Retrospectives on Three Decades of Safe Clinical Experience with Allogeneic Dermal Progenitor Fibroblasts: High Versatility in Topical Cytotherapeutic Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Overview of the Current Global Cytotherapy Ecosystem
2.2. Retrospective Analysis of the Clinical Work on Dermal Progenitor Fibroblasts and Derivatives under the Swiss Progenitor Cell Transplantation Program
3. Results
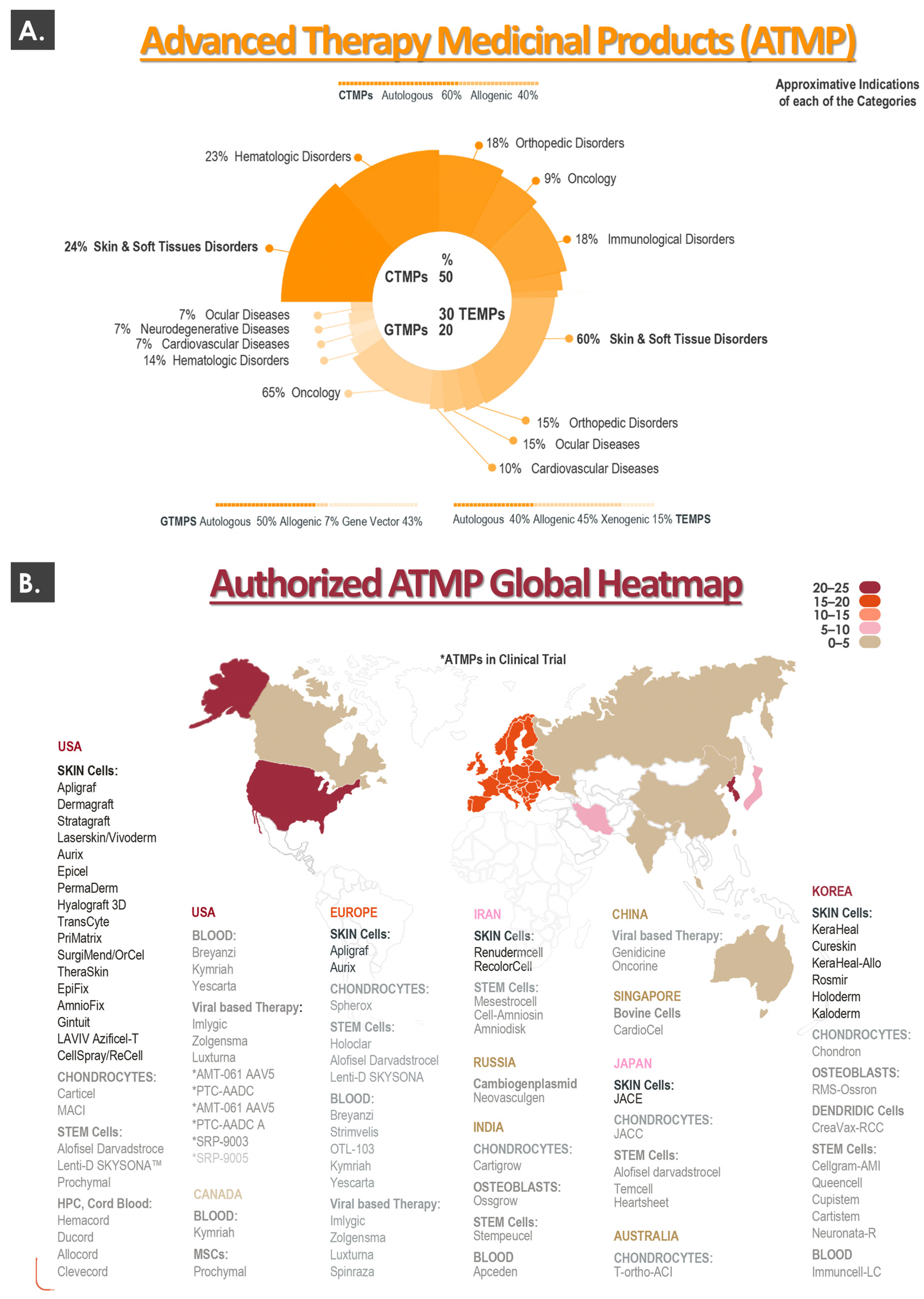
3.1. The Current Global Cytotherapeutic Ecosystem: Summarized Geographical Distribution of Approved Advanced Therapy Medicinal Products
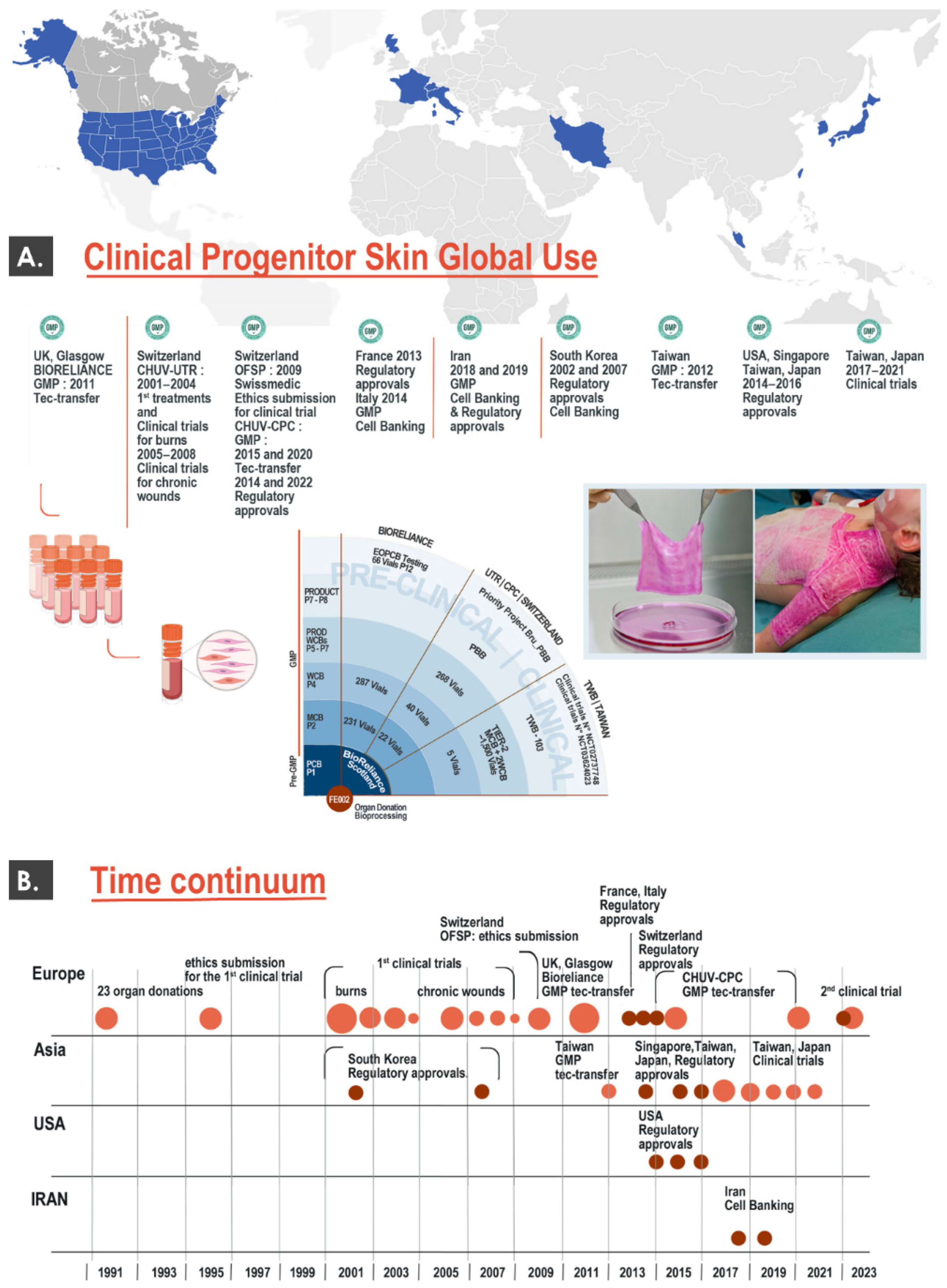
3.2. Global Clinical Work around Dermal Progenitor Cells: International Milestones for the Swiss Progenitor Cell Transplantation Program and Other Clinical Groups
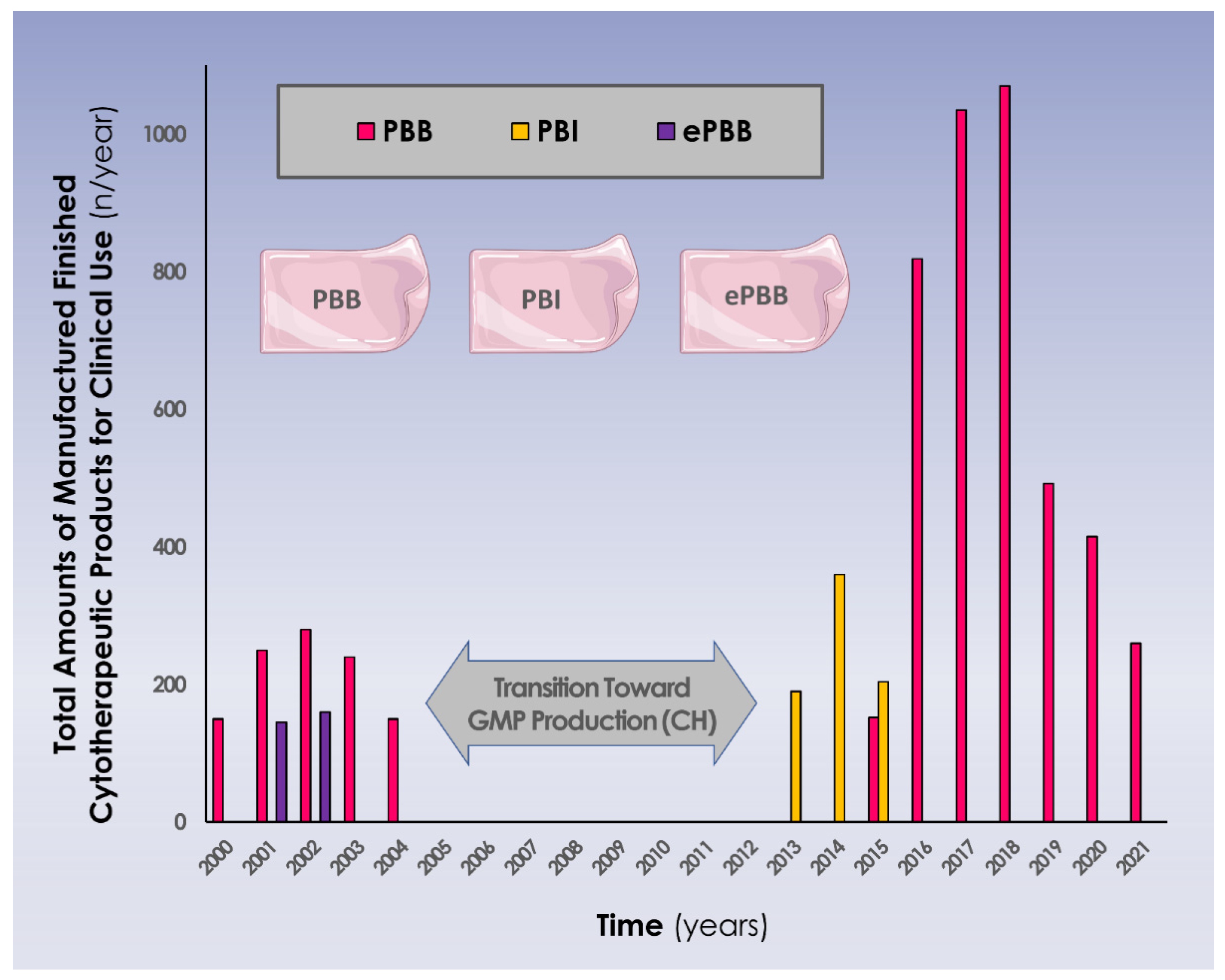
3.3. Three Decades of Clinical Work around Allogeneic Dermal Progenitor Fibroblasts and Derivatives in Switzerland
4. Discussion
4.1. High Versatility of Dermal Progenitor Fibroblasts and Derivatives for Topical Therapeutic Application
