Magnetoresponsive Functionalized Nanocomposite Aggregation Kinetics and Chain Formation at the Targeted Site during Magnetic Targeting
Abstract
:1. Introduction
- To investigate the arterial depositions and distribution of PEG_MNCs following local delivery in a stented artery model in a uniform magnetic field produced by a regionally positioned permanent magnet.
- To create a novel concept using permanent magnet systems to guide and target the functionalized nanocomposite around the stent to test the approach’s effectiveness for treating coronary heart disease (CHD).
- PEG_MNCs aggregation or chain formation in and around the implanted stent (in the presence of the external magnetic field).
2. Materials and Methods
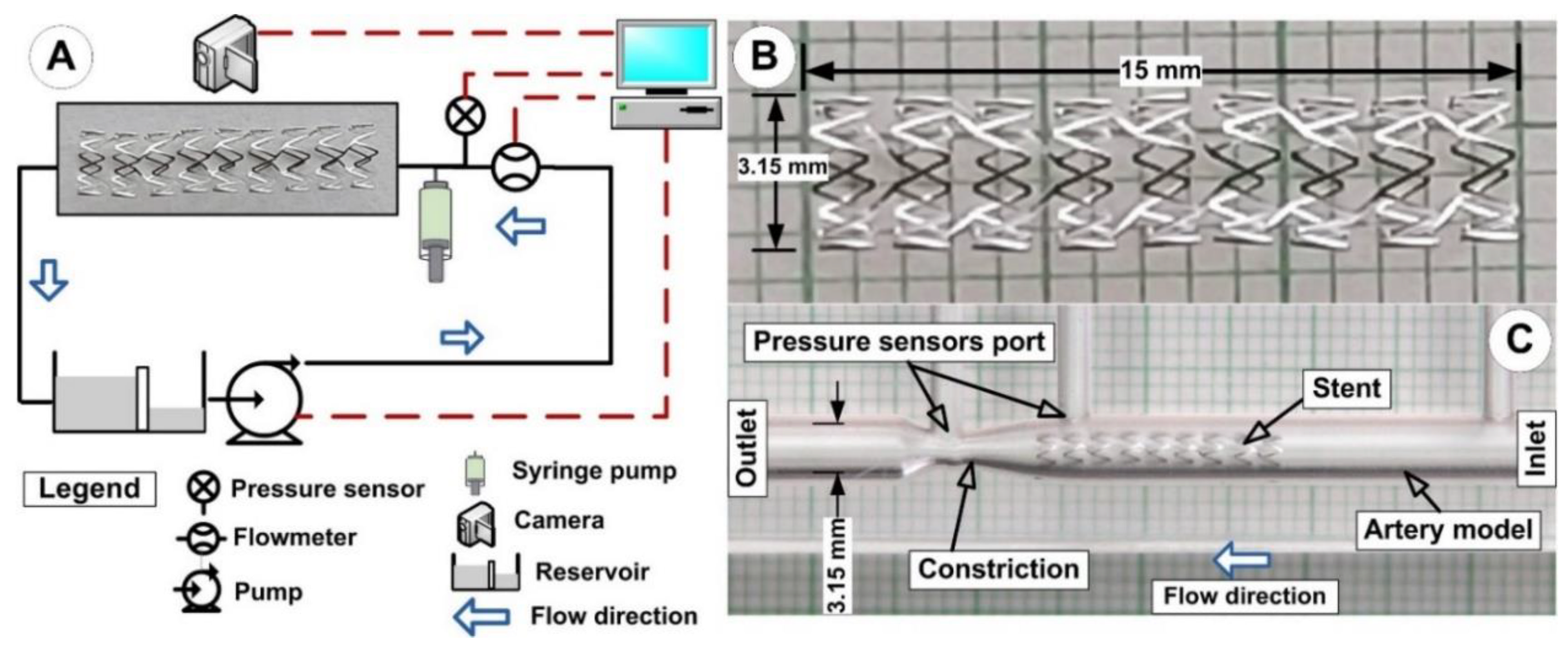
2.1. Experimental Setup
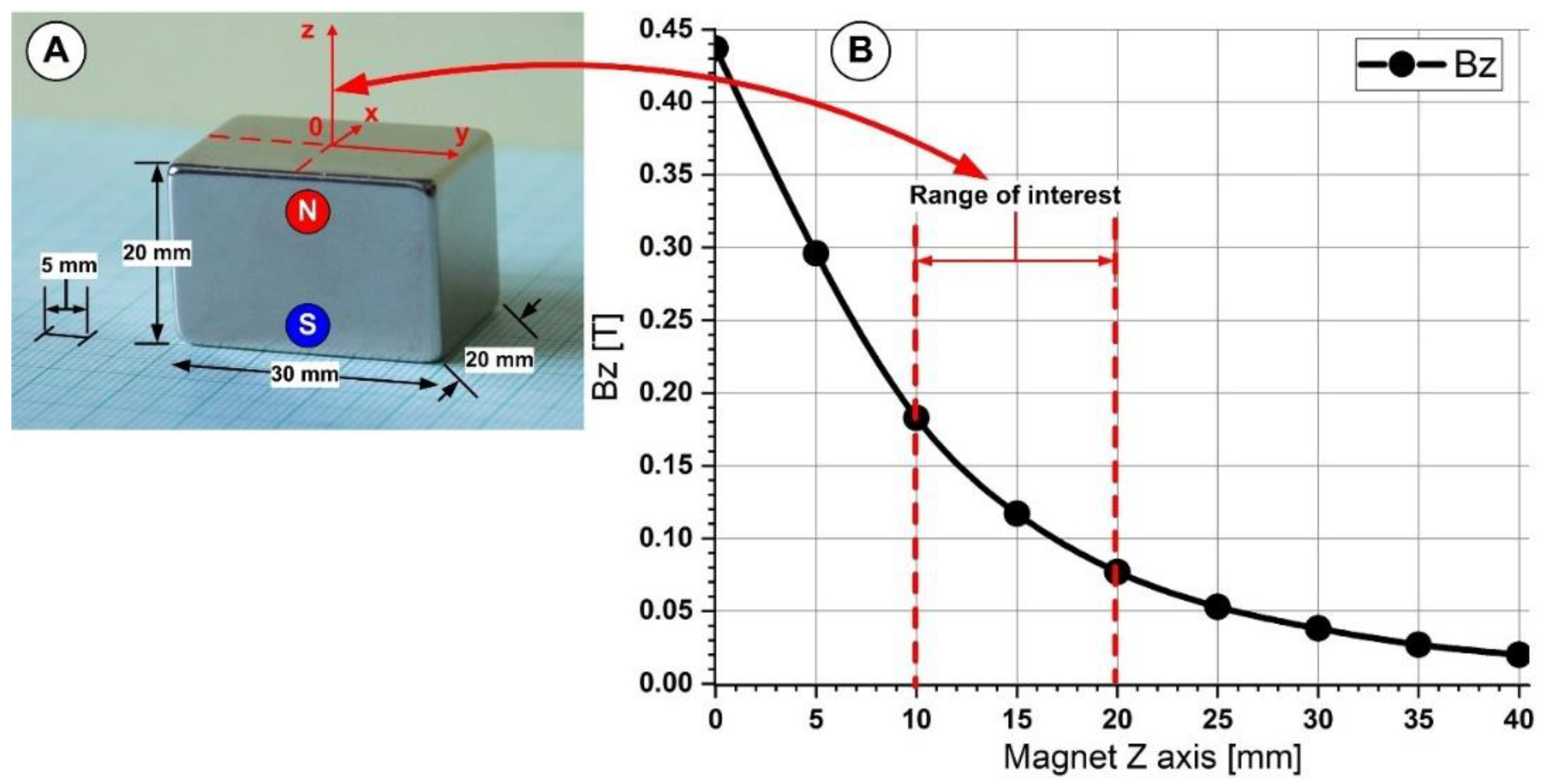
2.2. Magnetic Field Generation
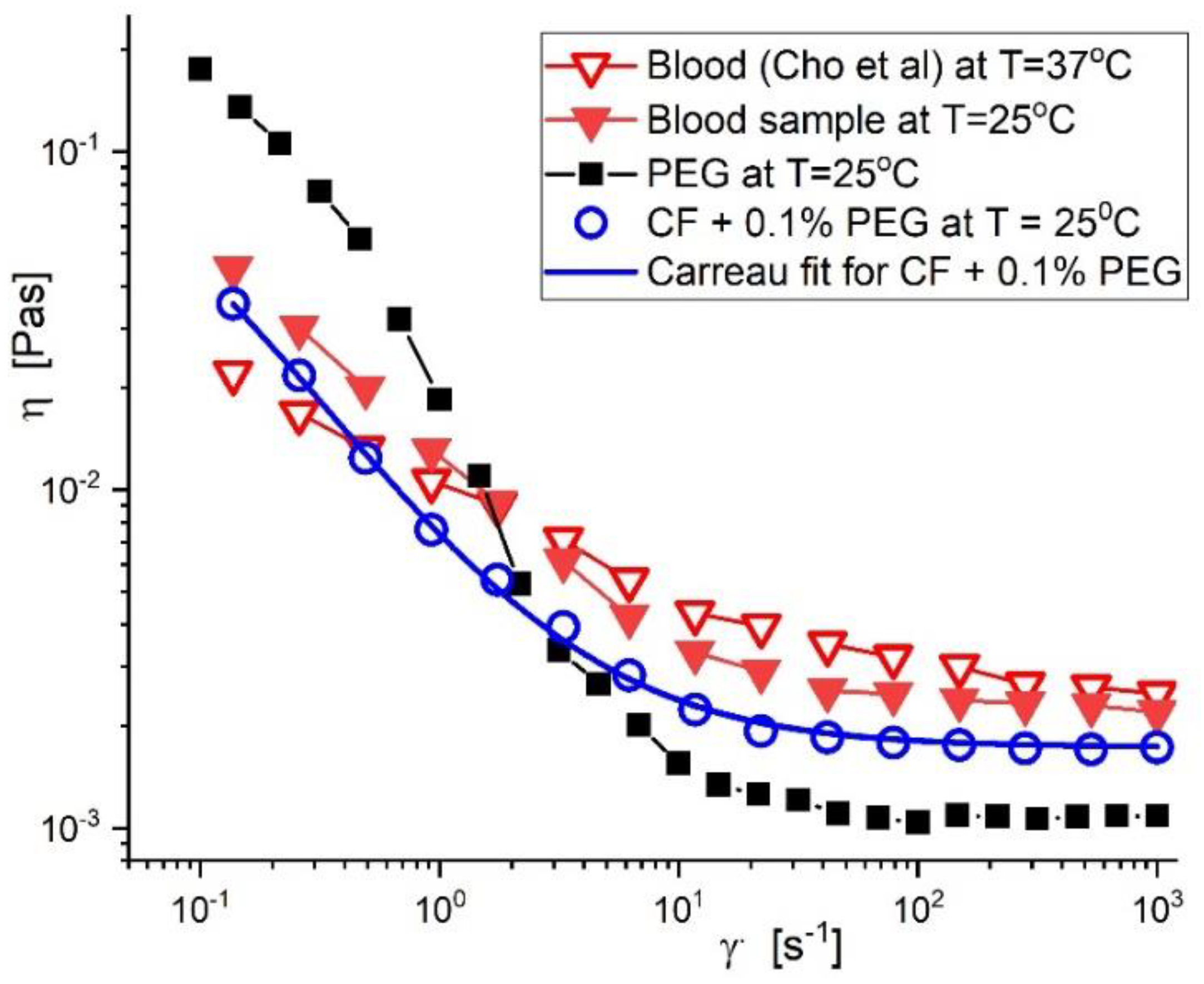
2.3. Blood Analogue Fluid, Preparation, and Rheological Properties
3. Results
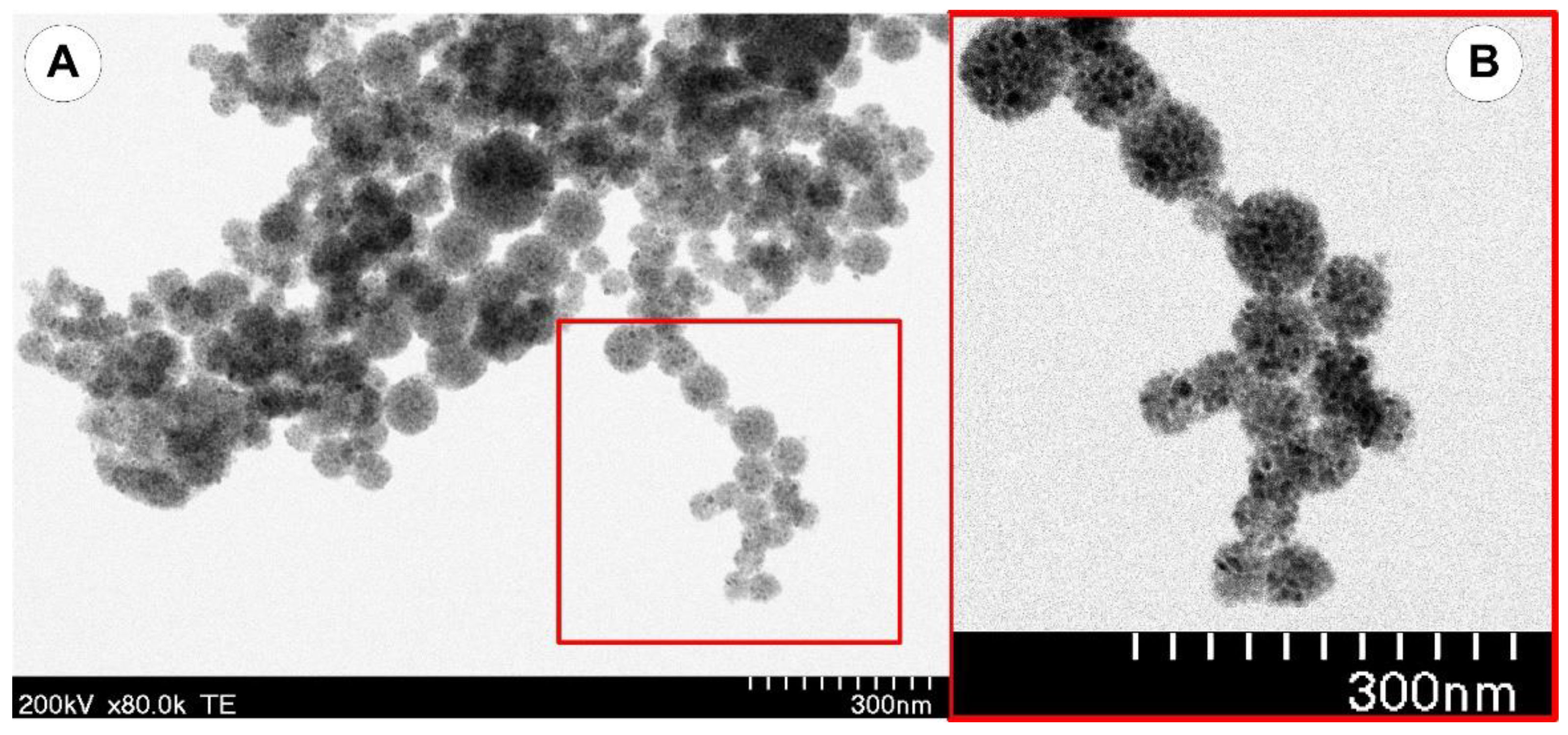
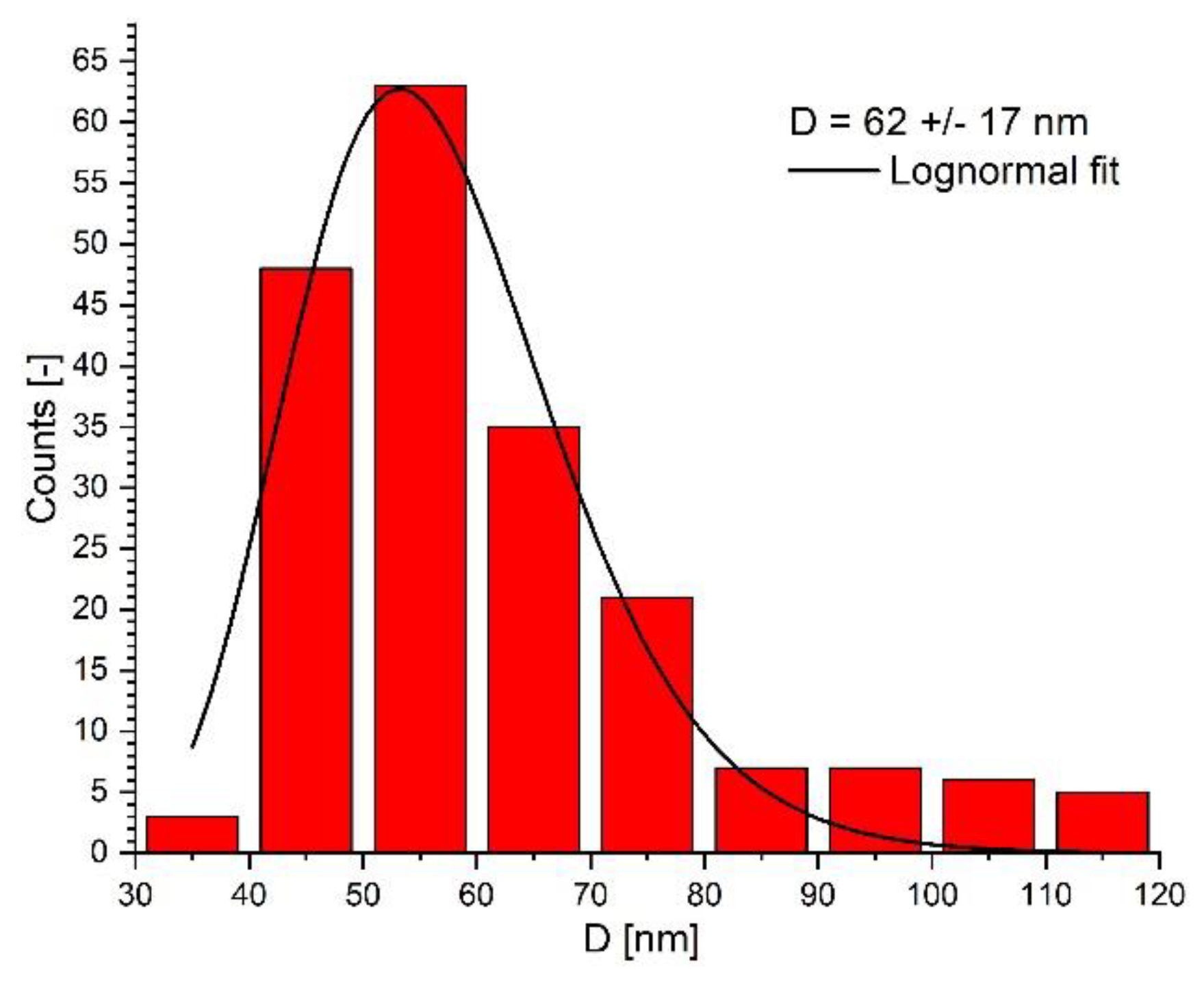
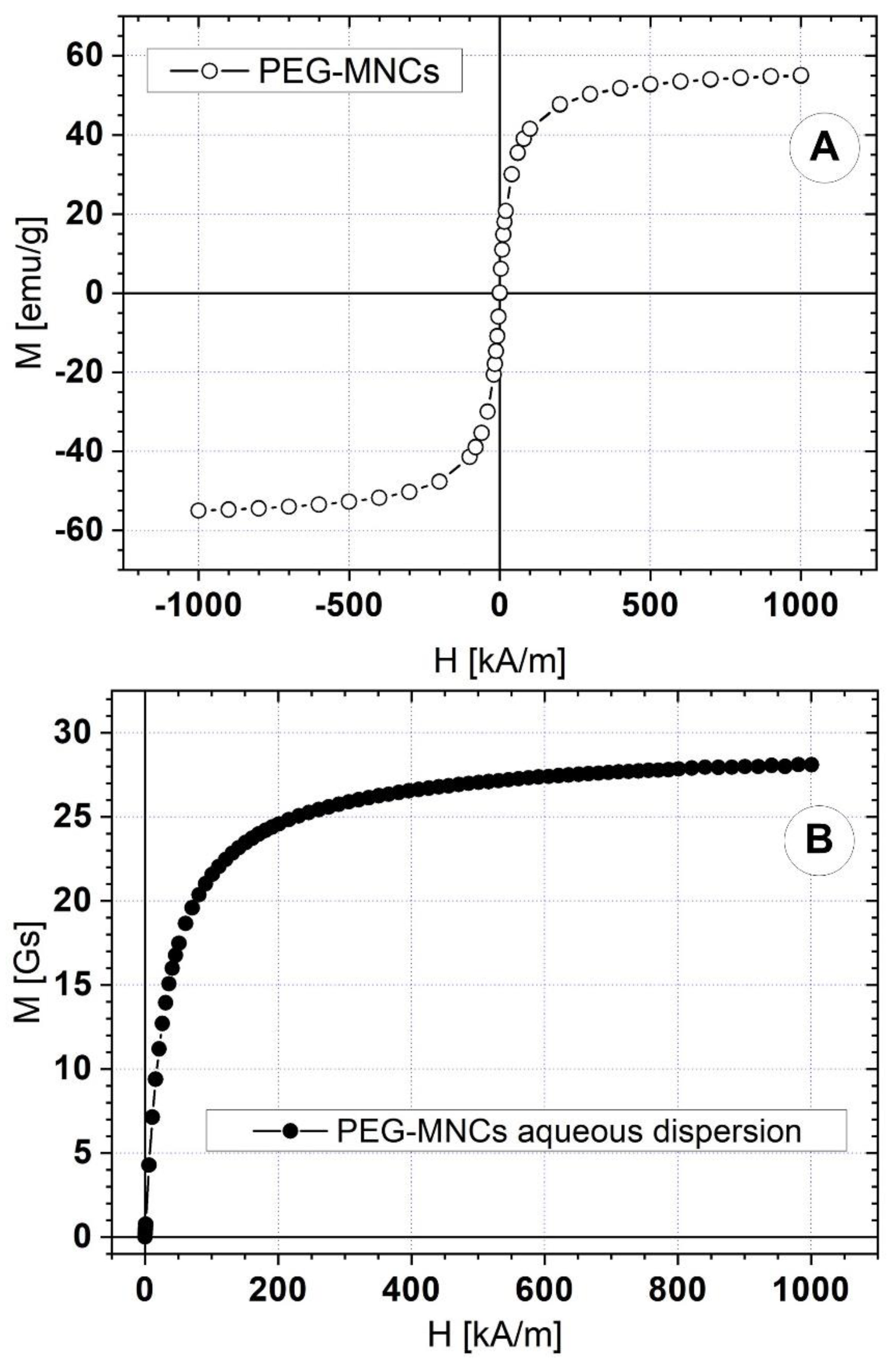
3.1. Synthesis and Characterization of the PEG-Functionalized Magnetic Nanocomposite Clusters (PEG_MNC)
3.2. Rheological Properties of the PEG_MNC Aqueous Dispersion
Viscosity Curves of PEG_MNC Aqueous Dispersions
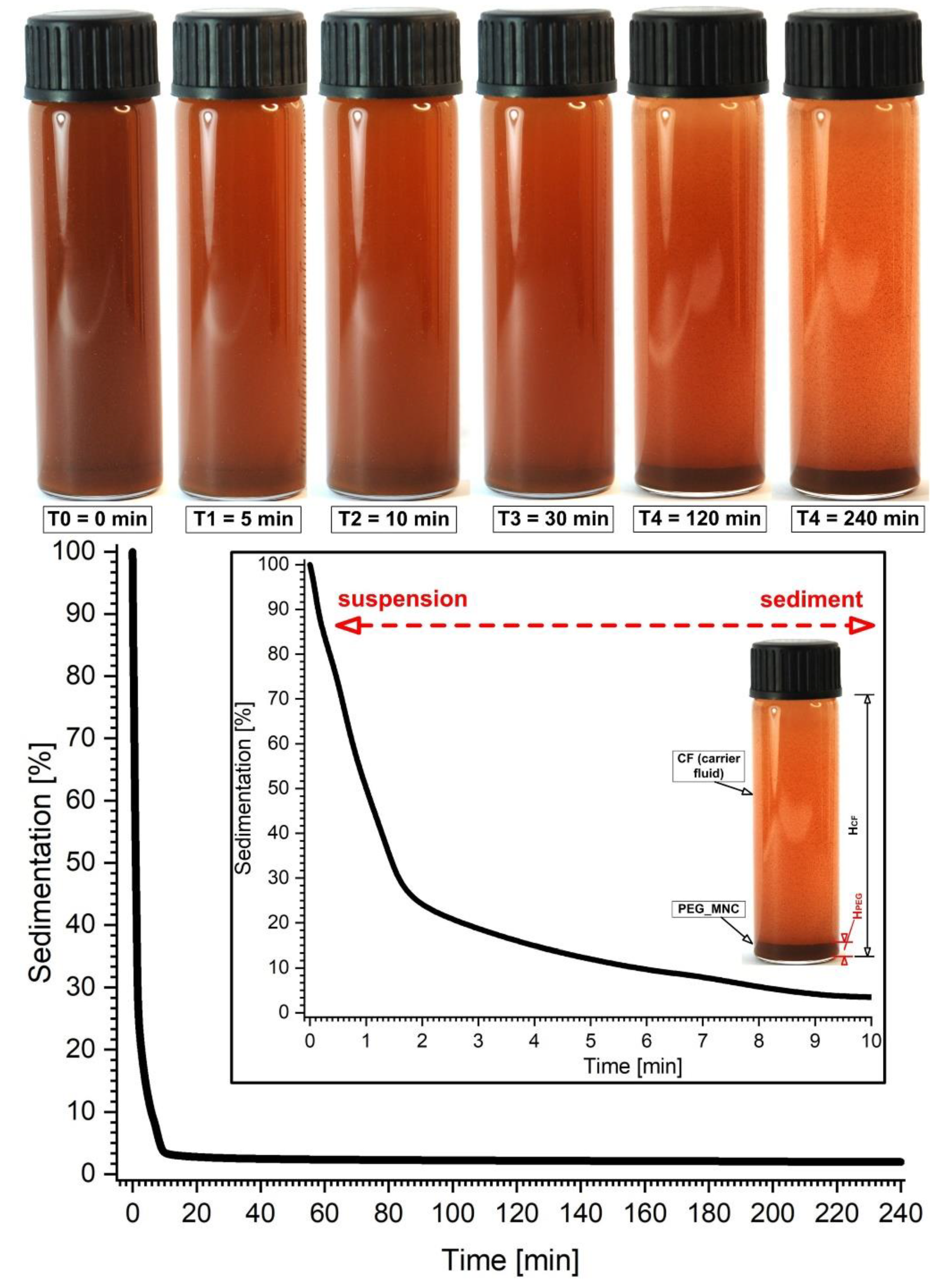
3.3. PEG_MNC Sedimentation
3.4. PEG_MNC Delivery at the Targeted Site
3.5. PEG_MNC Aggregation and Chain Formation Inside the Expanded Stent
3.6. Suspension Model’s Aggregation Process in the Magnetic Field
3.7. Colloidal Stability of the PEG_MNCs
3.8. Colloidal Interactions among the PEG_MNCs
4. Discussion
4.1. Clinical Perspective
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garg, S.; Serruys, P.W. Coronary stents current status. J. Am. Col. Cardiol. 2010, 56, S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, G.L.; Chieffo, A.; Colombo, A. Is there still a survival advantage to bypass surgery over percutaneous intervention in the modern era? Prog. Cardiovasc. Dis. 2015, 58, 335–341. [Google Scholar] [CrossRef]
