Testing the Protective Effects of Sulfobutylether-Βeta-Cyclodextrin (SBECD) and Sugammadex against Chlorpromazine-Induced Acute Toxicity in SH-SY5Y Cell Line and in NMRI Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Experiments
2.3. Animal Experiments
3. Results
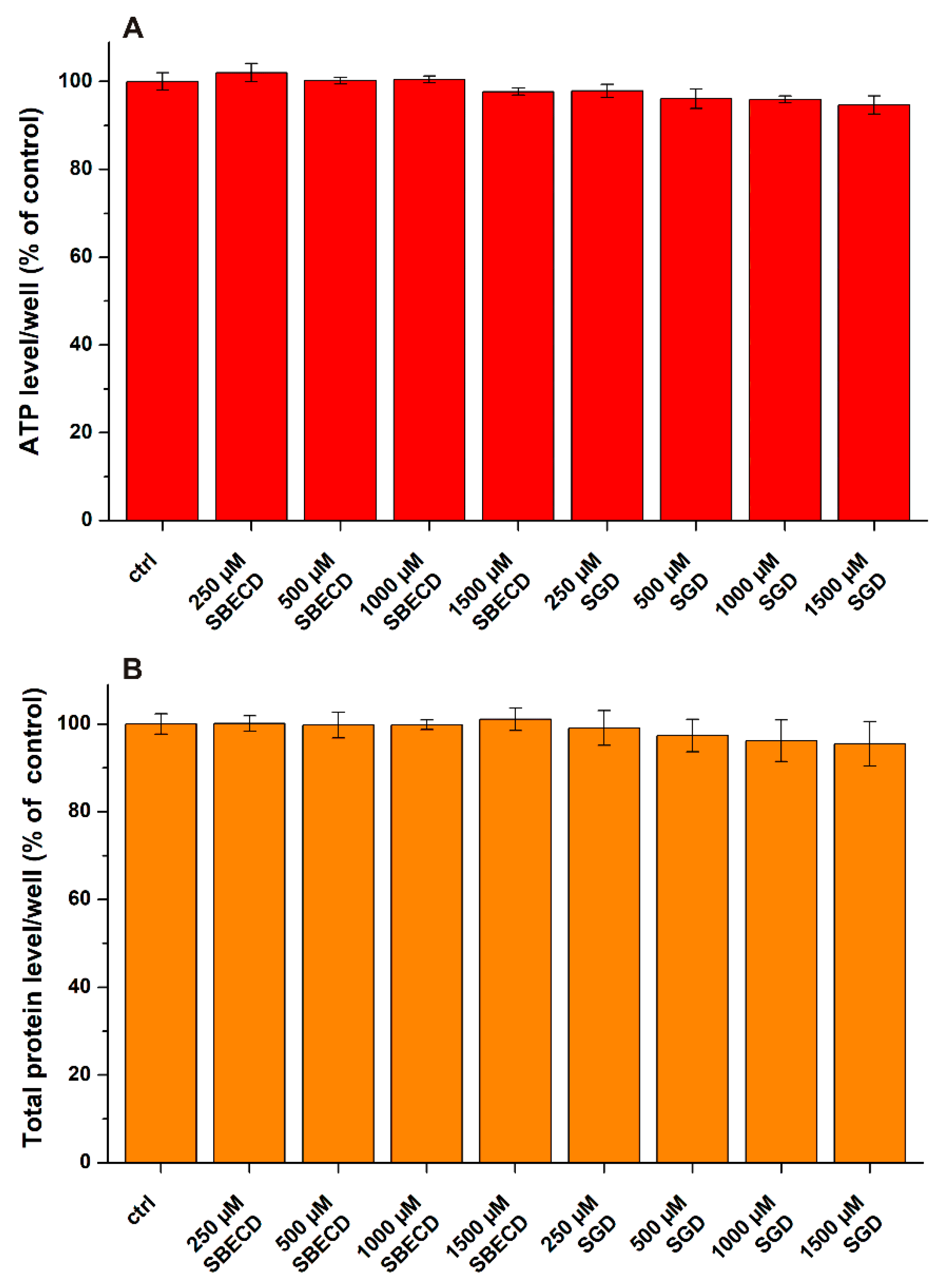
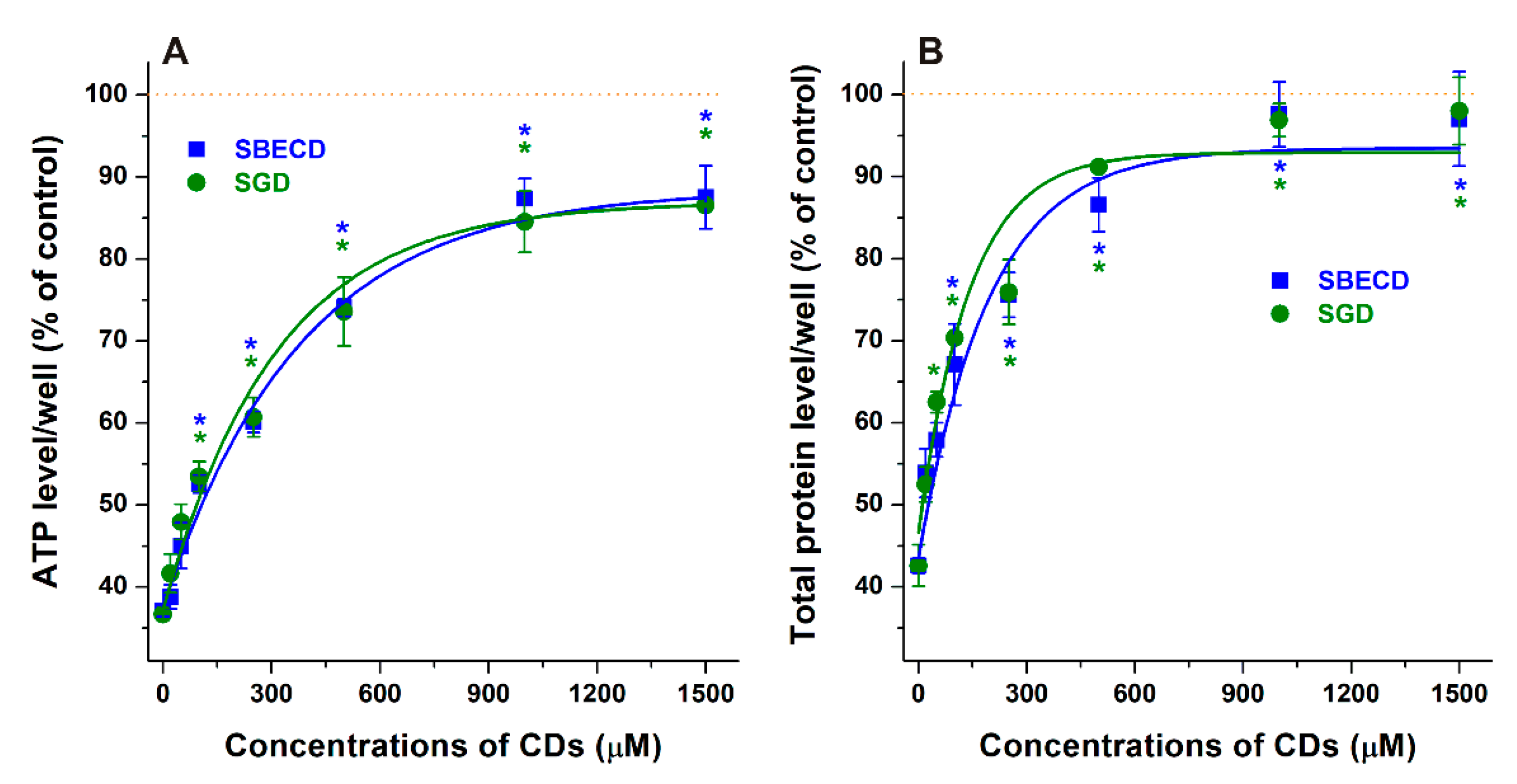
3.1. Effects of CDs on CPZ-Induced Decrease in Cell Viability
3.2. Effects of CDs on CPZ-Induced 24 h Mortality in Female NMRI Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyd-Kimball, D.; Gonczy, K.; Lewis, B.; Mason, T.; Siliko, N.; Wolfe, J. Classics in Chemical Neuroscience: Chlorpromazine. ACS Chem. Neurosci. 2019, 10, 79–88. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Alamo, C.; Cuenca, E.; Shen, W.W.; Clervoy, P.; Rubio, G. History of the discovery and clinical introduction of chlorpromazine. Ann. Clin. Psychiatry 2005, 17, 113–135. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Model List of Essential Medicines: 22nd List (2021); World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Saha, K.B.; Bo, L.; Zhao, S.; Xia, J.; Sampson, S.; Zaman, R.U. Chlorpromazine versus atypical antipsychotic drugs for schizophrenia. Cochrane Database Syst. Rev. 2016, 4, CD010631. [Google Scholar] [CrossRef] [PubMed]
- Samara, M.T.; Cao, H.; Helfer, B.; Davis, J.M.; Leucht, S. Chlorpromazine versus every other antipsychotic for schizophrenia: A systematic review and meta-analysis challenging the dogma of equal efficacy of antipsychotic drugs. Eur. Neuropsychopharmacol. 2014, 24, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Otręba, M.; Kośmider, L.; Rzepecka-Stojko, A. Antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards RNA-viruses. A review. Eur. J. Pharmacol. 2020, 887, 173553. [Google Scholar] [CrossRef] [PubMed]