4.2. Extensive/Long-Term Clinical Use of Allogeneic Progenitor Cytotherapies Has Demonstrated Safety and Utility in Complex Cutaneous Wound Care
4.3. High Patient Needs and Clinical Demand Remain for Complex Cutaneous Affections: Necessity for Novel and Integrative Biological-Based Therapeutic Solutions
- Intercellular contacts within patient tissues and cells
- Reversal of apoptotic mechanisms and signals resulting from tissular and cellular trauma
- Release of progenitor cell secretomes and related vesicles with signaling functions
- Production and local deposition of extracellular matrix in the wound
- Environment-related specific cellular functions and structural orchestration
- Paracrine modulation (e.g., stimulation of cell proliferation and migration) or trophic action on patient cells and tissues
- Anti-inflammatory and pro-angiogenic effects
- Scavenging of oxidative stress sources
4.4. Navigating the Evolving Swiss Regulatory Ecosystem for the Provision of Safe and Standardized Allogeneic Progenitor Cytotherapies for Burns and Wounds
4.5. Technical Limitations and Clinical Hindsight on Topical Progenitor Cytotherapeutics: Pharmaceutical Solutions and Margins of Optimization for Future Work
4.6. Next Generations of Clinical Progenitor Cell-Based Cytotherapeutics and Derivatives: Improving Stability, Fighting Patient Infection, and Reducing Product Degradation
- Second-generation PBBs (PBI): Collagen scaffolds seeded with viable and growth-arrested (i.e., γ-irradiated) dermal progenitor fibroblasts (i.e., clinical stage, currently discontinued).
- Third-generation PBBs: Similar to the first generation, with addition of antimicrobial dendrimers, for combination of intended effects and management of the infectious risk (i.e., preclinical stage in large animal model) [60].
- Fourth-generation PBBs: Appropriate vehicle yielding temperature-stabilized non-viable dermal progenitor fibroblasts, for an off-the-shelf availability (i.e., development phase) [38].
- Fifth-generation PBBs: Appropriate vehicle yielding cell-derived cell-free and temperature-stabilized therapeutic extracts, for an off-the-shelf availability (i.e., development phase).
