Pharmaceutical Systems as a Strategy to Enhance the Stability of Oxytetracycline Hydrochloride Polymorphs in Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
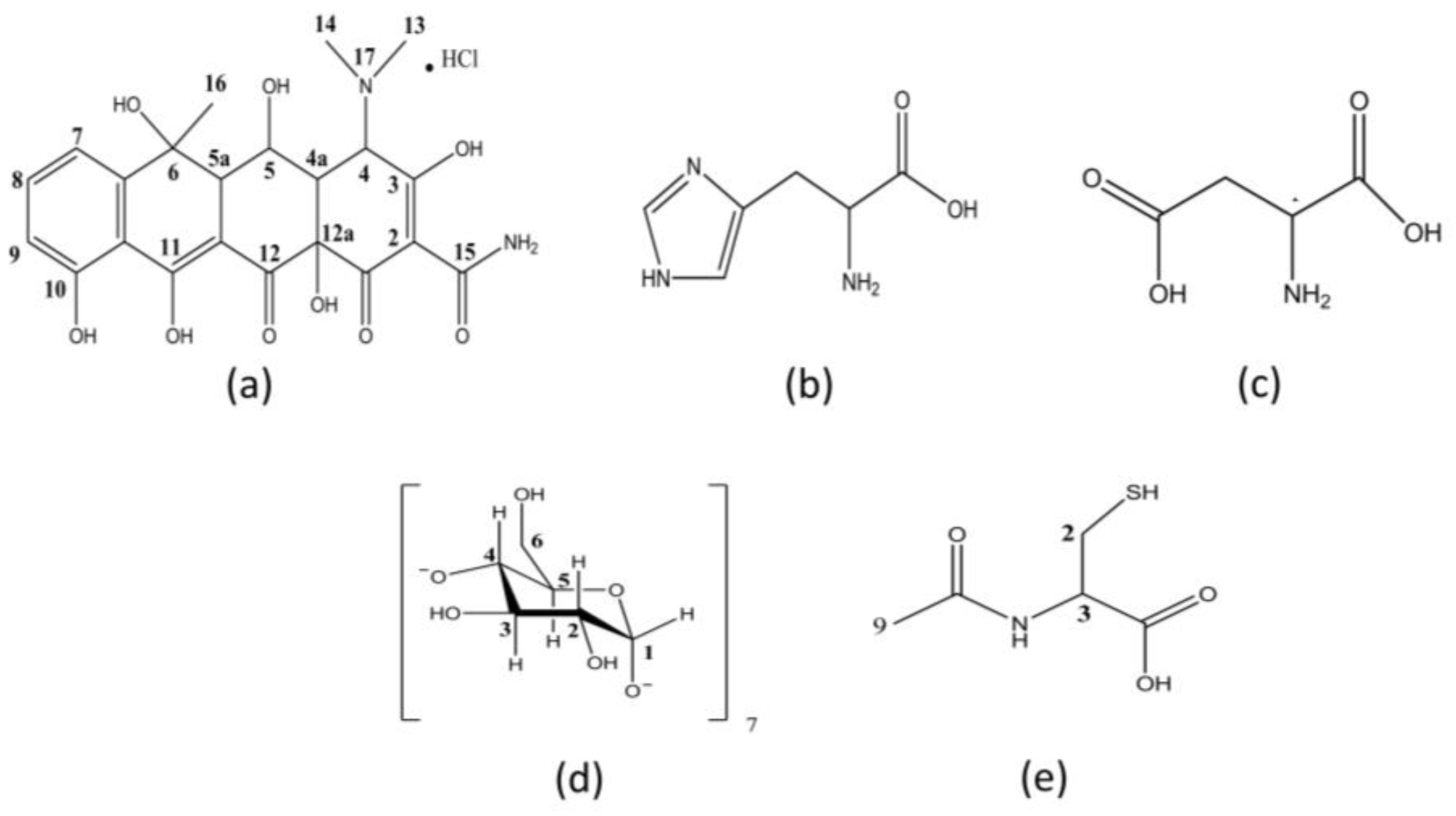
2.2. Obtaining the Solid Forms of Oxytetracycline Hydrochloride
2.3. Preparation of Binary Systems
2.4. Determination of Association Constants from UV-Visible Spectroscopy
2.4.1. Scott’s Plot Method
2.4.2. Statistical Treatment of Data
2.5. Stability Study Design
2.5.1. Chemical Stability Study
2.5.2. Chromatographic Conditions
2.5.3. Validation of Chromatographic Method
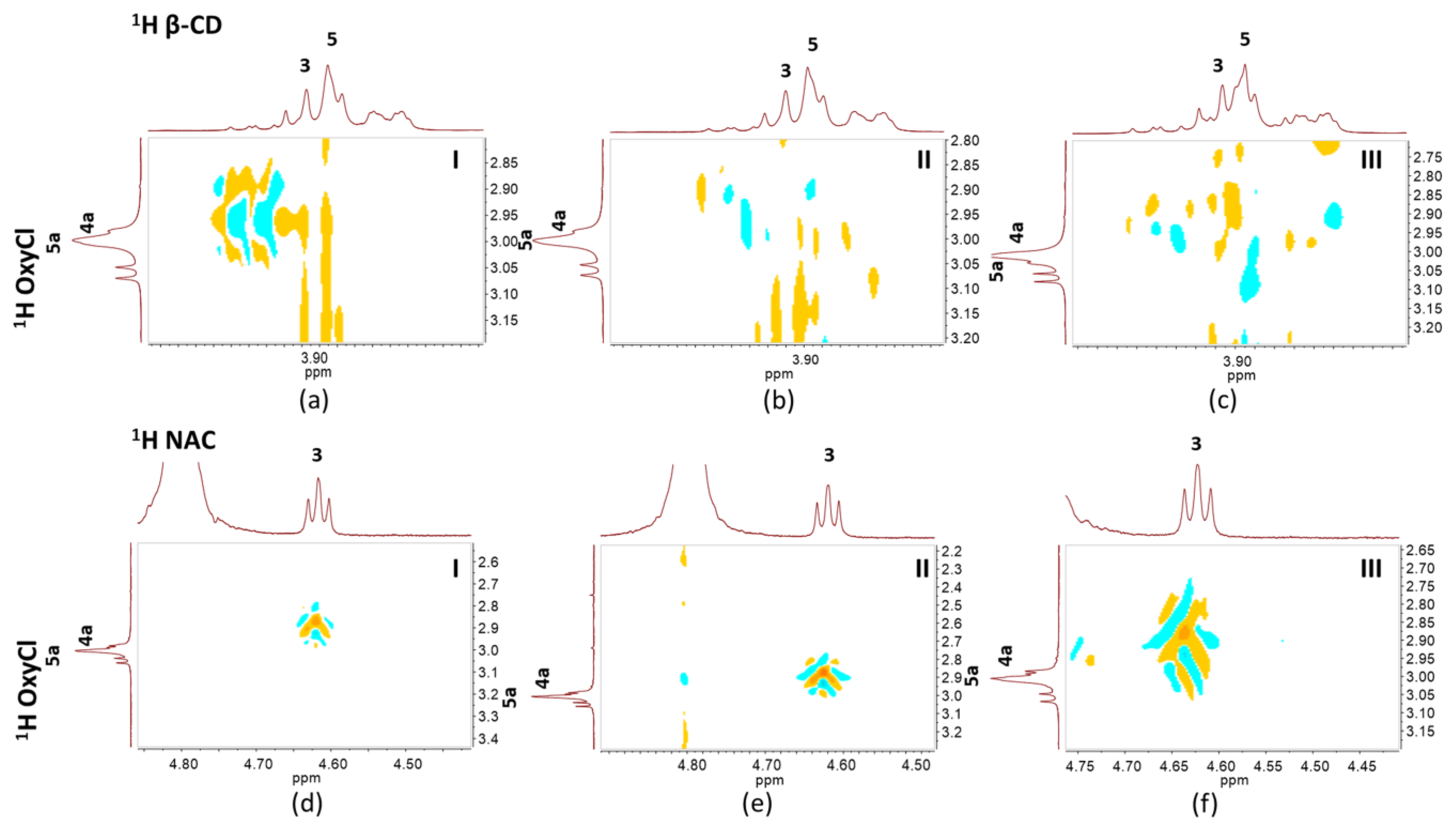
2.6. Nuclear Magnetic Resonance Spectroscopy Study
3. Results and Discussion
3.1. Determination of the Apparent Binding Constants
3.2. Stability Studies
3.2.1. Validation of Chromatographic Method
3.2.2. Degradation Studies
3.3. Nuclear Magnetic Resonance Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iram, F.; Iram, H.; Iqbal, A.; Husaín, A. Forced Degradation Studies. J. Anal. Pharm. Res. 2016, 3, 00073. [Google Scholar] [CrossRef]
- Rehman, Q.; Akash, M.S.H.; Imran, I.; Rehman, K. Stability of Pharmaceutical Products. In Drug Stability and Chemical Kinetics; Akash, M.S.H., Rehman, K., Eds.; Springer: Singapore, 2020; pp. 147–154. ISBN 978-981-15-6426-0. [Google Scholar]
- Kaur, M.; Kaur, G.; Kaur, H.; Sharma, S. Overview on stability studies. Int. J. Pharm. Chem. Sci. 2013, 3, 1231–1241. [Google Scholar]
- Stability Testing of New Drug Substances and Products Q1A (R2). In Proceedings of the International Conference on Harmonization of Technical Requirements for registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 2003.
