In-Depth Analysis of Physiologically Based Pharmacokinetic (PBPK) Modeling Utilization in Different Application Fields Using Text Mining Tools
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Method
2.2. Eligibility Criteria
2.3. Text Preprocessing
2.4. Widgets to Determine Word Counts, Frequency and Significance
2.5. Topic Modeling
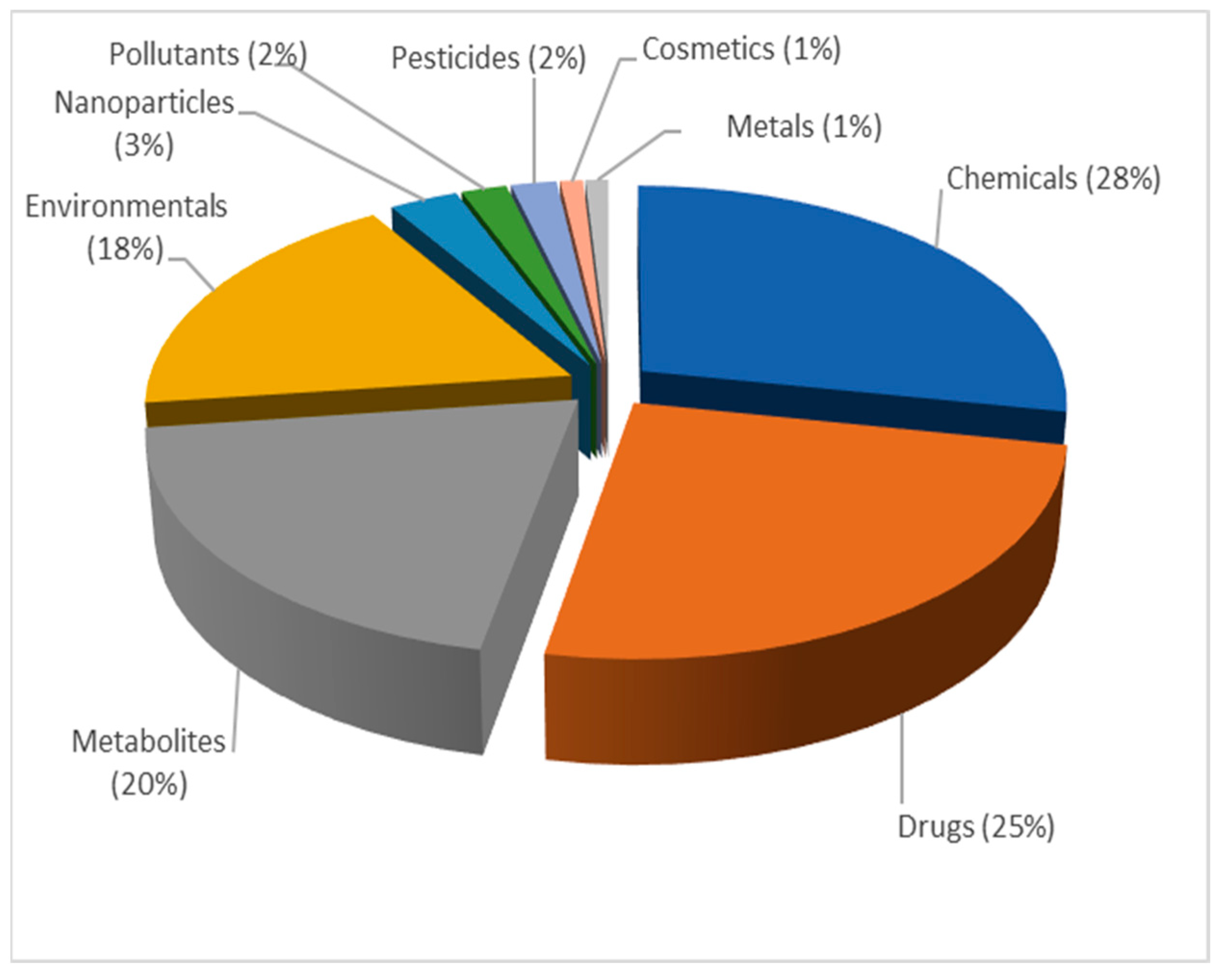
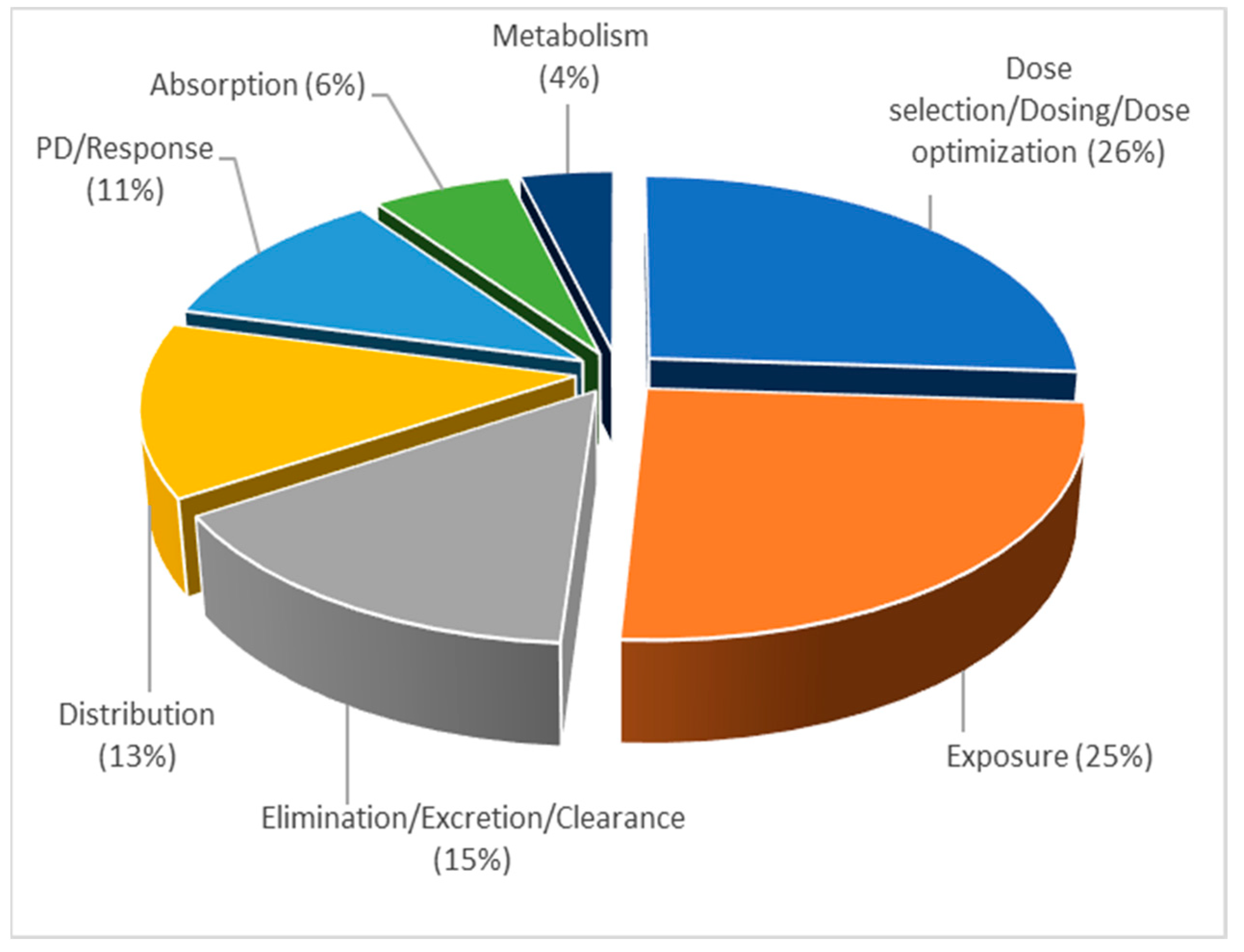
3. Results and Discussion
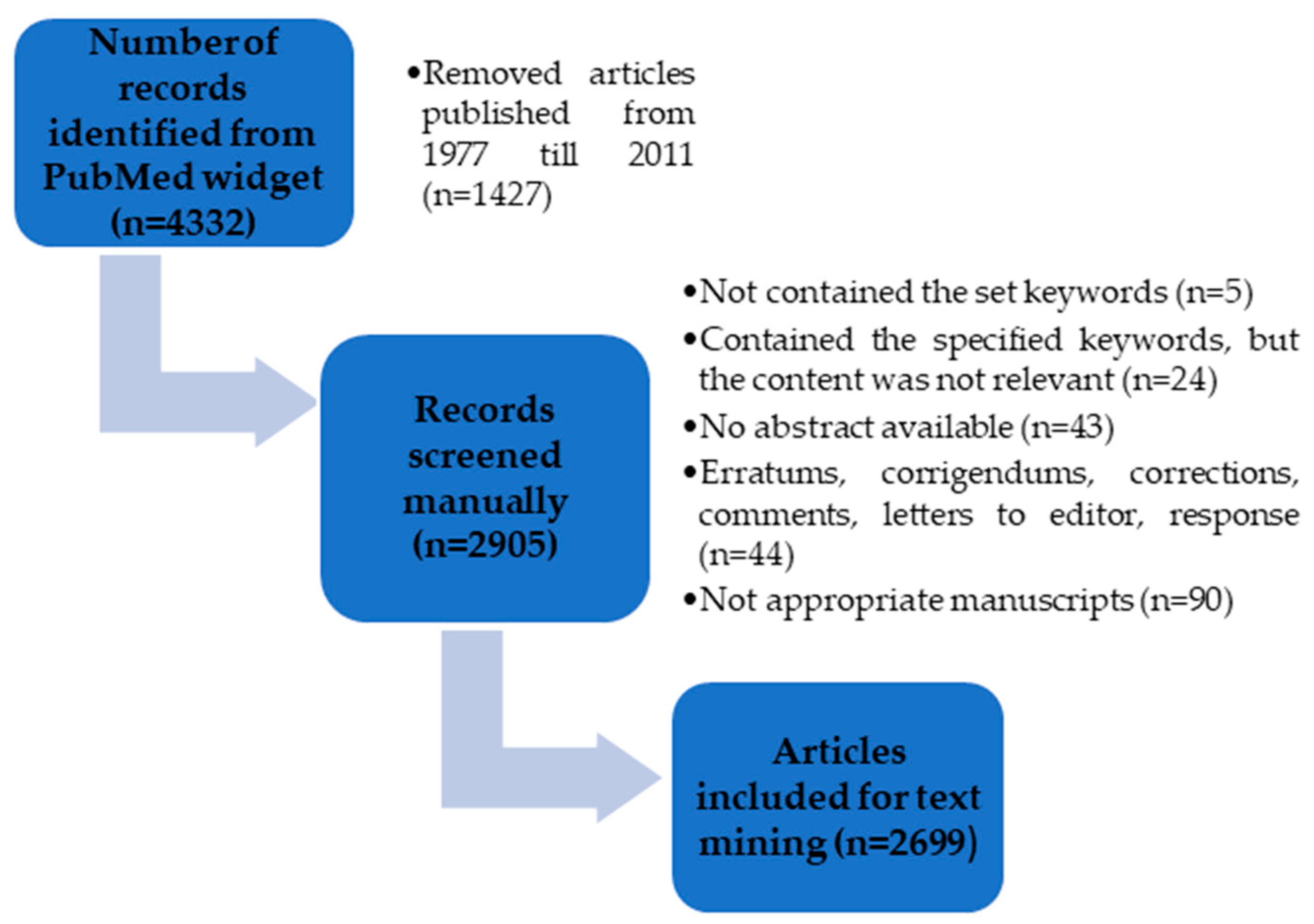
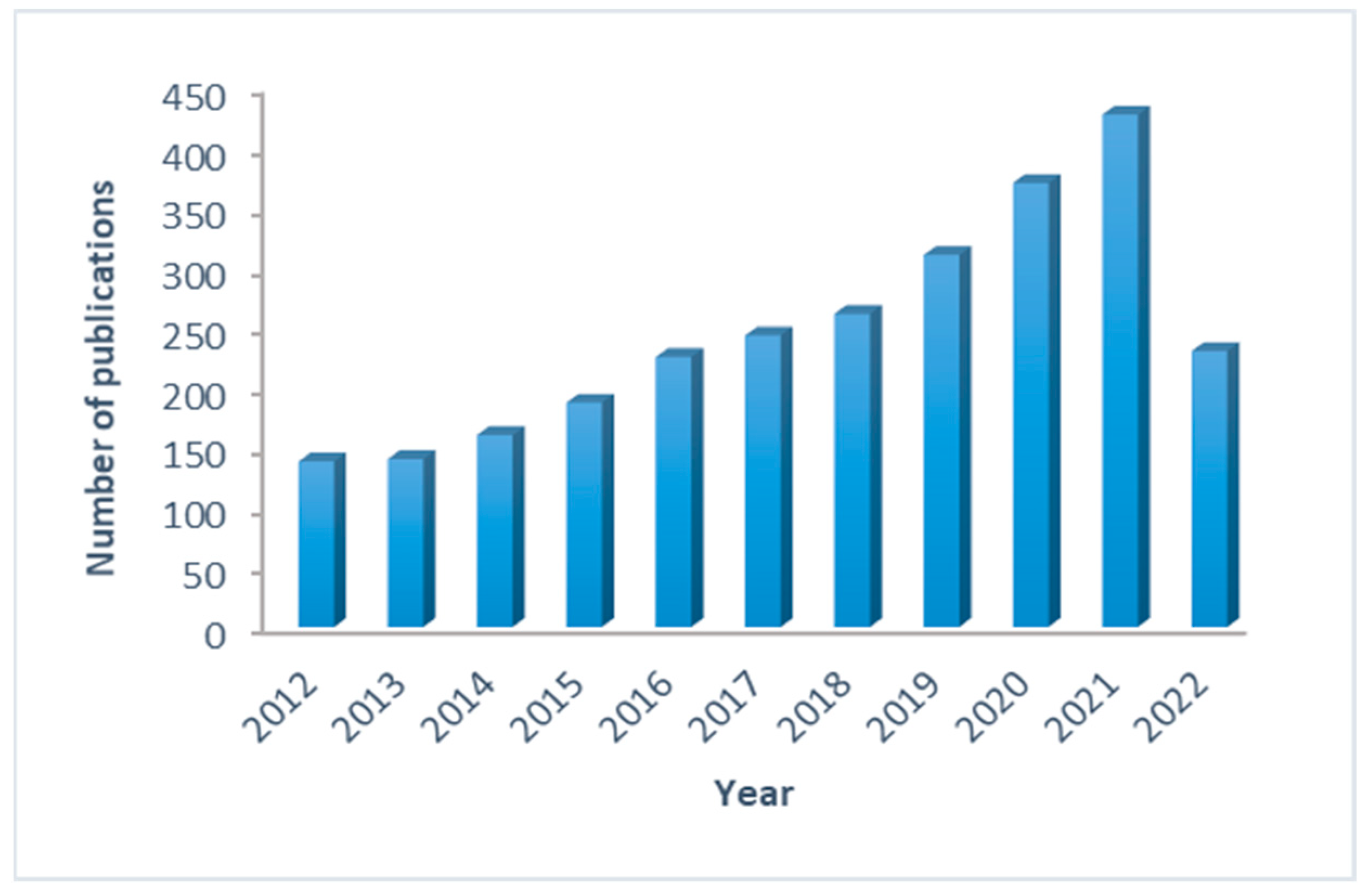
3.1. PubMed Search
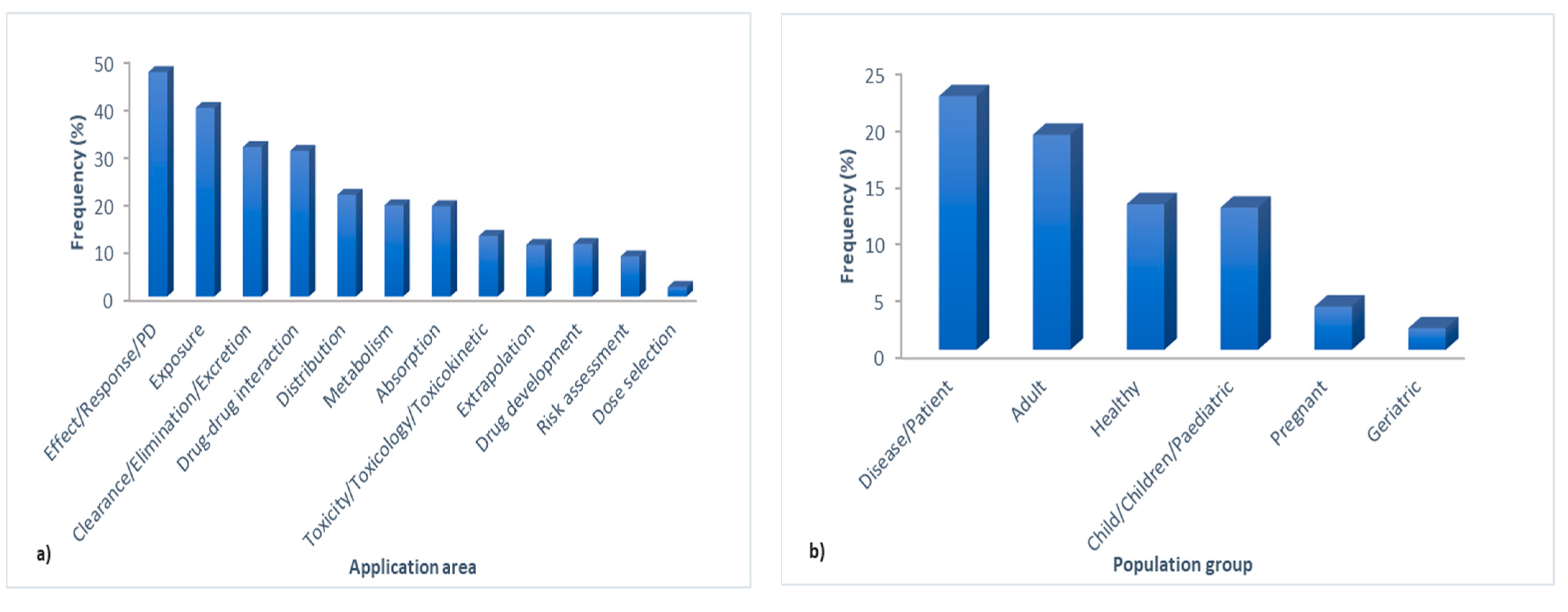
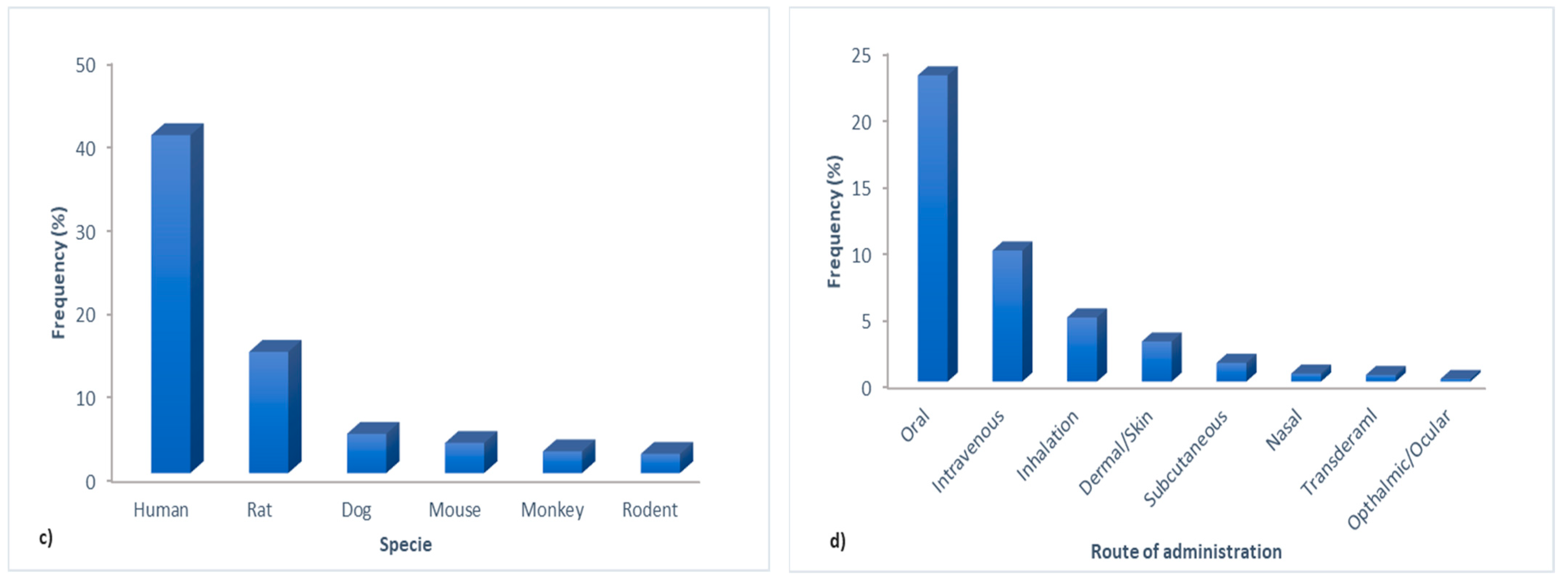
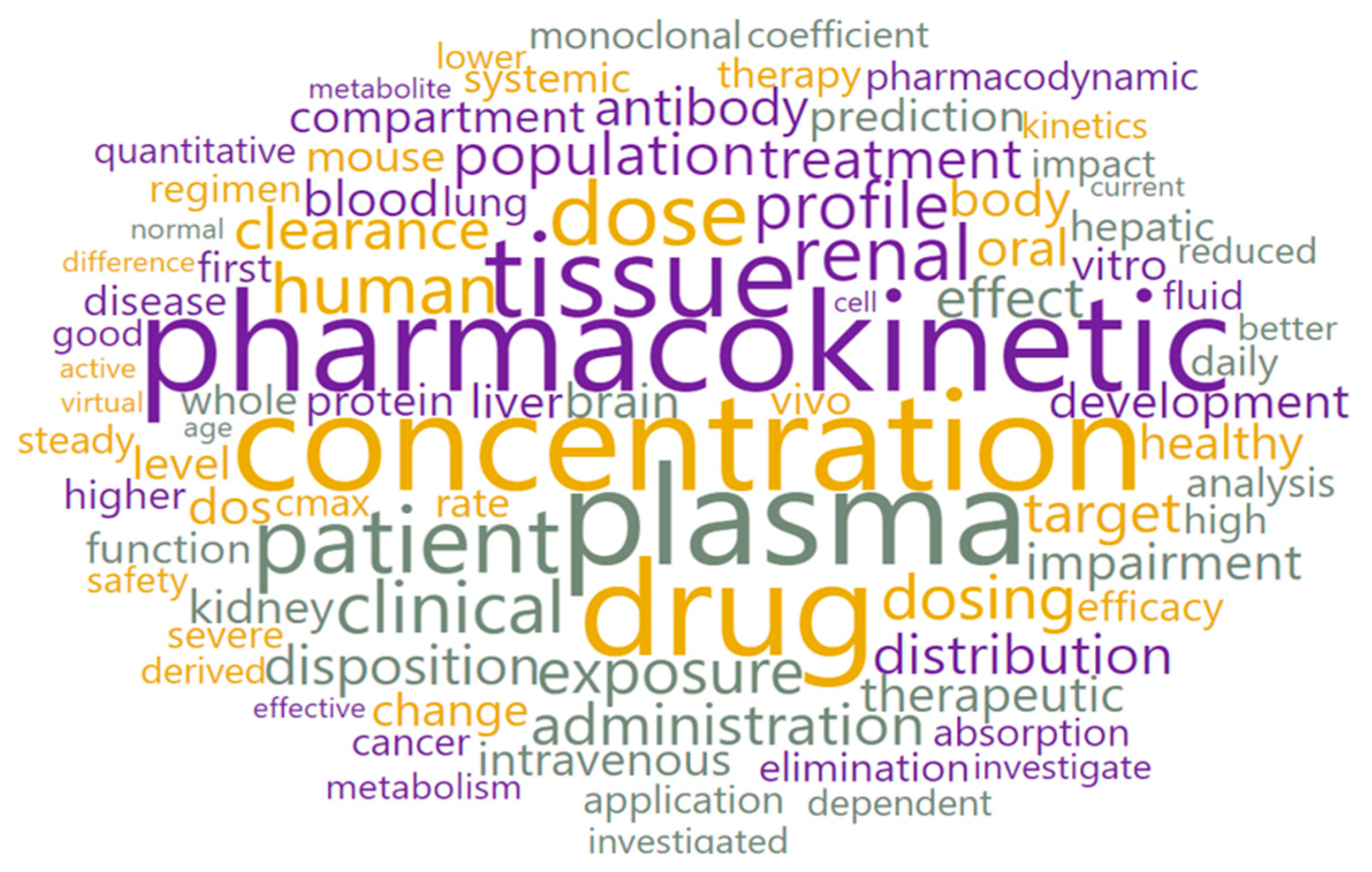
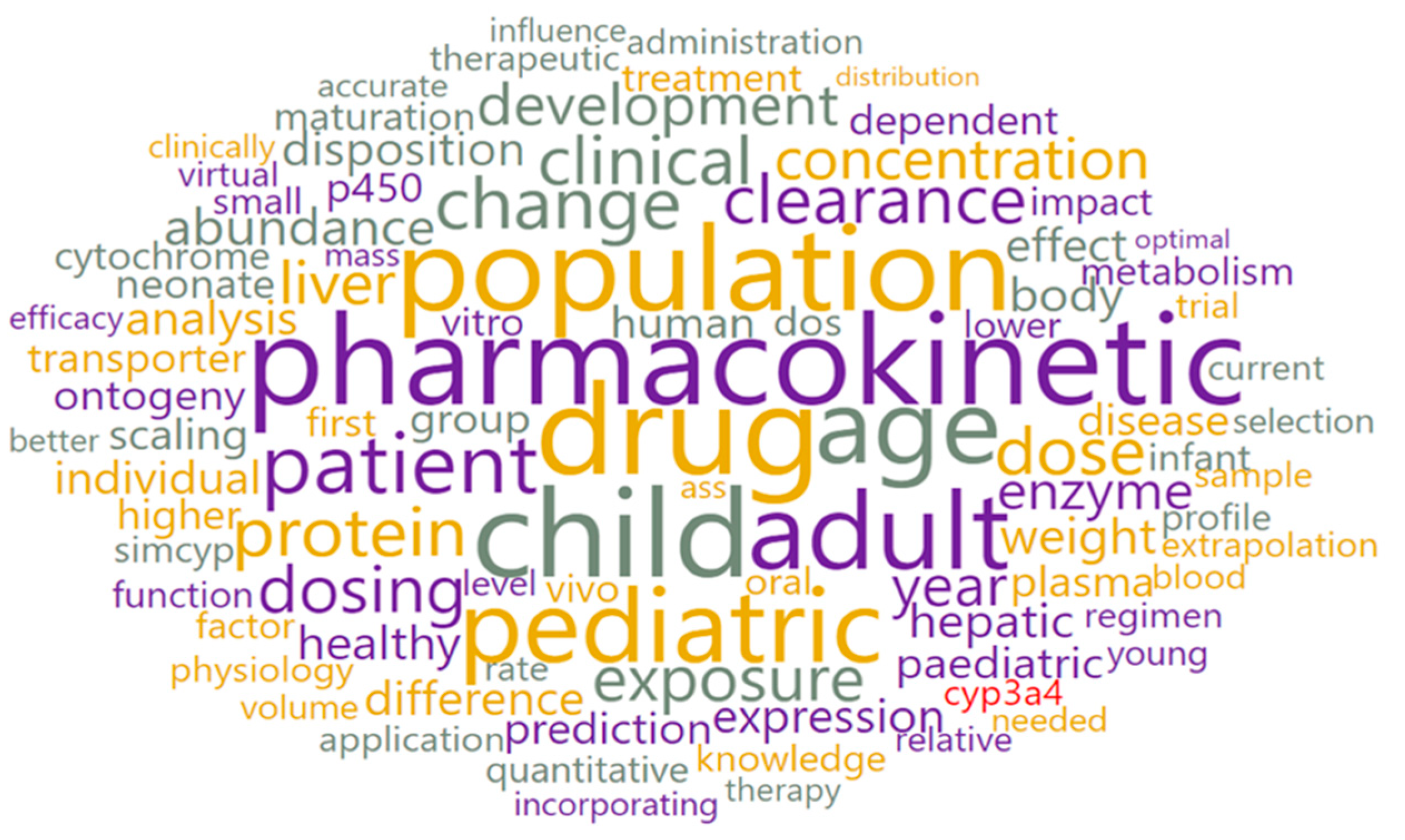
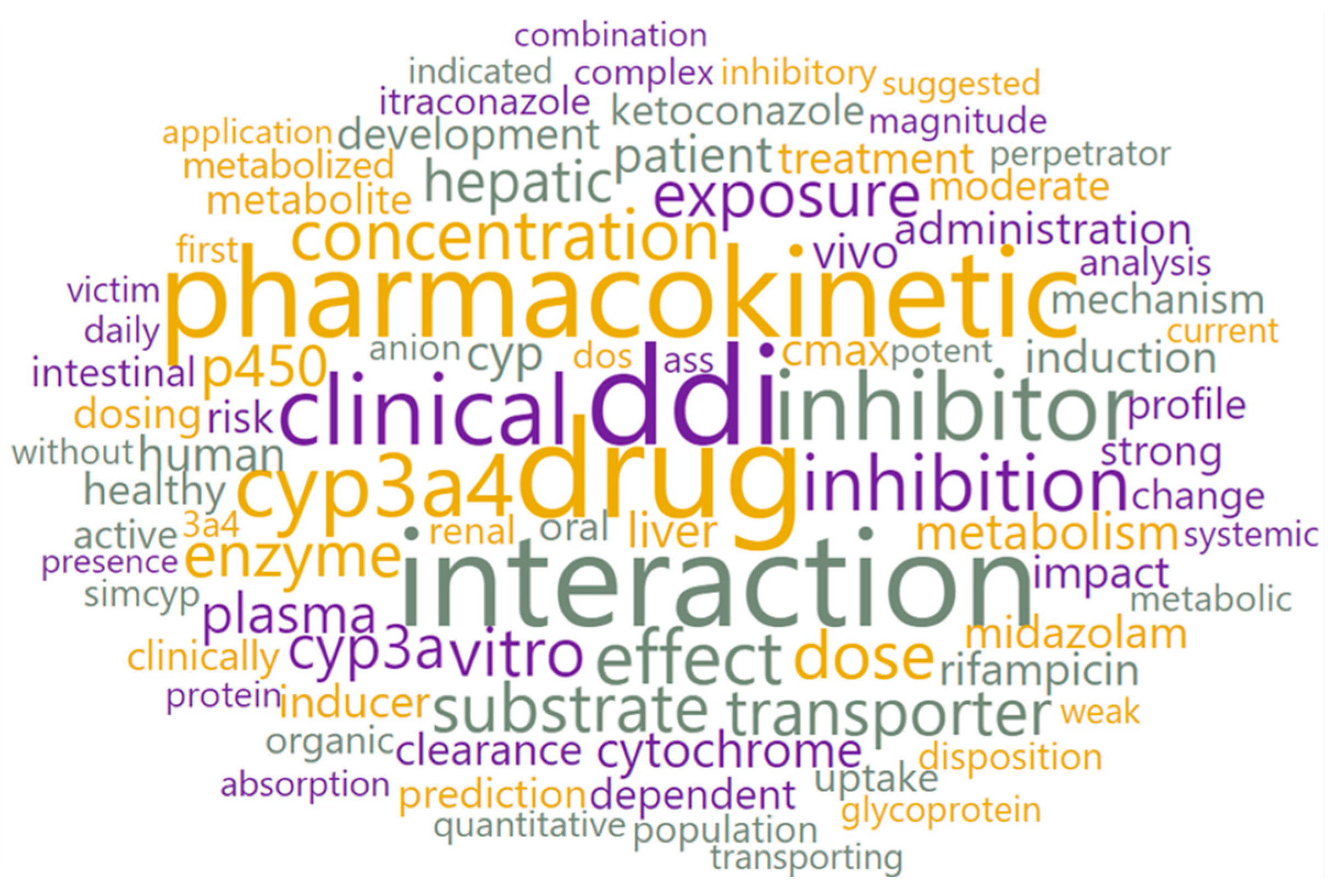
3.2. Text Preprocessing and Word Cloud Generation
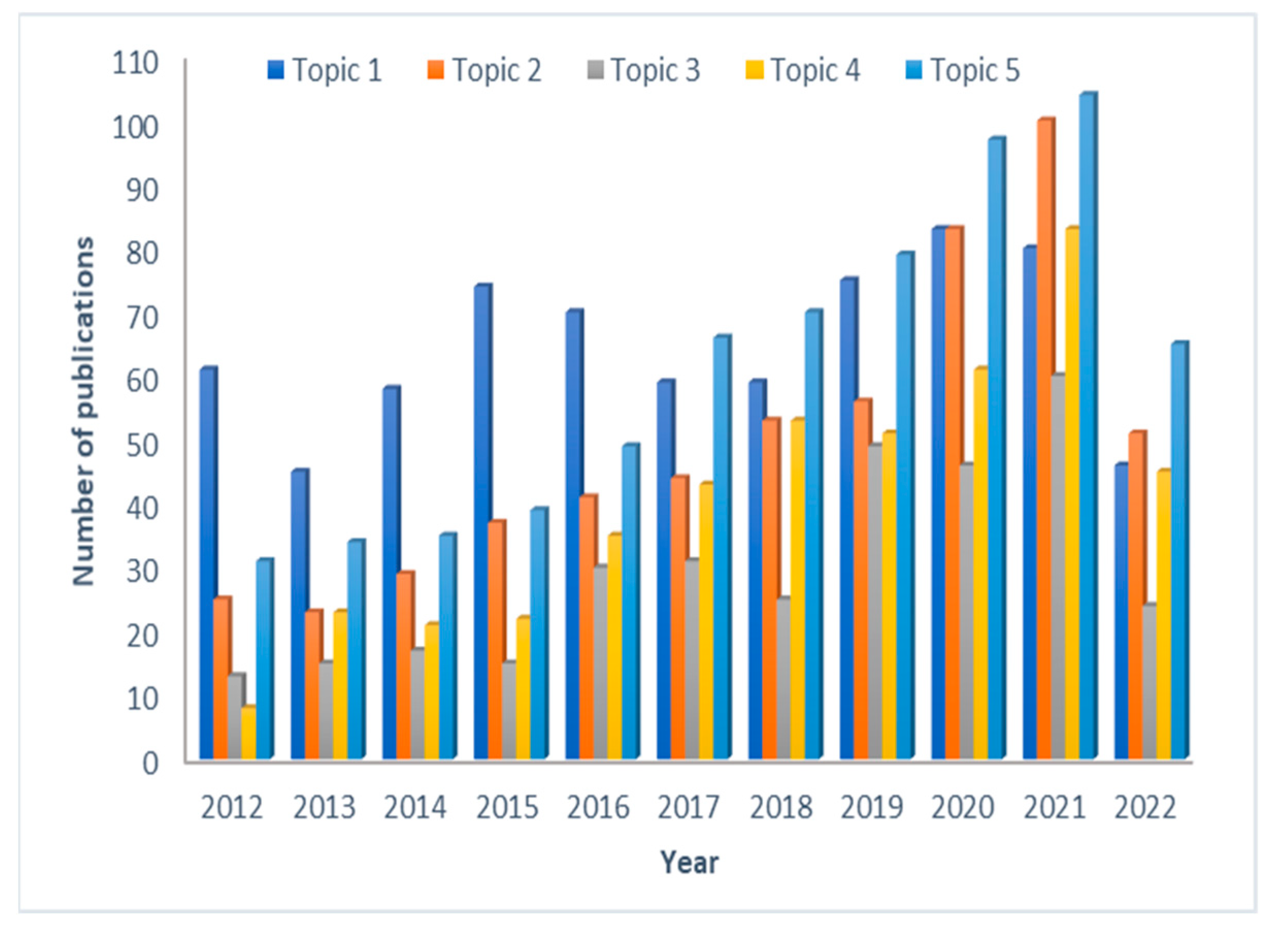
3.3. Topic Modeling
3.3.1. Topic 1—Early Drug Development, Risk Assessment and Toxicity Assessment
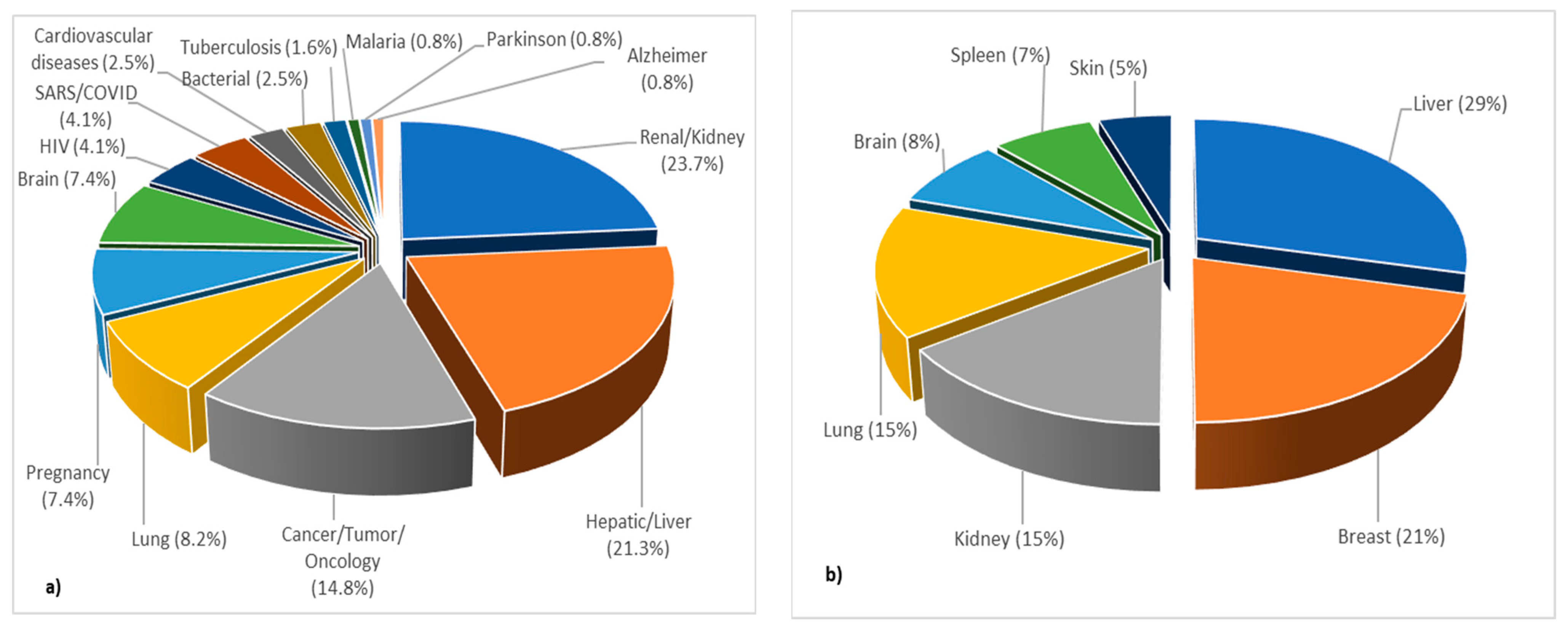
3.3.2. Topic 2—PBPK Modeling in Specific/Diseased Populations
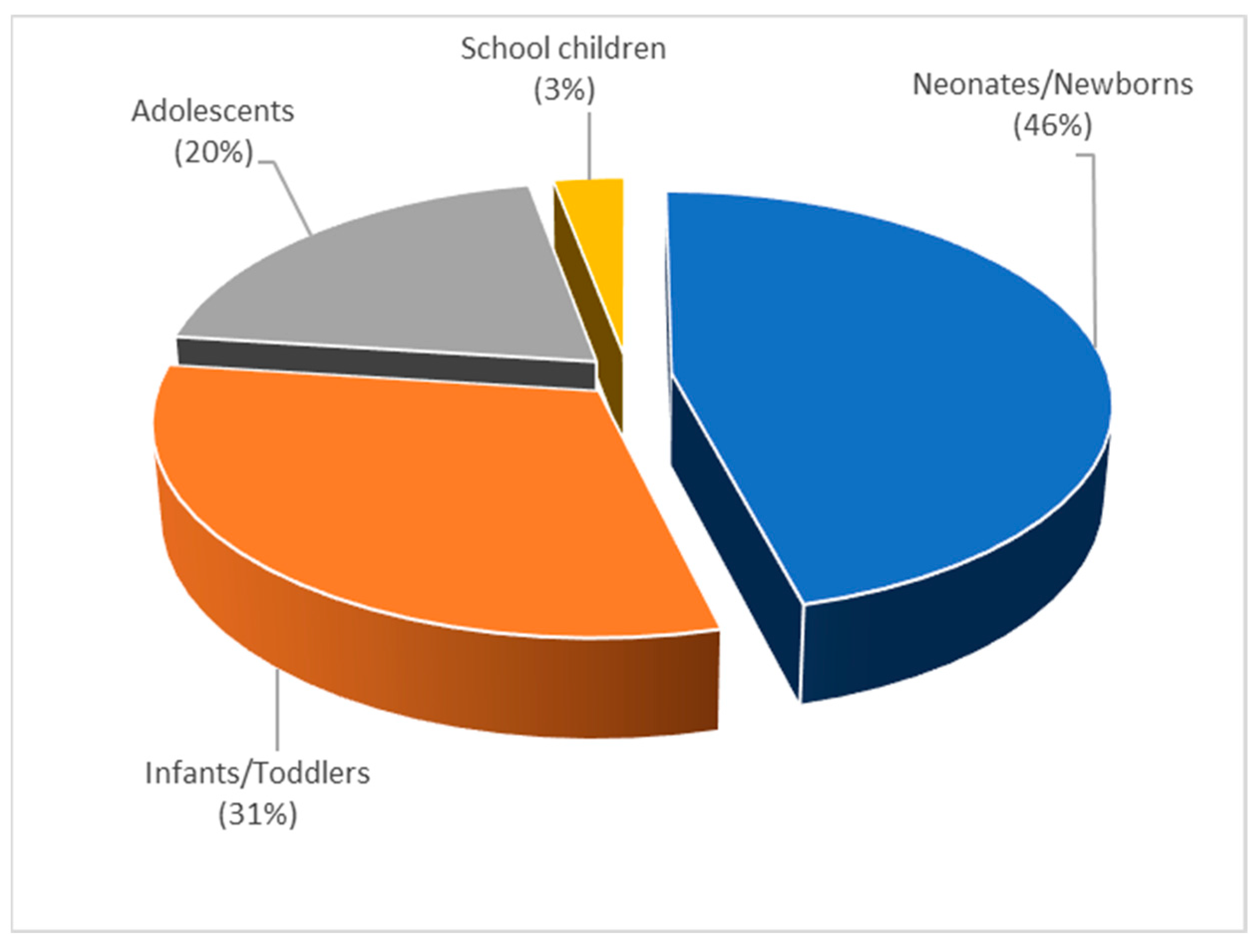
3.3.3. Topic 3—Pediatric PBPK (P-PBPK) Modeling
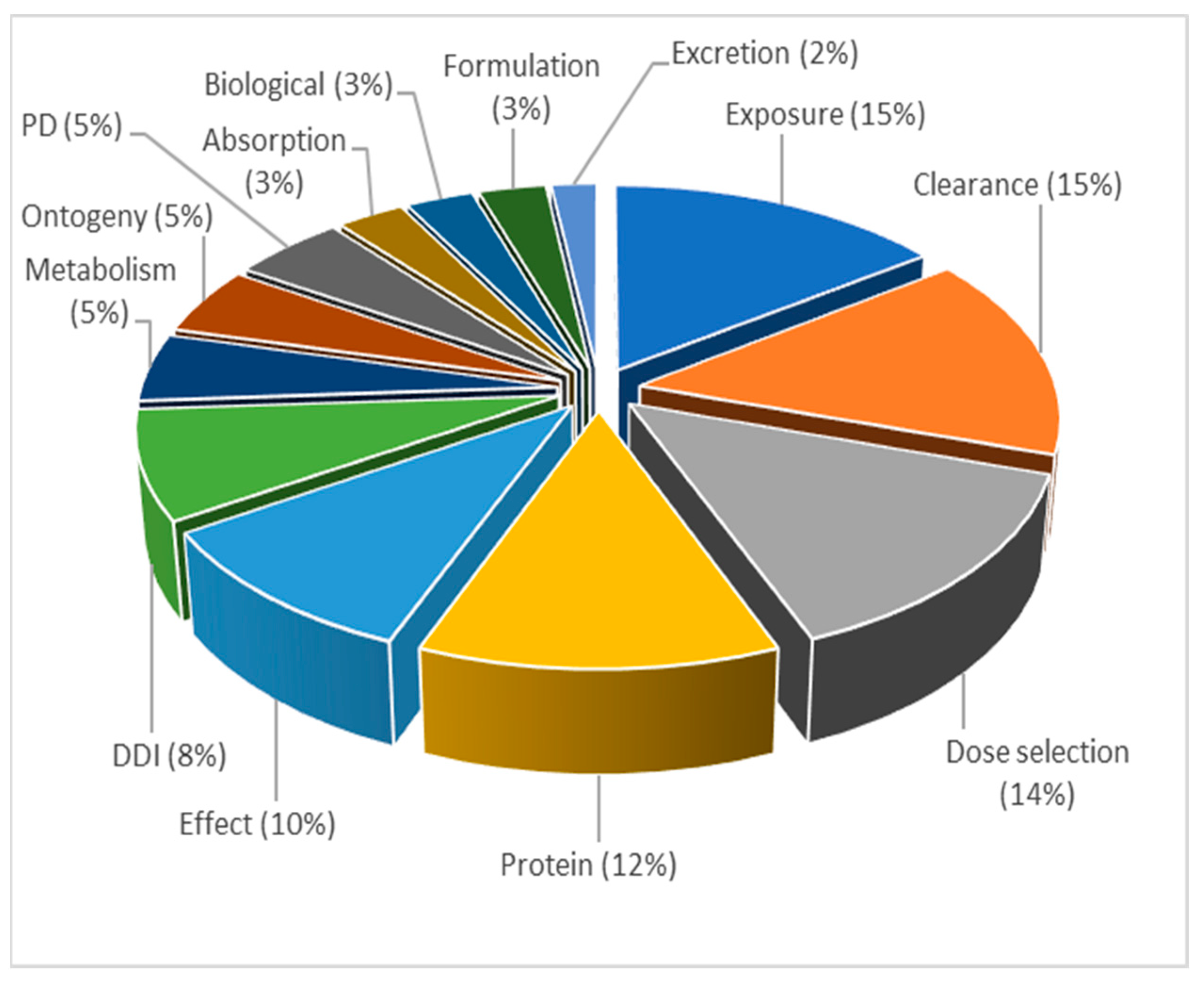
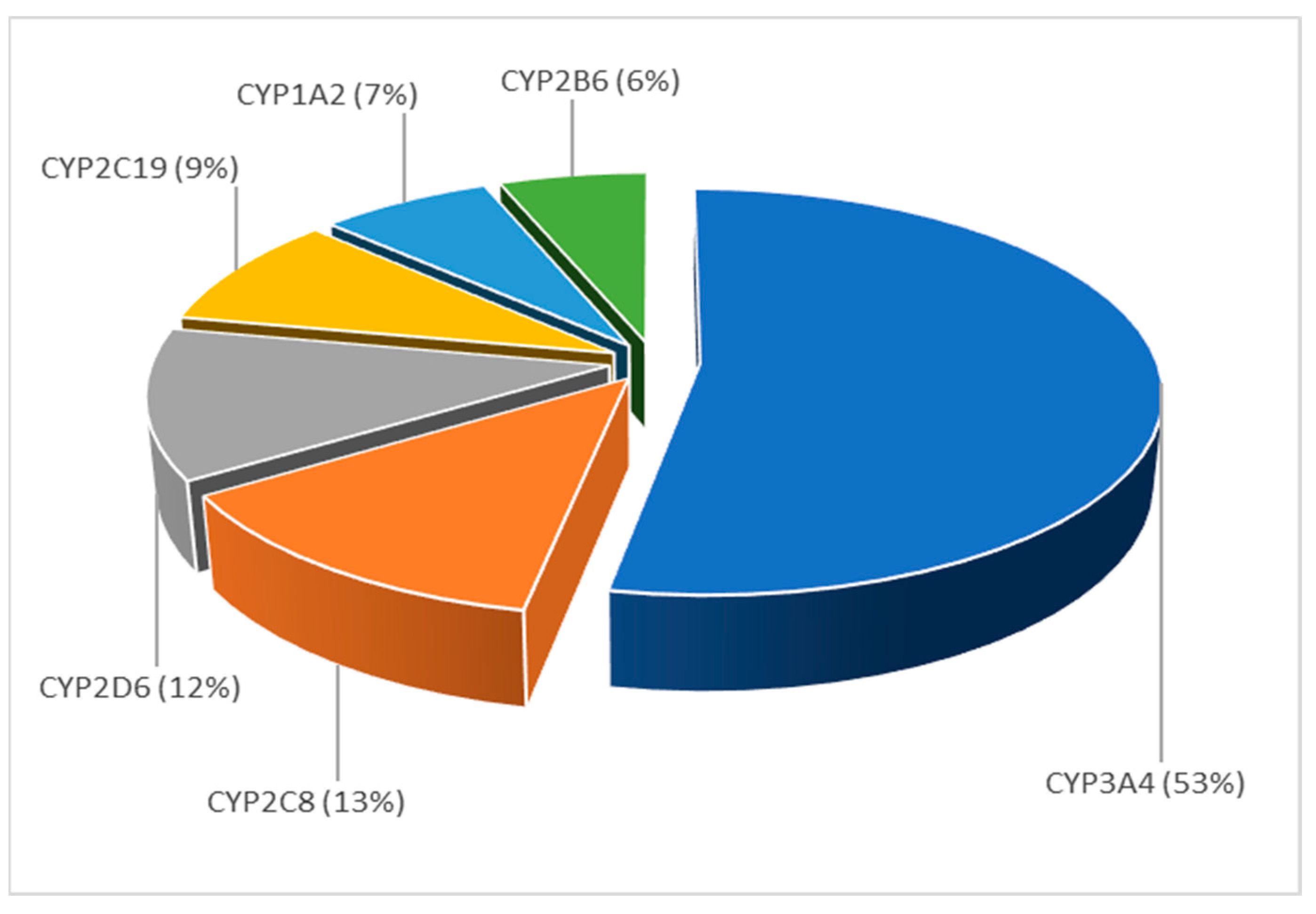
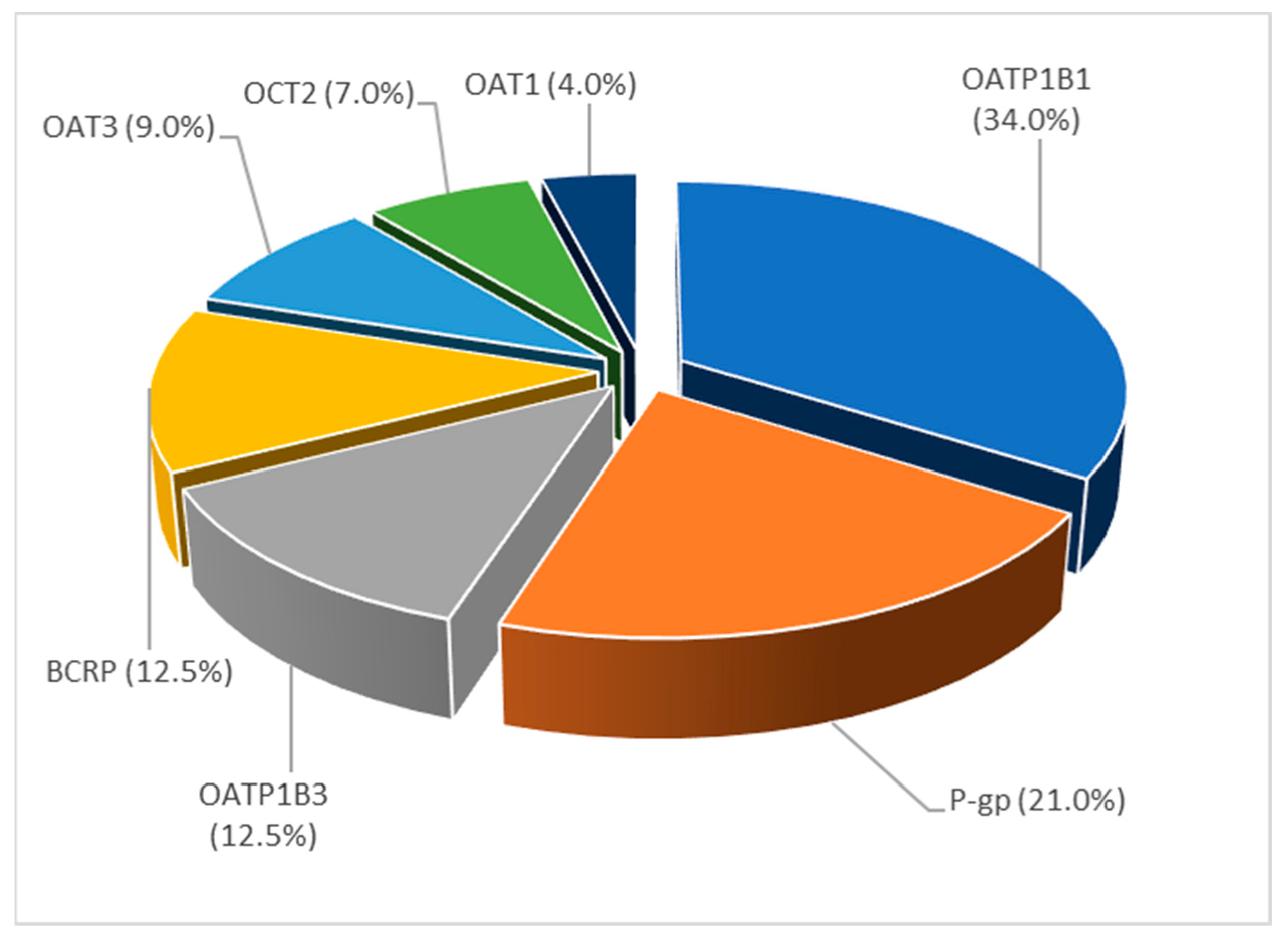
3.3.4. Topic 4—Drug-Drug Interactions
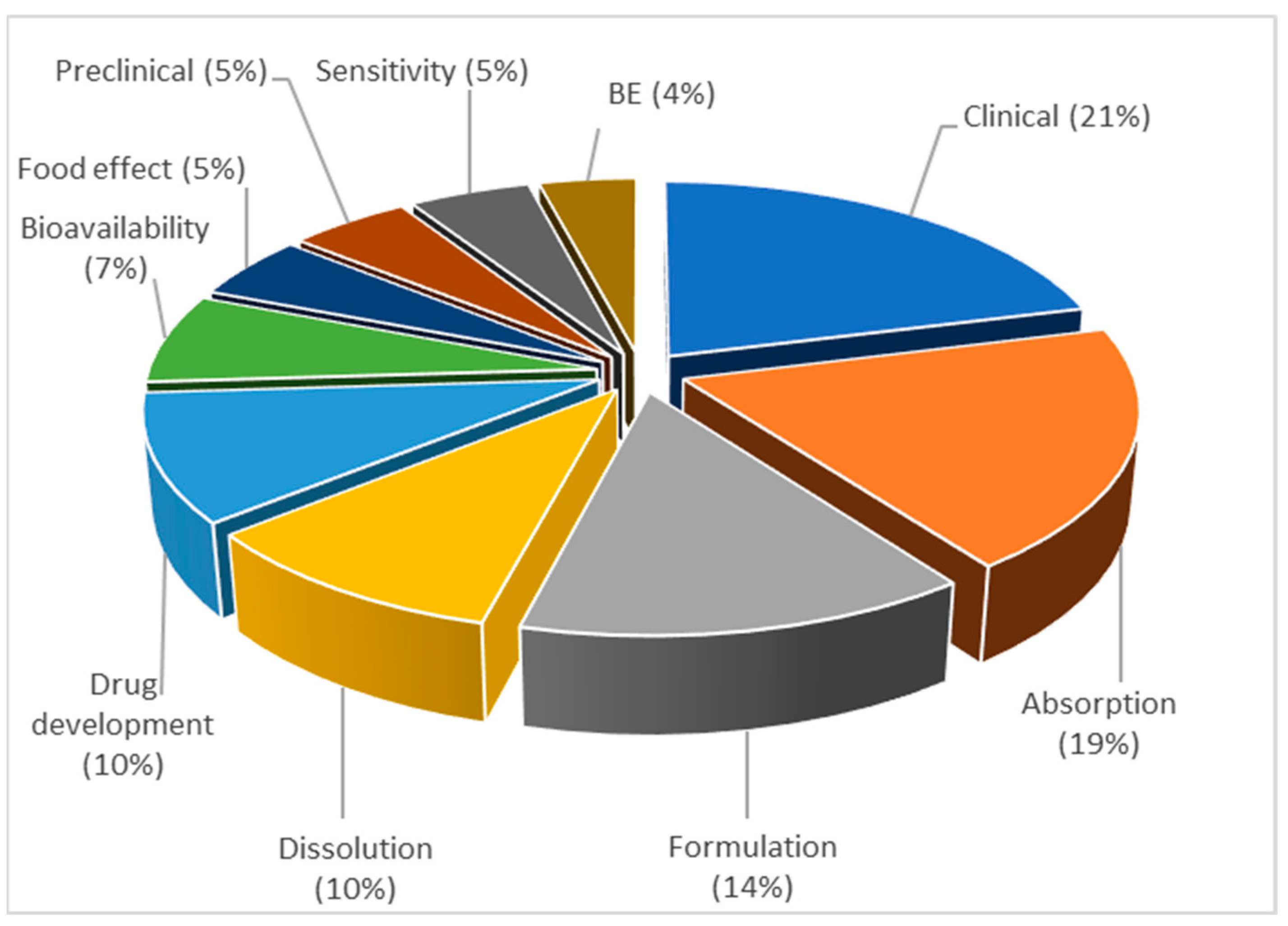
3.3.5. Topic 5—Drug Absorption Modeling and Physiologically Based Biopharmaceutics Modeling (PBBM)
4. Limitations of the Applied Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, P.; Zhang, L.; Grillo, J.A.; Liu, Q.; Bullock, J.M.; Moon, Y.J.; Song, P.; Brar, S.S.; Madabushi, R.; Wu, T.C.; et al. Applications of Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation during Regulatory Review. Clin. Pharmacol. Ther. 2011, 89, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Yarmush, M.L.; Maguire, T. Physiologically Based Pharmacokinetic Models: Integration of In Silico Approaches with Micro Cell Culture Analogues. Curr. Drug Metab. 2012, 13, 863–880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rowland, M.; Peck, C.; Tucker, G. Physiologically-Based Pharmacokinetics in Drug Development and Regulatory Science. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 45–73. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.M.; Rowland-Yeo, K. Basic Concepts in Physiologically Based Pharmacokinetic Modeling in Drug Discovery and Development. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e63. [Google Scholar] [CrossRef]