4.7. Current Status of the Clinical Work around Progenitor Biological Bandages in Switzerland: Local Perspectives of Clinical Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statements
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATMP | advanced therapy medicinal product |
| cATMP | combined advanced therapy medicinal product |
| CHUV | centre hospitalier universitaire vaudois |
| CTMP | cell therapy medicinal product |
| DSW | donor-site wound |
| FDA | US Food and Drug Administration |
| FE002-SK2 | primary skin-derived progenitor cell type |
| GLP | good laboratory practices |
| GMP | good manufacturing practices |
| GTMP | gene therapy medicinal product |
| IB | investigator’s brochure |
| IMPD | investigational medicinal product dossier |
| MSC | mesenchymal stem cell |
| NCT | clinical study unique identification code |
| PBB | progenitor biological bandage |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| TEM | transmission electron microscopy |
| TEMP | tissue engineered medicinal product |
| TFDA | Taiwan Food and Drug Administration |
| UK | United Kingdom |
| USA | United States of America |
References
- Green, H.; Kehinde, O.; Thomas, J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA 1979, 76, 5665–5668. [Google Scholar] [CrossRef]
- Gallico, G.G., 3rd; O’Connor, N.E.; Compton, C.C.; Kehinde, O.; Green, H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N. Engl. J. Med. 1984, 311, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M. Autologous chondrocyte transplantation. Clin. Orthop. Rel. Res. 1999, 367, S147–S155. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Paplham, P.; McCarthy, P.L. Remestemcel-L for acute graft-versus-host disease therapy. Exp. Opin. Biol. Ther. 2014, 14, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Lin, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; She, B.R.; Applegate, L.A. Bringing safe and standardized cell therapies to industrialized processing for burns and wounds. Front. Bioeng. Biotechnol. 2020, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Rezaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two decades of global progress in authorized advanced therapy medicinal products: An emerging revolution in therapeutic strategies. Front. Cell Develop. Biol. 2020, 8, 547653. [Google Scholar] [CrossRef]
- Hunsberger, J.; Harrysson, O.; Shirwaiker, R.; Starly, B.; Wysk, R.; Cohen, P.; Allikson, J.; Yoo, J.; Atala, A. Manufacturing road map for tissue engineering and regenerative medicine technologies. Stem. Cells Transl. Med. 2015, 4, 130–135. [Google Scholar] [CrossRef]
- Johnson, P.C.; Bertram, T.A.; Tawil, B.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A survey of North American academia and industry. Tissue Eng. Part A 2011, 17, 5–15. [Google Scholar] [CrossRef]
- Bertram, T.A.; Tentoff, E.; Johnson, P.C.; Tawil, B.; Van Dyke, M.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A pilot survey of governmental funding agencies and the financial industry. Tissue Eng. Part A 2012, 18, 2187–2194. [Google Scholar] [CrossRef]
- Abdel-Sayed, P.; Michetti, M.; Scaletta, C.; Flahaut, M.; Hirt-Burri, N.; De Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Cell therapies for skin regeneration: An overview of 40 years of experience in burn units. Swiss Med. Wkly. 2019, 149, w20079. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Orthopaedic gene therapy: Twenty-five years on. JBJS Rev. 2021, 9, e20.00220. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Vanderkelen, A.; De Vos, D.; Draye, J.P.; Rose, T.; Ceulemans, C.; Ectors, N.; Huys, I.; Jennes, S.; Verbeken, G. Business oriented EU human cell and tissue product legislation will adversely impact Member States’ health care systems. Cell. Tissue Bank. 2013, 14, 525–560. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.F.; Hildebrandt, M.; Greinix, H.; Scheding, S.; Koehl, U.; Worel, N.; Apperley, J.; Edinger, M.; Hauser, A.; Mischak-Weissinger, E.; et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy 2014, 16, 289–297. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, S.; Veltrop-Duits, L.; Hoozemans-Strik, M.; Ras, T.; Blom-Veenman, J.; Guchelaar, H.J.; Zandvliet, M.; Meij, P. Hurdles in clinical implementation of academic advanced therapy medicinal products: A national evaluation. Cytotherapy 2016, 18, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, J.; de Buys Roessingh, A.S.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue engineered fetal skin constructs for pediatric burns. Lancet 2005, 366, 840–842. [Google Scholar] [CrossRef]
- Al-Dourobi, K.; Laurent, A.; Deghayli, L.; Flahaut, M.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; Waselle, L.; Simon, J.P.; Ezzi, O.E.; et al. Retrospective evaluation of progenitor biological bandage use: A complementary and safe therapeutic management option for prevention of hypertrophic scarring in pediatric burn care. Pharmaceuticals 2021, 14, 201. [Google Scholar] [CrossRef]