- Sengupta, P.; Chatterjee, B.; Tekade, K.R. Current regulatory requirements and practical approaches for stability analysis of pharmaceutical products: A comprehensive review. Int. J. Pharm. 2018, 543, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Haider, K.; Akash, M.S.H.; Faheem, A.; Rehman, K. Guidelines for Drug Stability and Stability Testing. In Drug Stability and Chemical Kinetics; Akash, M.S.H., Rehman, K., Eds.; Springer: Singapore, 2020; pp. 19–29. ISBN 978-981-15-6426-0. [Google Scholar]
- Zilker, M.; Sörgel, F.; Holzgrabe, U. A systematic review of the stability of finished pharmaceutical products and drug substances beyond their labeled expiry dates. J. Pharm. Biomed. Anal. 2019, 166, 222–235. [Google Scholar] [CrossRef]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Liu, Y. Characterization and stability of beta-acids/hydroxypropyl-β-cyclodextrin inclusion complex. J. Mol. Struct. 2020, 1201, 127–159. [Google Scholar] [CrossRef]
- Minecka, A.; Tarnacka, M.; Jurkiewicz, K.; Hacula, B.; Wrzalik, R.; Bródka, A.; Kaminska, E. The impact of the size of acetylated cyclodextrin on the stability of amorphous metronidazole. Int. J. Pharm. 2022, 624, 122025. [Google Scholar] [CrossRef]
- Jansook, P.; Hnin, M.H.; Praphanwittaya, P.; Loftsson, T.; Stefansson, E. Effect of salt formation on γ-cyclodextrin solubilization of irbesartan and candesartan and the chemical stability of their ternary complexes. J. Drug Deliv. Sci. Technol. 2022, 67, 102980. [Google Scholar] [CrossRef]
- Li, J.; Pan, D.; Yi, J.; Hao, L.; Kang, Q.; Liu, X.; Lu, L.; Lu, J. Protective effect of β-cyclodextrin on stability of nisin and corresponding interactions involved. Carbohydr. Polym. 2019, 233, 115115. [Google Scholar] [CrossRef]
- Samuelsen, L.; Holm, R.; Lathuile, A.; Schönbeck, C. Correlation between the stability constant and pH for β-Cyclodextrin complexes. Int. J. Pharm. 2019, 568, 118523. [Google Scholar] [CrossRef]
- Jiang, L.; Xia, N.; Wang, F.; Xie, C.; Ye, R.; Tang, H.; Zhang, H.; Liu, Y. Preparation and characterization of curcumin/β-cyclodextrin nanoparticles by nanoprecipitation to improve the stability and bioavailability of curcumin. LWT 2022, 171, 114149. [Google Scholar] [CrossRef]
- Altamimi, M.; Elzayat, E.; Alhowyan, A.; Alshehri, S.; Shakeel, F. Effect of β-cyclodextrin and different surfactants on solubility, stability, and permeability of hydrochlorothiazide. J. Mol. Liq. 2018, 250, 323–328. [Google Scholar] [CrossRef]
- Sousa Gramoza, A.; Santana, V.; Nadvorny, D.; da Rocha Passos, T.; Felts de La Roca Soares, M.; Soares-Sobrinho, J. Influence of cyclodextrin on posaconazole stability, release and activity: Improve the utility of the drug. J. Drug Deliv. Sci. Technol. 2019, 53, 101153. [Google Scholar] [CrossRef]
- Pamudji, J.S.; Mauludin, R.; Nurhabibah. Influence of β-Cyclodextrin on Cefixime Stability in Liquid Suspension Dosage Form. Procedia Chem. 2014, 13, 119–127. [Google Scholar] [CrossRef]
