison with Sterile Water for Ophthalmic Administration: Formulation and Stability Study. Pharmaceutics 2023, 15, 208. https://doi.org/10.3390/pharmaceutics15010208
Leanpolchareanchai J, Tangteerakoon P, Supapsophon P, Sukavatcharin S, Simaroj P, Suksiriworapong J. Extemporaneous Preparation of 20 mg/mL Ganciclovir in Artificial Tears in Comparison with Sterile Water for Ophthalmic Administration: Formulation and Stability Study. Pharmaceutics. 2023; 15(1):208. https://doi.org/10.3390/pharmaceutics15010208
Chicago/Turabian StyleLeanpolchareanchai, Jiraporn, Patamaporn Tangteerakoon, Patcharin Supapsophon, Somsiri Sukavatcharin, Pornchai Simaroj, and Jiraphong Suksiriworapong. 2023. "Extemporaneous Preparation of 20 mg/mL Ganciclovir in Artificial Tears in Comparison with Sterile Water for Ophthalmic Administration: Formulation and Stability Study" Pharmaceutics 15, no. 1: 208. https://doi.org/10.3390/pharmaceutics15010208
APA StyleLeanpolchareanchai, J., Tangteerakoon, P., Supapsophon, P., Sukavatcharin, S., Simaroj, P., & Suksiriworapong, J. (2023). Extemporaneous Preparation of 20 mg/mL Ganciclovir in Artificial Tears in Comparison with Sterile Water for Ophthalmic Administration: Formulation and Stability Study. Pharmaceutics, 15(1), 208. https://doi.org/10.3390/pharmaceutics15010208