- Applegate, L.A.; Weber, D.; Simon, J.P.; Scaletta, C.; Hirt-Burri, N.; de Buys Roessingh, A.S.; Raffoul, W. Organ donation and whole-cell bioprocessing in the Swiss fetal progenitor cell transplantation platform. In Organ Donation and Organ Donors; Saidi, R.F., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 125–147. ISBN 978-1-62618-853-2. [Google Scholar]
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 557758. [Google Scholar] [CrossRef]
- Dimitropoulos, G.; Jafari, P.; de Buys Roessingh, A.; Hirt-Burri, N.; Raffoul, W.; Applegate, L.A. Burn patient care lost in good manufacturing practices? Ann. Burn. Fire Disasters 2016, 29, 111–115. [Google Scholar] [PubMed]
- Hirt-Burri, N.; Scaletta, C.; Gerber, S.; Pioletti, D.P.; Applegate, L.A. Wound-healing gene family expression differences between fetal and foreskin cells used for bioengineered skin substitutes. Atif. Organs 2008, 32, 509–518. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Mohd Abdul Kader Jailani, M.S.; Badrul Hisham, M.; Sunthar Raj, N.; Shamsuddin, S.A.; Ng, M.H.; Fauzi, M.B.; Maarof, M. Cell secretomes for wound healing and tissue regeneration: Next generation acellular based tissue engineered products. J. Tissue Eng. 2022, 13, 20417314221114273. [Google Scholar] [CrossRef]
- Alrubaiy, L.; Al-Rubaiy, K.K. Skin substitutes: A brief review of types and clinical applications. Oman Med. J. 2009, 24, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Wang, Y.; Farinelli, W.A.; Jiménez-Lozano, J.; Franco, W.; Sakamoto, F.H.; Cheung, E.J.; Purschke, M.; Doukas, A.G.; Anderson, R.R. Fractional skin harvesting: Autologous skin grafting without donor-site morbidity. Plast Reconstr. Surg. Glob. Open 2013, 1, e47. [Google Scholar] [CrossRef] [PubMed]
- Kadam, D. Novel expansion techniques for skin grafts. Indian J. Plast Surg. 2016, 49, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Momeni, M.; Fallah, N.; Bajouri, A.; Bagheri, T.; Orouji, Z.; Pahlevanpour, P.; Shafieyan, S.; Sodeifi, N.; Alizadeh, A.; Aghdami, N.; et al. A randomized, double-blind, phase I clinical trial of fetal cell-based skin substitutes on healing of donor sites in burn patients. Burns 2019, 45, 914–922. [Google Scholar] [CrossRef]
- Poinas, A.; Perrot, P.; Lorant, J.; Nerrière, O.; Nguyen, J.M.; Saiagh, S.; Frenard, C.; Leduc, A.; Malard, O.; Espitalier, F.; et al. CICAFAST: Comparison of a biological dressing composed of fetal fibroblasts and keratinocytes on a split-thickness skin graft donor site versus a traditional dressing: A randomized controlled trial. Trials 2019, 20, 612. [Google Scholar] [CrossRef]
- De Buys Roessingh, A.S.; Hohlfeld, J.; Scaletta, C.; Hirt-Burri, N.; Gerber, S.; Hohlfeld, P.; Gebbers, J.O.; Applegate, L.A. Development, characterization, and use of a fetal skin cell bank for tissue engineering in wound healing. Cell Transpl. 2006, 15, 823–834. [Google Scholar] [CrossRef]
- Quintin, A.; Hirt-Burri, N.; Scaletta, C.; Schizas, C.; Pioletti, D.P.; Applegate, L.A. Consistency and safety of cell banks for research and clinical use: Preliminary analysis of fetal skin banks. Cell Transpl. 2007, 16, 675–684. [Google Scholar] [CrossRef]
- Applegate, L.A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; Pioletti, D.P. Whole-cell bioprocessing of human fetal cells for tissue engineering of skin. Skin Pharmacol. Physiol. 2009, 22, 63–73. [Google Scholar] [CrossRef]
- Laurent, A.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; de Buys Roessingh, A.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Biotechnology and cytotherapeutics: The Swiss progenitor-cell transplantation program. Encyclopedia 2022, 2, 336–364. [Google Scholar] [CrossRef]
- Laurent-Applegate, L.A.; Hohlfeld, P. Compositions Comprising Undifferentiated Fetal Cells for the Treatment of Skin Disorders; WIPO: Karlsruhe, Germany, 2003; WIPO, WO03068287A1. [Google Scholar]
- Laurent-Applegate, L.A.; Hohlfeld, P. Fetal Skin Cell Protein Compositions for the Treatment of Skin Conditions, Disorders or Diseases and Methods of Making and Using the Same; WIPO: Karlsruhe, Germany, 2006; WIPO, WO2006092668A2. [Google Scholar]
- De Buys Roessingh, A.S.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Applegate, L.A. A decade after foetal skin progenitor cell therapy in pediatric burn treatment. J. Regen. Med. 2015, 4, 1. [Google Scholar] [CrossRef]
- Ramelet, A.A.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Pioletti, D.P.; Offord, E.; Mansourian, R.; Applegate, L.A. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp. Gerontol. 2009, 44, 208–218. [Google Scholar] [CrossRef]
- Laurent, A.; Darwiche, S.E.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; Laurent, P.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Banking progenitor cells for hippiatric regenerative medicine: Optimized establishment of safe and consistent cell sources for standardized veterinary therapeutic protocols. Am. J. Biomed. Sci. Res. 2020, 8, 252–271. [Google Scholar] [CrossRef]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354 (Suppl. S1), S32–S34. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rasool, B.K.; Al Mahri, N.; Alburaimi, N.; Abdallah, F.; Shamma, A.S.B. A narrative review of the potential roles of lipid-based vesicles (vesiculosomes) in burn management. Sci. Pharm. 2022, 90, 39. [Google Scholar] [CrossRef]