- Stip, E.; Rizvi, T.A.; Mustafa, F.; Javaid, S.; Aburuz, S.; Ahmed, N.N.; Aziz, K.A.; Arnone, D.; Subbarayan, A.; Al Mugaddam, F.; et al. The Large Action of Chlorpromazine: Translational and Transdisciplinary Considerations in the Face of COVID-19. Front. Pharmacol. 2020, 11, 577678. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Murru, A.; Pacchiarotti, I.; Undurraga, J.; Veronese, N.; Fornaro, M.; Stubbs, B.; Monaco, F.; Vieta, E.; Seeman, M.V.; et al. Safety, tolerability, and risks associated with first-and secondgeneration antipsychotics: A state-of-the-art clinical review. Therapeut. Clin. Risk Manag. 2017, 13, 757–777. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the impact of cavity size, occupancy, and substitutions on cytotoxicity and cholesterol homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug. Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J.; Osa, T. Preparation of Cyclodextrin Complexes. In Comprehensive Supramolecular Chemistry, (Cyclodextrins); Szejtli, J., Osa, T., Eds.; Pergamon: Oxford, UK, 1996; Volume 3, pp. 243–251. [Google Scholar]
- Daruházi, A.E.; Szente, L.; Balogh, B.; Mátyus, P.; Béni, S.; Takács, M.; Gergely, A.; Horváth, P.; Szoke, E.; Lemberkovics, E. Utility of cyclodextrins in the formulation of genistein part 1. Preparation and physicochemical properties of genistein complexes with native cyclodextrins. J. Pharm. Biomed. Anal. 2008, 48, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. Preparation of non-steroidal anti-inflammatory drug/β-cyclodextrin inclusion complexes by supercritical antisolvent process. J. CO2 Util. 2021, 44, 101397. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Cyclodextrins in peptide and protein delivery. Adv. Drug Deliv. Rev. 1999, 36, 101–123. [Google Scholar] [CrossRef]
- Redenti, E.; Pietra, C.; Gerloczy, A.; Szente, L. Cyclodextrins in oligonucleotide delivery. Adv. Drug Deliv. Rev. 2001, 53, 235–244. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Sali, N.; Kőszegi, T.; Szente, L.; Peles-Lemli, B. Interactions of zearalenone with native and chemically modified cyclodextrins and their potential utilization. J. Photochem. Photobiol. B 2015, 151, 63–68. [Google Scholar] [CrossRef]