- Clare, J.; Ganly, J.; Bursill, C.A.; Sumer, H.; Kingshott, P.; de Haan, J.B. The Mechanisms of Restenosis and Relevance to Next Generation Stent Design. Biomolecules 2022, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Jacobin-Valat, M.J.; Laroche-Traineau, J.; Larivière, M.; Mornet, S.; Sanchez, S.; Biran, M.; Lebaron, C.; Boudon, J.; Lacomme, S.; Cérutti, M.; et al. Nanoparticles functionalised with an anti-platelet human antibody for in vivo detection of atherosclerotic plaque by magnetic resonance imaging. Nanomed. NBM 2015, 11, 927–937. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Muktabar, A.; Tang, I.; Dravid, V.P.; Thaxton, C.S.; Venkatraman, S.; Woei, K. Engineered nanoparticles for the detection, treatment and prevention of atherosclerosis: How close are we? Drug Discov. Today 2017, 22, 1438–1446. [Google Scholar] [CrossRef]
- Garello, F.; Svenskaya, Y.; Parakhonskiy, B.; Filippi, M. Micro/Nanosystems for Magnetic Targeted Delivery of Bioagents. Pharmaceutics 2022, 14, 1132. [Google Scholar] [CrossRef] [PubMed]
- Moulton, K.S.; Heller, E.; Konerding, M.A.; Flynn, E.; Palinski, W.; Folkman, J. Angiogenesis inhibitors endostatin or TNP–470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation 1999, 99, 1726–1732. [Google Scholar] [CrossRef]
- Marx, S.O.; Totary-Jain, H.; Marks, A.R. Vascular smooth muscle cell proliferation in restenosis. Circ. Cardiovasc. Interv. 2011, 4, 104–111. [Google Scholar] [CrossRef]
- Unverdorben, M.; Vallbracht, C.; Cremers, B.; Heuer, H.; Hengstenberg, C.; Maikowski, C.; Werner, G.S.; Antoni, D.; Kleber, F.X.; Bocksch, W.; et al. Paclitaxelcoated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation 2009, 119, 2986–2994. [Google Scholar] [CrossRef]
- Sella, G.; Gandelman, G.; Teodorovich, N.; Tuvali, O.; Ayyad, O.; Abu Khadija, H.; Haberman, D.; Poles, L.; Jonas, M.; Volodarsky, I.; et al. Mid-Term Clinical Outcomes Following Drug-Coated Balloons in Coronary Artery Disease. J. Clin. Med. 2022, 11, 1859. [Google Scholar] [CrossRef]