- Naga, D.; Parrott, N.; Ecker, G.F.; Olivares-Morales, A. Evaluation of the Success of High-Throughput Physiologically Based Pharmacokinetic (HT-PBPK) Modeling Predictions to Inform Early Drug Discovery. Mol. Pharm. 2022, 19, 2203–2216. [Google Scholar] [CrossRef]
- Jones, H.M.; Dickins, M.; Youdim, K.; Gosset, J.R.; Attkins, N.J.; Hay, T.L. Application of PBPK Modelling in Drug Discovery and Development at Pfizer. Xenobiotica 2021, 42, 94–106. [Google Scholar] [CrossRef]
- Miller, N.A.; Reddy, M.B.; Heikkinen, A.T.; Lukacova, V.; Parrott, N. Physiologically Based Pharmacokinetic Modelling for First-In-Human Predictions: An Updated Model Building Strategy Illustrated with Challenging Industry Case Studies. Clin. Pharm. 2019, 58, 727–746. [Google Scholar] [CrossRef]
- Li, C.; Liu, B.; Chang, J.; Groessl, T.; Zimmerman, M.; He, Y.Q.; Isbell, J.; Tuntland, T. A Modern in Vivo Pharmacokinetic Paradigm: Combining Snapshot, Rapid and Full PK Approaches to Optimize and Expedite Early Drug Discovery. Drug Discov. Today 2013, 18, 71–78. [Google Scholar] [CrossRef]
- Chen, E.P.; Bondi, R.W.; Michalski, P.J. Model-Based Target Pharmacology Assessment (MTPA): An Approach Using PBPK/PD Modeling and Machine Learning to Design Medicinal Chemistry and DMPK Strategies in Early Drug Discovery. J. Med. Chem. 2021, 64, 3185–3196. [Google Scholar] [CrossRef]
- Glassman, P.M.; Balthasar, J.P. Physiologically-Based Modeling of Monoclonal Antibody Pharmacokinetics in Drug Discovery and Development. Drug Metab. Pharmacokinet. 2019, 34, 3–13. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Y.; Li, L.; Shen, X. Computational Approaches in Preclinical Studies on Drug Discovery and Development. Front. Chem. 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Polak, S.; Tylutki, Z.; Holbrook, M.; Wiśniowska, B. Better Prediction of the Local Concentration–Effect Relationship: The Role of Physiologically Based Pharmacokinetics and Quantitative Systems Pharmacology and Toxicology in the Evolution of Model-Informed Drug Discovery and Development. Drug Discov. Today 2019, 24, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Templeton, I.E.; Jones, N.S.; Musib, L. Pediatric Dose Selection and Utility of PBPK in Determining Dose. AAPS J. 2018, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Michelet, R.; Bocxlaer, J.V.; Vermeulen, A. PBPK in Preterm and Term Neonates: A Review. Curr. Pharm. Des. 2017, 23, 5943–5954. [Google Scholar] [CrossRef] [PubMed]
