The Trimeric Artesunate Analog TF27, a Broadly Acting Anti-Infective Model Drug, Exerts Pronounced Anti-SARS-CoV-2 Activity Spanning Variants and Host Cell Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Antiviral Compounds
2.2. Cultured Cells, SARS-CoV-2 Isolates and Reporter Viruses, and Fluorescence-Based and Multi-Readout Replication Assays
2.3. Assessment of Drug Interactions Using the Loewe Additivity Fixed Dose Method
3. Results
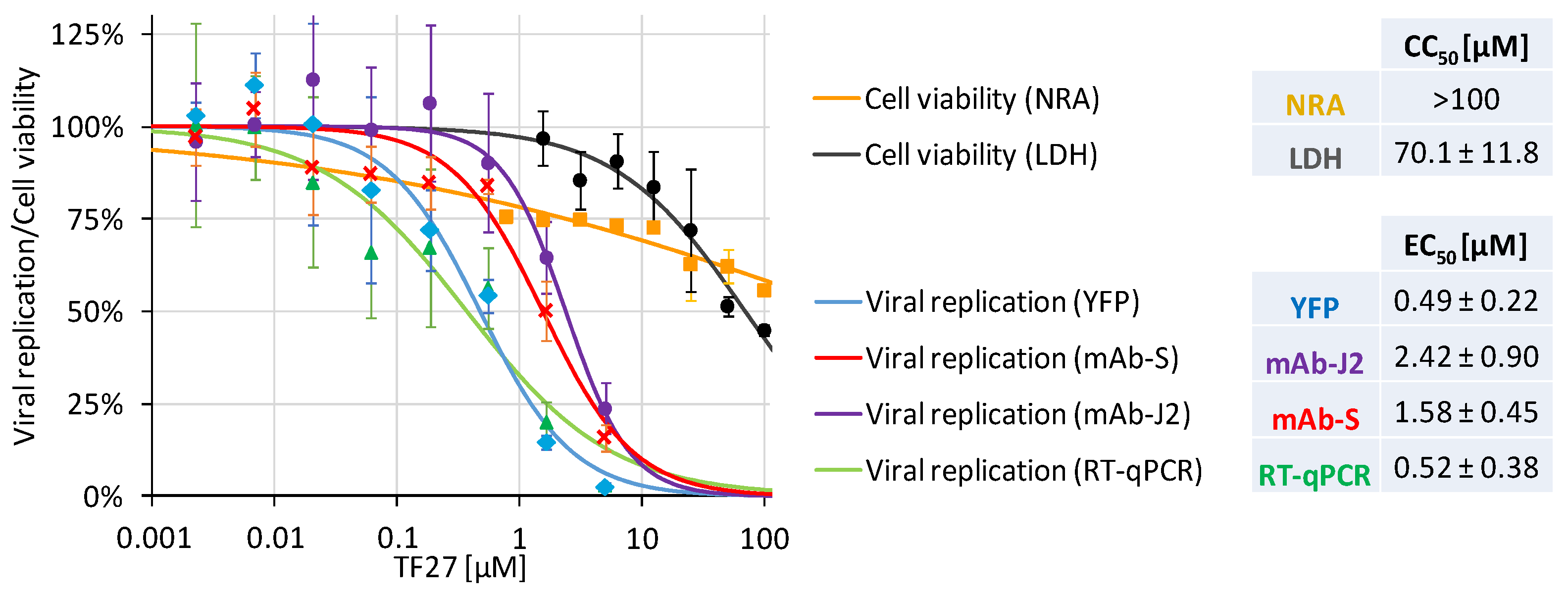
3.1. The Trimeric Artesunate Analog TF27 Inhibits SARS-CoV-2 Replication in Caco-2 Cells
3.2. Pretreatment Efficacy Demonstrates the Targeting of Host Cell Proteins
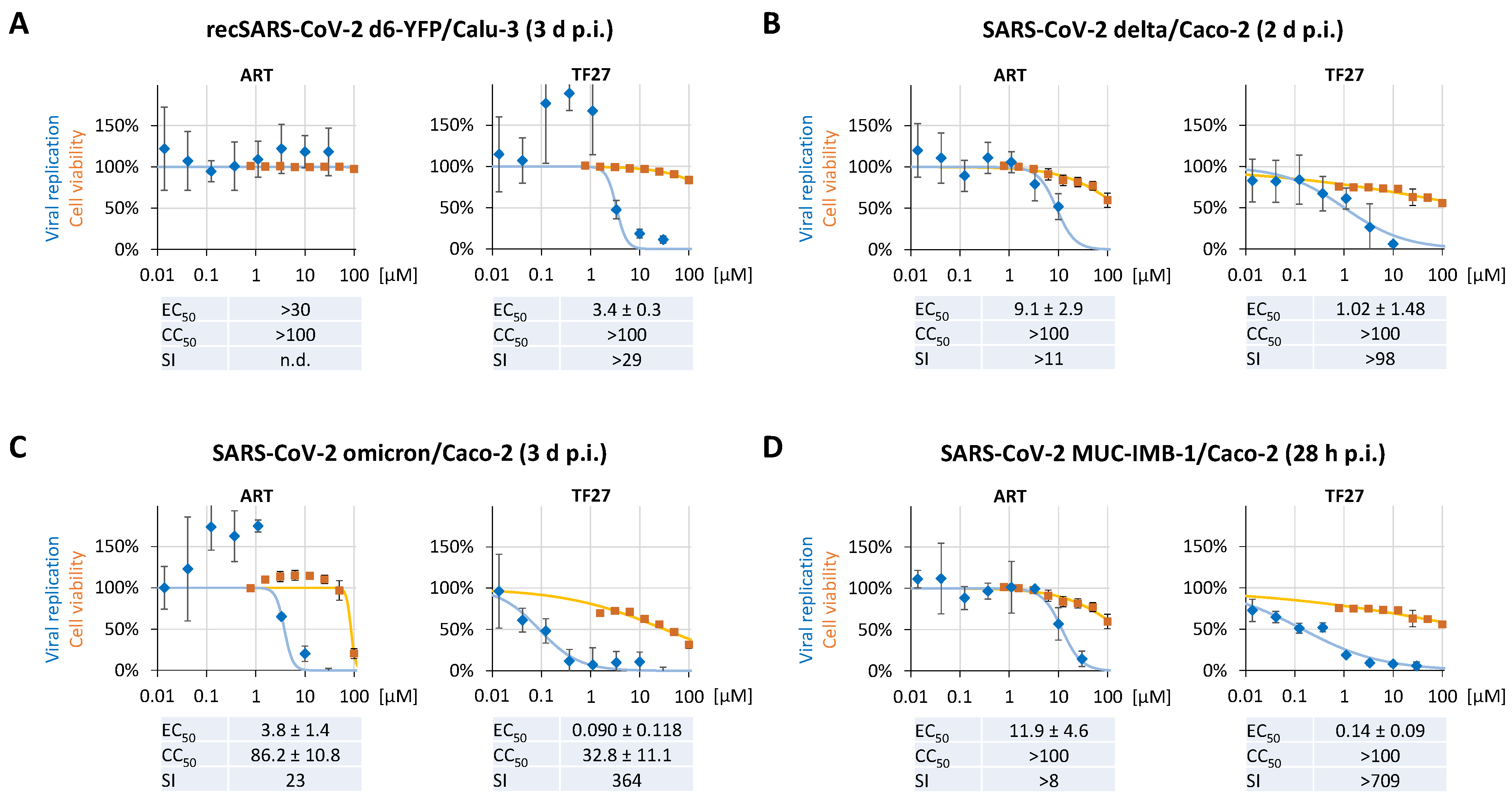
3.3. TF27 Inhibits SARS-CoV-2 Replication in Calu-3 Human Lung Cells and Is Active against Clinical Isolates including Delta and Omicron Variants
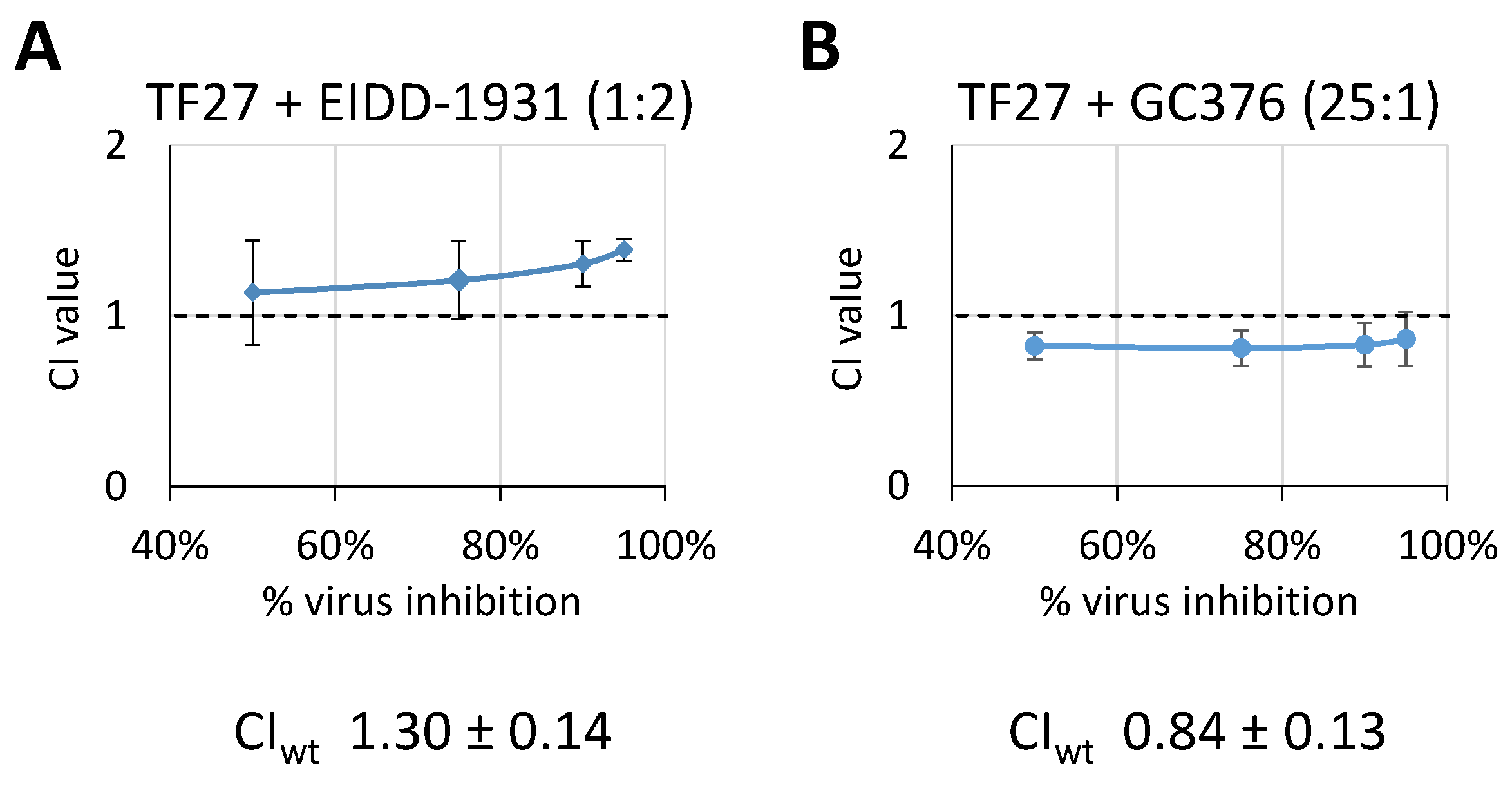
3.4. TF27 Exhibits Synergistic Interaction with GC376 but Antagonistic Interaction with EIDD-1931
4. Discussion
4.1. The Broad-Spectrum Antiviral Potential of Monomeric ART and Trimeric TF27 and Their Putative Relevance for the Development of Novel Anti-SARS-CoV-2 Treatment Strategies
4.2. Mechanistic Properties Based on the Host-Directed Mode of Antiviral Activity of Trimeric TF27, Parental ART, and Related Compounds
4.3. The Chances of Nominating a New Candidate for Studying Antiviral Properties in Clinical Settings and the Question of TF27-Induced Viral Drug Resistance
4.4. The Potential Benefit of TF27 as Part of a Combination Therapy to Achieve Improved Antiviral Efficacy by Exploiting Synergistic Drug Interactions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agostini, M.L.; Pruijssers, A.J.; Chappell, J.D.; Gribble, J.; Lu, X.; Andres, E.L.; Bluemling, G.R.; Lockwood, M.A.; Sheahan, T.P.; Sims, A.C.; et al. Small-Molecule Antiviral beta-d-N (4)-Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance. J. Virol. 2019, 93, e01348-19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hill, C.S.; Sarkar, S.; Tse, L.V.; Woodburn, B.M.D.; Schinazi, R.F.; Sheahan, T.P.; Baric, R.S.; Heise, M.T.; Swanstrom, R. beta-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. J. Infect. Dis. 2021, 224, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Casteels, T.; Frogne, T.; Ingvorsen, C.; Honore, C.; Courtney, M.; Huber, K.V.M.; Schmitner, N.; Kimmel, R.A.; Romanov, R.A.; et al. Artemisinins Target GABAA Receptor Signaling and Impair alpha Cell Identity. Cell 2017, 168, 86–100.e15. [Google Scholar] [CrossRef]
- Augustin, Y.; Staines, H.M.; Krishna, S. Artemisinins as a novel anti-cancer therapy: Targeting a global cancer pandemic through drug repurposing. Pharmacol. Ther. 2020, 216, 107706. [Google Scholar] [CrossRef]
- Khanal, P. Antimalarial and anticancer properties of artesunate and other artemisinins: Current development. Monatsh. Chem. 2021, 152, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wan, J.; Zhang, M.; Li, C.; Lin, J. Artesunate: A review of its therapeutic insights in respiratory diseases. Phytomedicine 2022, 104, 154259. [Google Scholar] [CrossRef]