- Schiele, T.M.; Krotz, F.; Klauss, V. Vascular restenosis—striving for therapy. Expert Opin. Pharmacother. 2004, 5, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Abbasnezhad, N.; Zirak, N.; Champmartin, S.; Shirinbayan, M.; Bakir, F. An Overview of In Vitro Drug Release Methods for Drug-Eluting Stents. Polymers 2022, 14, 2751. [Google Scholar] [CrossRef] [PubMed]
- Gazeau, F. Cooperative organization in iron oxide multicore nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents. ACS Nano. 2012, 6, 10935–10949. [Google Scholar] [CrossRef]
- Liu, X.; Wang, N.; Liu, X.; Deng, R.; Kang, R.; Xie, L. Vascular Repair by Grafting Based on Magnetic Nanoparticles. Pharmaceutics 2022, 14, 1433. [Google Scholar] [CrossRef] [PubMed]
- George, T.A.; Hsu, C.-C.; Meeson, A.; Lundy, D.J. Nanocarrier-Based Targeted Therapies for Myocardial Infarction. Pharmaceutics 2022, 14, 930. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, E.; Turcu, R.; Socoliuc, V.; Vékás, L. Magnetic iron oxide nanoparticles: Recent trends in design and synthesis of magnetoresponsive nanosystems. Biochem Biophys Res Commun. 2015, 468, 442–453. [Google Scholar] [CrossRef]
- Hu, M.; Butt, H.-J.; Landfester, K.; Bannwarth, M.B.; Wooh, S.; Therien-Aubin, H. Shaping the Assembly of Superparamagnetic Nanoparticles. ACS Nano. 2019, 13, 3015–3022. [Google Scholar] [CrossRef]
- Kappes, M.; Bernhard, F.; Pfister, F.; Huber, C.; Friedrich, P.R.; Stein, R.; Braun, C.; Band, J.; Schreiber, E.; Alexiou, C.; et al. Superparamagnetic Iron Oxide Nanoparticles for Targeted Cell Seeding: Magnetic Patterning and Magnetic 3D Cell Culture. Adv. Funct. Mater. 2022, 2203672. [Google Scholar] [CrossRef]
- Gaëtan, M.; Van de Walle, A.; Perez, J.E.; Ukai, T.; Maekawa, T.; Luciani, N.; Wilhelm, C. High-Throughput Differentiation of Embryonic Stem Cells into Cardiomyocytes with a Microfabricated Magnetic Pattern and Cyclic Stimulation. Adv. Funct. Mater. 2020, 30, 2002541. [Google Scholar] [CrossRef]
- Dutz, S.; Clement, J.H.; Eberbeck, D.; Gelbrich, T.; Hergt, R.; Müller, R.; Wotschadlo, J.; Zeisberger, M. Ferrofluids of magnetic multicore nanoparticles for biomedical applications. J. Mag. Magn. Mater. 2009, 321, 1501–1504. [Google Scholar] [CrossRef]