- Ince, I.; Solodenko, J.; Frechen, S.; Dallmann, A.; Niederalt, C.; Schlender, J.; Burghaus, R.; Lippert, J.; Willmann, S. Predictive Pediatric Modeling and Simulation Using Ontogeny Information. J. Clin. Pharmacol. 2019, 59, S95–S103. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, K.; Wei, X.; Li, Y.; Wang, T.; Song, Y. Physiologically Based Pharmacokinetic Models Are Effective Support for Pediatric Drug Development. AAPS PharmSciTech 2021, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Rohou, C.S.; Cheung, S.Y.A. Industry Perspective on Using MIDD for Pediatric Studies Requiring Integration of Ontogeny. J. Clin. Pharmacol. 2019, 59, S112–S119. [Google Scholar] [CrossRef]
- Chetty, M.; Johnson, T.N.; Polak, S.; Salem, F.; Doki, K.; Rostami-Hodjegan, A. Physiologically Based Pharmacokinetic Modelling to Guide Drug Delivery in Older People. Adv. Drug. Deliv. 2018, 135, 85–96. [Google Scholar] [CrossRef]
- Schlender, J.F.; Meyer, M.; Thelen, K.; Krauss, M.; Willmann, S.; Eissing, T.; Jaehde, U. Development of a Whole-Body Physiologically Based Pharmacokinetic Approach to Assess the Pharmacokinetics of Drugs in Elderly Individuals. Clin. Pharm. 2016, 55, 1573–1589. [Google Scholar] [CrossRef]
- Stader, F.; Siccardi, M.; Battegay, M.; Kinvig, H.; Penny, M.A.; Marzolini, C. Repository Describing an Aging Population to Inform Physiologically Based Pharmacokinetic Models Considering Anatomical, Physiological, and Biological Age-Dependent Changes. Clin. Pharmacokinet. 2019, 58, 483–501. [Google Scholar] [CrossRef]
- Rhee, S.J.; Chung, H.; Yi, S.; Yu, K.S.; Chung, J.Y. Physiologically Based Pharmacokinetic Modelling and Prediction of Metformin Pharmacokinetics in Renal/Hepatic-Impaired Young Adults and Elderly Populations. Eur. J. Drug. Metab. Pharm. 2017, 42, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.C.; Shah, M.; Rytting, E.; Nanovskaya, T.N.; Wang, X.; Clark, S.M.; Ahmed, M.S.; Hankins, G.D.V.; Caritis, S.N.; Venkataramanan, R. Prediction of Maternal and Fetal Pharmacokinetics of Indomethacin in Pregnancy. Br. J. Clin. Pharmacol. 2022, 88, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Rimawi, B.H.; Johnson, E.; Rajakumar, A.; Tao, S.; Jiang, Y.; Gillespie, S.; Schinazi, R.F.; Mirochnick, M.; Badell, M.L.; Chakraborty, R. Pharmacokinetics and Placental Transfer of Elvitegravir, Dolutegravir, and Other Antiretrovirals during Pregnancy. Antimicrob. Agents Chemother. 2017, 61, 2213–2216. [Google Scholar] [CrossRef] [PubMed]
- Szeto, K.X.; Le Merdy, M.; Dupont, B.; Bolger, M.B.; Lukacova, V. PBPK Modeling Approach to Predict the Behavior of Drugs Cleared by Kidney in Pregnant Subjects and Fetus. AAPS J. 2021, 23, 89. [Google Scholar] [CrossRef]
