Therapeutic Potential of Controlled Delivery Systems in Asthma: Preclinical Development of Flavonoid-Based Treatments
Abstract
:1. Introduction
2. Asthma Physiopathology, Current Treatments, and Related Challenges
3. Flavonoids: Chemistry and Biological Actions
4. Controlled Flavonoid Release Systems in Asthma Treatment
5. Perspectives of Novel Formulations Containing Flavonoids for the Treatment of Asthma, and Improving Pre-Clinical Data towards Successful Human Translation
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Pereira, A.M.; Morais-Almeida, M. Asthma costs and social impact. Asthma Res. Pract. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghoubi, M.; Adibi, A.; Safari, A.; FitzGerald, J.M.; Sadatsafavi, M. The Projected Economic and Health Burden of Uncontrolled Asthma in the United States. Am. J. Respir. Crit. Care Med. 2019, 200, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Garner, O.; Ramey, J.S.; Hanania, N.A. Management of Life-Threatening Asthma: Severe Asthma Series. Chest 2022, 162, 747–756. [Google Scholar] [CrossRef]
- McCracken, J.L.; Veeranki, S.P.; Ameredes, B.T.; Calhoun, W.J. Diagnosis and Management of Asthma in Adults: A Review. JAMA 2017, 318, 279–290. [Google Scholar] [CrossRef]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef]
- Borges, R.C.; Alith, M.B.; Nascimento, O.A.; Jardim, J.R. Gender differences in the perception of asthma respiratory symptoms in five Latin American countries. J. Asthma 2022, 59, 1030–1040. [Google Scholar] [CrossRef]
- Sharma, S.; Hashmi, M.F.; Chakraborty, R.K. Asthma Medications; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Verri, W.A., Jr.; Vicentini, F.T.M.C.; Baracat, M.M.; Georgetti, S.R.; Cardoso, R.D.; Cunha, M.T.; Ferreira, S.H.; Cunha, F.Q.; Fonseca, M.J.; Casagrande, R. Flavonoids as Anti-Inflammatory and Analgesic Drugs: Mechanisms of Action and Perspectives in the Development of Pharmaceutical Forms. In Studies in Natural Products Chemistry, 1st ed.; Rahman, A.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 36, pp. 297–330. [Google Scholar]
- Rashid, M.; Fareed, M.; Rashid, H.; Aziz, H.; Ehsan, N.; Khalid, S.; Ghaffar, I.; Ali, R.; Gul, A.; Hakeem, K.R. Flavonoids and Their Biological Secrets. In Plant and Human Health; Ozturk, M., Hakeem, K.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2, pp. 579–605. [Google Scholar]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef]
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent Research on Flavonoids and their Biomedical Applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar] [CrossRef]
- De Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Sansone, F.; Picerno, P.; Mencherini, T.; Villecco, F.; D’Ursi, A.M.; Aquino, R.P.; Lauro, M.R. Flavonoid microparticles by spray-drying: Influence of enhancers of the dissolution rate on properties and stability. J. Food Eng. 2011, 103, 188–196. [Google Scholar] [CrossRef]
- Grgic, J.; Selo, G.; Planinic, M.; Tisma, M.; Bucic-Kojic, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Guazelli, C.F.; Fattori, V.; Colombo, B.B.; Georgetti, S.R.; Vicentini, F.T.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J. Nat. Prod. 2013, 76, 200–208. [Google Scholar] [CrossRef]
- Dias, D.R.; Botrel, D.A.; Fernandes, R.V.B.; Borges, S.V. Encapsulation as a tool for bioprocessing of functional foods. Curr. Opin. Food Sci. 2017, 13, 31–37. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Jyothi, N.V.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef]
- Jia, Z.; Dumont, M.J.; Orsat, V. Encapsulation of phenolic compounds present in plants using protein matrices. Food Biosci. 2016, 15, 87–104. [Google Scholar] [CrossRef]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. Am. J. Respir. Crit. Care Med. 2022, 205, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.L.; Ling, M.F.; Adams, D.C.; Faustino, L.; Islam, S.A.; Afshar, R.; Griffith, J.W.; Harris, R.S.; Ng, A.; Radicioni, G.; et al. Allergic asthma is distinguished by sensitivity of allergen-specific CD4+ T cells and airway structural cells to type 2 inflammation. Sci. Transl. Med. 2016, 8, 359ra132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, G.G.; James, A.; Harkness, L.; Wark, P.A.B. Pathophysiology of severe asthma: We’ve only just started. Respirology 2018, 23, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Kay, A.B. The role of T lymphocytes in asthma. Chem. Immunol. Allergy 2006, 91, 59–75. [Google Scholar] [CrossRef]
- Kemeny, D.M. The role of the T follicular helper cells in allergic disease. Cell Mol. Immunol. 2012, 9, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Johnston, L.K.; Hsu, C.L.; Krier-Burris, R.A.; Chhiba, K.D.; Chien, K.B.; McKenzie, A.; Berdnikovs, S.; Bryce, P.J. IL-33 Precedes IL-5 in Regulating Eosinophil Commitment and Is Required for Eosinophil Homeostasis. J. Immunol. 2016, 197, 3445–3453. [Google Scholar] [CrossRef] [Green Version]
- Fulkerson, P.C.; Schollaert, K.L.; Bouffi, C.; Rothenberg, M.E. IL-5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J. Immunol. 2014, 193, 4043–4052. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, K.A.; O’Neill, L.A.J.; Gearing, A.J.H.; Callard, R.E. Eotaxin 1. In The Cytokine FactsBook and Webfacts, 2nd ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 213–216. [Google Scholar]
- Kuperman, D.A.; Huang, X.; Koth, L.L.; Chang, G.H.; Dolganov, G.M.; Zhu, Z.; Elias, J.A.; Sheppard, D.; Erle, D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002, 8, 885–889. [Google Scholar] [CrossRef]
- Ulrik, C.S.; Backer, V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur. Respir. J. 1999, 14, 892–896. [Google Scholar] [CrossRef] [Green Version]
- Hanania, N.A.; Moore, R.H.; Zimmerman, J.L.; Miller, C.T.; Bag, R.; Sharafkhaneh, A.; Dickey, B.F. The role of intrinsic efficacy in determining response to a beta2-agonist in acute severe asthma. Respir. Med. 2007, 101, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Ling, J.; Kumar, R. Crosstalk between NFkB and glucocorticoid signaling: A potential target of breast cancer therapy. Cancer Lett. 2012, 322, 119–126. [Google Scholar] [CrossRef]