- Socoliuc, V.; Avdeev, M.V.; Kuncser, V.; Turcu, R.; Tombácz, E.; Vékás, L. Ferrofluids and bio-ferrofluids: Looking back and stepping forward. Nanoscale 2022, 14, 4786–4886. [Google Scholar] [CrossRef] [PubMed]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Farkas, K.; Földesi, I.; Szabó, Á.; Iván, B.; Tombácz, E. PEGylation of Superparamagnetic Iron Oxide Nanoparticles with Self-Organizing Polyacrylate-PEG Brushes for Contrast Enhancement in MRI Diagnosis. Nanomaterials 2018, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Illés, E.; Szekeres, M.; Kupcsik, E.; Tóth, I.Y.; Farkas, K.; Jedlovszky Hajdú, A.; Tombácz, E. PEGylation of surfacted magnetite core-shell nanoparticles for biomedical application. Colloids Surf. A 2014, 460, 429–440. [Google Scholar] [CrossRef]
- Tóth, I.Y.; Illés, E.; Bauer, R.A.; Nesztor, D.; Szekeres, M.; Zupkó, I.; Tombácz, E. Designed polyelectrolyte shell on magnetite nanocore for dilution-resistant biocompatible magnetic fluids. Langmuir 2012, 28, 16638–16646. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; McMillan, J.M.; Liu, X.M.; Vishwasrao, H.M.; Kabanov, A.V.; Sokolsky-Papkov, M.; Gendelman, H.E. Formulation design facilitates magnetic nanoparticle delivery to diseased cells and tissues. Nanomedicine 2014, 9, 469–485. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Nukolova, N.V.; Kabanov, A.V.; Bronich, T.K. Nanocarriers for delivery of platinum anticancer drugs. Adv. Drug Del. Rev. 2013, 65, 1667–1685. [Google Scholar] [CrossRef]
- Lim, E.K.; Jang, E.; Lee, K.; Haam, S.; Huh, Y.M. Delivery of cancer therapeutics using nanotechnology. Pharmaceutics 2013, 5, 294. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles Cancer Imaging and Therapy. Theranostic 2012, 2, 3–44. [Google Scholar] [CrossRef]
- Bernad, S.I.; Craciunescu, I.; Sandhu, G.S.; Dragomir-Daescu, D.; Tombacz, E.; Vekas, L.; Turcu, R. Fluid Targeted delivery of functionalized magnetoresponsive nanocomposite particles to a ferromagnetic stent. J. Magn. Magn. Mater. 2021, 519, 167489. [Google Scholar] [CrossRef]