- Rasool, M.F.; Ali, S.; Khalid, S.; Khalid, R.; Majeed, A.; Imran, I.; Saeed, H.; Usman, M.; Ali, M.; Alali, A.S.; et al. Development and Evaluation of Physiologically Based Pharmacokinetic Drug-Disease Models for Predicting Captopril Pharmacokinetics in Chronic Diseases. Sci. Rep. 2021, 11, 8589. [Google Scholar] [CrossRef]
- Zhai, J.; Ji, B.; Cai, L.; Liu, S.; Sun, Y.; Wang, J. Physiologically-based Pharmacokinetic Modeling as a Treatment for Malaria and Optimized Dosing Regimens for Different Populations. J. Pers. Med. 2022, 12, 796. [Google Scholar] [CrossRef]
- Montanha, M.C.; Cottura, N.; Booth, M.; Hodge, D.; Bunglawala, F.; Kinvig, H.; Grañana-Castillo, S.; Lloyd, A.; Khoo, S.; Siccardi, M. PBPK Modelling of Dexamethasone in Patients With COVID-19 and Liver Disease. Front. Pharmacol. 2022, 13, 814134. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Y.; Guo, N.; Wang, X.; Han, B.; Xiang, X. Application of Physiologically Based Pharmacokinetic Modeling to Evaluate the Drug–Drug and Drug–Disease Interactions of Apatinib. Front. Pharmacol. 2021, 12, 780937. [Google Scholar] [CrossRef]
- Hanke, N.; Frechen, S.; Moj, D.; Britz, H.; Eissing, T.; Wendl, T.; Lehr, T. PBPK Models for CYP3A4 and P-Gp DDI Prediction: A Modeling Network of Rifampicin, Itraconazole, Clarithromycin, Midazolam, Alfentanil, and Digoxin. CPT Pharmacomet. Syst. 2018, 7, 647–659. [Google Scholar] [CrossRef]
- Wu, F.; Krishna, G.; Surapaneni, S. Physiologically Based Pharmacokinetic Modeling to Assess Metabolic Drug–Drug Interaction Risks and Inform the Drug Label for Fedratinib. Cancer Chemother. Pharmacol. 2020, 86, 461–473. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Jin, Z.; Dai, Y.; Lin, H.; Jiao, Z.; Ma, G.; Cai, W.; Han, B.; Xiang, X. Prediction of Pharmacokinetic Drug-Drug Interactions Causing Atorvastatin-Induced Rhabdomyolysis Using Physiologically Based Pharmacokinetic Modelling. Biomed. Pharm. 2019, 119, 109416. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, M.; Blumenstein, L.; Kourentas, A.; Mueller-Zsigmondy, M.; Lorenzo, S.; Sinn, A.; Velinova, M.; Heimbach, T. Physiologically Based Pharmacokinetic Modeling of Oral Absorption, PH, and Food Effect in Healthy Volunteers to Drive Alpelisib Formulation Selection. AAPS J. 2020, 22, 134. