Enteric Polymer–Based Amorphous Solid Dispersions Enhance Oral Absorption of the Weakly Basic Drug Nintedanib via Stabilization of Supersaturation
Abstract
1. Introduction
2. Materials and Methods
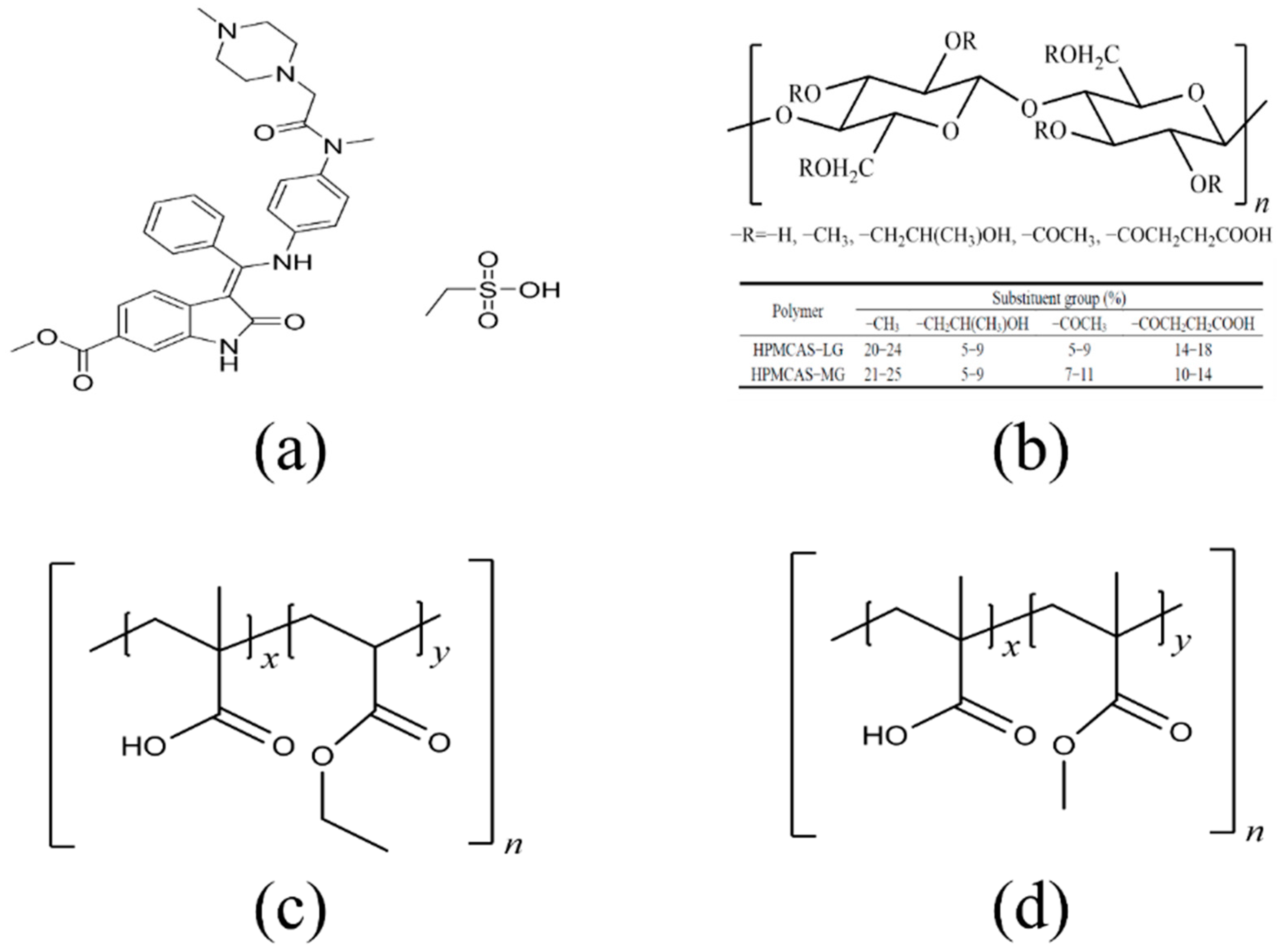
2.1. Materials