- Laurent, A.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Simon, J.P.; de Buys Roessingh, A.S.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Optimized manufacture of lyophilized dermal fibroblasts for next-generation off-the-shelf progenitor biological bandages in topical post-burn regenerative medicine. Biomedicines 2021, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Kagan, R.J.; Peck, M.D.; Ahrenholz, D.H.; Hickerson, W.L.; Holmes, J.; Korentager, R.; Kraatz, J.; Pollock, K.; Kotoski, G. Surgical management of the burn wound and use of skin substitutes. J. Burn Care Res. 2013, 34, e60–e79. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Herndon, D.N. Burns in children: Standard and new treatments. Lancet 2014, 383, 1168–1178. [Google Scholar] [CrossRef]
- De Graaf, E.; Van Baar, M.E.; Baartmans, M.G.A.; Scholten-Jaegers, S.M.; Nieuwenhuis, M.K.; Eshuis, J.; Hidding, J.; Beerthuizen, G.I.; Van der Vlies, G.H.; Dutch Burn Repository Group; et al. Partial-thickness scalds in children: A comparison of different treatment strategies. Burns 2017, 43, 733–740. [Google Scholar] [CrossRef]
- Hartmann-Fritsch, F.; Marino, D.; Reichmann, E. About ATMPs, SOPs and GMP: The hurdles to produce novel skin grafts for clinical use. Transfus. Med. Hemother. 2016, 43, 344–352. [Google Scholar] [CrossRef]
- Esteban-Vives, R.; Corcos, A.; Choi, M.S.; Young, M.T.; Over, P.; Ziembicki, J.; Gerlach, J.C. Cell-spray auto-grafting technology for deep partial-thickness burns: Problems and solutions during clinical implementation. Burns 2018, 44, 549–559. [Google Scholar] [CrossRef]
- Zuliani, T.; Saiagh, S.; Knol, A.C.; Esbelin, J.; Dréno, B. Fetal fibroblasts and keratinocytes with immunosuppressive properties for allogeneic cell-based wound therapy. PLoS ONE 2013, 8, e70408. [Google Scholar] [CrossRef] [PubMed]
- Meuli, M.; Hartmann-Fritsch, F.; Hüging, M.; Marino, D.; Saglini, M.; Hynes, S.; Neuhaus, K.; Manuel, E.; Middelkoop, E.; Reichmann, E.; et al. A cultured autologous dermo-epidermal skin substitute for full-thickness skin defects: A Phase I, open, prospective clinical trial in children. Plast. Reconstr. Surg. 2019, 144, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Moiemen, N.; Schiestl, C.; Hartmann-Fritsch, F.; Neuhaus, K.; Reichmann, E.; Löw, A.; Stenger, C.; Böttcher-Haberzeth, S.; Meuli, M. First time compassionate use of laboratory engineered autologous Zurich skin in a massively burned child. Burn. Open 2021, 5, 113–117. [Google Scholar] [CrossRef]
- Schiestl, C.; Meuli, M.; Vojvodic, M.; Pontiggia, L.; Neuhaus, D.; Brotschi, B.; Reichmann, E.; Böttcher-Haberzeth, S.; Neuhaus, K. Expanding into the future: Combining a novel dermal template with distinct variants of autologous cultured skin substitutes in massive burns. Burn. Open 2021, 5, 145–153. [Google Scholar] [CrossRef]
- Wong, T.; McGrath, J.A.; Navsaria, H. The role of fibroblasts in tissue engineering and regeneration. Br. J. Dermatol. 2007, 156, 1149–1155. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Marston, W.A.; Snyder, R.J.; Lee, T.D.; Cargill, D.I.; Slade, H.B. Spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: A phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2012, 380, 977–985. [Google Scholar] [CrossRef]
- Chinnici, C.M.; Amico, G.; Monti, M.; Motta, S.; Casalone, R.; Petri, S.L.; Spada, M.; Gridelli, B.; Conaldi, P.G. Isolation and characterization of multipotent cells from human fetal dermis. Cell Transpl. 2014, 23, 1169–1185. [Google Scholar] [CrossRef]
- Markeson, D.; Pleat, J.M.; Sharpe, J.R.; Harris, A.L.; Seifalian, A.M.; Watt, S.M. Scarring, stem cells, scaffolds and skin repair. J. Tissue Eng. Regen. Med. 2015, 9, 649–668. [Google Scholar] [CrossRef]
- Haddad, A.G.; Giatsidis, G.; Orgill, D.P.; Halvorson, E.G. Skin substitutes and bioscaffolds: Temporary and permanent coverage. Clin. Plast. Surg. 2017, 44, 627–634. [Google Scholar] [CrossRef]
- Goodarzi, P.; Falahzadeh, K.; Aghayan, H.; Jahani, F.M.; Payab, M.; Gilany, K.; Rahim, F.; Larijani, B.; Beik, A.T.; Adibi, H.; et al. GMP-compliant human fetal skin fibroblasts for wound healing. Arch. Neurosci. 2018, 5, e68497. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Park, I.S.; Truong, M.D.; Park, D.Y.; Park, S.H.; Min, B.H. Conditioned media derived from human fetal progenitor cells improves skin regeneration in burn wound healing. Cell Tissue Res. 2022, 389, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Gosiewska, A.; Yi, C.F.; Brown, L.J.; Cullen, B.; Silcock, D.; Geesin, J.C. Differential expression and regulation of extracellular matrix-associated genes in fetal and neonatal fibroblasts. Wound Repair Regen. 2001, 9, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.K.; Salgado, G.; Connolly, J.E.; Chan, J.K.; Lane, E.B. Characterization of fetal keratinocytes, showing enhanced stem cell-like properties: A potential source of cells for skin reconstruction. Stem Cell Rep. 2014, 3, 324–338. [Google Scholar] [CrossRef]