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.C.Y.; Talattof, A.; Tsakalozou, E.; Fan, J.; Zhao, L.; Zhang, X. Using Physiologically Based Pharmacokinetic (PBPK) Modeling to Evaluate the Impact of Pharmaceutical Excipients on Oral Drug Absorption: Sensitivity Analyses. AAPS J. 2016, 18, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wong, H. Predicting Oral Drug Absorption: Mini Review on Physiologically-Based Pharmacokinetic Models. Pharmaceutics 2017, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Thakur, P.S.; Shete, G.; Gangwal, R.; Sangamwar, A.T.; Bansal, A.K. Understanding the Oral Absorption of Irbesartan Using Biorelevant Dissolution Testing and PBPK Modeling. AAPS PharmSciTech 2020, 21, 102. [Google Scholar] [CrossRef]
- Jamie, M.; Marciniak, S.; Edwards, D.; Wragg, K.; Feng, K.; Barnett, A.; Hodjegan-Rostami, A. The Simcyp Population Based Simulator: Architecture, Implementation and Quality Assurance. Silico Pharmacol. 2013, 1, 9. [Google Scholar]
- El-Khateeb, E.; Burkhill, S.; Murby, S.; Amirat, H.; Rostami-Hodjegan, A.; Ahmad, A. Physiological-Based Pharmacokinetic Modeling Trends in Pharmaceutical Drug Development over the Last 20-Years; in-Depth Analysis of Applications, Organizations, and Platforms. Biopharm. Drug Dispos. 2021, 42, 107–117. [Google Scholar] [CrossRef]
- Medina-Franco, J.; Martinez-Mayorga, K.; Fernandez-de Gortari, E.; Kirchmair, J.; Bajorath, J. Rationality over fashion and hype in drug design. F1000Research 2021, 10, 397. [Google Scholar] [CrossRef]
- Food and Drug Administration Guidance for Industry. Physiologically Based Pharmacokinetic Analyses—Format and Content. 2018. Available online: https://www.fda.gov/media/101469/download (accessed on 9 December 2022).
- European Medicines Agency Guidance: Guideline on the Reporting of Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation. 2018. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation_en.pdf (accessed on 1 October 2022).
- Sager, E.J.; Jingjing, Y.; Ragueneau-Majlessi, I.; Isoherranen, N. Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation Approaches: A Systematic Review of Published Models, Applications and Model Verification. Drug Metab. Dispos. 2015, 43, 1823–1837. [Google Scholar] [CrossRef]
- Wu, D.; Li, M. Current State and Challenges of Physiologically Based Biopharmaceutics Modeling (PBBM) in Oral Drug Product Development. Pharm. Res. 2022. [Google Scholar] [CrossRef]