2.2. Determination of Equilibrium Solubility and Apparent n–Octanol/Water Partition Coefficient of Nintedanib
2.3. Solubility Measurement of Nintedanib in Polymer Solution
2.4. In Vitro Supersaturation Studies
2.5. Polarized Light Microscopy
2.6. Preparation and Characterization of Amorphous Solid Dispersions
2.6.1. Preparation of Amorphous Solid Dispersions
2.6.2. Scanning Electron Microscopy (SEM)
2.6.3. Differential Scanning Calorimetry (DSC)
2.6.4. Powder X-ray Diffraction (PXRD)
2.6.5. Fourier Transform Infrared Spectroscopy (FT–IR)
2.7. pH Shift Dissolution Studies of Amorphous Solid Dispersions
2.8. Stability Study
2.9. In Vivo Pharmacokinetic Study
3. Results and Discussion
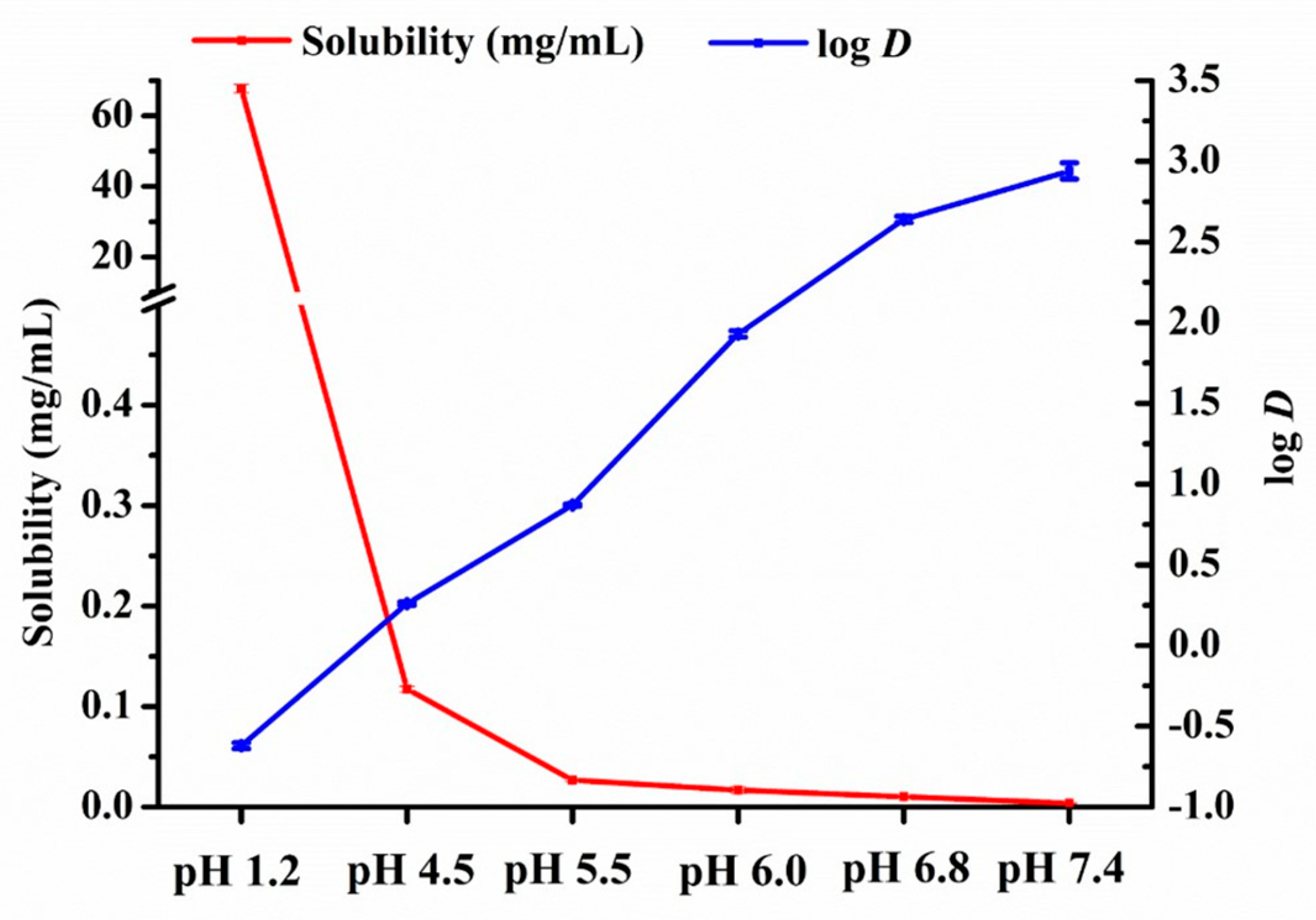