- Leung, D.Y.; Bloom, J.W. Update on glucocorticoid action and resistance. J. Allergy Clin. Immunol. 2003, 111, 3–22; quiz 23. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef]
- Barshes, N.R.; Goodpastor, S.E.; Goss, J.A. Pharmacologic immunosuppression. Front. Biosci. 2004, 9, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Widdifield, J.; Bernatsky, S.; Paterson, J.M.; Gunraj, N.; Thorne, J.C.; Pope, J.; Cividino, A.; Bombardier, C. Serious infections in a population-based cohort of 86,039 seniors with rheumatoid arthritis. Arthritis Care Res. 2013, 65, 353–361. [Google Scholar] [CrossRef]
- Yasir, M.; Goyal, A.; Sonthalia, S. Corticosteroid Adverse Effects; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Parsons, J.P.; Hallstrand, T.S.; Mastronarde, J.G.; Kaminsky, D.A.; Rundell, K.W.; Hull, J.H.; Storms, W.W.; Weiler, J.M.; Cheek, F.M.; Wilson, K.C.; et al. An official American Thoracic Society clinical practice guideline: Exercise-induced bronchoconstriction. Am. J. Respir. Crit. Care Med. 2013, 187, 1016–1027. [Google Scholar] [CrossRef]
- Suissa, S.; Ernst, P.; Benayoun, S.; Baltzan, M.; Cai, B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N. Engl. J. Med. 2000, 343, 332–336. [Google Scholar] [CrossRef]
- Sin, D.D.; Tu, J.V. Inhaled corticosteroid therapy reduces the risk of rehospitalization and all-cause mortality in elderly asthmatics. Eur. Respir. J. 2001, 17, 380–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suissa, S.; Ernst, P.; Kezouh, A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax 2002, 57, 880–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, S.P.; Kunselman, S.J.; Icitovic, N.; Moore, W.C.; Pascual, R.; Ameredes, B.T.; Boushey, H.A.; Calhoun, W.J.; Castro, M.; Cherniack, R.M.; et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N. Engl. J. Med. 2010, 363, 1715–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, G.; Lumry, W.; Wolfe, J.; Given, J.; White, M.V.; Woodring, A.; Baitinger, L.; House, K.; Prillaman, B.; Shah, T. Combined salmeterol 50 microg and fluticasone propionate 250 microg in the diskus device for the treatment of asthma. Am. J. Respir. Crit. Care Med. 2000, 161, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Tamm, M.; Richards, D.H.; Beghe, B.; Fabbri, L. Inhaled corticosteroid and long-acting beta2-agonist pharmacological profiles: Effective asthma therapy in practice. Respir Med. 2012, 106 (Suppl. S1), S9–S19. [Google Scholar] [CrossRef] [Green Version]
- Reiss, T.F.; Chervinsky, P.; Dockhorn, R.J.; Shingo, S.; Seidenberg, B.; Edwards, T.B. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: A multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch. Intern. Med. 1998, 158, 1213–1220. [Google Scholar] [CrossRef] [Green Version]
- Israel, E.; Cohn, J.; Dube, L.; Drazen, J.M. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zileuton Clinical Trial Group. JAMA 1996, 275, 931–936. [Google Scholar] [CrossRef]
- Virchow, J.C., Jr.; Prasse, A.; Naya, I.; Summerton, L.; Harris, A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am. J. Respir. Crit. Care Med. 2000, 162, 578–585. [Google Scholar] [CrossRef]
- Esposito, R.; Spaziano, G.; Giannattasio, D.; Ferrigno, F.; Liparulo, A.; Rossi, A.; Roviezzo, F.; Sessa, M.; Falciani, M.; Berrino, L.; et al. Montelukast Improves Symptoms and Lung Function in Asthmatic Women Compared with Men. Front. Pharmacol. 2019, 10, 1094. [Google Scholar] [CrossRef]
- Spahn, J.D.; Covar, R.A.; Jain, N.; Gleason, M.; Shimamoto, R.; Szefler, S.J.; Gelfand, E.W. Effect of montelukast on peripheral airflow obstruction in children with asthma. Ann. Allergy Asthma Immunol. 2006, 96, 541–549. [Google Scholar] [CrossRef]
- Montuschi, P. Role of Leukotrienes and Leukotriene Modifiers in Asthma. Pharmaceuticals 2010, 3, 1792–1811. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef]
- Ukena, D.; Harnest, U.; Sakalauskas, R.; Magyar, P.; Vetter, N.; Steffen, H.; Leichtl, S.; Rathgeb, F.; Keller, A.; Steinijans, V.W. Comparison of addition of theophylline to inhaled steroid with doubling of the dose of inhaled steroid in asthma. Eur. Respir. J. 1997, 10, 2754–2760. [Google Scholar] [CrossRef] [Green Version]
- Walker, S.; Monteil, M.; Phelan, K.; Lasserson, T.J.; Walters, E.H. Anti-IgE for chronic asthma. Cochrane Database Syst. Rev. 2003, 4, CD003559. [Google Scholar] [CrossRef]
- Hanania, N.A.; Wenzel, S.; Rosen, K.; Hsieh, H.J.; Mosesova, S.; Choy, D.F.; Lal, P.; Arron, J.R.; Harris, J.M.; Busse, W. Exploring the effects of omalizumab in allergic asthma: An analysis of biomarkers in the EXTRA study. Am. J. Respir. Crit. Care Med. 2013, 187, 804–811. [Google Scholar] [CrossRef]
- Castro, M.; Zangrilli, J.; Wechsler, M.E.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.; Korn, S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef]
- Nowak, R.M.; Parker, J.M.; Silverman, R.A.; Rowe, B.H.; Smithline, H.; Khan, F.; Fiening, J.P.; Kim, K.; Molfino, N.A. A randomized trial of benralizumab, an antiinterleukin 5 receptor alpha monoclonal antibody, after acute asthma. Am. J. Emerg. Med. 2015, 33, 14–20. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Ortega, H.G.; Yancey, S.W.; Mayer, B.; Gunsoy, N.B.; Keene, O.N.; Bleecker, E.R.; Brightling, C.E.; Pavord, I.D. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: A secondary analysis of the DREAM and MENSA studies. Lancet Respir. Med. 2016, 4, 549–556. [Google Scholar] [CrossRef]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D.; SIRIUS Investigators. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef]
- Casale, T.B.; Pacou, M.; Mesana, L.; Farge, G.; Sun, S.X.; Castro, M. Reslizumab Compared with Benralizumab in Patients with Eosinophilic Asthma: A Systematic Literature Review and Network Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2019, 7, 122–130.e121. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Diver, S.; Khalfaoui, L.; Emson, C.; Wenzel, S.E.; Menzies-Gow, A.; Wechsler, M.E.; Johnston, J.; Molfino, N.; Parnes, J.R.; Megally, A.; et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 1299–1312. [Google Scholar] [CrossRef]