- Chou, S.; Marousek, G.; Auerochs, S.; Stamminger, T.; Milbradt, J.; Marschall, M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antivir. Res 2011, 92, 364–368. [Google Scholar] [CrossRef]
- Kaptein, S.J.; Efferth, T.; Leis, M.; Rechter, S.; Auerochs, S.; Kalmer, M.; Bruggeman, C.A.; Vink, C.; Stamminger, T.; Marschall, M. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antivir. Res. 2006, 69, 60–69. [Google Scholar] [CrossRef]
- Hutterer, C.; Niemann, I.; Milbradt, J.; Frohlich, T.; Reiter, C.; Kadioglu, O.; Bahsi, H.; Zeittrager, I.; Wagner, S.; Einsiedel, J.; et al. The broad-spectrum antiinfective drug artesunate interferes with the canonical nuclear factor kappa B (NF-kappaB) pathway by targeting RelA/p65. Antivir. Res. 2015, 124, 101–109. [Google Scholar] [CrossRef]
- Auerochs, S.; Korn, K.; Marschall, M. A reporter system for Epstein-Barr virus (EBV) lytic replication: Anti-EBV activity of the broad anti-herpesviral drug artesunate. J. Virol. Methods 2011, 173, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Bertzbach, L.D.; Conradie, A.M.; Hahn, F.; Wild, M.; Marschall, M.; Kaufer, B.B. Artesunate derivative TF27 inhibits replication and pathogenesis of an oncogenic avian alphaherpesvirus. Antivir. Res. 2019, 171, 104606. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.N.; Marschall, M.; Henriksen, S.; Rinaldo, C.H. Antiviral effects of artesunate on polyomavirus BK replication in primary human kidney cells. Antimicrob. Agents Chemother. 2014, 58, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.N.; Marschall, M.; Rinaldo, C.H. Antiviral effects of artesunate on JC polyomavirus replication in COS-7 cells. Antimicrob. Agents Chemother. 2014, 58, 6724–6734. [Google Scholar] [CrossRef]
- Milbradt, J.; Auerochs, S.; Korn, K.; Marschall, M. Sensitivity of human herpesvirus 6 and other human herpesviruses to the broad-spectrum antiinfective drug artesunate. J. Clin. Virol. 2009, 46, 24–28. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Maseko, R.B.; Aderibigbe, B.A. Recent Advances in the Therapeutic Efficacy of Artesunate. Pharmaceutics 2022, 14, 504. [Google Scholar] [CrossRef]
- Obeid, S.; Alen, J.; Nguyen, V.H.; Pham, V.C.; Meuleman, P.; Pannecouque, C.; Le, T.N.; Neyts, J.; Dehaen, W.; Paeshuyse, J. Artemisinin analogues as potent inhibitors of in vitro hepatitis C virus replication. PLoS ONE 2013, 8, e81783. [Google Scholar] [CrossRef]
- Frohlich, T.; Hahn, F.; Belmudes, L.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Coute, Y.; Marschall, M.; Tsogoeva, S.B. Synthesis of Artemisinin-Derived Dimers, Trimers and Dendrimers: Investigation of Their Antimalarial and Antiviral Activities Including Putative Mechanisms of Action. Chemistry 2018, 24, 8103–8113. [Google Scholar] [CrossRef]