- Perry, C.; Davis, G.; Conner, T.M.; Zhang, T. Utilization of Physiologically Based Pharmacokinetic Modeling in Clinical Pharmacology and Therapeutics: An Overview. Curr. Pharmacol. Rep. 2020, 6, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.V.; Firman, J.W.; Goldsmith, M.R.; Grulke, C.M.; Tan, Y.M.; Paini, A.; Penson, P.E.; Sayre, R.R.; Webb, S.; Madden, J.C. A Systematic Review of Published Physiologically-Based Kinetic Models and an Assessment of Their Chemical Space Coverage. Altern. Lab. Anim. 2021, 49, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Taskar, K.S.; Reddy, V.P.; Burt, H.; Posada, M.M.; Varma, M.; Zheng, M.; Ullah, M.; Riedmaier, A.E.; Umehara, K.; Snoeys, J.; et al. Physiologically-Based Pharmacokinetic Models for Evaluating Membrane Transporter Mediated Drug–Drug Interactions: Current Capabilities, Case Studies, Future Opportunities, and Recommendations. Clin. Pharmacol. Ther. 2020, 107, 1082–1115. [Google Scholar] [CrossRef]
- Läer, S.; Khalil, F. Physiologically Based Pharmacokinetic Modeling: Methodology, Applications, and Limitations with a Focus on Its Role in Pediatric Drug Development. BioMed Res. Int. 2011, 2011, 907461. [Google Scholar] [CrossRef]
- Saeheng, T.; Na-Bangchang, K.; Karbwang, J. Utility of Physiologically Based Pharmacokinetic (PBPK) Modeling in Oncology Drug Development and Its Accuracy: A Systematic Review. Eur. J. Clin. Pharmacol. 2018, 74, 1365–1376. [Google Scholar] [CrossRef]
- Huang, S.M.; Abernethy, D.R.; Wang, Y.; Zhao, P.; Zineh, I. The Utility of Modeling and Simulation in Drug Development and Regulatory Review. J. Pharm. Sci. 2013, 102, 2912–2923. [Google Scholar] [CrossRef]
- Jamei, M. Recent Advances in Development and Application of Physiologically-Based Pharmacokinetic (PBPK) Models: A Transition from Academic Curiosity to Regulatory Acceptance. Curr. Pharmacol. Rep. 2016, 2, 161–169. [Google Scholar] [CrossRef]
- Lin, W.; Chen, Y.; Unadkat, J.D.; Zhang, X.; Wu, D.; Heimbach, T. Applications, Challenges, and Outlook for PBPK Modeling and Simulation: A Regulatory, Industrial and Academic Perspective. Pharm. Res. 2022, 39, 1701–1731. [Google Scholar] [CrossRef]
- Jabeen, R. Text Mining Methods for Biomedical Data Analysis. Master’s Thesis, Linkoping University, Linköping, Sweden, 2021. [Google Scholar]
- Wang, L.L.; Lo, K. Text Mining Approaches for Dealing with the Rapidly Expanding Literature on COVID-19. Brief. Bioinform. 2021, 22, 781–799. [Google Scholar] [CrossRef]
- Tao, D.; Yang, P.; Feng, H. Utilization of Text Mining as a Big Data Analysis Tool for Food Science and Nutrition. Compr. Rev. Food Sci. Food Saf. 2020, 19, 875–894. [Google Scholar] [CrossRef]