- Pham, T.H.; Chen, C.; Colice, G.; Parnes, J.R.; Griffiths, J.M.; Cook, B. Tezepelumab normalizes serum interleukin-5 and -13 levels in patients with severe, uncontrolled asthma. Ann. Allergy Asthma Immunol. 2021, 127, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Parnes, J.R.; Molfino, N.A.; Colice, G.; Martin, U.; Corren, J.; Menzies-Gow, A. Targeting TSLP in Asthma. J. Asthma Allergy 2022, 15, 749–765. [Google Scholar] [CrossRef]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Passalacqua, G.; Canonica, G.W. A Critical Evaluation of Anti-IL-13 and Anti-IL-4 Strategies in Severe Asthma. Int. Arch. Allergy Immunol. 2016, 170, 122–131. [Google Scholar] [CrossRef]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Chen, R.; Wei, J.; Guan, G.; Zhou, M.; Dong, N.; Cao, Y. Meta-analysis of randomized controlled trials for the efficacy and safety of anti-interleukin-13 therapy with lebrikizumab in patients with uncontrolled asthma. Allergy Asthma Proc. 2018, 39, 332–337. [Google Scholar] [CrossRef]
- Austin, C.D.; Gonzalez Edick, M.; Ferrando, R.E.; Solon, M.; Baca, M.; Mesh, K.; Bradding, P.; Gauvreau, G.M.; Sumino, K.; FitzGerald, J.M.; et al. A randomized, placebo-controlled trial evaluating effects of lebrikizumab on airway eosinophilic inflammation and remodelling in uncontrolled asthma (CLAVIER). Clin. Exp. Allergy 2020, 50, 1342–1351. [Google Scholar] [CrossRef]
- Corren, J.; Lemanske, R.F.; Hanania, N.A.; Korenblat, P.E.; Parsey, M.V.; Arron, J.R.; Harris, J.M.; Scheerens, H.; Wu, L.C.; Su, Z.; et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011, 365, 1088–1098. [Google Scholar] [CrossRef]
- Papi, A.; Blasi, F.; Canonica, G.W.; Morandi, L.; Richeldi, L.; Rossi, A. Treatment strategies for asthma: Reshaping the concept of asthma management. Allergy Asthma Clin. Immunol. 2020, 16, 75. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Jenkins, C.; Bateman, E.D. The paradoxes of asthma management: Time for a new approach? Eur. Respir. J. 2017, 50, 1701103. [Google Scholar] [CrossRef] [Green Version]
- Bleecker, E.R.; Menzies-Gow, A.N.; Price, D.B.; Bourdin, A.; Sweet, S.; Martin, A.L.; Alacqua, M.; Tran, T.N. Systematic Literature Review of Systemic Corticosteroid Use for Asthma Management. Am. J. Respir. Crit. Care Med. 2020, 201, 276–293. [Google Scholar] [CrossRef]
- Zeiger, R.S.; Schatz, M.; Li, Q.; Chen, W.; Khatry, D.B.; Tran, T.N. Burden of Chronic Oral Corticosteroid Use by Adults with Persistent Asthma. J. Allergy Clin. Immunol. Pract. 2017, 5, 1050–1060.e1059. [Google Scholar] [CrossRef]
- Bourdin, A.; Molinari, N.; Vachier, I.; Pahus, L.; Suehs, C.; Chanez, P. Mortality: A neglected outcome in OCS-treated severe asthma. Eur. Respir. J. 2017, 50, 1701486. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids—Food Sources, Health Benefits, and Mechanisms Involved. Rocz. Panstw. Zakl. Hig. 2017, 65, 79–85. [Google Scholar]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Machado, H.; Nagem, T.J.; Peters, V.M.; Fonseca, C.S.; Oliveira, T.T. Flavonoids and potential therapeutic. Bol. Centro Biol. Reprod. 2008, 27, 33–39. [Google Scholar]
- Yamagata, K. Metabolic Syndrome: Preventive Effects of Dietary Flavonoids. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 60, pp. 1–28. [Google Scholar]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Schmidt, S.; Buttle, I.; Akkar, A.; Schmitt, J.; Bromer, S. SolEmuls-novel technology for the formulation of i.v. emulsions with poorly soluble drugs. Int. J. Pharm. 2004, 269, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczynska, A.; Malecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, D.; Freitas, M.; Lima, J.L.; Fernandes, E. Proinflammatory Pathways: The Modulation by Flavonoids. Med. Res. Rev. 2015, 35, 877–936. [Google Scholar] [CrossRef]
- Krajka-Kuzniak, V.; Baer-Dubowska, W. Modulation of Nrf2 and NF-kappaB Signaling Pathways by Naturally Occurring Compounds in Relation to Cancer Prevention and Therapy. Are Combinations Better Than Single Compounds? Int. J. Mol. Sci. 2021, 22, 8223. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tome, S.M.; Silva, A.M.; Laufer, S.; Lima, J.L.; Fernandes, E. Flavonoids inhibit COX-1 and COX-2 enzymes and cytokine/chemokine production in human whole blood. Inflammation 2015, 38, 858–870. [Google Scholar] [CrossRef]
- Hohmann, M.S.; Zaninelli, T.H.; Staurengo-Ferrari, L.; Manchope, M.F.; Badaro-Garcia, S.; de Freitas, A.; Casagrande, R.; Verri, W.A.J. Nrf2 in Immune Responses During Inflammation. In Nrf2 and Its Modulation in Inflammation; Deng, H., Ed.; Springer: Cham, Switzerland, 2020; pp. 23–50. [Google Scholar]
- Staurengo-Ferrari, L.; Badaro-Garcia, S.; Hohmann, M.S.N.; Manchope, M.F.; Zaninelli, T.H.; Casagrande, R.; Verri, W.A., Jr. Contribution of Nrf2 Modulation to the Mechanism of Action of Analgesic and Anti-inflammatory Drugs in Pre-clinical and Clinical Stages. Front. Pharmacol. 2018, 9, 1536. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Takahashi, R. Flavonoids and asthma. Nutrients 2013, 5, 2128–2143. [Google Scholar] [CrossRef]
- Toledo, A.C.; Sakoda, C.P.; Perini, A.; Pinheiro, N.M.; Magalhaes, R.M.; Grecco, S.; Tiberio, I.F.; Camara, N.O.; Martins, M.A.; Lago, J.H.; et al. Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model. Br. J. Pharmacol. 2013, 168, 1736–1749. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.H.; Shin, D.; Han, S.Y.; Kim, J.L.; Kang, Y.H. Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J. Nutr. 2012, 142, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Li, M.L.; Xia, M.Y.; Shao, J.Y. Fisetin-treatment alleviates airway inflammation through inhbition of MyD88/NF-kappaB signaling pathway. Int. J. Mol. Med. 2018, 42, 208–218. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Dai, J.; Liu, H.; Li, R.R.; Sun, P.L.; Du, Q.; Pang, L.L.; Chen, Z.; Yin, K.S. Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-kappaB activity in a murine model of asthma. Can. J. Physiol. Pharmacol. 2009, 87, 729–735. [Google Scholar] [CrossRef]