- Ishii, T.; Eto, K. Fetal stem cell transplantation: Past, present, and future. World J. Stem Cells 2014, 6, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Akershoek, J.J.; Vlig, M.; Talhout, W.; Boekema, B.K.; Richters, C.D.; Beelen, R.H.; Brouwer, K.M.; Middelkoop, E.; Ulrich, M.M. Cell therapy for full-thickness wounds: Are fetal dermal cells a potential source? Cell Tissue Res. 2016, 364, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, P.; Kaeppeli, A.; Siriwardena, T.; Darbre, T.; Perron, K.; Jafari, P.; Reymond, J.; Pioletti, D.; Applegate, L.A. Anti-microbial dendrimers against multidrug-resistant P. aeruginosa enhance the angiogenic effect of biological burn-wound bandages. Sci. Rep. 2016, 6, 22020. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Mills, S.J.; Smith, L.E.; Strudwick, X.L.; Ting, A.E.; Vaes, B.; Cowin, A.J. Human multipotent adult progenitor cell-conditioned medium improves wound healing through modulating inflammation and angiogenesis in mice. Stem Cell Res. Ther. 2020, 11, 299. [Google Scholar] [CrossRef]
- An, Y.H.; Kim, D.H.; Lee, E.J.; Lee, D.; Park, M.J.; Ko, J.; Kim, D.W.; Koh, J.; Hong, H.S.; Son, Y.; et al. High-efficient production of adipose-derived stem cell (ADSC) secretome through maturation process and its non-scarring wound healing applications. Front. Bioeng. Biotechnol. 2021, 9, 681501. [Google Scholar] [CrossRef]
- Bian, D.; Wu, Y.; Song, G.; Azizi, R.; Zamani, A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: A comprehensive review. Stem Cell Res. Ther. 2022, 13, 24. [Google Scholar] [CrossRef]
- Hacker, S.; Mittermayr, R.; Nickl, S.; Haider, T.; Lebherz-Eichinger, D.; Beer, L.; Mitterbauer, A.; Leiss, H.; Zimmermann, M.; Schweiger, T.; et al. Paracrine factors from irradiated peripheral blood mononuclear cells improve skin regeneration and angiogenesis in a porcine burn model. Sci. Rep. 2016, 6, 25168. [Google Scholar] [CrossRef] [PubMed]
- Klas, K.; Ondracek, A.S.; Hofbauer, T.M.; Mangold, A.; Pfisterer, K.; Laggner, M.; Copic, D.; Direder, M.; Bormann, D.; Ankersmit, H.J.; et al. The effect of paracrine factors released by irradiated peripheral blood mononuclear cells on neutrophil extracellular trap formation. Antioxidants 2022, 11, 1559. [Google Scholar] [CrossRef] [PubMed]
- Laggner, M.; Copic, D.; Nemec, L.; Vorstandlechner, V.; Gugerell, A.; Gruber, F.; Peterbauer, A.; Ankersmit, H.J.; Mildner, M. Therapeutic potential of lipids obtained from γ-irradiated PBMCs in dendritic cell-mediated skin inflammation. EBioMedicine 2020, 55, 102774. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.X.; Chang, T.; Lin, X. Secretomes as an emerging class of bioactive ingredients for enhanced cosmeceutical applications. Exp. Dermatol. 2022, 31, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Traxler, D.; Simader, E.; Beer, L.; Narzt, M.S.; Gruber, F.; Madlener, S.; Laggner, M.; Erb, M.; Vorstandlechner, V.; et al. Different pro-angiogenic potential of γ-irradiated PBMC-derived secretome and its subfractions. Sci. Rep. 2018, 8, 18016. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Abdel-Sayed, P.; Scaletta, C.; Laurent, P.; Laurent, E.; Michetti, M.; de Buys Roessingh, A.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Back to the cradle of cytotherapy: Integrating a century of clinical research and biotechnology-based manufacturing for modern tissue-specific cellular treatments in Switzerland. Bioengineering 2021, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Abdel-Sayed, P.; Ducrot, A.; Hirt-Burri, N.; Scaletta, C.; Jaccoud, S.; Nuss, K.; de Buys Roessingh, A.S.; Raffoul, W.; Pioletti, D.; et al. Development of standardized fetal progenitor cell therapy for cartilage regenerative medicine: Industrial transposition and preliminary safety in xenogeneic transplantation. Biomolecules 2021, 11, 250. [Google Scholar] [CrossRef]
- Laurent, A.; Abdel-Sayed, P.; Grognuz, A.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Kronen, P.; Nuss, K.; et al. Industrial development of standardized fetal progenitor cell therapy for tendon regenerative medicine: Preliminary safety in xenogeneic transplantation. Biomedicines 2021, 9, 380. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Applegate, L.A. Preparation of parental cell bank from foetal tissue; WIPO: Karlsruhe, Germany, 2013; WIPO, WO2013008174A1. [Google Scholar]
| Clinical Study Name | Clinical Study Design | Patient Population Size | Patient Demographics | Type of Wound | Product Used, Cell Types, Formulation, Delivery System | Type of Comparison Treatment | Mean Healing Time | Follow-Up Time | Study Completion Date |
|---|---|---|---|---|---|---|---|---|---|
| “The effects of acellular amniotic membrane loaded by cultured fetal fibroblast cells in split thickness skin wound healing” | Study Type: Interventional Allocation: Randomized Masking: Double-blinded Phase: Phase 1 | 10 (the patient is his own control) | 12–60 years old Sexes: All No healthy volunteers accepted | DSW | Amniotic membrane seeded with fibroblasts; Acellular amniotic membrane; Temporary coverage | Amniotic membrane; vaseline gauze | 12.1 ± 3.1 days | 23 ± 5 days | June, 2015 |