- Lin, H.J.; Sheu, P.C.Y.; Tsai, J.J.P.; Wang, C.C.N.; Chou, C.Y. Text Mining in a Literature Review of Urothelial Cancer Using Topic Model. BMC Cancer 2020, 20, 462. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, I.; Rodríguez, J.V.; Shirvanizadeh, N.; Ortiz, A.; Pardo-Quiles, D.J. Applications of Artificial Intelligence, Machine Learning, Big Data and the Internet of Things to the COVID-19 Pandemic: A Scientometric Review Using Text Mining. Int. J. Environ. Res. Public Health 2021, 18, 8578. [Google Scholar] [CrossRef] [PubMed]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hočevar, T.; Milutinovič, M.; Možina, M.; Polajnar, M.; Toplak, M.; Starič, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach Lear Res. 2013, 14, 2349–2353. [Google Scholar]
- Daud, A.; Li, J.; Muhammad, F. Knowledge Discovery Through Directed Probabilistic Topic Models: A Survey. Front. Comput. Sci. China 2009, 4, 280–301. [Google Scholar] [CrossRef]
- Kumar, K. Evaluation of Topic Modeling: Topic Coherence. DataScience+ 2018. Available online: https://datascienceplus.com/evaluation-of-topic-modeling-topic-coherence/ (accessed on 6 October 2022).
- Food & Drug Administration Draft Guidance for Industry: The Use of Physiologically Based Pharmacokinetic Analyses—Biopharmaceutics Applications for Oral Drug Product Development, Manufacturing Changes and Controls. 2020. Available online: https://www.fda.gov/media/142500/download (accessed on 1 October 2022).
- Hines, D.; Bell, S.; Chang, X.; Mansouri, K.; Allen, D.; Kleinstreuer, N. Application of an Accessible Interface for Pharmacokinetic Modeling and In Vitro to In Vivo extrapolation. Front. Pharmacol. 2022, 13, 864742. [Google Scholar] [CrossRef]
- Heimbach, T.; Chen, Y.; Chen, J.; Dixit, V.; Parrott, N.; Peters, S.A.; Poggesi, I.; Sharma, P.; Snoeys, J.; Shebley, M.; et al. Physiologically-Based Pharmacokinetic Modeling in Renal and Hepatic Impairment Populations: A Pharmaceutical Industry Perspective. Clin. Pharmacol. Ther. 2021, 110, 21–25. [Google Scholar] [CrossRef]
- Food & Drug Administration Draft Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing. 2020. Available online: https://www.fda.gov/media/78573/download (accessed on 1 October 2022).
- European Medicines Agency Guidance: Guideline on the Evalaution of the Pharmacokinetics of Medicinal Products in Patients with Impaired Hepatic Function. 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-impaired-hepatic-function_en.pdf (accessed on 1 October 2022).
- European Medicines Agency Guidance: Guideline on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients with Decreased Renal Function. 2016. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-decreased-renal-function_en.pdf (accessed on 1 October 2022).