- Löbmann, K.; Grohganz, H.; Laitinen, R.; Strachan, C.; Rades, T. Amino acids as co-amorphous stabilizers for poorly watersoluble drugs–Part 1: Preparation, stability and dissolution enhancement. Eur. J. Pharm. Biopharm. 2013, 85, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Nugrahani, I.; Utamia, D.; Ibrahim, S.; Nugraha, Y.P.; Uekusa, H. Zwitterionic cocrystal of diclofenac and L-proline: Structure determination, solubility, kinetics of cocrystallization, and stability study. Eur. J. Pharm. Sci. 2018, 117, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Franҫa, M.T.; Marcos, M.T.; Pereira, R.F.; Stulzer, H.K. Could the small molecules such as amino acids improve aqueous solubility and stabilize amorphous systems containing Griseofulvin. Eur. J. Pharm. Sci. 2020, 143, 105178. [Google Scholar] [CrossRef]
- Bongioanni, A.; Bueno, M.S.; Mezzano, B.A.; Longhi, M.R.; Garnero, C. Amino acids and its pharmaceutical applications: A mini review. Int. J. Pharm. 2022, 613, 121375. [Google Scholar] [CrossRef]
- Wu, W.; Grohganz, H.; Rades, T.; Löbmann, K. Comparison of co-former performance in co-amorphous formulations: Single amino acids, amino acid physical mixtures, amino acids salts and dipeptides as co-formers. Eur. J. Pharm. Sci. 2021, 156, 105582. [Google Scholar] [CrossRef]
- Adhikari, B.R.; Sinha, S.; Gordon, K.C.; Das, S.C. Amino acids improve aerosolization and chemical stability of potential inhalable amorphous Spray-dried ceftazidime for Pseudomonas aeruginosa lung infection. Int. J. Pharm. 2022, 621, 121799. [Google Scholar] [CrossRef]
- Bueno, M.S.; Miñambres, G.G.; Bongioanni, A.; Chattah, A.K.; Aiassa, V.; Longhi, M.R.; Garnero, C. Exploring solid forms of Oxytetracycline hydrochloride. Int. J. Pharm. 2020, 585, 119496. [Google Scholar] [CrossRef]
- Jácome-Acatitlaa, G.; Tzompantzi, F.; López-González, R.; García-Mendoza, C.; Alvaro, J.M.; Gómez, R. Photodegradation of sodium naproxen and oxytetracycline hydrochloride in aqueous medium using as photocatalysts Mg-Al calcined hydrotalcites. J. Photochem. Photobiol. A 2014, 277, 82–89. [Google Scholar] [CrossRef]
- German, R.; Bukowska, B.; Pajchel, G.; Grzybowskaa, W.; Tyski, S. Extremely long time stability study of selected antibiotic standards. J. Pharm. Biomed. Anal. 2010, 51, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Bakkour, Y.; Vermeersch, G.; Morcellet, M.; Boschin, F.; Martel, B.; Azaroual, N. Formation of Cyclodextrin Inclusion Complexes with Doxycyclin-Hyclate: NMR Investigation of Their Characterisation and Stability. J. Incl. Phenom. Macrocycl. Chem. 2006, 54, 109–114. [Google Scholar] [CrossRef]
- Wang, Q.; Yates, S. Laboratory Study of Oxytetracycline Degradation Kinetics in Animal Manure and Soil. J. Agric. Food Chem. 2008, 56, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Validation of Analytical Procedures: Text and Methodology Q2 (R1). In Proceedings of the International Conference on Harmonization of Technical Requirements for registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 2005.