- Sankar, D.S.; Hemalatha, K. Pulsatile flow of Herschel–Bulkley fluid through stenosed arteries—A mathematical model. Int. J. Non-Linear Mech. 2006, 41, 979–990, 2006. [Google Scholar] [CrossRef]
- Bernad, S.I.; Susan-Resiga, D.; Bernad, E. Hemodynamic Effects on Particle Targeting in the Arterial Bifurcation for Different Magnet Positions. Molecules 2019, 24, 2509. [Google Scholar] [CrossRef] [PubMed]
- Bernad, S.I.; Susan-Resiga, D.; Vekas, L.; Bernad, E.S. Drug targeting investigation in the critical region of the arterial bypass graft. J. Magn. Magn. Mater. 2019, 475, 14–23. [Google Scholar] [CrossRef]
- Torii, R.; Wood, N.B.; Hadjiloizou, N.; Dowsey, A.W.; Wright, A.R.; Hughes, A.D.; Davies, J.; Francis, D.P.; Mayet, J.; Yang, G.; et al. Stress phase angle depicts differences in coronary artery hemodynamics due to changes in flow and geometry after percutaneous coronary intervention. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H765–H776. [Google Scholar] [CrossRef] [PubMed]
- Uthamaraj, S.; Tefft, B.J.; Klabusay, M.; Hlinomaz, O.; Sandhu, G.; Dragomir-Daescu, D. Design and validation of a novel ferromagnetic bare metal stent capable of capturing and retaining endothelial cells. ABME 2014, 24, 2416. [Google Scholar] [CrossRef] [PubMed]
- Tefft, B.J.; Uthamaraj, S.; Harbuzariu, A.; Harburn, J.J.; Witt, T.A.; Newman, B.; Psaltis, P.J.; Hlinomaz, O.; Holmes, D.R., Jr.; Gulati, R.; et al. Nanoparticle-Mediated Cell Capture Enables Rapid Endothelialization of a Novel Bare Metal Stent. Tissue Eng. Part A 2018, 24, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.I.; Kensey, K.R. Effects of the non-Newtonian viscosity of blood on flows in a diseased arterial vessel. Part 1: Steady flows. Biorheology 1991, 28, 241–262. [Google Scholar] [CrossRef]
- Mandal, P.K. An unsteady analysis of non-Newtonian blood flow through tapered arteries with a stenosis. Non. Linear Mech. 2005, 40, 151–164, 2005. [Google Scholar] [CrossRef]