- Willman, S.; Coboeken, K.; Zhang, Y.; Mayer, H.; Ince, I.; Mesic, E.; Thelen, K.; Kubitza, D.; Lensing, A.W.A.; Yang, H. Population Pharmacokinetic Analysis of Rivaroxaban in Children and Comparison to Prospective Physiologically-Based Pharmacokinetic Predictions. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 1195–1207. [Google Scholar] [CrossRef]
- Yu, Y.; DuBois, S.; Wetmore, C.; Khosravan, R. Physiologically Based Pharmacokinetic Modeling and Simulation of Sunitinib in Pediatric. AAPS J. 2020, 22, 31. [Google Scholar] [CrossRef]
- Rabbie, C.S.; Zhou, L.; Vishwanathan, K.; Wild, M.; Xu, S.; Freshwater, T.; Jain, L.; Schalkwijk, S.; Tomkinson, H.; Zhou, D. Physiologically Based Pharmacokinetic Modeling for Selumetinib to Evaluate Drug-Drug Interactions and Pediatric Dose Regimens. J. Clin. Pharmacol. 2021, 61, 1493–1504. [Google Scholar] [CrossRef]
- Jo, H.; Reddy, P.V.; Parkinson, J.; Boulton, D.; Tang, W. Model-Informed Pediatric Dose Selection for Dapaglifozin by Incorporating Developmental Changes. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 108–118. [Google Scholar] [CrossRef]
- Hanke, N.; Claudia, K.; Thiemann, M.; Fricke, H.; Lehr, T. Translational PBPK Modeling of the Protein Therapeutic and CD95L Inhibitor Asunercept to Develop Dose Recommendations for its First Use in Pediatric Glioblastoma Patients. Pharmaceutics 2019, 11, 152. [Google Scholar] [CrossRef]
- Li, A.; Yeo, K.; Welty, D.; Rong, H. Development of Guanfacine Extended-Release Dosing Strategies in Children and Adolescents with ADHD Using a Physiologically Based Pharmacokinetic Model to Predict Drug-Drug Interactions with Moderate CYP3A4 Inhibitors or Inducers. Paediatr. Drugs 2018, 20, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Johson, T.; Small, G.B.; Yeo, R.K. Increasing Application of Pediatric Physiologically Based Pharmacokinetic Models Across Academic and Industry Organizations. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Food & Drug Administration Draft Guidance for Industry: General Clinical Pharmacology Considerations for Pediatric Studies of Drugs, Including Biological Products. 2022. Available online: https://www.fda.gov/media/90358/download (accessed on 1 October 2022).
- European Medicines Agency Guidance: Guideline on the Investigation of Drug Interactions. 2012. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf (accessed on 1 October 2022).
- Food & Drug Administration Guidance for Industry: Clinical Drug-Interaction Studies-Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions. 2020. Available online: https://www.fda.gov/media/134581/download (accessed on 1 October 2022).
- Grimstein, M.; Yang, Y.; Zhang, X.; Grillo, J.; Huang, M.S.; Zineh, I.; Wang, Y. Physiologically Based Pharmacokinetic Modeling in Regulatory Science: An Update From the U.S Food and Drug Administration’s Office of Clinical Pharmacology. J. Pharm. Sci. 2019, 108, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Mukherjee, D.; Salem, H.A.; Miles, D.; Menon, M.R.; Gibbs, P.J. Dose Adjustment of Venetoclax when Co-Administered with Posaconazole: Clinical Drug-Drug Interaction Predictions Using a PBPK Approach. Cancer Chemother. Pharmacol. 2021, 87, 465–474. [Google Scholar] [CrossRef]
- Wang, K.; Yao, X.; Zhang, M.; Liu, D.; Gao, Y.; Sahasranaman, S.; Ou, C.Y. Comprehensive PBPK Model to Predict Drug Interaction Potential of Zanubrutinib as a Victim or Perpetrator. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 441–454. [Google Scholar] [CrossRef]
- Willmann, S.; Coboeken, K.; Kapsa, S.; Thelen, K.; Mundhenke, M.; Fischer, K.; Hugl, B.; Wolfgang, M. Applications of Physiologically Based Pharmacokinetic Modeling of Rivaroxaban—Renal and Hepatic Impairment and Drug-Drug Interaction Potential. J. Clin. Pharmacol. 2021, 61, 656–665. [Google Scholar] [CrossRef]
- Food & Drug Administration Guidance for Industry: In Vitro Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions. 2020. Available online: https://www.fda.gov/media/134582/download (accessed on 1 October 2022).
- Bermejo, M.; Hens, B.; Dickens, J.; Mudie, D.; Paixão, P.; Tsume, Y.; Shedden, K.; Amidon, L.G. A Mechanistic Physiologically based Biopharmaceutics Modeling (PBBM) Approach to Assess the In Vivo Performance of an Orally Administered Drug Product: From IVIVC to IVIVP. Pharmaceutics 2019, 12, 74. [Google Scholar] [CrossRef]
- Cvijić, S.; Ignjatović, J.; Parojčić, J.; Ibrić, S. The Emerging Role of Physiologically-Based Pharmacokinetic/Biopharmaceutics Modeling in Formulation Development. Arh. Za Farm. 2021, 71, 318–335. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Chung, J.; Asperen, J.; Heimbach, T. Physiologically Based Absorption Modeling to Impact Biopharmaceutics and Formulation Strategies in Drug Development—Industry Case studies. J. Pharm. Sci. 2016, 105, 2723–2734. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Balakrishnan, A.; Manser, K. Utility of PBPK Absorption Modeling to Guide Modified Release Formulation Development of Gaboxadol, a Highly Soluble Compound with Region-Dependent Absorption. J. Pharm. Sci. 2016, 105, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Duan, J.; Kesisoglou, F.; Novakovic, J.; Amidon, G.L.; Jamie, M.; Lukacova, V.; Eissing, T.; Tsakalozou, E.; Zhao, L.; et al. Mechanistic Oral Absorption Modeling and Simulation for Formulation Development and Bioequivalence Evaluation: Report of an FDA Public Workshop. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, T.; Kesisoglou, F.; Novakovic, J.; Tistaert, C.; Zsigmondy, M.M.; Kollipara, S.; Ahmed, T.; Mitra, A.; Sharp, S.S. Establishing the Bioequivalence Safe Space for Immediate Release Oral Dosage Forms using Physiologically Based Biopharmaceutics Modeling (PBBM): Case Studies. J. Pharm. Sci. 2021, 110, 3896–3906. [Google Scholar] [CrossRef] [PubMed]
- Stillhart, C.; Pepin, X.; Tistaert, C.; Good, D.; Bergh, V.D.A.; Parrot, N.; Kesisoglou, F. PBPK Absorption Modeling: Establishing the In Vitro-In vivo Link—Industry Perspective. AAPS J. 2019, 21, 19. [Google Scholar] [CrossRef]
- Food & Drug Administration Grant (1U01FD007320): Dermal Drug Product Quality and Bioequivalence Assessment through Advanced Mechanistic Absorption Modeling and PBPK Simulation. 2021. Available online: https://www.fda.gov/media/154295/download (accessed on 1 October 2022).
- Food & Drug Administration Grant (1U01FD007323): Progressing Integration of In Vitro Topical Formulation Characterization, Release and Permeation Data to the Next Level—PBPK Based Extrapolation to Bioequivalence Assessment in Virtual Populations. 2021. Available online: https://www.fda.gov/media/154295/download (accessed on 1 October 2022).
- Food & Drug Administration Grant (1UF01FD006521): Characterization of Key System Parameters of Mechanistic Dermal PBPK Models in Various Skin Diseases and Performance Verification of the Model Using Observed Local and Systemic Concentrations. 2020. Available online: https://www.fda.gov/media/146749/download (accessed on 1 October 2022).
- Food & Drug Administration Grant (1U01FD006927): Development and Validation of a PBPK/PD Modeling Strategy for Opthalmic Drug Products to Support Translation from Preclinical Species to Human. 2020. Available online: https://www.fda.gov/media/146749/download (accessed on 1 October 2022).
- Food & Drug Administration Grant (1U01FD006929): Computation Biology (Cobi) Tools as a Framework for Physiologically-Based Pharmacokinetic/Pharmacodynamic Model Extrapolation from Rabbit to Human for Ophthalmic Drug Products. 2020. Available online: https://www.fda.gov/media/146749/download (accessed on 1 October 2022).
- Food & Drug Administration Contract (75F40120C00150): Robust In Vitro/In Silico Model to Accelerate Generic Drug Product Development for the Oral Cavity Route of Administration. 2020. Available online: https://www.fda.gov/media/146749/download (accessed on 1 October 2022).
- Edginton, A.; Theil, F.P.; Schmitt, W.; Willmann, S. Whole Body Physiologically-Based Pharmacokinetic Models: Their Use in Clinical Drug Development. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 1143–1152. [Google Scholar] [CrossRef]




| Word | Weight |
|---|---|
| Drug | 6108 |
| Pharmacokinetic | 3552 |
| Concentration | 3251 |
| Exposure | 2642 |
| Human | 2567 |
| Plasma | 2094 |
| Vitro | 1892 |
| Clinical | 1785 |
| Dose | 1778 |
| Tissue | 1697 |
| Effect | 1482 |
| Vivo | 1429 |
| Population | 1347 |
| Development | 1196 |
| Oral | 1079 |
| Interaction | 1067 |
| Clearance | 1027 |
| Patient | 1016 |
| Rat | 949 |
| Absorption | 944 |
| DDI | 821 |
| Metabolism | 798 |
| Dosing | 821 |
| Risk | 738 |
| Age | 738 |
| Distribution | 711 |
| Child | 675 |
| Topic 1 | Topic 2 | Topic 3 | Topic 4 | Topic 5 |
|---|---|---|---|---|
| Human | Concentration | Population | Drug | Drug |
| Exposure | Plasma | Age | DDI | Vitro |
| Concentration | Tissue | Child | Interaction | Vivo |
| Rat | Pharmacokinetic | Drug | Clinical | Absorption |
| Liver | Dose | Pharmacokinetic | Pharmacokinetic | Development |
| Blood | Renal | Adult | Cyp3A4 | Pharmacokinetic |
| Pharmacokinetic | Drug | Protein | Inhibition | Clinical |
| Tissue | Patient | Change | Exposure | Oral |
| Risk | Dosing | Dosing | Effect | Silico |
| Specie | Clinical | Clearance | Inhibitor | Effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstevska, A.; Đuriš, J.; Ibrić, S.; Cvijić, S. In-Depth Analysis of Physiologically Based Pharmacokinetic (PBPK) Modeling Utilization in Different Application Fields Using Text Mining Tools. Pharmaceutics 2023, 15, 107. https://doi.org/10.3390/pharmaceutics15010107
Krstevska A, Đuriš J, Ibrić S, Cvijić S. In-Depth Analysis of Physiologically Based Pharmacokinetic (PBPK) Modeling Utilization in Different Application Fields Using Text Mining Tools. Pharmaceutics. 2023; 15(1):107. https://doi.org/10.3390/pharmaceutics15010107
Chicago/Turabian StyleKrstevska, Aleksandra, Jelena Đuriš, Svetlana Ibrić, and Sandra Cvijić. 2023. "In-Depth Analysis of Physiologically Based Pharmacokinetic (PBPK) Modeling Utilization in Different Application Fields Using Text Mining Tools" Pharmaceutics 15, no. 1: 107. https://doi.org/10.3390/pharmaceutics15010107
APA StyleKrstevska, A., Đuriš, J., Ibrić, S., & Cvijić, S. (2023). In-Depth Analysis of Physiologically Based Pharmacokinetic (PBPK) Modeling Utilization in Different Application Fields Using Text Mining Tools. Pharmaceutics, 15(1), 107. https://doi.org/10.3390/pharmaceutics15010107