- Granero, G.E.; Maitre, M.M.; Garnero, C.; Longhi, M.R. Synthesis, characterization and in vitro release studies of a new acetazolamide:HP-β-CD:TEA inclusion complex. Eur. J. Med. Chem. 2008, 43, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Toro, R.; Díaz de Delgado, G.; Bahsas, A.; Delgado, J.M. The presence of polymorphism in Oxytetracycline hydrochloride shown by X-ray powder diffraction techniques. Z. Kristallogr. 2007, 26, 563–568. [Google Scholar] [CrossRef]
- Cerutti, J.P.; Aiassa, V.; Fernández, M.A.; Longhi, M.R.; Quevedo, M.A.; Zoppi, A. Structural, physicochemical and biological characterization of chloramphenicol multicomponent complexes. J. Mol. Liq. 2021, 331, 115761. [Google Scholar] [CrossRef]
- Nunes de Melo, P.; Barbosa, E.G.; Basílio de Caland, L.; Carpegianni, H.; Garnero, C.; Longhi, M.; Fernandes-Pedrosa, M.; Silva-Júnior, A.A. Host-guest interactions between benznidazole and beta-cyclodextrin in multicomponent complex systems involving hydrophilic polymers and triethanolamine in aqueous solution. J. Mol. Liq. 2013, 186, 147–156. [Google Scholar] [CrossRef]
- Ma, S.X.; Chen, W.; Yang, X.D.; Zhang, N.; Wang, S.J.; Liu, L.; Yang, L.J. Alpinetin/hydroxypropyl-β-cyclodextrin host-guest system: Preparation, characterization, inclusion mode, solubilization and stability. J. Pharm. Biomed. Anal. 2012, 67–68, 193–200. [Google Scholar] [CrossRef]
- Fawaz, N.S.; Al-Heibshy, E.B.; Naile, Ö.; Müzeyyen, D. Preparation and In Vitro Characterization of Rosuvastatin Calcium Incorporated Methyl Beta Cyclodextrin and Captisol® Inclusion Complexes. Drug Dev. Ind. Pharm. 2020, 46, 1495–1506. [Google Scholar] [CrossRef]
- Tahir, M.N.; Cao, Y.; Azzouz, A.; Roy, R. Host-guest chemistry of the sulfasalazine-β-cyclodextrin inclusion complex. Tetrahedron 2021, 85, 132052. [Google Scholar] [CrossRef]
- Aiassa, V.; Zoppi, A.; Becerra, M.C.; Albesa, I.; Longhi, M.R. Enhanced inhibition of bacterial biofilm formation and reduced toxicity by chloramphenicol:β-cyclodextrin:N-acetylcysteine complex. Carbohydr. Polym. 2016, 152, 672–678. [Google Scholar] [CrossRef] [PubMed]
 ) OxyCl-I, (
) OxyCl-I, (  ) OxyCl-II, and (
) OxyCl-II, and (  ) OxyCl-III.
) OxyCl-III.
 ) OxyCl-I, (
) OxyCl-I, (  ) OxyCl-II, and (
) OxyCl-II, and (  ) OxyCl-III.