- Hahn, F.; Frohlich, T.; Frank, T.; Bertzbach, L.D.; Kohrt, S.; Kaufer, B.B.; Stamminger, T.; Tsogoeva, S.B.; Marschall, M. Artesunate-derived monomeric, dimeric and trimeric experimental drugs—Their unique mechanistic basis and pronounced antiherpesviral activity. Antivir. Res. 2018, 152, 104–110. [Google Scholar] [CrossRef]
- Reiter, C.; Frohlich, T.; Gruber, L.; Hutterer, C.; Marschall, M.; Voigtlander, C.; Friedrich, O.; Kappes, B.; Efferth, T.; Tsogoeva, S.B. Highly potent artemisinin-derived dimers and trimers: Synthesis and evaluation of their antimalarial, antileukemia and antiviral activities. Bioorg. Med. Chem. 2015, 23, 5452–5458. [Google Scholar] [CrossRef]
- Frohlich, T.; Kiss, A.; Wolfling, J.; Mernyak, E.; Kulmany, A.E.; Minorics, R.; Zupko, I.; Leidenberger, M.; Friedrich, O.; Kappes, B.; et al. Synthesis of Artemisinin-Estrogen Hybrids Highly Active against HCMV, P. falciparum, and Cervical and Breast Cancer. ACS Med. Chem. Lett. 2018, 9, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Hage, S.; Herrmann, A.; Wangen, C.; Kicuntod, J.; Jungnickl, D.; Tillmanns, J.; Muller, R.; Fraedrich, K.; Uberla, K.; et al. Methodological Development of a Multi-Readout Assay for the Assessment of Antiviral Drugs against SARS-CoV-2. Pathogens 2021, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, T.; Reiter, C.; Saeed, M.E.M.; Hutterer, C.; Hahn, F.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Marschall, M.; Efferth, T.; et al. Synthesis of Thymoquinone-Artemisinin Hybrids: New Potent Antileukemia, Antiviral, and Antimalarial Agents. ACS Med. Chem. Lett. 2018, 9, 534–539. [Google Scholar] [CrossRef]
- Capci Karagoz, A.; Reiter, C.; Seo, E.J.; Gruber, L.; Hahn, F.; Leidenberger, M.; Klein, V.; Hampel, F.; Friedrich, O.; Marschall, M.; et al. Access to new highly potent antileukemia, antiviral and antimalarial agents via hybridization of natural products (homo)egonol, thymoquinone and artemisinin. Bioorg. Med. Chem. 2018, 26, 3610–3618. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, A.C.; Leidenberger, M.; Hahn, F.; Hampel, F.; Friedrich, O.; Marschall, M.; Kappes, B.; Tsogoeva, S.B. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorg. Med. Chem. 2019, 27, 110–115. [Google Scholar] [CrossRef]
- Sonntag, E.; Hahn, F.; Bertzbach, L.D.; Seyler, L.; Wangen, C.; Muller, R.; Tannig, P.; Grau, B.; Baumann, M.; Zent, E.; et al. In vivo proof-of-concept for two experimental antiviral drugs, both directed to cellular targets, using a murine cytomegalovirus model. Antivir. Res. 2019, 161, 63–69. [Google Scholar] [CrossRef]
- Wild, M.; Bertzbach, L.D.; Tannig, P.; Wangen, C.; Muller, R.; Herrmann, L.; Frohlich, T.; Tsogoeva, S.B.; Kaufer, B.B.; Marschall, M.; et al. The trimeric artesunate derivative TF27 exerts strong anti-cytomegaloviral efficacy: Focus on prophylactic efficacy and oral treatment of immunocompetent mice. Antivir. Res. 2020, 178, 104788. [Google Scholar] [CrossRef]