- Faisal, Z.; Garai, E.; Csepregi, R.; Bakos, K.; Fliszár-Nyúl, E.; Szente, L.; Balázs, A.; Cserháti, M.; Kőszegi, T.; Urbányi, B.; et al. Protective effects of beta-cyclodextrins vs. zearalenone-induced toxicity in HeLa cells and Tg(vtg1:mCherry) zebrafish embryos. Chemosphere 2020, 240, 124948. [Google Scholar] [CrossRef]
- Keating, G.M. Sugammadex: A Review of Neuromuscular Blockade Reversal. Drugs 2016, 76, 1041–1052. [Google Scholar] [CrossRef]
- Bom, A.; Bradley, M.; Cameron, K.; Clark, J.K.; Van Egmond, J.; Feilden, H.; MacLean, E.J.; Muir, A.W.; Palin, R.; Rees, D.C.; et al. A novel concept of reversing neuromuscular block: Chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew. Chem. Int. Ed. Engl. 2002, 41, 266–270. [Google Scholar] [CrossRef]
- Davidson, J.; Molitor, E.; Moores, S.; Gale, S.E.; Subramanian, K.; Jiang, X.; Sidhu, R.; Kell, P.; Zhang, J.; Fujiwara, H.; et al. 2-Hydroxypropyl-b-cyclodextrin is the active component in a triple combination formulation for treatment of Niemann-Pick C1 disease. Biochim. Biophys. Acta 2019, 1864, 1545–1561. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yamada, Y.; Ishitsuka, Y.; Matsuo, M.; Shiraishi, K.; Wada, K.; Uchio, Y.; Kondo, Y.; Takeo, T.; Nakagata, N.; et al. Efficacy of 2-Hydroxypropyl-β-cyclodextrin in Niemann-Pick Disease Type C Model Mice and Its Pharmacokinetic Analysis in a Patient with the Disease. Biol. Pharm. Bull. 2015, 38, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chipot, C.; Cai, W.; Shao, X. Molecular dynamics study of the inclusion of cholesterol into cyclodextrins. J. Phys. Chem. B 2006, 110, 6372–6378. [Google Scholar] [CrossRef] [PubMed]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta 2007, 1768, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.W.; Gray, J.E.; Weaver, R.N. Cyclodextrin Nephrosis in the Rat. Am. J. Pathol. 1976, 83, 367–382. [Google Scholar]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Fliszár-Nyúl, E.; Bock, I.; Csepregi, R.; Szente, L.; Szabó, I.; Csenki, Z.; Poór, M. Testing the protective effects of cyclodextrins vs. alternariol-induced acute toxicity in HeLa cells and in zebrafish embryos. Environ. Toxicol. Pharmacol. 2022, 95, 103965. [Google Scholar] [CrossRef]