- Park, H.H.; Lee, S.; Son, H.Y.; Park, S.B.; Kim, M.S.; Choi, E.J.; Singh, T.S.; Ha, J.H.; Lee, M.G.; Kim, J.E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Drzewiecki, G.; Krishnarao, D. Quercetin: An inhibitor of antigen-induced human basophil histamine release. J. Immunol. 1981, 127, 546–550. [Google Scholar]
- Fewtrell, C.M.; Gomperts, B.D. Effect of flavone inhibitors of transport ATPases on histamine secretion from rat mast cells. Nature 1977, 265, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Kimata, M.; Shichijo, M.; Miura, T.; Serizawa, I.; Inagaki, N.; Nagai, H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy 2000, 30, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Higa, S.; Arimitsu, J.; Naka, T.; Shima, Y.; Ohshima, S.; Fujimoto, M.; Yamadori, T.; Kawase, I.; Tanaka, T. Flavonoids such as luteolin, fisetin and apigenin are inhibitors of interleukin-4 and interleukin-13 production by activated human basophils. Int. Arch. Allergy Immunol. 2004, 134, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.P.; Matteliano, M.L.; Middleton, E., Jr. Effect of quercetin on human polymorphonuclear leukocyte lysosomal enzyme release and phospholipid metabolism. Life Sci. 1982, 31, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, J.Y.; Cho, C.H.; Kim, C.J. Anti-asthmatic action of quercetin and rutin in conscious guinea-pigs challenged with aerosolized ovalbumin. Arch. Pharm. Res. 2007, 30, 1599–1607. [Google Scholar] [CrossRef]
- Gil, B.; Sanz, M.J.; Terencio, M.C.; Ferrandiz, M.L.; Bustos, G.; Paya, M.; Gunasegaran, R.; Alcaraz, M.J. Effects of flavonoids on Naja naja and human recombinant synovial phospholipases A2 and inflammatory responses in mice. Life Sci. 1994, 54, PL333–PL338. [Google Scholar] [CrossRef]
- Higa, S.; Hirano, T.; Kotani, M.; Matsumoto, M.; Fujita, A.; Suemura, M.; Kawase, I.; Tanaka, T. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J. Allergy Clin. Immunol. 2003, 111, 1299–1306. [Google Scholar] [CrossRef]
- Mastuda, H.; Morikawa, T.; Ueda, K.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of antigen-Induced degranulation, TNF-alpha and IL-4 production from RBL-2H3 cells. Bioorg. Med. Chem. 2002, 10, 3123–3128. [Google Scholar] [CrossRef]
- Leemans, J.; Cambier, C.; Chandler, T.; Billen, F.; Clercx, C.; Kirschvink, N.; Gustin, P. Prophylactic effects of omega-3 polyunsaturated fatty acids and luteolin on airway hyperresponsiveness and inflammation in cats with experimentally-induced asthma. Vet. J. 2010, 184, 111–114. [Google Scholar] [CrossRef]
- Choi, J.R.; Lee, C.M.; Jung, I.D.; Lee, J.S.; Jeong, Y.I.; Chang, J.H.; Park, H.J.; Choi, I.W.; Kim, J.S.; Shin, Y.K.; et al. Apigenin protects ovalbumin-induced asthma through the regulation of GATA-3 gene. Int. Immunopharmacol. 2009, 9, 918–924. [Google Scholar] [CrossRef]
- Wu, M.Y.; Hung, S.K.; Fu, S.L. Immunosuppressive effects of fisetin in ovalbumin-induced asthma through inhibition of NF-kappaB activity. J. Agric. Food Chem. 2011, 59, 10496–10504. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, C.M.; Jung, I.D.; Lee, J.S.; Jeong, Y.I.; Chang, J.H.; Chun, S.H.; Kim, M.J.; Choi, I.W.; Ahn, S.C.; et al. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmacol. 2009, 9, 261–267. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, B.K.; Lee, Y.C. Antiasthmatic effects of hesperidin, a potential Th2 cytokine antagonist, in a mouse model of allergic asthma. Mediat. Inflamm. 2011, 2011, 485402. [Google Scholar] [CrossRef]
- Shi, Y.; Tan, Y.; Mao, S.; Gu, W. Naringenin inhibits allergeninduced airway remodeling in a murine model of asthma. Mol. Med. Rep. 2014, 9, 1204–1208. [Google Scholar] [CrossRef] [Green Version]
- Amakura, Y.; Tsutsumi, T.; Sasaki, K.; Nakamura, M.; Yoshida, T.; Maitani, T. Influence of food polyphenols on aryl hydrocarbon receptor-signaling pathway estimated by in vitro bioassay. Phytochemistry 2008, 69, 3117–3130. [Google Scholar] [CrossRef]
- Hirano, T.; Arimitsu, J.; Higa, S.; Naka, T.; Ogata, A.; Shima, Y.; Fujimoto, M.; Yamadori, T.; Ohkawara, T.; Kuwabara, Y.; et al. Luteolin, a flavonoid, inhibits CD40 ligand expression by activated human basophils. Int. Arch. Allergy Immunol. 2006, 140, 150–156. [Google Scholar] [CrossRef]
- Kimata, M.; Inagaki, N.; Nagai, H. Effects of luteolin and other flavonoids on IgE-mediated allergic reactions. Planta Med. 2000, 66, 25–29. [Google Scholar] [CrossRef]
- Funaguchi, N.; Ohno, Y.; La, B.L.; Asai, T.; Yuhgetsu, H.; Sawada, M.; Takemura, G.; Minatoguchi, S.; Fujiwara, T.; Fujiwara, H. Narirutin inhibits airway inflammation in an allergic mouse model. Clin. Exp. Pharmacol. Physiol. 2007, 34, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Alexandrakis, M.; Kempuraj, D.; Lytinas, M. Anti-inflammatory actions of flavonoids and structural requirements for new design. Int. J. Immunopathol. Pharmacol. 2001, 14, 119–127. [Google Scholar] [PubMed]
- Wang, X.; Cao, Y.; Chen, S.; Lin, J.; Bian, J.; Huang, D. Anti-Inflammation Activity of Flavones and Their Structure-Activity Relationship. J. Agric. Food Chem. 2021, 69, 7285–7302. [Google Scholar] [CrossRef] [PubMed]
- Heng, P.W.S. Controlled release drug delivery systems. Pharm. Dev. Technol. 2018, 23, 833. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Tan, M.L.; Choong, P.F.; Dass, C.R. Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides 2010, 31, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielinska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Bala, R.; Sindhu, R.K.; Kaundle, B.; Madaan, R.; Cavalu, S. The prospective of liquid crystals in nano formulations for drug delivery systems. J. Mol. Struct. 2021, 1245, 131117. [Google Scholar] [CrossRef]
- Tran, T.T.D.; Tran, P.H.L. Controlled Release Film Forming Systems in Drug Delivery: The Potential for Efficient Drug Delivery. Pharmaceutics 2019, 11, 290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Wang, L.; Yang, F.; Wu, D. Controlled cross-linking strategy for formation of hydrogels, microgels and nanogels. J. Control. Release 2015, 213, e25. [Google Scholar] [CrossRef]
- Cao, Q.C.; Wang, X.; Wu, D.C. Controlled Cross-linking Strategy for Formation of Hydrogels, Microgels and Nanogels. Chin. J. Polym. Sci. 2018, 36, 8–17. [Google Scholar] [CrossRef]