- Hahn, F.; Hamilton, S.T.; Wangen, C.; Wild, M.; Kicuntod, J.; Bruckner, N.; Follett, J.E.L.; Herrmann, L.; Kheimar, A.; Kaufer, B.B.; et al. Development of a PROTAC-Based Targeting Strategy Provides a Mechanistically Unique Mode of Anti-Cytomegalovirus Activity. Int. J. Mol. Sci. 2021, 22, 12858. [Google Scholar] [CrossRef]
- Herrmann, A.; Jungnickl, D.; Cordsmeier, A.; Peter, A.S.; Uberla, K.; Ensser, A. Cloning of a Passage-Free SARS-CoV-2 Genome and Mutagenesis Using Red Recombination. Int. J. Mol. Sci. 2021, 22, 10188. [Google Scholar] [CrossRef]
- Peter, A.S.; Roth, E.; Schulz, S.R.; Fraedrich, K.; Steinmetz, T.; Damm, D.; Hauke, M.; Richel, E.; Mueller-Schmucker, S.; Habenicht, K.; et al. A pair of noncompeting neutralizing human monoclonal antibodies protecting from disease in a SARS-CoV-2 infection model. Eur. J. Immunol. 2022, 52, 770–783. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Hu, H.; Li, Y.; Wang, X.; Xu, M.; Liu, J.; Zhang, H.; Yan, Y.; Zhao, L.; Li, W.; et al. Anti-SARS-CoV-2 Potential of Artemisinins In Vitro. ACS Infect. Dis. 2020, 6, 2524–2531. [Google Scholar] [CrossRef]
- Gendrot, M.; Andreani, J.; Boxberger, M.; Jardot, P.; Fonta, I.; Le Bideau, M.; Duflot, I.; Mosnier, J.; Rolland, C.; Bogreau, H.; et al. Antimalarial drugs inhibit the replication of SARS-CoV-2: An in vitro evaluation. Travel Med. Infect. Dis. 2020, 37, 101873. [Google Scholar] [CrossRef]
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Polyak, S.J.; Wagoner, J.; Towler, M.J.; Weathers, P.J. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 2021, 274, 114016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gilmore, K.; Ramirez, S.; Settels, E.; Gammeltoft, K.A.; Pham, L.V.; Fahnoe, U.; Feng, S.; Offersgaard, A.; Trimpert, J.; et al. In vitro efficacy of artemisinin-based treatments against SARS-CoV-2. Sci. Rep. 2021, 11, 14571. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Trimpert, J.; Moon, S.; Haag, R.; Gilmore, K.; Kaufer, B.B.; Seeberger, P.H. In vitro efficacy of Artemisia extracts against SARS-CoV-2. Virol. J. 2021, 18, 182. [Google Scholar] [CrossRef]
- Touret, F.; Gilles, M.; Barral, K.; Nougairede, A.; van Helden, J.; Decroly, E.; de Lamballerie, X.; Coutard, B. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020, 10, 13093. [Google Scholar] [CrossRef]
- Held, F.E.; Guryev, A.A.; Frohlich, T.; Hampel, F.; Kahnt, A.; Hutterer, C.; Steingruber, M.; Bahsi, H.; von Bojnicic-Kninski, C.; Mattes, D.S.; et al. Facile access to potent antiviral quinazoline heterocycles with fluorescence properties via merging metal-free domino reactions. Nat. Commun. 2017, 8, 15071. [Google Scholar] [CrossRef]
- Jacquet, C.; Marschall, M.; Andouard, D.; El Hamel, C.; Chianea, T.; Tsogoeva, S.B.; Hantz, S.; Alain, S. A highly potent trimeric derivative of artesunate shows promising treatment profiles in experimental models for congenital HCMV infection in vitro and ex vivo. Antivir. Res. 2020, 175, 104700. [Google Scholar] [CrossRef]