- Akbar, N.S.; Nadeem, S. Carreau fluid model for blood flow through a tapered artery with a stenosis. Ain Shams Eng. J. 2014, 5, 1307–1316. [Google Scholar] [CrossRef]
- Nowak, J.; Wiekhorst, F.; Trahms, L.; Odenbach, S. The influence of hydrodynamic diameter and core composition on the magnetoviscous effect of biocompatible ferrofluids. J. Phys. Condens. Matter 2014, 26, 176004. [Google Scholar] [CrossRef]
- Vasilescu, C.; Latikka, M.; Knudsen, K.D.; Garamus, V.M.; Socoliuc, V.; Turcu, R.; Tombacz, E.; Susan-Resiga, D.; Ras, R.H.A.; Vekas, L. High concentration aqueous magnetic fluids: Structure, colloidal stability, magnetic and flow properties. Soft Matter 2018, 14, 6648–6666. [Google Scholar] [CrossRef] [PubMed]
- Carreau, P.J. Rheological Equations from Molecular Network Theories. Trans. Soc. Rheol. 1972, 16, 99–127. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Elimelech, M.; Borkovec, M. DLVO Interaction between Colloidal Particles: Beyond Derjaguin’s Approximation. Croat. Chem. Acta 1998, 71, 883–903. [Google Scholar]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J. Interaction of Unequal Double layers at Constant Charge. J. Colloids Interface Sci. 1975, 51, 44–51. [Google Scholar] [CrossRef]
- Ivanov, A.O.; Novak, E.V. Phase separation of ferrocolloids: The role of van der Waals interaction. Colloid J. 2007, 69, 332. [Google Scholar] [CrossRef]
- Ivanov, A.O.; Kuznetsova, O.B. Magnetic properties of dense ferrofluids: An influence of interparticle correlations. Phys. Rev. 2001, E 64, 041405. [Google Scholar] [CrossRef]
- Socoliuc, V.; Turcu, R. Large scale aggregation in magnetic colloids induced by high frequency magnetic fields. J. Magn. Magn. Mater. 2020, 500, 166348. [Google Scholar] [CrossRef]
- Ta, H.T.; Truong, N.P.; Whittaker, A.K.; Davis, T.P.; Peter, K. The effects of particle size, shape, density and flow characteristics on particle margination to vascular walls in cardiovascular diseases. Expert Opin. Drug Deliv. 2018, 15, 33–45. [Google Scholar] [CrossRef]