3.1. Equilibrium Solubility and Apparent n–Octanol/Water Partition Coefficient of Nintedanib
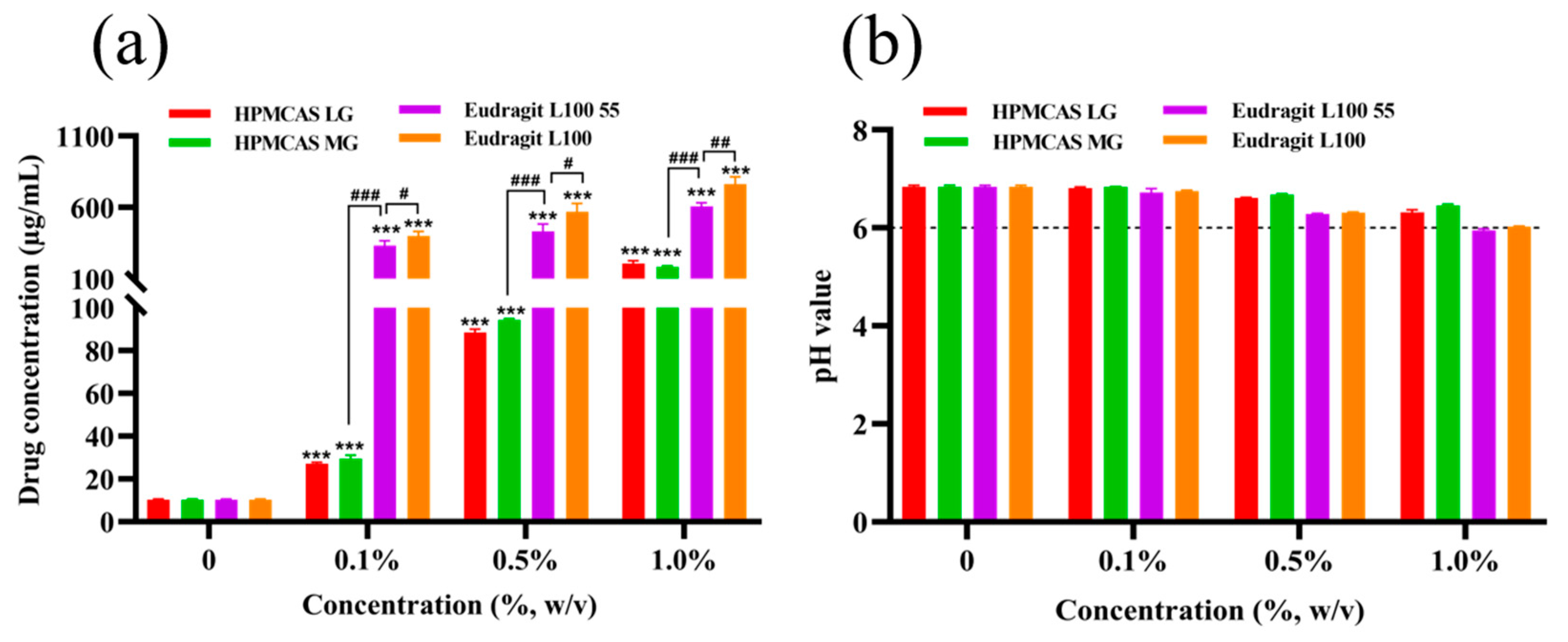
3.2. Effect of Enteric Polymers on Solubility of Nintedanib
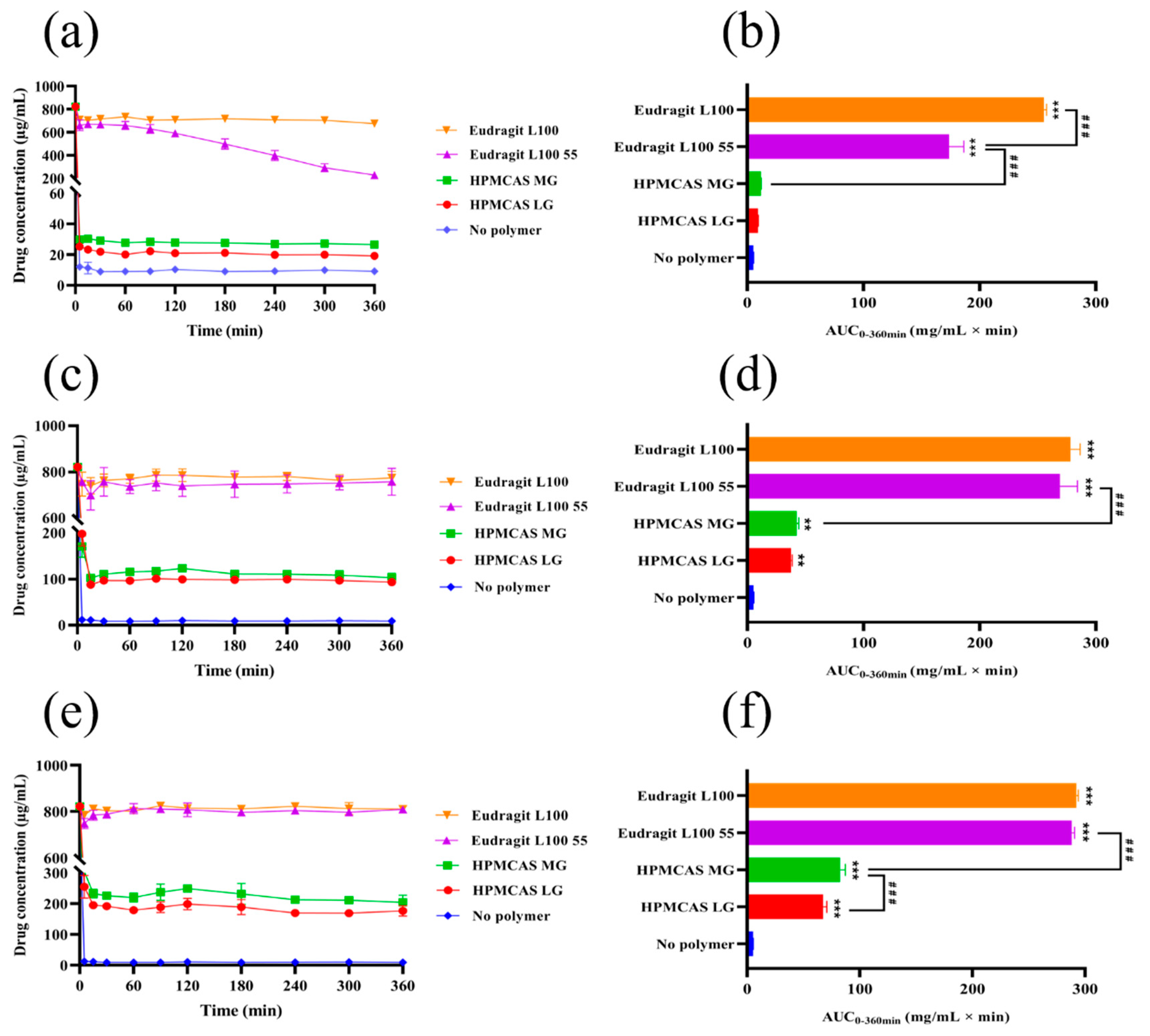
3.3. Supersaturation Kinetics of Nintedanib in the Presence of Enteric Polymers