- Li, Z.; Xu, D.; Yuan, Y.; Wu, H.; Hou, J.; Kang, W.; Bai, B. Advances of spontaneous emulsification and its important applications in enhanced oil recovery process. Adv. Colloid Interface Sci. 2020, 277, 102119. [Google Scholar] [CrossRef]
- Patel, D.; Sawant, K.K. Self micro-emulsifying drug delivery system: Formulation development and biopharmaceutical evaluation of lipophilic drugs. Curr. Drug Deliv. 2009, 6, 419–424. [Google Scholar] [CrossRef]
- Nava-Arzaluz, M.G.; Pinon-Segundo, E.; Ganem-Rondero, A.; Lechuga-Ballesteros, D. Single emulsion-solvent evaporation technique and modifications for the preparation of pharmaceutical polymeric nanoparticles. Recent Pat. Drug Deliv. Formul. 2012, 6, 209–223. [Google Scholar] [CrossRef]
- Moulik, S.P.; Rakshit, A.K.; Pan, A.; Naskar, B. An Overview of Coacervates: The Special Disperse State of Amphiphilic and Polymeric Materials in Solution. Colloids Interfaces 2022, 63, 45. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Garud, A.; Singh, D.; Garud, N. Solid Lipid Nanoparticles (SLN): Method, Characterization and Applications. Int. Curr. Pharm. J. 2012, 1, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Clarence, D.D.; Paudel, K.R.; Manandhar, B.; Singh, S.K.; Devkota, H.P.; Panneerselvam, J.; Gupta, V.; Chitranshi, N.; Verma, N.; Saad, S.; et al. Unravelling the Therapeutic Potential of Nano-Delivered Functional Foods in Chronic Respiratory Diseases. Nutrients 2022, 14, 3828. [Google Scholar] [CrossRef]
- Magar, R.T.; Sohng, J.K. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef]
- Tu, B.; Liu, Z.J.; Chen, Z.F.; Ouyanga, Y.; Hu, Y.J. Understanding the structure–activity relationship between quercetin and naringenin: In vitro. RSC Adv. 2015, 5, 106171–106181. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Food 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kilic, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Dora, C.L.; Andrade, E.L.; Chaves, J.S.; Silva, L.F.; Lemos-Senna, E.; Calixto, J.B. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol. Res. 2010, 61, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Kumar, S.; Gupta, R.K.; Sharma, A.; Verma, A.K.; Stalin, K.; Chaudhari, B.P.; Das, M.; Singh, S.P.; Dwivedi, P.D. Reversion of Asthmatic Complications and Mast Cell Signalling Pathways in BALB/c Mice Model Using Quercetin Nanocrystals. J. Biomed. Nanotechnol. 2016, 12, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Iwao, Y.; Ishida, H.; Kimura, S.I.; Wakimoto, T.; Kondo, H.; Itai, S.; Noguchi, S. Crystal Structures of Flavone C-Glycosides from Oolong Tea Leaves: Chafuroside A Dihydrate and Chafuroside B Monohydrate. Chem. Pharm. Bull. 2019, 67, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Niho, N.; Mutoh, M.; Sakano, K.; Takahashi, M.; Hirano, S.; Nukaya, H.; Sugimura, T.; Wakabayashi, K. Inhibition of intestinal carcinogenesis by a new flavone derivative, chafuroside, in oolong tea. Cancer Sci. 2006, 97, 248–251. [Google Scholar] [CrossRef]
- Onoue, S.; Matsui, T.; Aoki, Y.; Ishida, H.; Nukaya, H.; Kou, K.; Yamada, S. Self-assembled micellar formulation of chafuroside A with improved anti-inflammatory effects in experimental asthma/COPD-model rats. Eur. J. Pharm. Sci. 2012, 45, 184–189. [Google Scholar] [CrossRef]
- Xie, F.; Du, G.; Ma, S.; Li, Y.; Wang, R.; Guo, F. Structural elucidation of in vitro metabolites of bavachinin in rat liver microsomes by LC-ESI-MSn and chemical synthesis. Xenobiotica 2016, 46, 296–306. [Google Scholar] [CrossRef]
- Du, G.; Zhao, Y.; Feng, L.; Yang, Z.; Shi, J.; Huang, C.; Li, B.; Guo, F.; Zhu, W.; Li, Y. Design, Synthesis, and Structure-Activity Relationships of Bavachinin Analogues as Peroxisome Proliferator-Activated Receptor gamma Agonists. ChemMedChem 2017, 12, 183–193. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Wang, M.; Tian, Y.; Sun, X.; Sun, G.; Sun, X. Bavachinin Induces Oxidative Damage in HepaRG Cells through p38/JNK MAPK Pathways. Toxins 2018, 10, 154. [Google Scholar] [CrossRef] [Green Version]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef]
- Li, X.; Xing, H.; Qin, Z.; Yang, J.; Wang, P.; Zhang, X.; Yao, Z.; Yao, X. Potential metabolism determinants and drug-drug interactions of a natural flavanone bavachinin. RSC Adv. 2020, 10, 35141–35152. [Google Scholar] [CrossRef]
- Wang, K.; Feng, Y.; Li, S.; Li, W.; Chen, X.; Yi, R.; Zhang, H.; Hong, Z. Oral Delivery of Bavachinin-Loaded PEG-PLGA Nanoparticles for Asthma Treatment in a Murine Model. J. Biomed. Nanotechnol. 2018, 14, 1806–1815. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, Q.; Li, H. Targeted Therapeutic Effect of Bavachinin Nanospheres on Pathological Site of Chronic Asthmatic Mice Model. J. Nanosci. Nanotechnol. 2021, 21, 1085–1090. [Google Scholar] [CrossRef]
- Park, H.; Dao, T.T.; Kim, H.P. Synthesis and inhibition of PGE2 production of 6,8-disubstituted chrysin derivatives. Eur. J. Med. Chem. 2005, 40, 943–948. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.P.; He, J.; Liu, D.; Zhang, Q.Z.; Li, K.; Zheng, X.; Tang, G.T.; Guo, Y.; Liu, Y. The Relationship between Pharmacological Properties and Structure- Activity of Chrysin Derivatives. Mini Rev. Med. Chem. 2019, 19, 555–568. [Google Scholar] [CrossRef]
- Yao, W.; Cheng, J.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L.; Lu, G. Toxicological evaluation of a flavonoid, chrysin: Morphological, behavioral, biochemical and histopathological assessments in rats. Drug Chem. Toxicol. 2021, 44, 601–612. [Google Scholar] [CrossRef]
- Roy, S.; Manna, K.; Jha, T.; Saha, K.D. Chrysin-loaded PLGA attenuates OVA-induced allergic asthma by modulating TLR/NF-kappaB/NLRP3 axis. Nanomedicine 2020, 30, 102292. [Google Scholar] [CrossRef]
- Chen, Y.P.; Zhang, Z.Y.; Li, Y.P.; Li, D.; Huang, S.L.; Gu, L.Q.; Xu, J.; Huang, Z.S. Syntheses and evaluation of novel isoliquiritigenin derivatives as potential dual inhibitors for amyloid-beta aggregation and 5-lipoxygenase. Eur. J. Med. Chem. 2013, 66, 22–31. [Google Scholar] [CrossRef]