- Uribe, L.A.; Leonardo, S.; Nielsen, T.T.; Steinmann, C.; Campàs, M.; Fragoso, A. Supramolecular Complexes of Plant Neurotoxin Veratridine with Cyclodextrins and Their Antidote-like Effect on Neuro-2a Cell Viability. Pharmaceutics 2022, 14, 598. [Google Scholar] [CrossRef]
- da Silva, M.C.G.; da Silva, J.F.; Santos, T.P.; da Silva, N.P.C.; dos Santos, A.R.; de Andrade, A.L.C.; da Silva Souza, E.H.L.; Cadena, M.R.S.; de Sá, F.B.; da Silva Junior, V.A.; et al. The complexation of steroid hormones into cyclodextrin alters the toxic effects on the biological parameters of zebrafish (Danio rerio). Chemosphere 2019, 214, 330–340. [Google Scholar] [CrossRef]
- Weiss-Errico, M.; Berry, J.; O’Shea, K. β-Cyclodextrin Attenuates Perfluorooctanoic Acid Toxicity in the Zebrafish Embryo Model. Toxics 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.H.; Le, H.T.; Rodriguez, I.; Kim, E.Y.; Kim, K.; Jeong, S.Y.; Woo, S.H.; Lee, Y.R.; Castaneda, R.; Hong, J.; et al. Enhanced antidiabetic efficacy and safety of compound K⁄β-cyclodextrin inclusion complex in zebrafish. J. Ginseng Res. 2017, 41, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.H.; Engelke, A.; Wenz, G. Solubilizing steroidal drugs by β-cyclodextrin derivatives. Int. J. Pharm. 2017, 531, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Weiss-Errico, M.J.; O’Shea, K.E. Detailed NMR investigation of cyclodextrin-perfluorinated surfactant interactions in aqueous media. J. Hazard. Mater. 2017, 329, 57–65. [Google Scholar] [CrossRef]
- Igami, K.; Ozawa, M.; Inoue, S.; Iohara, D.; Miyazaki, T.; Shinoda, M.; Anraku, M.; Hirayama, F.; Uekama, K. The formation of an inclusion complex between a metabolite of ginsenoside, compound K and γ-cyclodextrin and its dissolution characteristics. J. Pharm. Pharmacol. 2016, 68, 646–654. [Google Scholar] [CrossRef]