- Bae, J.E.; Huh, M.I.; Ryu, B.K.; Do, J.Y.; Jin, S.U.; Moon, M.J.; Jung, J.C.; Chang, Y.; Kim, E.; Chi, S.G.; et al. The effect of static magnetic fields on the aggregation and cytotoxicity of magnetic nanoparticles. Biomaterials 2011, 32, 9401–9414. [Google Scholar] [CrossRef] [PubMed]
- van Vlerken, L.E.; Vyas, T.K.; Amiji, M.M. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm. Res. 2007, 24, 1405–1414. [Google Scholar] [CrossRef]
- Kanaras, A.G.; Kamounah, F.S.; Schaumburg, K.; Kiely, C.J.; Brust, M. Thioalkylated tetraethylene glycol: A new ligand for water soluble monolayer protected gold clusters. Chem. Commun. 2002, 20, 2294–2295. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.V.; Churaev, N.V.; Muller, V.M. The Derjaguin—Landau—Verwey—Overbeek (DLVO) Theory of Stability of Lyophobic Colloids. In Surface Forces; Springer: Boston, MA, USA, 1987. [Google Scholar] [CrossRef]
- Hermann, J.; DiStasio, R.A., Jr.; Tkatchenko, A. First-Principles Models for van der Waals Interactions in Molecules and Materials: Concepts, Theory, and Applications. Chem. Rev. 2017, 117, 4714–4758. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.W.; Casula, M.F.; Sim, J.H.; Kim, S.Y.; Cheon, J.; Alivisatos, A.P. Surfactant-assisted elimination of a high energy facet as a means of controlling the shapes of TiO2 nanocrystals. J. Am. Chem. Soc. 2003, 125, 15981–15985. [Google Scholar] [CrossRef] [PubMed]
- Förster, S.; Antonietti, M. Amphiphilic block copolymers in structure-controlled nanomaterial hybrids. Adv. Mat. 1998, 10, 195–217. [Google Scholar] [CrossRef]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Szabó, Á.; Iván, B.; Turcu, R.; Vékás, L.; Zupkó, I.; Jaics, G.; Tombácz, E. Multifunctional PEG-carboxylate copolymer coated superparamagnetic iron oxide nanoparticles for biomedical application. J. Mag. Magn. Mater. 2018, 451, 710–720. [Google Scholar] [CrossRef] [Green Version]

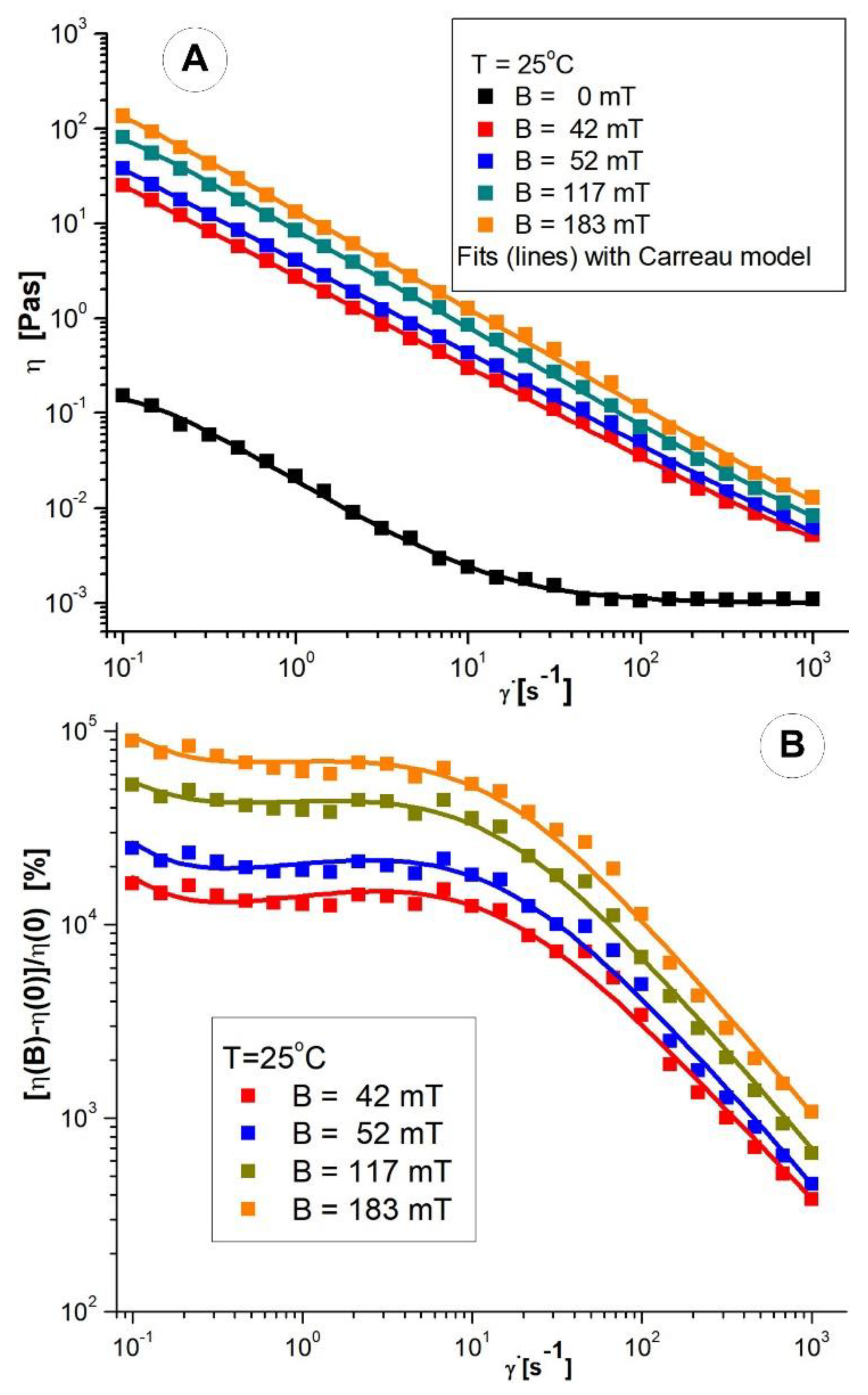
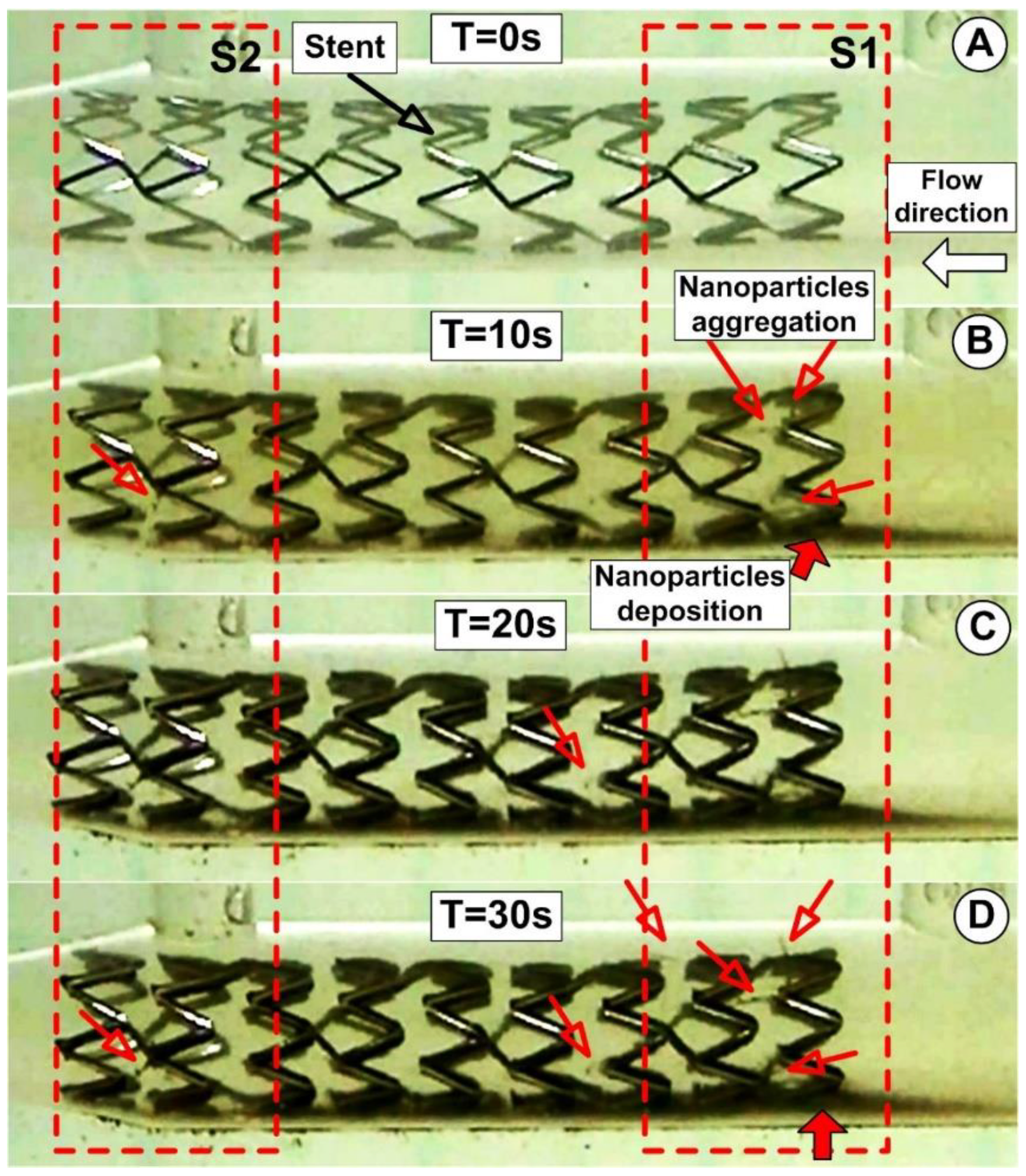
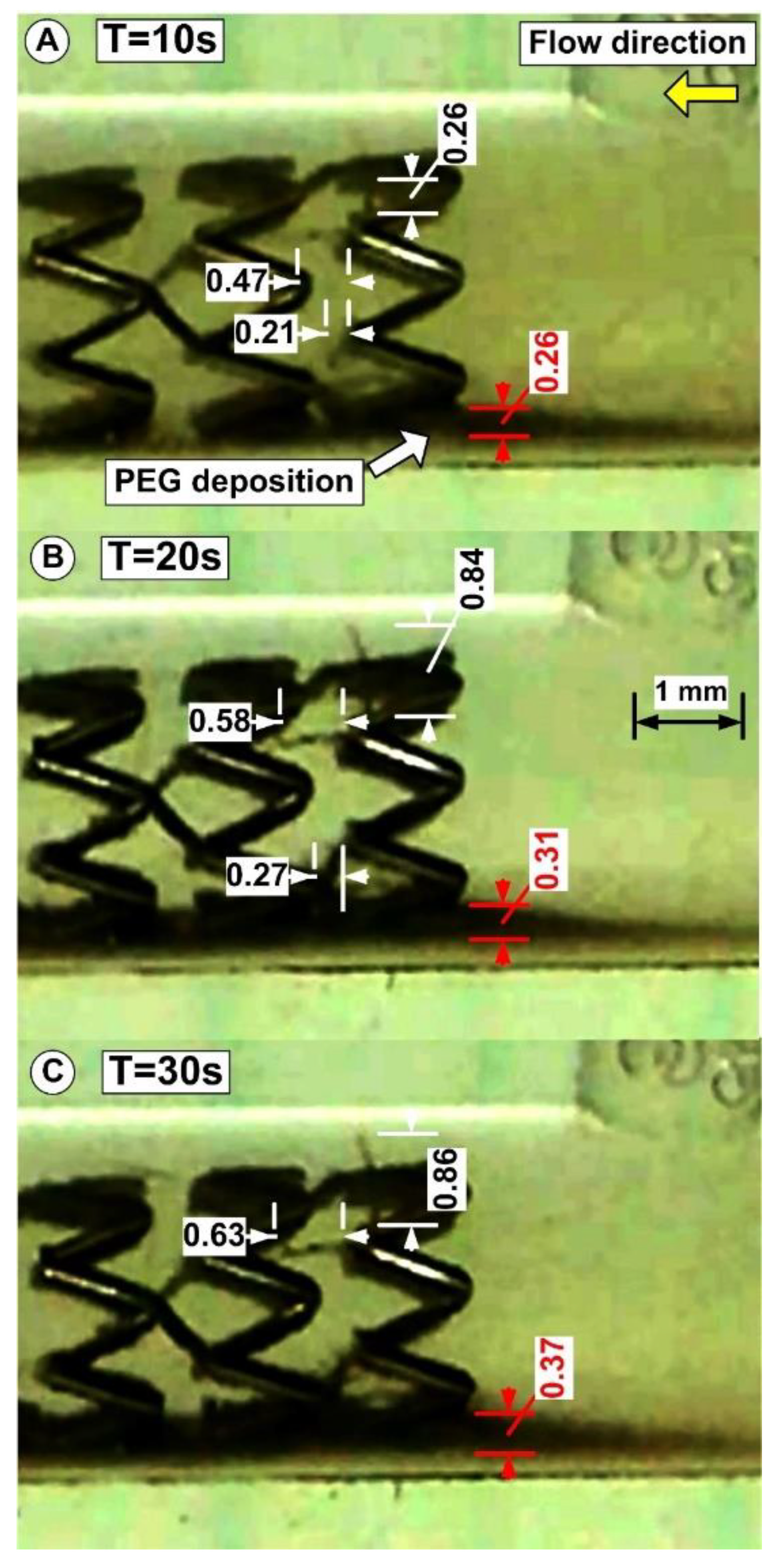

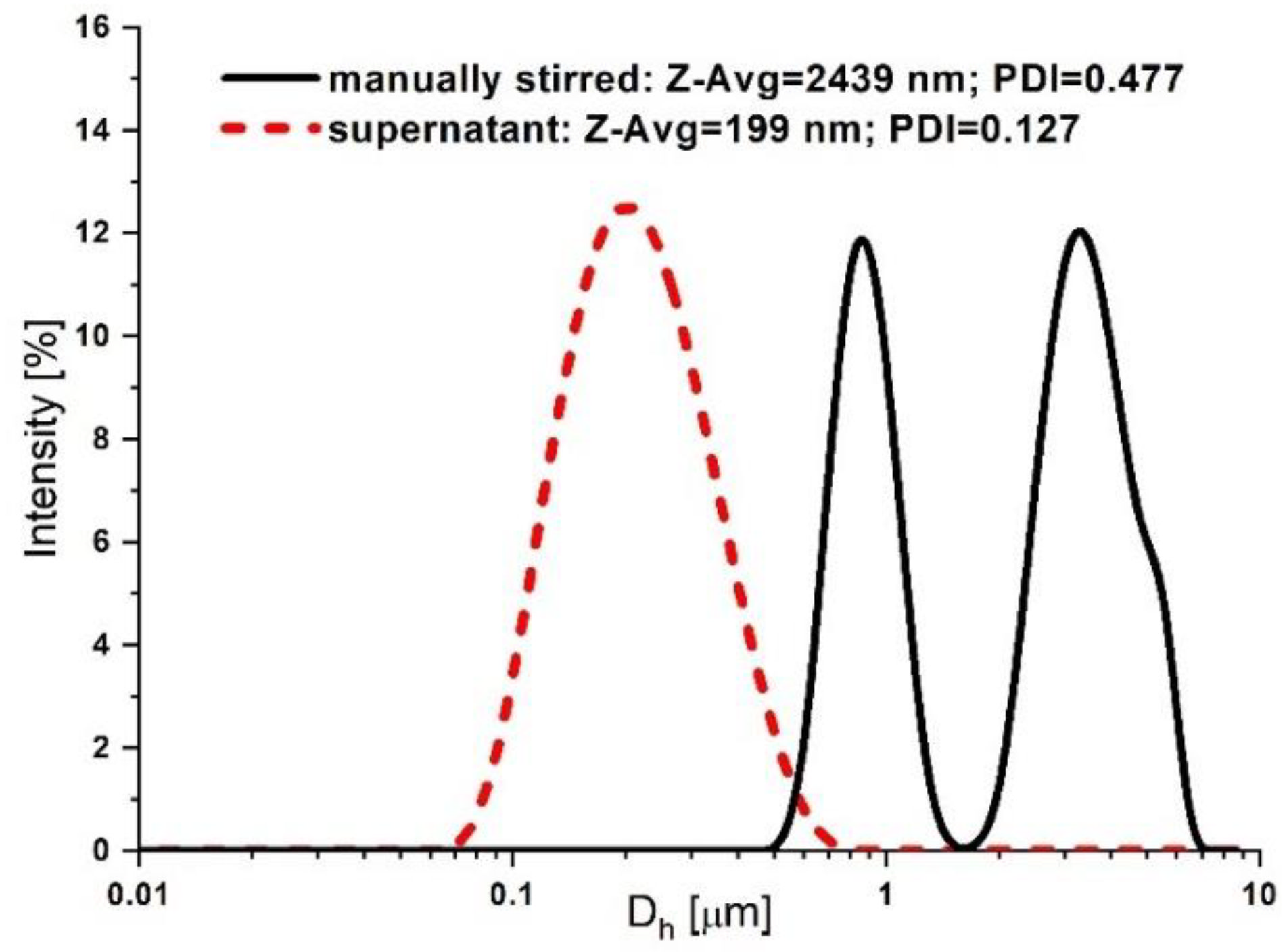
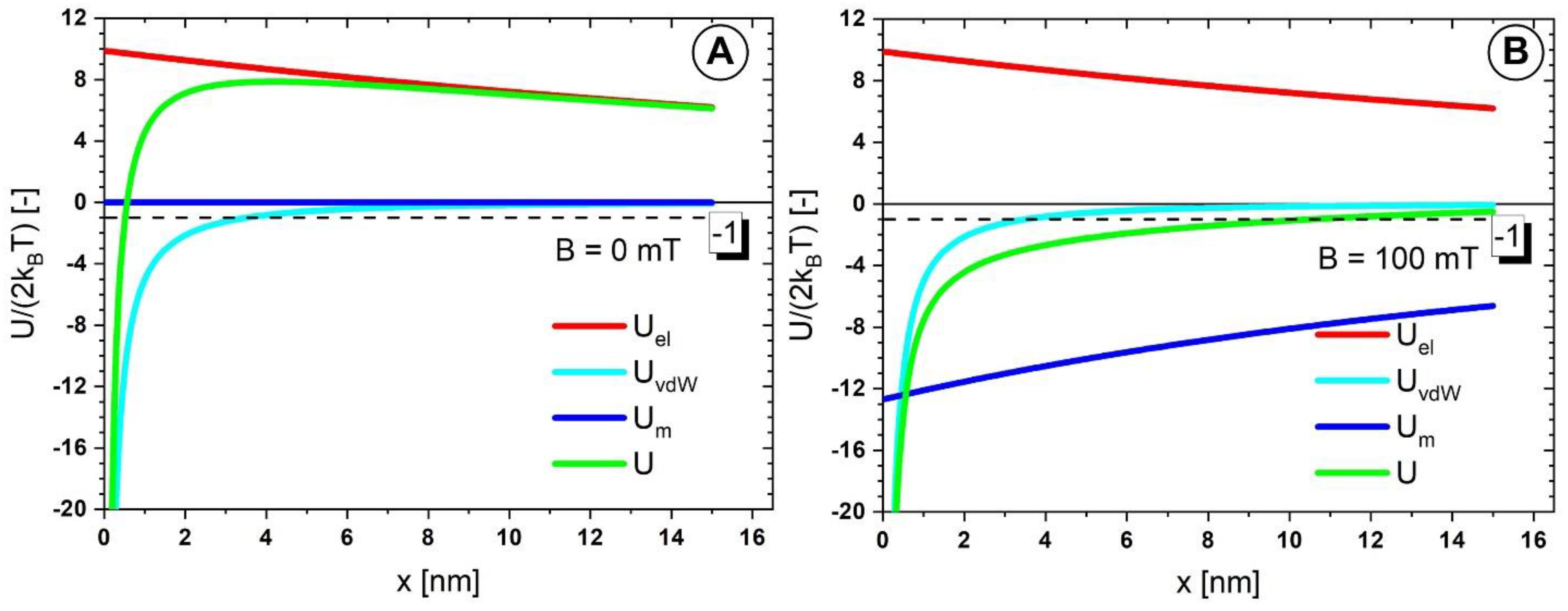
| Fluid | T [°C] | B [mT] | η∞ [Pas] | η0 [Pas] | C [s] | p [-] | r2 |
|---|---|---|---|---|---|---|---|