- Escobar, S.J.M.; Munoz, L.; Fong, G.; Winnischofer, S.M.B.; Dennis, J.M.; Rocha, M.E.M.; Witting, P.K. 273—The Flavonoid Isoliquiritigenin Is Toxic to Neuroblastoma Cells and Promotes Necroptosis. Free Radic Biol. Med. 2016, 100, S121. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.J.; Yun, K.J.; Cho, Y.W.; Park, H.J.; Lee, K.T. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages. Eur. J. Pharmacol. 2008, 584, 175–184. [Google Scholar] [CrossRef]
- Cao, M.; Zhan, M.; Wang, Z.; Wang, Z.; Li, X.M.; Miao, M. Development of an Orally Bioavailable Isoliquiritigenin Self-Nanoemulsifying Drug Delivery System to Effectively Treat Ovalbumin-Induced Asthma. Int. J. Nanomed. 2020, 15, 8945–8961. [Google Scholar] [CrossRef]
- Tu, B.; Li, R.R.; Liu, Z.J.; Chen, Z.F.; Ouyang, Y.; Hu, Y.J. Structure-activity relationship study between baicalein and wogonin by spectrometry, molecular docking and microcalorimetry. Food Chem. 2016, 208, 192–198. [Google Scholar] [CrossRef]
- Park, B.B.; Choi, J.W.; Park, D.; Choi, D.; Paek, J.; Kim, H.J.; Son, S.Y.; Mushtaq, A.U.; Shin, H.; Kim, S.H.; et al. Structure-Activity Relationships of Baicalein and its Analogs as Novel TSLP Inhibitors. Sci. Rep. 2019, 9, 8762. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Shi, A.; Pang, H.; Xue, W.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Li, K.; Xiao, W.; et al. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J. Ethnopharmacol. 2014, 156, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mehrabi Nasab, E.; Athari, S.S. Study effect of Baicalein encapsulated/loaded Chitosan-nanoparticle on allergic Asthma pathology in mouse model. Saudi J. Biol. Sci. 2021, 28, 4311–4317. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Akhtar, N.; Raish, M.; Aqil, M. Systemic delivery of beta-blockers via transdermal route for hypertension. Saudi Pharm. J. 2015, 23, 587–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tijani, A.O.; Nunez, E.; Singh, K.; Khanna, G.; Puri, A. Transdermal Route: A Viable Option for Systemic Delivery of Antidepressants. J. Pharm. Sci. 2021, 110, 3129–3149. [Google Scholar] [CrossRef] [PubMed]
- Tanner, T.; Marks, R. Delivering drugs by the transdermal route: Review and comment. Skin Res. Technol. 2008, 14, 249–260. [Google Scholar] [CrossRef]
- Peluso, I.; Palmery, M. Flavonoids at the pharma-nutrition interface: Is a therapeutic index in demand? Biomed. Pharmacother. 2015, 71, 102–107. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Zhang, M.; Sun, J. Nanocarriers for oral drug delivery. J. Drug Target. 2013, 21, 515–527. [Google Scholar] [CrossRef]
- De Souza, I.F.F.; Dos Santos, T.Q.; Placido, R.V.; Mangerona, B.A.; Carvalho, F.C.; Boralli, V.B.; Ruela, A.L.M.; Pereira, G.R. The liquid crystalline phase behaviour of a nasal formulation modifies the brain disposition of donepezil in rats in the treatment of Alzheimer’s disease. Colloids Surf. B Biointerfaces 2021, 203, 111721. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czock, D.; Keller, F.; Rasche, F.M.; Haussler, U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 2005, 44, 61–98. [Google Scholar] [CrossRef] [PubMed]
- Papiris, S.; Kotanidou, A.; Malagari, K.; Roussos, C. Clinical review: Severe asthma. Crit. Care 2002, 6, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Joskova, M.; Franova, S.; Sadlonova, V. Acute bronchodilator effect of quercetin in experimental allergic asthma. Bratisl. Lek. Listy 2011, 112, 9–12. [Google Scholar] [PubMed]
- Franova, S.; Molitorisova, M.; Kazimierova, I.; Joskova, M.; Forsberg, C.I.N.; Novakova, E.; Sutovska, M. Pharmacodynamic evaluation of dihydroxyflavone derivate chrysin in a guinea pig model of allergic asthma. J. Pharm. Pharmacol. 2021, 73, 233–240. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Wen, Q.; Li, Y. Isoliquiritigenin, a flavonoid from licorice, relaxes guinea-pig tracheal smooth muscle in vitro and in vivo: Role of cGMP/PKG pathway. Eur. J. Pharmacol. 2008, 587, 257–266. [Google Scholar] [CrossRef]
- Hazekamp, A.; Verpoorte, R.; Panthong, A. Isolation of a bronchodilator flavonoid from the Thai medicinal plant Clerodendrum petasites. J. Ethnopharmacol. 2001, 78, 45–49. [Google Scholar] [CrossRef]
- Ghayur, M.N.; Khan, H.; Gilani, A.H. Antispasmodic, bronchodilator and vasodilator activities of (+)-catechin, a naturally occurring flavonoid. Arch. Pharm. Res. 2007, 30, 970–975. [Google Scholar] [CrossRef]
- Khan, A.U.; Gilani, A.H. Selective bronchodilatory effect of Rooibos tea (Aspalathus linearis) and its flavonoid, chrysoeriol. Eur. J. Nutr. 2006, 45, 463–469. [Google Scholar] [CrossRef]
- Jasemi, S.V.; Khazaei, H.; Fakhri, S.; Mohammadi-Noori, E.; Farzaei, M.H. Naringenin Improves Ovalbumin-Induced Allergic Asthma in Rats through Antioxidant and Anti-Inflammatory Effects. Evid.-Based Complement. Alternat. Med. 2022, 2022, 9110798. [Google Scholar] [CrossRef]
- Medeiros, K.C.; Faustino, L.; Borduchi, E.; Nascimento, R.J.; Silva, T.M.; Gomes, E.; Piuvezam, M.R.; Russo, M. Preventive and curative glycoside kaempferol treatments attenuate the TH2-driven allergic airway disease. Int. Immunopharmacol. 2009, 9, 1540–1548. [Google Scholar] [CrossRef]
- Park, S.H.; Gong, J.H.; Choi, Y.J.; Kang, M.K.; Kim, Y.H.; Kang, Y.H. Kaempferol Inhibits Endoplasmic Reticulum Stress-Associated Mucus Hypersecretion in Airway Epithelial Cells And Ovalbumin-Sensitized Mice. PLoS ONE 2015, 10, e0143526. [Google Scholar] [CrossRef] [Green Version]
- Goh, F.Y.; Upton, N.; Guan, S.; Cheng, C.; Shanmugam, M.K.; Sethi, G.; Leung, B.P.; Wong, W.S. Fisetin, a bioactive flavonol, attenuates allergic airway inflammation through negative regulation of NF-kappaB. Eur. J. Pharmacol. 2012, 679, 109–116. [Google Scholar] [CrossRef]
- Huang, W.C.; Liu, C.Y.; Shen, S.C.; Chen, L.C.; Yeh, K.W.; Liu, S.H.; Liou, C.J. Protective Effects of Licochalcone A Improve Airway Hyper-Responsiveness and Oxidative Stress in a Mouse Model of Asthma. Cells 2019, 8, 617. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.L.; Wang, C.H.; Lin, C.H.; Zhou, N.; Kao, S.T.; Wu, D.C. Luteolin Attenuates Airway Mucus Overproduction via Inhibition of the GABAergic System. Sci. Rep. 2016, 6, 32756. [Google Scholar] [CrossRef]