- Uekama, K.; Irie, T.; Sunada, M.; Otagiri, M.; Iwasaki, K.; Okano, Y.; Miyata, T.; Kasé, Y. Effects of cyclodextrins on chlorpromazine-induced haemolysis and central nervous system responses. J. Pharm. Pharmacol. 1981, 33, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Uekama, K. Protection against the photosensitized skin irritancy of chlorpromazine by cyclodextrin complexation. J. Pharmacobiodyn. 1985, 8, 788–791. [Google Scholar] [CrossRef]
- Irie, T.; Kuwahara, S.; Otagiri, M.; Uekama, K.; Iwamasa, T. Reduction in the local tissue toxicity of chlorpromazine by beta-cyclodextrin complexation. J. Pharmacobiodyn. 1983, 6, 790–792. [Google Scholar] [CrossRef]
- Svendsen, O. β-Cyclodextrin and Local Muscle Toxicity of Intramuscular Drug Formulations. In The Target Organ and the Toxic Process; Chambers, P.L., Chambers, C.M., Dirheimer, G., Eds.; Archives of Toxicology; Springer: Berlin/Heidelberg, Germany, 1988; Volume 12. [Google Scholar] [CrossRef]
- Wang, Z.; Landy, D.; Sizun, C.; Cézard, C.; Solgadi, A.; Przybylski, C.; de Chaisemartin, L.; Herfindal, L.; Barratt, G.; Legrand, F.-X. Cyclodextrin complexation studies as the first step for repurposing of chlorpromazine. Int. J. Pharmaceut. 2020, 584, 119391. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell proliferation and cytotoxicity assays. Curr. Pharmaceut. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Karatas, E.; Caglar, H.; Bulut, H. Dose-dependent proliferative and cytotoxic effects of melatonin on human epidermoid carcinoma and normal skin fibroblast cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 829–830, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, R.; Temesfői, V.; Poór, M.; Faust, Z.; Kőszegi, T. Green fluorescent protein-based viability assay in a multiparametric configuration. Molecules 2018, 23, 1575. [Google Scholar] [CrossRef] [PubMed]
- Csenki, Z.; Garai, E.; Faisal, Z.; Csepregi, R.; Garai, K.; Kánainé Sipos, D.; Szabó, I.; Kőszegi, T.; Czéh, Á.; Czömpöly, T.; et al. The individual and combined effects of ochratoxin A with citrinin and their metabolites (ochratoxin B, ochratoxin C, and dihydrocitrinone) on 2D/3D cell cultures, and zebrafish embryo models. Food Chem. Toxicol. 2021, 158, 112674. [Google Scholar] [CrossRef] [PubMed]