- Wild, M.; Hahn, F.; Grau, B.; Herrmann, L.; Niesar, A.; Schutz, M.; Lorion, M.M.; Ackermann, L.; Tsogoeva, S.B.; Marschall, M. The Artemisinin-Derived Autofluorescent Compound BG95 Exerts Strong Anticytomegaloviral Activity Based on a Mitochondrial Targeting Mechanism. Int. J. Mol. Sci. 2020, 21, 5578. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Niesar, A.; Wangen, C.; Wild, M.; Grau, B.; Herrmann, L.; Capci, A.; Adrait, A.; Coute, Y.; Tsogoeva, S.B.; et al. Target verification of artesunate-related antiviral drugs: Assessing the role of mitochondrial and regulatory proteins by click chemistry and fluorescence labeling. Antivir. Res. 2020, 180, 104861. [Google Scholar] [CrossRef] [PubMed]
- Wild, M.; Kicuntod, J.; Seyler, L.; Wangen, C.; Bertzbach, L.D.; Conradie, A.M.; Kaufer, B.B.; Wagner, S.; Michel, D.; Eickhoff, J.; et al. Combinatorial Drug Treatments Reveal Promising Anticytomegaloviral Profiles for Clinically Relevant Pharmaceutical Kinase Inhibitors (PKIs). Int. J. Mol. Sci. 2021, 22, 575. [Google Scholar] [CrossRef]
- Schönborn, J.; Oberstrass, J.; Breyel, E.; Tittgen, J.; Schumacher, J.; Lukacs, N. Monoclonal antibodies to double-stranded RNA as probes of RNA structure in crude nucleic acid extracts. Nucleic Acids Res. 1991, 19, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.C.; Ho, W.E.; Cheng, C.; Godoy, L.C.; Wishnok, J.S.; Ong, C.N.; Wong, W.S.; Wogan, G.N.; Tannenbaum, S.R. Untargeted Proteomics and Systems-Based Mechanistic Investigation of Artesunate in Human Bronchial Epithelial Cells. Chem. Res. Toxicol. 2015, 28, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Xiao, Y. Profiling of Multiple Targets of Artemisinin Activated by Hemin in Cancer Cell Proteome. ACS Chem. Biol. 2016, 11, 882–888. [Google Scholar] [CrossRef]
- Wu, W.M.; Chen, Y.L.; Zhai, Z.; Xiao, S.H.; Wu, Y.L. Study on the mechanism of action of artemether against schistosomes: The identification of cysteine adducts of both carbon-centred free radicals derived from artemether. Bioorg. Med. Chem. Lett. 2003, 13, 1645–1647. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.J.; Chia, W.N.; Loh, C.C.; Li, Z.; Lee, Y.M.; He, Y.; Yuan, L.X.; Lim, T.K.; Liu, M.; et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015, 6, 10111. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Asawamahasakda, W.; Meshnick, S.R. Alkylation of human albumin by the antimalarial artemisinin. Biochem. Pharmacol. 1993, 46, 336–339. [Google Scholar] [CrossRef]
- Mautner, L.; Hoyos, M.; Dangel, A.; Berger, C.; Ehrhardt, A.; Baiker, A. Replication kinetics and infectivity of SARS-CoV-2 variants of concern in common cell culture models. Virol. J. 2022, 19, 76. [Google Scholar] [CrossRef]
- Bojkova, D.; Reus, P.; Panosch, L.; Bechtel, M.; Rothenburger, T.; Kandler, J.; Pfeiffer, A.; Wagner, J.U.G.; Shumliakivska, M.; Dimmeler, S.; et al. Identification of novel antiviral drug candidates using an optimized SARS-CoV-2 phenotypic screening platform. bioRxiv 2022. [Google Scholar] [CrossRef]