- Das, M.; Ram, A.; Ghosh, B. Luteolin alleviates bronchoconstriction and airway hyperreactivity in ovalbumin sensitized mice. Inflamm. Res. 2003, 52, 101–106. [Google Scholar] [CrossRef]
- Li, R.R.; Pang, L.L.; Du, Q.; Shi, Y.; Dai, W.J.; Yin, K.S. Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma. Immunopharmacol. Immunotoxicol. 2010, 32, 364–370. [Google Scholar] [CrossRef]
- Iwamura, C.; Shinoda, K.; Yoshimura, M.; Watanabe, Y.; Obata, A.; Nakayama, T. Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. Allergol. Int. 2010, 59, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Wei, D.; Bian, T.; Xie, P.; Zou, J.; Mu, H.; Zhang, B.; Zhou, X. Genistein attenuated allergic airway inflammation by modulating the transcription factors T-bet, GATA-3 and STAT-6 in a murine model of asthma. Pharmacology 2012, 89, 229–236. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; da Silva, E.V.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef]
- Liu, Y.N.; Zha, W.J.; Ma, Y.; Chen, F.F.; Zhu, W.; Ge, A.; Zeng, X.N.; Huang, M. Galangin attenuates airway remodelling by inhibiting TGF-beta1-mediated ROS generation and MAPK/Akt phosphorylation in asthma. Sci Rep. 2015, 5, 11758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.Y.; Jeong, G.S.; Lee, H.S.; Kwon, K.S.; Lee, S.M.; Park, J.W.; Kim, Y.C.; Park, B.H. Sulfuretin attenuates allergic airway inflammation in mice. Biochem. Biophys. Res. Commun. 2010, 400, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A. Pharmacological Strategies and Recent Advancement in Nano-Drug Delivery for Targeting Asthma. Life 2022, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, Y.; Ishimoto, Y.; Hotta, Y.; Hosoda, A.; Yoshikawa, H.; Akamatsu, M.; Tamura, H. Effect of flavonoids on androgen and glucocorticoid receptors based on in vitro reporter gene assay. Bioorg. Med. Chem. Lett. 2009, 19, 4706–4710. [Google Scholar] [CrossRef]
- Aun, M.V.; Bonamichi-Santos, R.; Arantes-Costa, F.M.; Kalil, J.; Giavina-Bianchi, P. Animal models of asthma: Utility and limitations. J. Asthma Allergy 2017, 10, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Chapman, D.G.; Tully, J.E.; Nolin, J.D.; Janssen-Heininger, Y.M.; Irvin, C.G. Animal models of allergic airways disease: Where are we and where to next? J. Cell Biochem. 2014, 115, 2055–2064. [Google Scholar] [CrossRef] [Green Version]
- Hesselmar, B.; Enelund, A.C.; Eriksson, B.; Padyukov, L.; Hanson, L.A.; Aberg, N. The heterogeneity of asthma phenotypes in children and young adults. J. Allergy 2012, 2012, 163089. [Google Scholar] [CrossRef] [Green Version]
- Sagar, S.; Akbarshahi, H.; Uller, L. Translational value of animal models of asthma: Challenges and promises. Eur. J. Pharmacol. 2015, 759, 272–277. [Google Scholar] [CrossRef]
- Shin, Y.S.; Takeda, K.; Gelfand, E.W. Understanding asthma using animal models. Allergy Asthma Immunol. Res. 2009, 1, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.N.; Chorawala, M.R. Animal models of asthma. Pharm. Res. Opin. 2014, 1, 139–147. [Google Scholar]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef]
- Alessandrini, F.; Musiol, S.; Schneider, E.; Blanco-Perez, F.; Albrecht, M. Mimicking Antigen-Driven Asthma in Rodent Models-How Close Can We Get? Front. Immunol. 2020, 11, 575936. [Google Scholar] [CrossRef]
- Kim, C.H.; Ahn, J.H.; Kim, S.J.; Lee, S.Y.; Kim, Y.K.; Kim, K.H.; Moon, H.S.; Song, J.S.; Park, S.H.; Kwon, S.S. Co-administration of vaccination with DNA encoding T cell epitope on the Der p and BCG inhibited airway remodeling in a murine model of chronic asthma. J. Asthma 2006, 43, 345–353. [Google Scholar] [CrossRef]
- Barrett, N.A.; Maekawa, A.; Rahman, O.M.; Austen, K.F.; Kanaoka, Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J. Immunol. 2009, 182, 1119–1128. [Google Scholar] [CrossRef]
- Debeuf, N.; Haspeslagh, E.; van Helden, M.; Hammad, H.; Lambrecht, B.N. Mouse Models of Asthma. Curr. Protoc. Mouse Biol. 2016, 6, 169–184. [Google Scholar] [CrossRef]
- Yasuda, Y.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Group 2 Innate Lymphoid Cells and the House Dust Mite-Induced Asthma Mouse Model. Cells 2020, 9, 1178. [Google Scholar] [CrossRef]
- McKinley, L.; Alcorn, J.F.; Peterson, A.; Dupont, R.B.; Kapadia, S.; Logar, A.; Henry, A.; Irvin, C.G.; Piganelli, J.D.; Ray, A.; et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J. Immunol. 2008, 181, 4089–4097. [Google Scholar] [CrossRef] [Green Version]
- Manni, M.L.; Mandalapu, S.; McHugh, K.J.; Elloso, M.M.; Dudas, P.L.; Alcorn, J.F. Molecular Mechanisms of Airway Hyperresponsiveness in a Murine Model of Steroid-Resistant Airway Inflammation. J. Immunol. 2016, 196, 963–977. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Oh, S.Y.; Jeon, S.G.; Park, H.W.; Lee, S.Y.; Chun, E.Y.; Bang, B.; Lee, H.S.; Oh, M.H.; Kim, Y.S.; et al. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J. Immunol. 2007, 178, 5375–5382. [Google Scholar] [CrossRef] [Green Version]
- Stein, J.; Maxeiner, J.H.; Montermann, E.; Hohn, Y.; Raker, V.; Taube, C.; Sudowe, S.; Reske-Kunz, A.B. Non-eosinophilic airway hyper-reactivity in mice, induced by IFN-gamma producing CD4+ and CD8+ lung T cells, is responsive to steroid treatment. Scand. J. Immunol. 2014, 80, 327–338. [Google Scholar] [CrossRef]
- Yu, Q.L.; Chen, Z. Establishment of different experimental asthma models in mice. Exp. Ther. Med. 2018, 15, 2492–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ober, C.; Thompson, E.E. Rethinking genetic models of asthma: The role of environmental modifiers. Curr. Opin. Immunol. 2005, 17, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef]
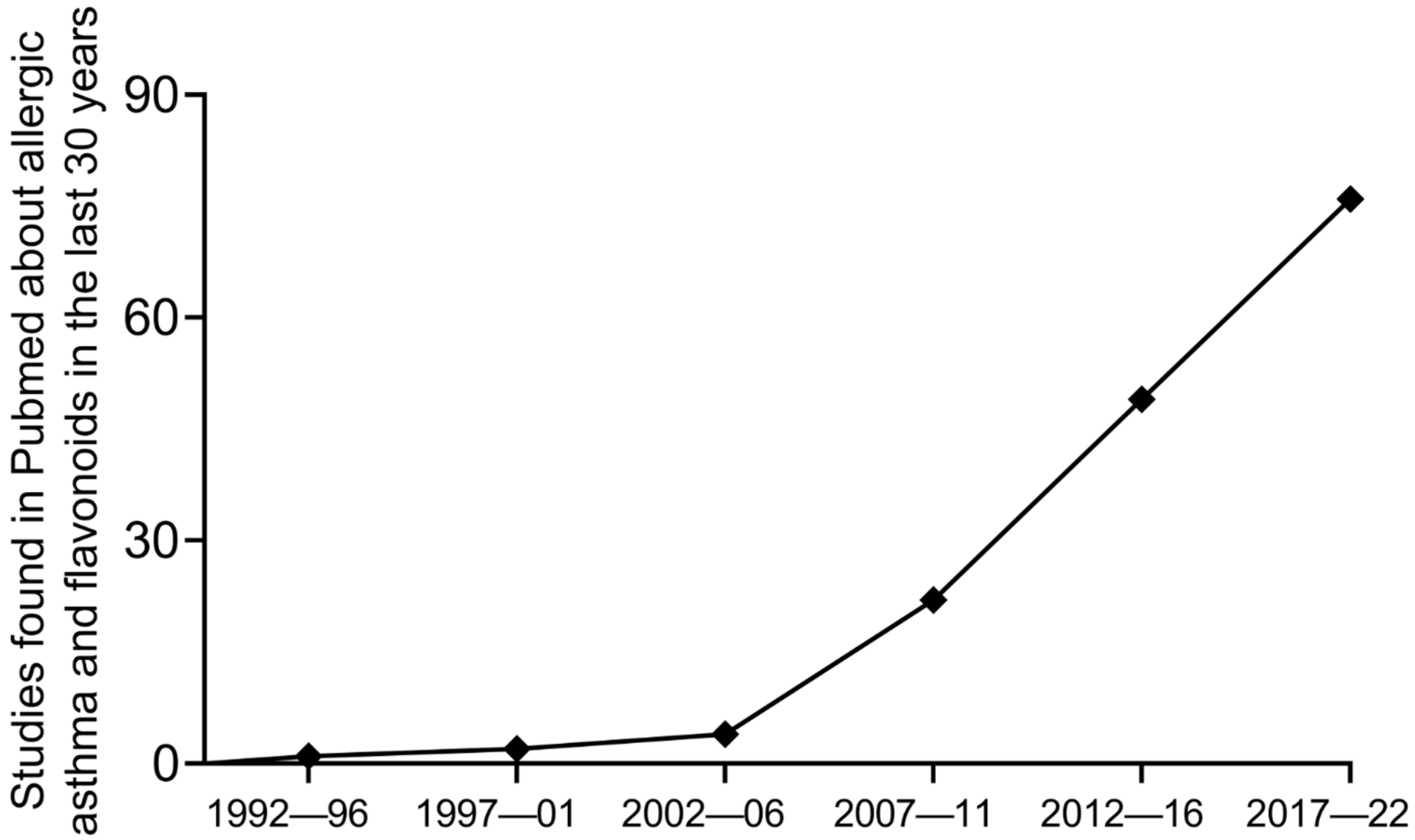
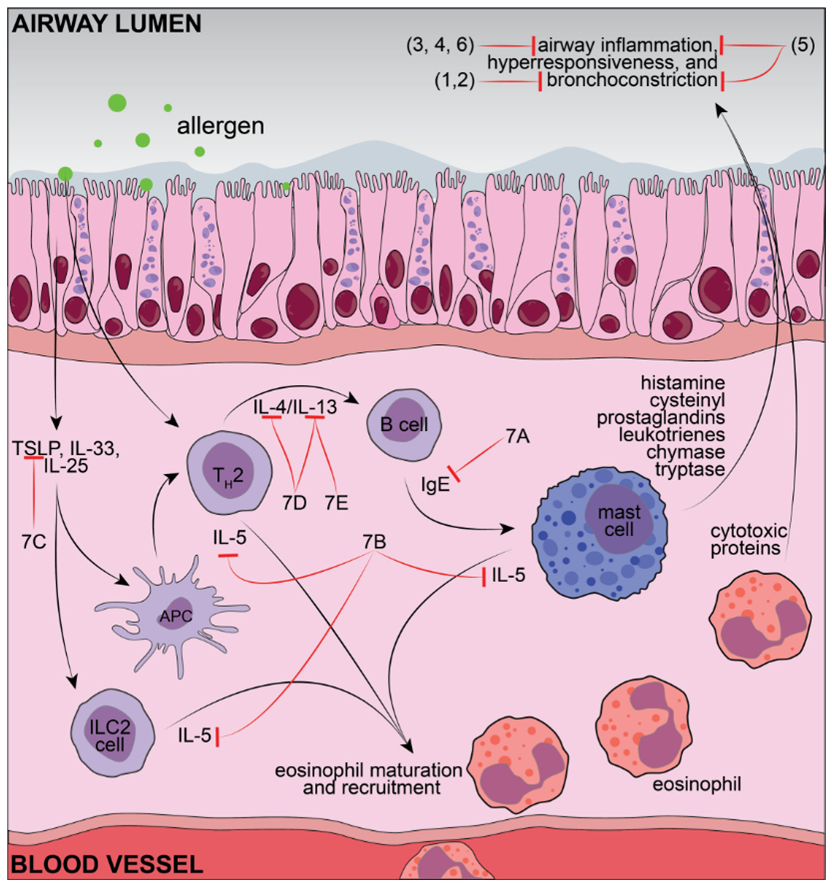
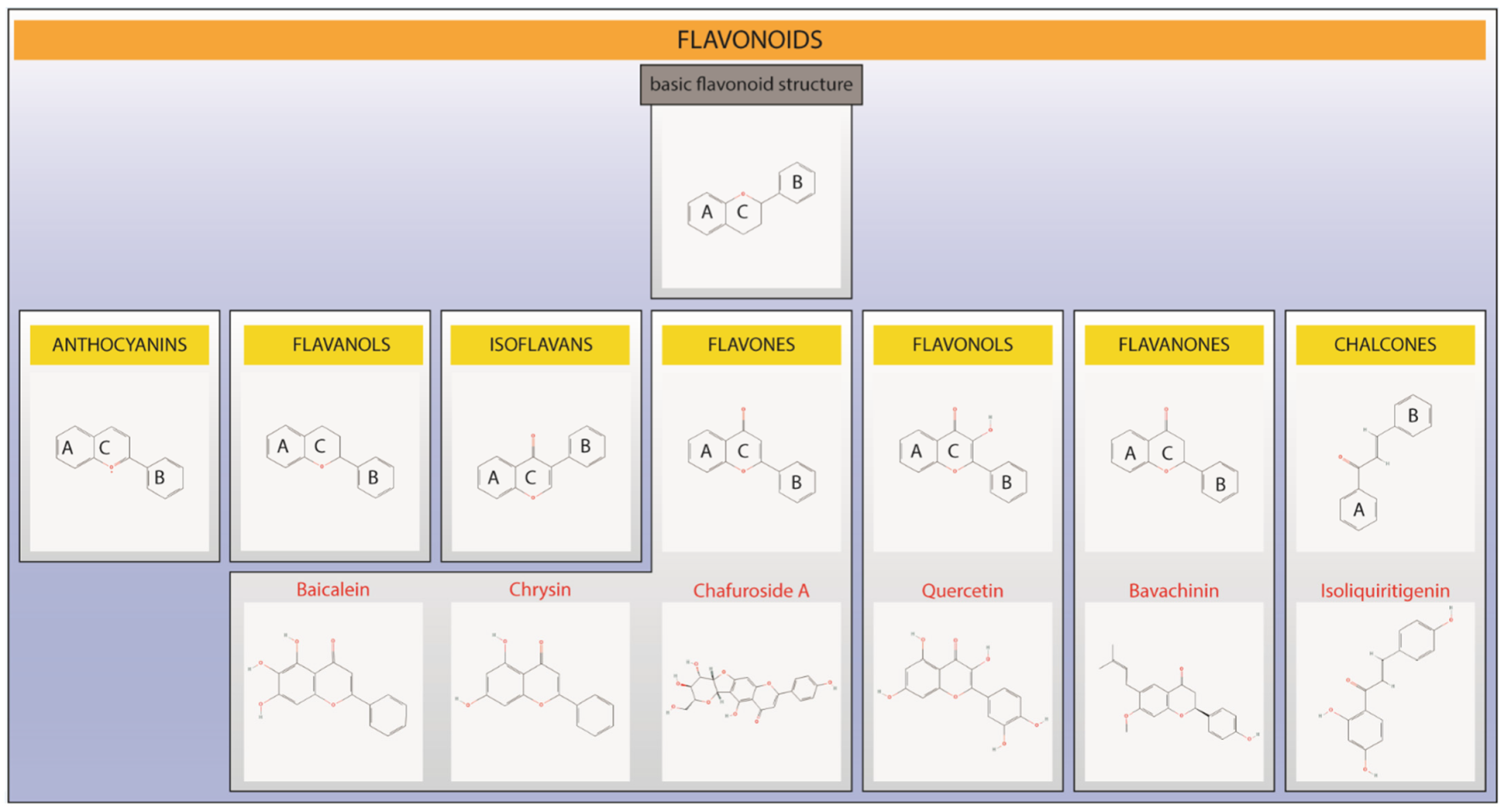
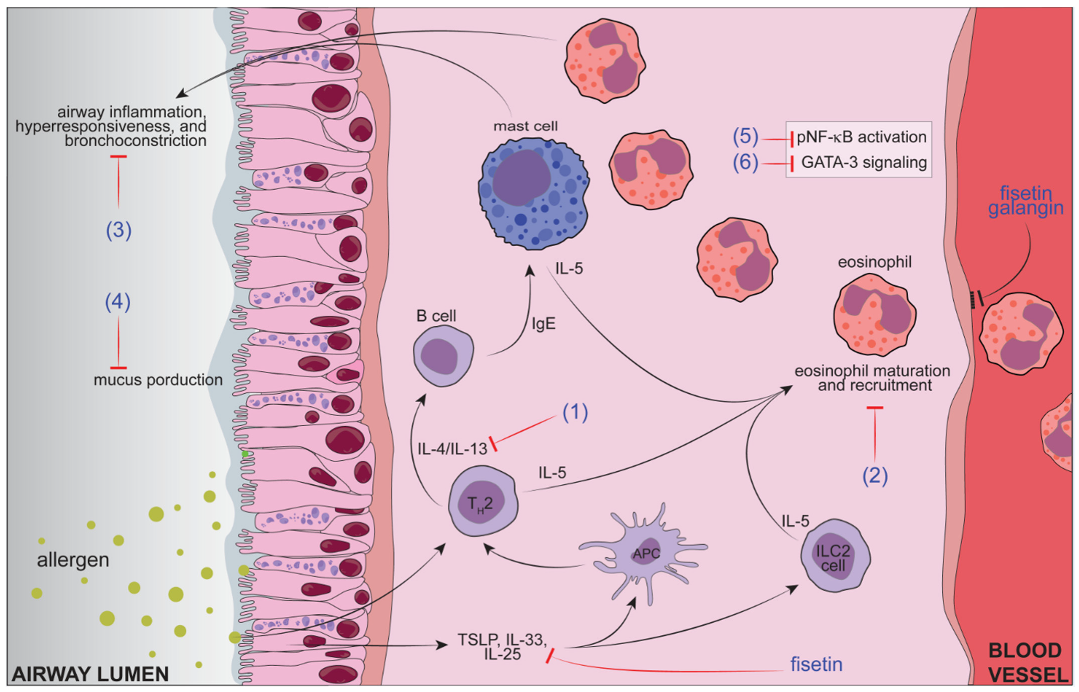
| Flavonoid (Respective Class) | Dose (Route of Administration) | Type of Controlled Delivery System (Better or Not than Free Forms) | Asthma Model and Species (Observed Parameters) |
|---|---|---|---|