- Sali, N.; Nagy, S.; Poór, M.; Kőszegi, T. Multiparametric luminescent cell viability assay in toxicology models: A critical evaluation. J. Pharmacol. Toxicol. Methods 2016, 79, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bruce, R.D. An up-and-down procedure for acute toxicity testing. Fundam. Appl. Toxicol. 1985, 5, 151–157. [Google Scholar] [CrossRef]
- Dixon, W.J. The Up-and-Down Method for Small Samples. J. Am. Stat. Assoc. 1965, 60, 967–978. [Google Scholar] [CrossRef]
- Kovacs, K.; Ancha, M.; Jane, M.; Lee, S.; Angalakurthi, S.; Negrito, M.; Rasheed, S.; Nwaneri, A.; Petrikovics, I. Identification, solubility enhancement and in vivo testing of a cyanide antidote candidate. Eur. J. Pharmaceut. Sci. 2013, 49, 352–358. [Google Scholar] [CrossRef]
- Kovacs, K.; Duke, A.C.; Shifflet, M.; Winner, B.; Lee, S.A.; Rockwood, G.A.; Petrikovics, I. Parenteral dosage form development and testing of dimethyl trisulfide, as an antidote candidate to combat cyanide intoxication. Pharm. Dev. Technol. 2017, 22, 958–963. [Google Scholar] [CrossRef]
- Campbell, D.E.S.; Richter, W. An Observational Method Estimating Toxicity and Drug. Actions in Mice applied to 68 Reference Drugs. Acta Pharmacol. Toxicol. 1967, 25, 345–363. [Google Scholar] [CrossRef]
- Dandiya, P.C.; Johnson, G.; Sellers, E.A. Influence of variation in environmental temperature on the acute toxicity of reserpine and chlorpromazine in mice. Can. J. Biochem. Physiol. 1960, 38, 591. [Google Scholar] [CrossRef]
- Hoshino, T.; Ishida, K.; Irie, T.; Uekama, K.; Ono, T. An attempt to reduce the photosensitizing potential of chlorpromazine with the simultaneous use of beta- and dimethyl-beta-cyclodextrins in guinea pigs. Arch. Dermatol. Res. 1989, 281, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Roubein, I.F.; Samuelly, M.; Keup, W. The toxicity of chlorpromazine and mescaline on mouse cerebellum and fibroblast cells in culture. Acta Pharmacol. Toxicol. 1973, 33, 326–329. [Google Scholar] [CrossRef]