- Drouot, E.; Piret, J.; Boivin, G. Artesunate demonstrates in vitro synergism with several antiviral agents against human cytomegalovirus. Antivir. Ther. 2016, 21, 535–539. [Google Scholar] [CrossRef]
- He, R.; Park, K.; Cai, H.; Kapoor, A.; Forman, M.; Mott, B.; Posner, G.H.; Arav-Boger, R. Artemisinin-derived dimer diphenyl phosphate is an irreversible inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 2012, 56, 3508–3515. [Google Scholar] [CrossRef]
- Oiknine-Djian, E.; Bar-On, S.; Laskov, I.; Lantsberg, D.; Haynes, R.K.; Panet, A.; Wolf, D.G. Artemisone demonstrates synergistic antiviral activity in combination with approved and experimental drugs active against human cytomegalovirus. Antivir. Res. 2019, 172, 104639. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Forman, M.; Mott, B.T.; Venkatadri, R.; Posner, G.H.; Arav-Boger, R. Unique and highly selective anticytomegalovirus activities of artemisinin-derived dimer diphenyl phosphate stem from combination of dimer unit and a diphenyl phosphate moiety. Antimicrob. Agents Chemother. 2013, 57, 4208–4214. [Google Scholar] [CrossRef] [PubMed]
- Oiknine-Djian, E.; Weisblum, Y.; Panet, A.; Wong, H.N.; Haynes, R.K.; Wolf, D.G. The Artemisinin Derivative Artemisone Is a Potent Inhibitor of Human Cytomegalovirus Replication. Antimicrob. Agents Chemother. 2018, 62, e00288-18. [Google Scholar] [CrossRef]
- Cai, H.; Kapoor, A.; He, R.; Venkatadri, R.; Forman, M.; Posner, G.H.; Arav-Boger, R. In vitro combination of anti-cytomegalovirus compounds acting through different targets: Role of the slope parameter and insights into mechanisms of Action. Antimicrob. Agents Chemother. 2014, 58, 986–994. [Google Scholar] [CrossRef]
- Wang, Y.; Mukhopadhyay, R.; Roy, S.; Kapoor, A.; Su, Y.P.; Charman, S.A.; Chen, G.; Wu, J.; Wang, X.; Vennerstrom, J.L.; et al. Inhibition of Cytomegalovirus Replication with Extended-Half-Life Synthetic Ozonides. Antimicrob. Agents Chemother. 2019, 63, e01735-18. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Fuzimoto, A.D. An overview of the anti-SARS-CoV-2 properties of Artemisia annua, its antiviral action, protein-associated mechanisms, and repurposing for COVID-19 treatment. J. Integr. Med. 2021, 19, 375–388. [Google Scholar] [CrossRef]
- Wagoner, J.; Herring, S.; Hsiang, T.Y.; Ianevski, A.; Biering, S.B.; Xu, S.; Hoffmann, M.; Pohlmann, S.; Gale, M., Jr.; Aittokallio, T.; et al. Combinations of Host- and Virus-Targeting Antiviral Drugs Confer Synergistic Suppression of SARS-CoV-2. Microbiol. Spectr. 2022, 10, e0333122. [Google Scholar] [CrossRef] [PubMed]
- Bafna, K.; White, K.; Harish, B.; Rosales, R.; Ramelot, T.A.; Acton, T.B.; Moreno, E.; Kehrer, T.; Miorin, L.; Royer, C.A.; et al. Hepatitis C virus drugs that inhibit SARS-CoV-2 papain-like protease synergize with remdesivir to suppress viral replication in cell culture. Cell Rep. 2021, 35, 109133. [Google Scholar] [CrossRef] [PubMed]