| Quercetin (flavonol) [140] | 3 and 10 mg/kg (oral) | Microemulsions (yes for eosinophils recruitment) | OVA-induced asthma, BALB/c mice and Wistar rats (↓ eosinophils recruitment, NFκB p65, IL-4, IL-5, and mucus production) |
| Quercetin (flavonol) [141] | 1 mg/kg (intra-peritoneal) | Nanocrystal (not evaluated) | OVA-induced asthma, BALB/c mice (↓ sIgE, IL-4, IL-5, and activation of mast cells) |
| Chafuroside A (flavone) [142] | 0.1 mg/animal (oral) | Micelle (yes) | OVA-induced asthma, Sprague–Dawley rats (↓ eosinophils, macrophage, and neutrophils recruitment) |
| Bavachinin (flavanone) [143] | 50 mg/kg (oral) | Nanoparticle (not evaluated) | OVA-induced asthma, BALB/c mice (↓ IL-4, IL-5, IL-13, and the % of cytokine-producing CD4+ T cells) |
| Bavachinin (flavanone) [144] | We did not have access to full manuscript | Nanosphere (not evaluated) | (↓ Th2 cytokines and inflammatory infiltrate) |
| Chrysin (flavone) [145] | 50 mg/kg (oral) | Nanoparticle (yes) | OVA-induced asthma, BALB/c mice (↓ eosinophils, macrophage, neutrophils, and lymphocytes recruitment, IgE, NFκB p65, IL-4, IL-5, and IL-13) |
| Isoliquiritigenin (chalcone) [146] | 5 and 10 mg/kg (intragastric) | Nanoemulsions (yes) | OVA-induced asthma, Sprague–Dawley rats (↓ eosinophils, neutrophils, and lymphocytes recruitment, sIgE, IL-4, and IL-5) |
| Baicalein (flavone) [147] | 1 mg/mL (intranasal) | Loaded or emulsified nanoparticle (not evaluated) | OVA-induced asthma, BALB/c mice (↓ IL-5, mucus production, and AHR) |
| Flavonoid (Respective Class) | Dose (Route of Administration) | Adverse Effects/Toxicity | Asthma Model and Species (Observed Parameters) |
|---|---|---|---|
| Naringenin (flavanone) [153] | 20 and 40 mg/kg (oral) | Not evaluated | OVA-induced asthma, Wistar rats (↓ eosinophils recruitment, IL-4 and IL-13, and oxidative stress) |
| Kaempferol (flavonol) [154,155] | 3, 30, and 90 mg/kg (subcutaneous) [154] 10 and 20 mg/kg (oral) [155] | Not evaluated in both | OVA-induced asthma, BALB/c mice (↓ eosinophils and total leukocyte recruitment, IL-5 and IL-13, ER-mediated stress response, AHR, and mucus production) |
| Fisetin (flavonol) [118,156] | 1 and 3 mg/kg (intraperitoneal) [118] 0.3, 1, and 3 mg/kg (intravenous) [156] | Not evaluated in both | OVA-induced asthma, BALB/c mice (↓ adhesion molecules, eotaxin, TSLP, eosinophils, macrophages, neutrophils, and lymphocytes recruitment, NFκB p65, GATA-3, IL-33, IL-4, IL-5, and IL-13, AHR, and mucus production |
| Licochalcone A (chalcone) [157] | 5 and 10 mg/kg (intraperitoneal) | 33% mortality rate with 50 mg/kg dose | OVA-induced asthma, BALB/c mice (↓ eosinophils and lymphocytes recruitment, IgE, CCL11, IL-4, IL-5, and IL-13, AHR, and oxidative stress) |
| Luteolin (flavone) [158,159] | 0.1, 1, and 10 mg/kg (intraperitoneal) [158] 0.1, 1, and 10 mg/kg (oral) [159] | Not evaluated in both | OVA-induced asthma, BALB/c mice (↓ GABA signaling, IgE, IL-4, IL-5, and IL-13, AHR, and mucus production) |
| Rutin (flavonol) [112] | 7.5, 15, and 30 mg/kg (oral) | Not evaluated | OVA-induced asthma, Dunkin-Hartley guinea pig (↓ eosinophils and neutrophils recruitment, histamine, and AHR) |
| Apigenin (flavone) [117,160] | 5 and 10 mg/kg (intraperitoneal) [117] 2 and 20 mg/kg (intraperitoneal) [160] | Not evaluated in both | OVA-induced asthma, BALB/c mice (↓ eosinophils recruitment, IgE, GATA-3, IL-4, IL-5, and AHR) |
| Hesperidin (flavanone) [120] | 1 and 5 mg/kg (oral) | Not evaluated | OVA-induced asthma, BALB/c mice (↓ eotaxin, eosinophils recruitment, sIgE, GATA-3, IL-5, IL-17, and AHR) |
| Sakuranetin (flavanone) [102] | 20 mg/kg (10 µL intranasal) | Not evaluated | OVA-induced asthma, BALB/c mice and Wistar rats (↓ eotaxin, eosinophils recruitment, sIgE, IL-5, NFκB, AHR, and oxidative stress) |
| Naringenin chalcone (chalcone) [161] | 0.8 mg/kg (oral) | Not evaluated | OVA-induced asthma, BALB/c mice (↓ eosinophils recruitment, IL-4, IL-5, and IL-13, and AHR) |
| Genistein (isoflavone) [162] | 20 and 40 mg/kg (intraperitoneal) | Not evaluated | OVA-induced asthma, BALB/c mice (↓ eosinophils and lymphocytes recruitment, GATA-3, STAT-6, IL-4, and IL-5) |
| Isoquercitrin (flavonol) [163] | 15 mg/kg (oral) | Not evaluated | OVA-induced asthma, BALB/c mice (↓ eosinophils recruitment, and IL-5) |
| Galangin (flavonol) [164] | 0.1 and 0.5 mg/kg (intraperitoneal) | Low toxicity with low dose (10 µM) in ASMCs human cells | OVA-induced asthma, BALB/c mice (↓ eosinophils and neutrophils recruitment, sIgE, TGF-β1, VEGF, and mucus production) |
| Sulfuretin (aurone) [165] | 0.04 mg/kg (intraperitoneal) | Not evaluated | OVA-induced asthma, BALB/c mice (↓ eotaxin, eosinophils and lymphocytes recruitment, NFκB p65, IL-5, IL-3, and AHR) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghi, S.M.; Zaninelli, T.H.; Carra, J.B.; Heintz, O.K.; Baracat, M.M.; Georgetti, S.R.; Vicentini, F.T.M.C.; Verri, W.A.; Casagrande, R. Therapeutic Potential of Controlled Delivery Systems in Asthma: Preclinical Development of Flavonoid-Based Treatments. Pharmaceutics 2023, 15, 1. https://doi.org/10.3390/pharmaceutics15010001
Borghi SM, Zaninelli TH, Carra JB, Heintz OK, Baracat MM, Georgetti SR, Vicentini FTMC, Verri WA, Casagrande R. Therapeutic Potential of Controlled Delivery Systems in Asthma: Preclinical Development of Flavonoid-Based Treatments. Pharmaceutics. 2023; 15(1):1. https://doi.org/10.3390/pharmaceutics15010001
Chicago/Turabian StyleBorghi, Sergio M., Tiago H. Zaninelli, Jéssica B. Carra, Olivia K. Heintz, Marcela M. Baracat, Sandra R. Georgetti, Fabiana T. M. C. Vicentini, Waldiceu A. Verri, and Rubia Casagrande. 2023. "Therapeutic Potential of Controlled Delivery Systems in Asthma: Preclinical Development of Flavonoid-Based Treatments" Pharmaceutics 15, no. 1: 1. https://doi.org/10.3390/pharmaceutics15010001
APA StyleBorghi, S. M., Zaninelli, T. H., Carra, J. B., Heintz, O. K., Baracat, M. M., Georgetti, S. R., Vicentini, F. T. M. C., Verri, W. A., & Casagrande, R. (2023). Therapeutic Potential of Controlled Delivery Systems in Asthma: Preclinical Development of Flavonoid-Based Treatments. Pharmaceutics, 15(1), 1. https://doi.org/10.3390/pharmaceutics15010001