- Shenoy, M.A.; Biaglow, J.E.; Varnes, M.E.; Daniel, J.W. A biochemical basis for the radiosensitizing and cytotoxic effects of chlorpromazine hydrochloride in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 725–728. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, K.S.; Choi, Y.-K.; Lim, H.J.; Lee, H.G.; Lim, Y.; Lee, Y.H. The antipsychotic agent chlorpromazine induces autophagic cell death by inhibiting the Akt/mTOR pathway in human U-87MG glioma cells. Carcinogenesis 2013, 34, 2080–2089. [Google Scholar] [CrossRef]
- Okimoto, K.; Ohike, A.; Ibuki, R.; Aoki, O.; Ohnishi, N.; Irie, T.; Uekama, K.; Rajewski, R.A.; Stella, V.J. Design and evaluation of an osmotic pump tablet (OPT) for chlorpromazine using (SBE)7m-beta-CD. Pharm. Res. 1999, 16, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Szmeja, S.; Gubica, T.; Ostrowski, A.; Zalewska, A.; Szeleszczuk, Ł.; Zawada, K.; Zielińska-Pisklak, M.; Skowronek, K.; Wiweger, M. Caffeine-Cyclodextrin Complexes as Solids: Synthesis, Biological and Physicochemical Characterization. Int. J. Mol. Sci. 2021, 22, 4191. [Google Scholar] [CrossRef]
- Prabu, S.; Swaminathan, M.; Sivakumar, K.; Rajamohan, R. Preparation, characterization and molecular modeling studies of the inclusion complex of Caffeine with Beta-cyclodextrin. J. Mol. Struct. 2015, 1099, 616–624. [Google Scholar] [CrossRef]
- Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Szente, L.; Poór, M. Interactions of Mycotoxin Alternariol with Cyclodextrins and its Removal from Aqueous Solution by Beta-Cyclodextrin Bead Polymer. Biomolecules 2019, 9, 428. [Google Scholar] [CrossRef]
- Mottram, A.R.; Bryant, S.M.; Aks, S.E. Effect of Cyclodextrin Infusion in a Rat Model of Verapamil Toxicity. Am. J. Ther. 2011, 18, 371–374. [Google Scholar] [CrossRef]
- Mottram, A.R.; Bryant, S.M.; Aks, S.E. Dose-Dependent Response to Cyclodextrin Infusion in a Rat Model of Verapamil Toxicity. West. J. Emerg. Med. 2012, 13, 63–67. [Google Scholar] [CrossRef][Green Version]