| “TWB-103 for adult patients with split-thickness skin graft donor site wounds” 1 | Study Type: Interventional Phase: Phase 1 and 2 Allocation: Randomized Intervention Model: Parallel Assignment Masking: Triple (Participant, Investigator, Outcomes Assessor) | 48 (24 controls) | 20–65 years old Sexes: All No healthy volunteers accepted | DSW | TWB-103 add-on Tegaderm; Hydrogel seeded with fibroblasts; Temporary coverage | Placebo hydrogel; Tegaderm | From DSW creation to the first 100% re- epithelialization, D42 or earlier | 1 year | 7 May 2021 |
| “Controlled comparison of a traditional dressing versus a biologic dressing composed of fetal fibroblasts and keratinocytes in association with a collagen matrix on skin donor sites (CICAFAST)” | Study Type: Interventional Phase: Phase 1 and 2 Allocation: Randomized Intervention Model: Crossover Assignment Masking: None (Open Label) | 38 (the patient is his own control) | >18 years old Sexes: All No healthy volunteers accepted | DSW | Biological dressing CICAFAST; Bovine collagen matrix of 100 cm2 seeded with fibroblasts and keratinocytes; Temporary coverage | Paraffin gauze; Jelonet | Healing at D8 (or D11 or D15 if the healing is not completed) | 6 months | 16 November 2023 (estimated) |
| “Evaluation of the safety and effectiveness of progenitor biological bandages in burn care” 1 | Study Type: Interventional Phase: Phase 1 and 2 Allocation: Randomized Intervention Model: Parallel Assignment Masking: Single (Participant) | 76 (estimated) | Child, adult, older adult Sexes: All No healthy volunteers accepted | DSW | Progenitor biological bandages (PBB); Equine collagen matrix of 108 cm2 seeded with fibroblasts; Temporary coverage | Jelonet | Maximum of 15 ± 1 days | 5 years | 1 May 2023 (estimated) |
| Type of Biological Bandage | Treatment Indication | Years of Clinical Application | Implicated Clinical Centers | Patients Treated (n) | Clinical Trials & Literature References |
|---|---|---|---|---|---|
| PBB | Primary burn wounds | 2001–Present | CHUV, Lausanne, Switzerland | >100 | NCT05339490 [15,16,30,33] |
| Donor-site wounds | 2001–Present | CHUV, Lausanne, Switzerland | >50 | NCT05339490 [30] | |
| Chronic lower-limb ulcers | 2001–2005 | CHUV, Lausanne, Switzerland; Private medical practice, Switzerland | >15 | [34] | |
| PBI | Primary burn wounds | 2013–2015 | CHUV, Lausanne, Switzerland | 22 | NA (unpublished results) |
| Donor-site wounds | 2013–2015 | CHUV, Lausanne, Switzerland | >10 | NA (unpublished results) | |
| ePBB | Traumatic wounds 1 | 2000–2003 | Private veterinary practice, Switzerland | 4 | [35] |
| Year | Type of Biological Bandage | Number of Patients Treated (n) | Mean PBB Amount/Bandage Exchange Procedure (n) | Mean Total PBB Amount/Treated Patient (n) | ||||
|---|---|---|---|---|---|---|---|---|
| PBB | PBI | Pediatric | Adult | Pediatric | Adult | Pediatric | Adult | |
| 2013 | NA | 40 | 1 | 1 | 20.0 | 20.0 | 20.0 | 20.0 |
| 2014 | NA | 350 | 9 | 1 | 15.7 | 10.0 | 36.7 | 20.0 |
| 2015a | NA | 174 | 2 | 8 | 14.0 | 8.7 | 35.0 | 13.0 |
| 2015b | 152 | NA | 5 | 4 | 11.9 | 11.3 | 21.4 | 11.3 |
| 2016 | 819 | NA | 8 | 9 | 12.8 | 30.9 | 36.8 | 58.3 |
| 2017 1 | 1015 | NA | 6 | 9 | 17.9 | 34.8 | 41.7 | 85.0 |
| 2018 2 | 830 | NA | 4 | 7 | 30.9 | 27.2 | 85.0 | 70.0 |
| 2019 | 492 | NA | 11 | 4 | 8.4 | 19.1 | 29.1 | 43.0 |
| 2020 | 415 | NA | 6 | 5 | 10.9 | 26.7 | 29.2 | 48.0 |
| 2021 | 164 | NA | 10 | 0 | 7.1 | NA | 16.4 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurent, A.; Rey, M.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Raffoul, W.; de Buys Roessingh, A.; Hirt-Burri, N.; Applegate, L.A. Retrospectives on Three Decades of Safe Clinical Experience with Allogeneic Dermal Progenitor Fibroblasts: High Versatility in Topical Cytotherapeutic Care. Pharmaceutics 2023, 15, 184. https://doi.org/10.3390/pharmaceutics15010184
Laurent A, Rey M, Scaletta C, Abdel-Sayed P, Michetti M, Flahaut M, Raffoul W, de Buys Roessingh A, Hirt-Burri N, Applegate LA. Retrospectives on Three Decades of Safe Clinical Experience with Allogeneic Dermal Progenitor Fibroblasts: High Versatility in Topical Cytotherapeutic Care. Pharmaceutics. 2023; 15(1):184. https://doi.org/10.3390/pharmaceutics15010184
Chicago/Turabian StyleLaurent, Alexis, Marina Rey, Corinne Scaletta, Philippe Abdel-Sayed, Murielle Michetti, Marjorie Flahaut, Wassim Raffoul, Anthony de Buys Roessingh, Nathalie Hirt-Burri, and Lee Ann Applegate. 2023. "Retrospectives on Three Decades of Safe Clinical Experience with Allogeneic Dermal Progenitor Fibroblasts: High Versatility in Topical Cytotherapeutic Care" Pharmaceutics 15, no. 1: 184. https://doi.org/10.3390/pharmaceutics15010184
APA StyleLaurent, A., Rey, M., Scaletta, C., Abdel-Sayed, P., Michetti, M., Flahaut, M., Raffoul, W., de Buys Roessingh, A., Hirt-Burri, N., & Applegate, L. A. (2023). Retrospectives on Three Decades of Safe Clinical Experience with Allogeneic Dermal Progenitor Fibroblasts: High Versatility in Topical Cytotherapeutic Care. Pharmaceutics, 15(1), 184. https://doi.org/10.3390/pharmaceutics15010184