| CF + 0.1% PEG_MC | 25 | 0 | 0.00154 | 0.095 | 18.08 | 0.664 | 0.999 |
| T [°C] | B [mT] | η∞ [Pas] | η0 [Pas] | C [s] | p [-] | r2 |
|---|---|---|---|---|---|---|
| 25 °C | 0 | 0.001 | 0.182 | 7.645 | 0.559 | 0.986 |
| 42 | 0.001 | 86.6 | 12.20 | 0.478 | 0.996 | |
| 52 | 0.001 | 98.00 | 12.31 | 0.486 | 0.994 | |
| 117 | 0.001 | 155.35 | 17.00 | 0.513 | 0.996 | |
| 183 | 0.001 | 322.20 | 21.26 | 0.517 | 0.991 |
| Time [s] | Chain 1 [mm] | Chain 2 [mm] | Chain 3 [mm] |
|---|---|---|---|
| 0 (start) | 0 | 0 | 0 |
| 10 | 0.26 | 0.47 | 0.21 |
| 20 | 0.84 | 0.58 | 0.27 |
| 30 (end) | 0.86 | 0.63 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernad, S.I.; Socoliuc, V.; Susan-Resiga, D.; Crăciunescu, I.; Turcu, R.; Tombácz, E.; Vékás, L.; Ioncica, M.C.; Bernad, E.S. Magnetoresponsive Functionalized Nanocomposite Aggregation Kinetics and Chain Formation at the Targeted Site during Magnetic Targeting. Pharmaceutics 2022, 14, 1923. https://doi.org/10.3390/pharmaceutics14091923
Bernad SI, Socoliuc V, Susan-Resiga D, Crăciunescu I, Turcu R, Tombácz E, Vékás L, Ioncica MC, Bernad ES. Magnetoresponsive Functionalized Nanocomposite Aggregation Kinetics and Chain Formation at the Targeted Site during Magnetic Targeting. Pharmaceutics. 2022; 14(9):1923. https://doi.org/10.3390/pharmaceutics14091923
Chicago/Turabian StyleBernad, Sandor I., Vlad Socoliuc, Daniela Susan-Resiga, Izabell Crăciunescu, Rodica Turcu, Etelka Tombácz, Ladislau Vékás, Maria C. Ioncica, and Elena S. Bernad. 2022. "Magnetoresponsive Functionalized Nanocomposite Aggregation Kinetics and Chain Formation at the Targeted Site during Magnetic Targeting" Pharmaceutics 14, no. 9: 1923. https://doi.org/10.3390/pharmaceutics14091923
APA StyleBernad, S. I., Socoliuc, V., Susan-Resiga, D., Crăciunescu, I., Turcu, R., Tombácz, E., Vékás, L., Ioncica, M. C., & Bernad, E. S. (2022). Magnetoresponsive Functionalized Nanocomposite Aggregation Kinetics and Chain Formation at the Targeted Site during Magnetic Targeting. Pharmaceutics, 14(9), 1923. https://doi.org/10.3390/pharmaceutics14091923