) OxyCl-III.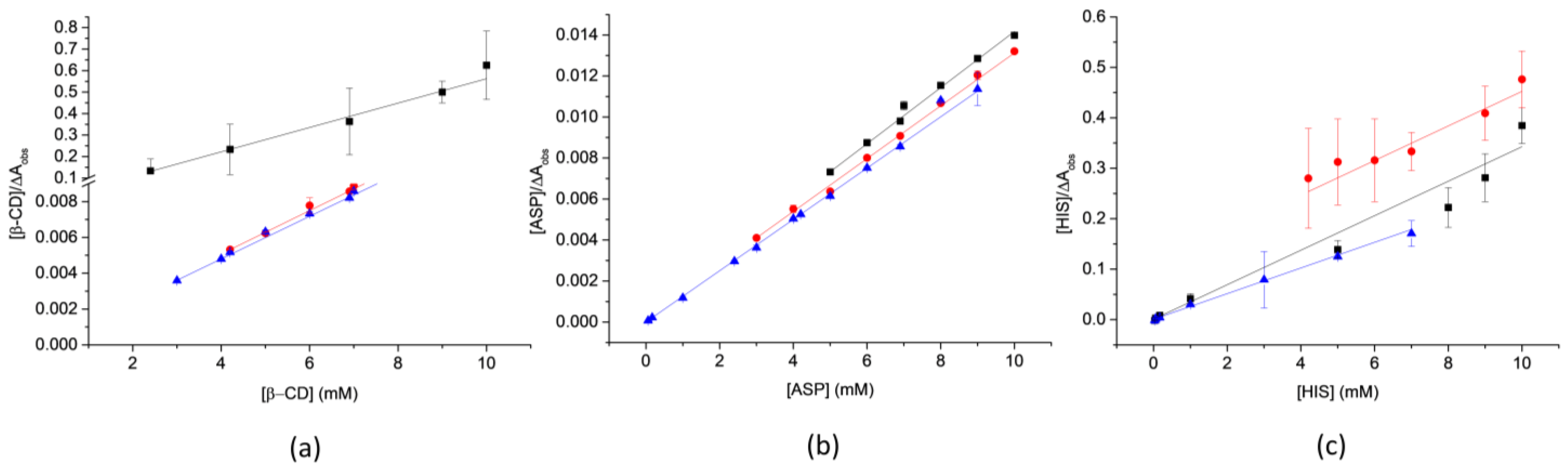
| Polymorph | Ligands | |||
|---|---|---|---|---|
| β-CD | ASP | HIS | NAC | |
| OxyCl-I | 3761 | 34,645 | 224 | 2042 |
| OxyCl-II | 15,425 | 3063 | 323 | 4582 |
| OxyCl-III | 3607 | 1771 | 8247 | 3897 |
| Parameters | Results | |
|---|---|---|
| Linearity | Regression equation a | Y = 14.544 X − 2.603 |
| Correlation coefficient (r2) | 0.994 | |
| Linear range (μg/mL) | 5.0–40.0 | |
| Detection limit | 0.38 (μg/mL) | |
| Quantification limit | 1.15 (μg/mL) | |
| Nominal Concentration (μg/mL) | Measured Concentration (μg/mL) | Accuracy (% Recovery) | Precision (% R.S.D.) |
|---|---|---|---|
| Intra-day (repeatability) | |||
| 20 | 20.1 ± 0.2 | 100.36 | 1.26 |
| 25.2 | 25.2 ± 0.4 | 100.22 | 1.73 |
| 30.2 | 30.2 ± 0.1 | 99.86 | 0.32 |
| Inter-day (intermediate precision) | |||
| 20.2 | 20.5 ± 0.1 | 101.38 | 0.55 |
| 25.5 | 26.0 ± 0.3 | 101.93 | 1.33 |
| 29.9 | 30.5 ± 0.1 | 101.96 | 0.42 |
| Solids | k0 (h−1) | kobs (h−1) | t50 (h) | t90 (h) | k0/kobs |
|---|---|---|---|---|---|
| OxyCl-I | 0.0102 | 68 | 10 | ||
| OxyCl-I:β-CD | 0.0134 | 52 | 8 | 0.76 | |
| OxyCl-I:ASP | 0.0143 | 48 | 7 | 0.71 | |
| OxyCl-I: NAC | 0.0093 | 74 | 11 | 1.10 | |
| OxyCl-II | 0.0121 | 57 | 9 | ||
| OxyCl-II:β-CD | 0.0132 | 53 | 8 | 0.92 | |
| OxyCl-II:ASP | 0.0142 | 49 | 7 | 0.85 | |
| OxyCl-II:NAC | 0.0079 | 73 | 11 | 1.53 | |
| OxyCl-III | 0.0116 | 60 | 9 | ||
| OxyCl-III:β-CD | 0.0087 | 80 | 12 | 1.33 | |
| OxyCl-III:ASP | 0.0300 | 23 | 4 | 0.39 | |
| OxyCl-III:NAC | 0.0095 | 87 | 13 | 1.22 |
| Solids | k0 (h−1) | kobs (h−1) | t50 (h) | t90 (h) | k0/kobs |
| OxyCl-I | 0.009 | 81 | 12 | ||
| OxyCl-I:NAC | 0.012 | 58 | 9 | 0.75 | |
| OxyCl-II | 0.005 | 139 | 21 | ||
| OxyCl-II:NAC | 0.022 | 32 | 5 | 0.22 | |
| OxyCl-III | 0.007 | 102 | 15 | ||
| OxyCl-III:NAC | 0.013 | 53 | 8 | 0.54 |
| δ (ppm) | ||||||
|---|---|---|---|---|---|---|
| Assignment | Polymorph | Assignment | Ligand | |||
| OxyCl-I | OxyCl-II | OxyCl-III | NAC | β-CD | ||
| H4 | 4.167 | 4.280 | 4.167 | H2 | 2.992 | |
| H13-14 | 3.054 | 3.054 | 3.061 | H3 | 4.569 | |
| H4a | 2.843 | 2.939 | 2.843 | H9 | 2.099 | |
| H5 | 3.961 | 3.949 | 3.964 | |||
| H5a | 2.927 | 2.968 | 2.927 | H1 | 5.068 | |
| H16 | 1.814 | 1.803 | 1.821 | H2 | 3.647 | |