- Gidari, A.; Sabbatini, S.; Schiaroli, E.; Bastianelli, S.; Pierucci, S.; Busti, C.; Comez, L.; Libera, V.; Macchiarulo, A.; Paciaroni, A.; et al. The Combination of Molnupiravir with Nirmatrelvir or GC376 Has a Synergic Role in the Inhibition of SARS-CoV-2 Replication In Vitro. Microorganisms 2022, 10, 1475. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Lavrijsen, M.; Lamers, M.M.; de Vries, A.C.; Rottier, R.J.; Bruno, M.J.; Peppelenbosch, M.P.; Haagmans, B.L.; Pan, Q. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022, 32, 322–324. [Google Scholar] [CrossRef] [PubMed]
- De Forni, D.; Poddesu, B.; Cugia, G.; Chafouleas, J.; Lisziewicz, J.; Lori, F. Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients. PLoS ONE 2022, 17, e0276751. [Google Scholar] [CrossRef]
- Jin, W.; Stokes, J.M.; Eastman, R.T.; Itkin, Z.; Zakharov, A.V.; Collins, J.J.; Jaakkola, T.S.; Barzilay, R. Deep learning identifies synergistic drug combinations for treating COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105070118. [Google Scholar] [CrossRef]
- Bojkova, D.; Stack, R.; Rothenburger, T.; Kandler, J.D.; Ciesek, S.; Wass, M.N.; Michaelis, M.; Cinatl, J., Jr. Synergism of interferon-beta with antiviral drugs against SARS-CoV-2 variants. J. Infect. 2022, 85, 573–607. [Google Scholar] [CrossRef]
- Hahn, F.; Wangen, C.; Hage, S.; Peter, A.S.; Dobler, G.; Hurst, B.; Julander, J.; Fuchs, J.; Ruzsics, Z.; Uberla, K.; et al. IMU-838, a Developmental DHODH Inhibitor in Phase II for Autoimmune Disease, Shows Anti-SARS-CoV-2 and Broad-Spectrum Antiviral Efficacy In Vitro. Viruses 2020, 12, 1394. [Google Scholar] [CrossRef]
- Schultz, D.C.; Johnson, R.M.; Ayyanathan, K.; Miller, J.; Whig, K.; Kamalia, B.; Dittmar, M.; Weston, S.; Hammond, H.L.; Dillen, C.; et al. Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2. Nature 2022, 604, 134–140. [Google Scholar] [CrossRef]
- Stegmann, K.M.; Dickmanns, A.; Heinen, N.; Blaurock, C.; Karrasch, T.; Breithaupt, A.; Klopfleisch, R.; Uhlig, N.; Eberlein, V.; Issmail, L.; et al. Inhibitors of dihydroorotate dehydrogenase cooperate with molnupiravir and N4-hydroxycytidine to suppress SARS-CoV-2 replication. iScience 2022, 25, 104293. [Google Scholar] [CrossRef]
- Min, L.; Sun, Q. A promising strategy against SARS-CoV-2: Pyrimidine inhibitors synergize with nucleoside analogues. Signal Transduct. Target. Ther. 2022, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Bobrowski, T.; Chen, L.; Eastman, R.T.; Itkin, Z.; Shinn, P.; Chen, C.Z.; Guo, H.; Zheng, W.; Michael, S.; Simeonov, A.; et al. Synergistic and Antagonistic Drug Combinations against SARS-CoV-2. Mol. Ther. 2021, 29, 873–885. [Google Scholar] [CrossRef] [PubMed]


| Trioxane Compounds | Type of Investigation | Antiviral Activity Analyzed | References |
|---|---|---|---|
| Artesunate and artemisinin | Review article | Various anti-herpesviral and non-herpesviral activities | [6] |
| Artesunate | HHV-6A in cultured cells | Anti-HHV-6 activity | [15] |