- Ozbilgin, S.; Ozbilgin, M.; Kucukoztas, B.; Kamaci, G.; Unek, T.; Yurtlu, B.S.; Güneli, M.E.; Hanci, V.; Gunerli, A. Evaluation of the effectiveness of sugammadex for verapamil intoxication. Basic Clin. Pharmacol. Toxicol. 2013, 113, 280–285. [Google Scholar] [CrossRef] [PubMed]


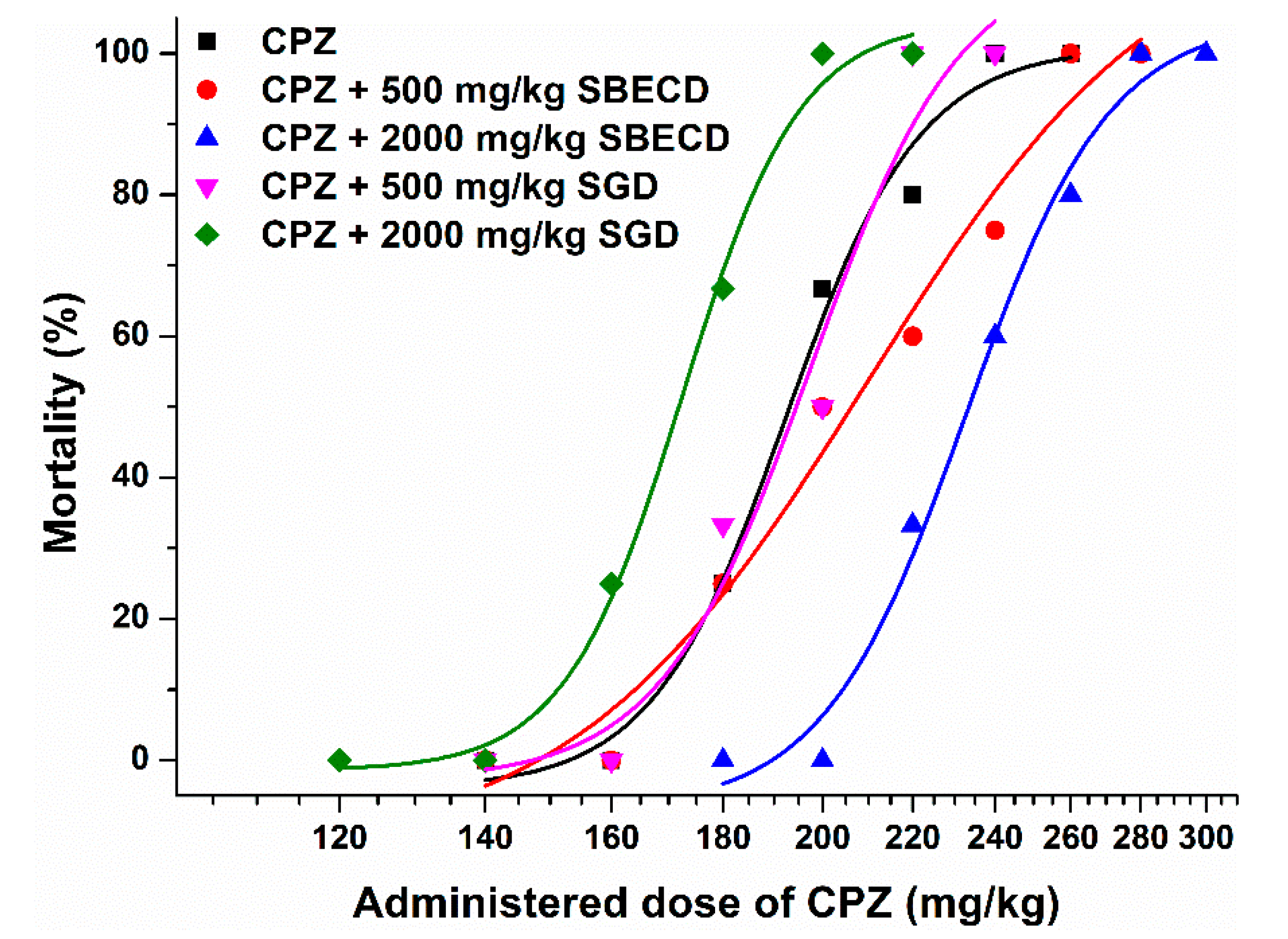
| CPZ (Control) | CPZ + 500 mg/kg SBECD | CPZ + 2000 mg/kg SBECD | CPZ + 500 mg/kg SGD | CPZ + 2000 mg/kg SGD | |
|---|---|---|---|---|---|
| LD50 (mg/kg, based on 24 h mortality) | 194.0 | 206.2 | 232.8 | 200.0 | 171.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fliszár-Nyúl, E.; Csepregi, R.; Benkovics, G.; Szente, L.; Poór, M. Testing the Protective Effects of Sulfobutylether-Βeta-Cyclodextrin (SBECD) and Sugammadex against Chlorpromazine-Induced Acute Toxicity in SH-SY5Y Cell Line and in NMRI Mice. Pharmaceutics 2022, 14, 1888. https://doi.org/10.3390/pharmaceutics14091888
Fliszár-Nyúl E, Csepregi R, Benkovics G, Szente L, Poór M. Testing the Protective Effects of Sulfobutylether-Βeta-Cyclodextrin (SBECD) and Sugammadex against Chlorpromazine-Induced Acute Toxicity in SH-SY5Y Cell Line and in NMRI Mice. Pharmaceutics. 2022; 14(9):1888. https://doi.org/10.3390/pharmaceutics14091888
Chicago/Turabian StyleFliszár-Nyúl, Eszter, Rita Csepregi, Gábor Benkovics, Lajos Szente, and Miklós Poór. 2022. "Testing the Protective Effects of Sulfobutylether-Βeta-Cyclodextrin (SBECD) and Sugammadex against Chlorpromazine-Induced Acute Toxicity in SH-SY5Y Cell Line and in NMRI Mice" Pharmaceutics 14, no. 9: 1888. https://doi.org/10.3390/pharmaceutics14091888
APA StyleFliszár-Nyúl, E., Csepregi, R., Benkovics, G., Szente, L., & Poór, M. (2022). Testing the Protective Effects of Sulfobutylether-Βeta-Cyclodextrin (SBECD) and Sugammadex against Chlorpromazine-Induced Acute Toxicity in SH-SY5Y Cell Line and in NMRI Mice. Pharmaceutics, 14(9), 1888. https://doi.org/10.3390/pharmaceutics14091888