| H7 | 7.254 | 7.246 | 7.261 | H3 | 3.965 | |
| H8 | 7.624 | 7.617 | 7.631 | H4 | 3.582 | |
| H9 | 7.037 | 7.026 | 7.044 | H5 | 3.870 | |
| NH2 | 8.352 | 8.254 | 8.360 | H6 | 3.908 | |
| Δδ = δsystem − δfree | ||||||
|---|---|---|---|---|---|---|
| Assignment | OxyCl-I:β-CD | OxyCl-II:β-CD | OxyCl-III:β-CD | OxyCl-I:NAC | OxyCl-II:NAC | OxyCl-III:NAC |
| H4 | 0.152 | 0.044 | 0.179 | 0.172 | 0.06 | 0.178 |
| H13-14 | 0.005 | 0.009 | 0.009 | −0.007 | −0.004 | 0.002 |
| H4a | 0.095 | 0.045 | 0.187 | 0.143 | 0.049 | 0.147 |
| H5 | 0.015 | 0.032 | 0.016 | −0.007 | 0.008 | 0.011 |
| H5a | 0.071 | 0.035 | 0.085 | 0.076 | 0.037 | 0.078 |
| H16 | 0.001 | 0.046 | 0.027 | −0.005 | 0.008 | 0.007 |
| H7 | 0.008 | 0.021 | 0.009 | −0.005 | 0.005 | 0.005 |
| H8 | 0.011 | 0.022 | 0.012 | −0.007 | 0.003 | 0.005 |
| H9 | 0.004 | 0.018 | 0.005 | −0.006 | −0.003 | 0.014 |
| Ligands | ||||||
| β-CD | ||||||
| H1 | 0.002 | 0.007 | 0.007 | |||
| H2 | 0.009 | 0.014 | 0.019 | |||
| H3 | −0.05 | −0.045 | −0.04 | |||
| H4 | 0.001 | 0.006 | 0.006 | |||
| H5 | −0.036 | −0.024 | −0.027 | |||
| H6 | 0.007 | 0.012 | 0.005 | |||
| NAC | ||||||
| H2 | −0.012 | −0.006 | −0.016 | |||
| H3 | 0.047 | 0.05 | 0.054 | |||
| H9 | −0.012 | −0.009 | −0.009 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, M.S.; Longhi, M.R.; Garnero, C. Pharmaceutical Systems as a Strategy to Enhance the Stability of Oxytetracycline Hydrochloride Polymorphs in Solution. Pharmaceutics 2023, 15, 192. https://doi.org/10.3390/pharmaceutics15010192
Bueno MS, Longhi MR, Garnero C. Pharmaceutical Systems as a Strategy to Enhance the Stability of Oxytetracycline Hydrochloride Polymorphs in Solution. Pharmaceutics. 2023; 15(1):192. https://doi.org/10.3390/pharmaceutics15010192
Chicago/Turabian StyleBueno, Maria S., Marcela R. Longhi, and Claudia Garnero. 2023. "Pharmaceutical Systems as a Strategy to Enhance the Stability of Oxytetracycline Hydrochloride Polymorphs in Solution" Pharmaceutics 15, no. 1: 192. https://doi.org/10.3390/pharmaceutics15010192
APA StyleBueno, M. S., Longhi, M. R., & Garnero, C. (2023). Pharmaceutical Systems as a Strategy to Enhance the Stability of Oxytetracycline Hydrochloride Polymorphs in Solution. Pharmaceutics, 15(1), 192. https://doi.org/10.3390/pharmaceutics15010192