| Artesunate | EBV reporter system | Anti-EBV activity | [11] |
| Artesunate and derivatives | Antiviral/mechanistic study | Broad-spectrum and NK-κB targeting | [10] |
| Hybrid compounds | Chemistry, confocal imaging | Anti-HCMV and intracellular trafficking | [39] |
| Trimers, dimers, and monomers | Antiviral/mechanistic study | TF27 unique, and strongest anti-HCMV drug | [19] |
| TF27, analogs/dendrimers | Comparing bioactivities | TF27 strongest antiviral activity, and target ID | [18] |
| TF27 | MDV/chicken model | Inhibits MDV replication and tumorigenesis | [12] |
| TF27 | MCMV/mouse model | Intraperitoneal MCMV treatment efficacy | [26] |
| TF27 | cCMV ex vivo model | Anti-cCMV high efficacy | [40] |
| TF27 | MCMV/mouse model | Anti-MCMV oral prophylactic efficacy | [27] |
| Autofluorescent BG95 | Confocal imaging a.o. | Anti-HCMV and mitochondrial targeting | [41] |
| Linker model compounds | Target ID and verification | Mitochondrial/regulatory proteins as targets | [42] |
| TF27 | Drug combination assessment | No true synergy TF27 + GCV | [43] |
| TF27 compared to artesunate | Multi-readout system | Strong anti-SARS-CoV-2 activity | Present study |
| HCMV/HFFs | SARS-CoV-2/Caco-2 | |||||||
|---|---|---|---|---|---|---|---|---|
| EC50 [µM] | CC50 [µM] | SI | Fold Increase Relative to ART | EC50 [µM] | CC50 [µM] | SI | Fold Increase Relative to ART | |
| ART | 5.4 ± 0.6 | >10 | >2 | 1 | >40 | >100 | n.d. | 1 |
| TF27 | 0.04 ± 0.01 | >10 | >250 | 113 | 0.46 ± 0.20 | >100 | >185 | >87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, F.; Wangen, C.; Häge, S.; Herrmann, L.; Herrmann, A.; Tsogoeva, S.B.; Marschall, M. The Trimeric Artesunate Analog TF27, a Broadly Acting Anti-Infective Model Drug, Exerts Pronounced Anti-SARS-CoV-2 Activity Spanning Variants and Host Cell Types. Pharmaceutics 2023, 15, 115. https://doi.org/10.3390/pharmaceutics15010115
Hahn F, Wangen C, Häge S, Herrmann L, Herrmann A, Tsogoeva SB, Marschall M. The Trimeric Artesunate Analog TF27, a Broadly Acting Anti-Infective Model Drug, Exerts Pronounced Anti-SARS-CoV-2 Activity Spanning Variants and Host Cell Types. Pharmaceutics. 2023; 15(1):115. https://doi.org/10.3390/pharmaceutics15010115
Chicago/Turabian StyleHahn, Friedrich, Christina Wangen, Sigrun Häge, Lars Herrmann, Alexandra Herrmann, Svetlana B. Tsogoeva, and Manfred Marschall. 2023. "The Trimeric Artesunate Analog TF27, a Broadly Acting Anti-Infective Model Drug, Exerts Pronounced Anti-SARS-CoV-2 Activity Spanning Variants and Host Cell Types" Pharmaceutics 15, no. 1: 115. https://doi.org/10.3390/pharmaceutics15010115
APA StyleHahn, F., Wangen, C., Häge, S., Herrmann, L., Herrmann, A., Tsogoeva, S. B., & Marschall, M. (2023). The Trimeric Artesunate Analog TF27, a Broadly Acting Anti-Infective Model Drug, Exerts Pronounced Anti-SARS-CoV-2 Activity Spanning Variants and Host Cell Types. Pharmaceutics, 15(1), 115. https://doi.org/10.3390/pharmaceutics15010115