3.4. Crystallization Kinetics of Nintedanib in the Presence of Enteric Polymers
3.5. Characterization of Amorphous Solid Dispersions
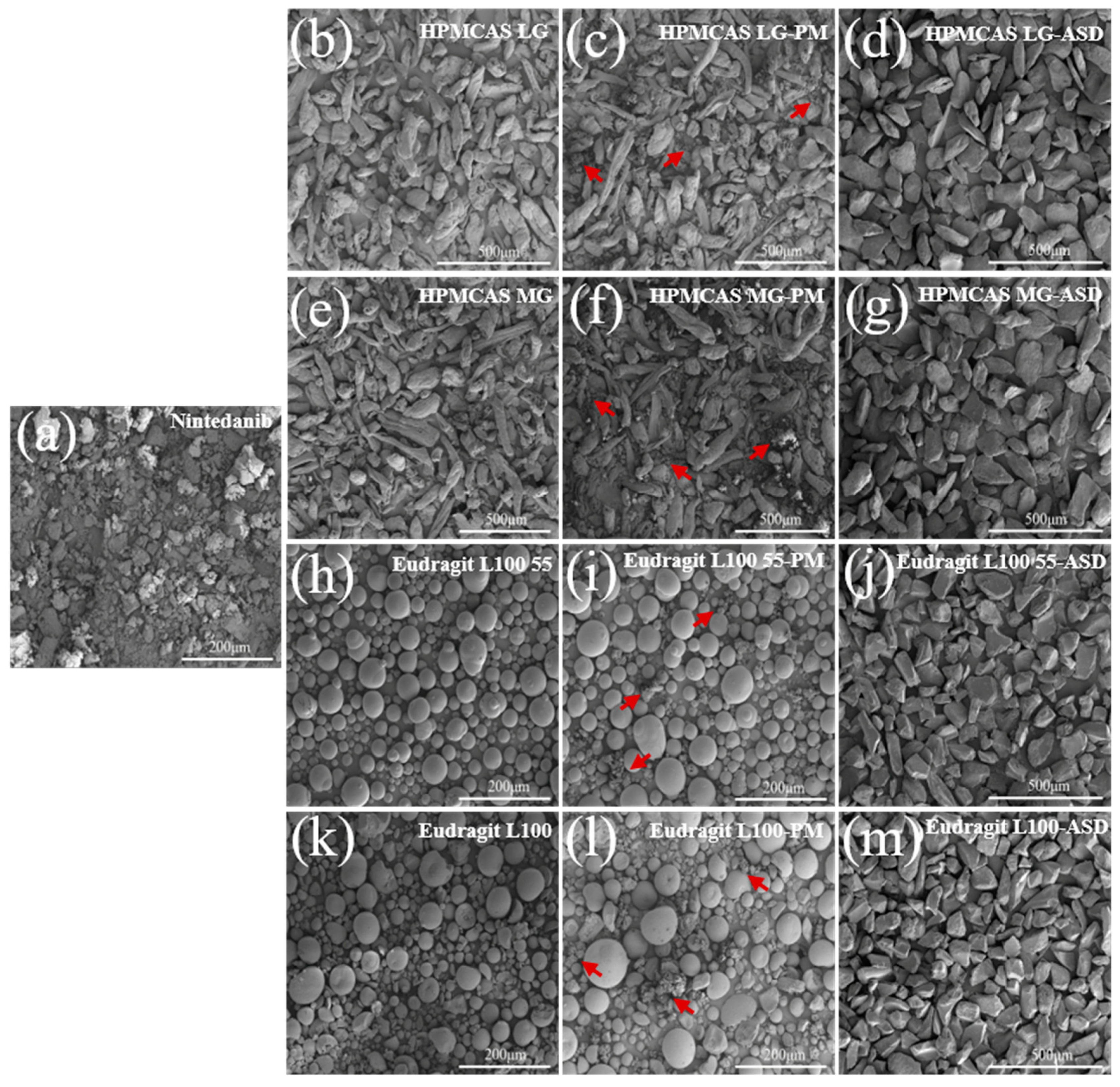
3.5.1. SEM
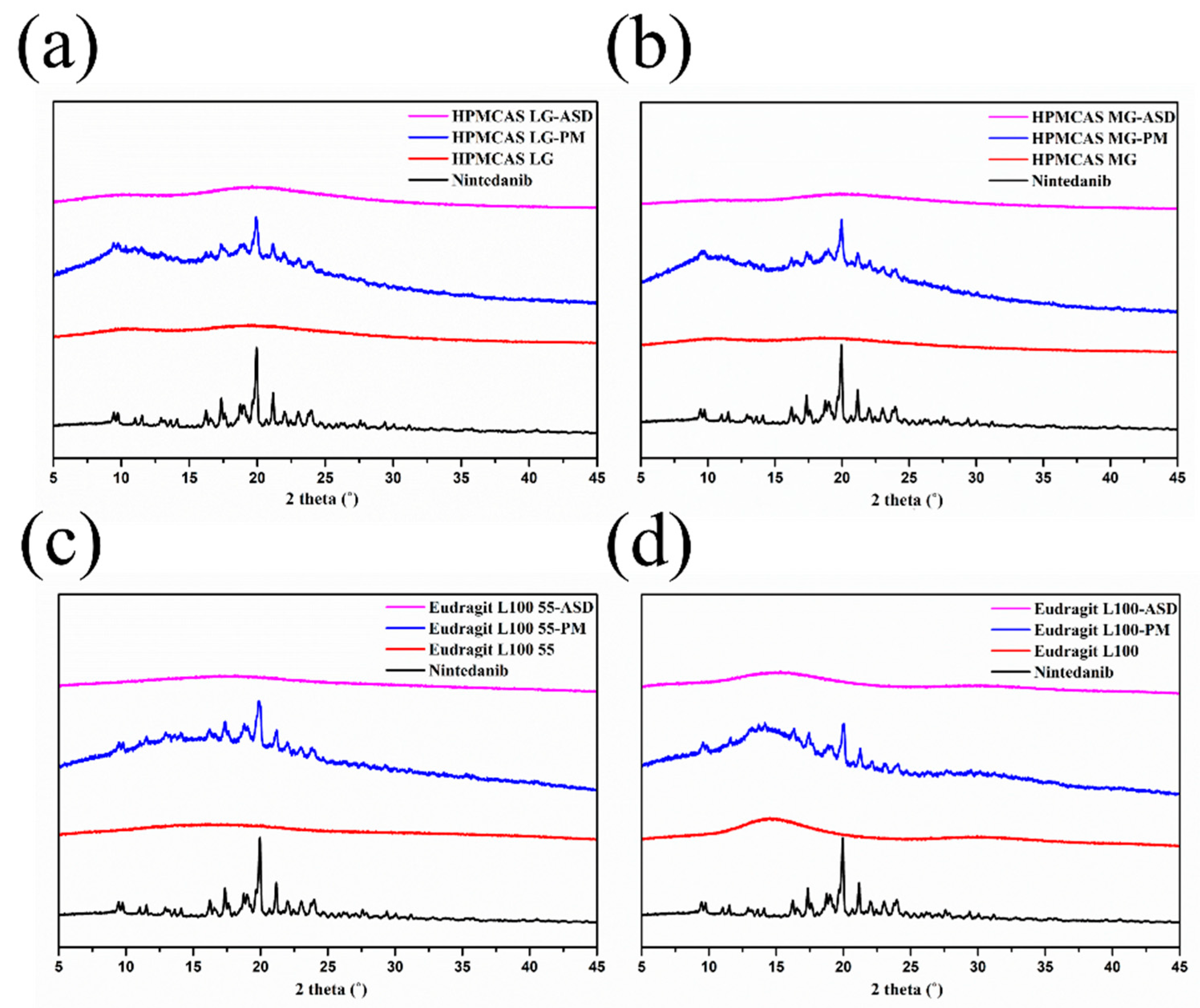
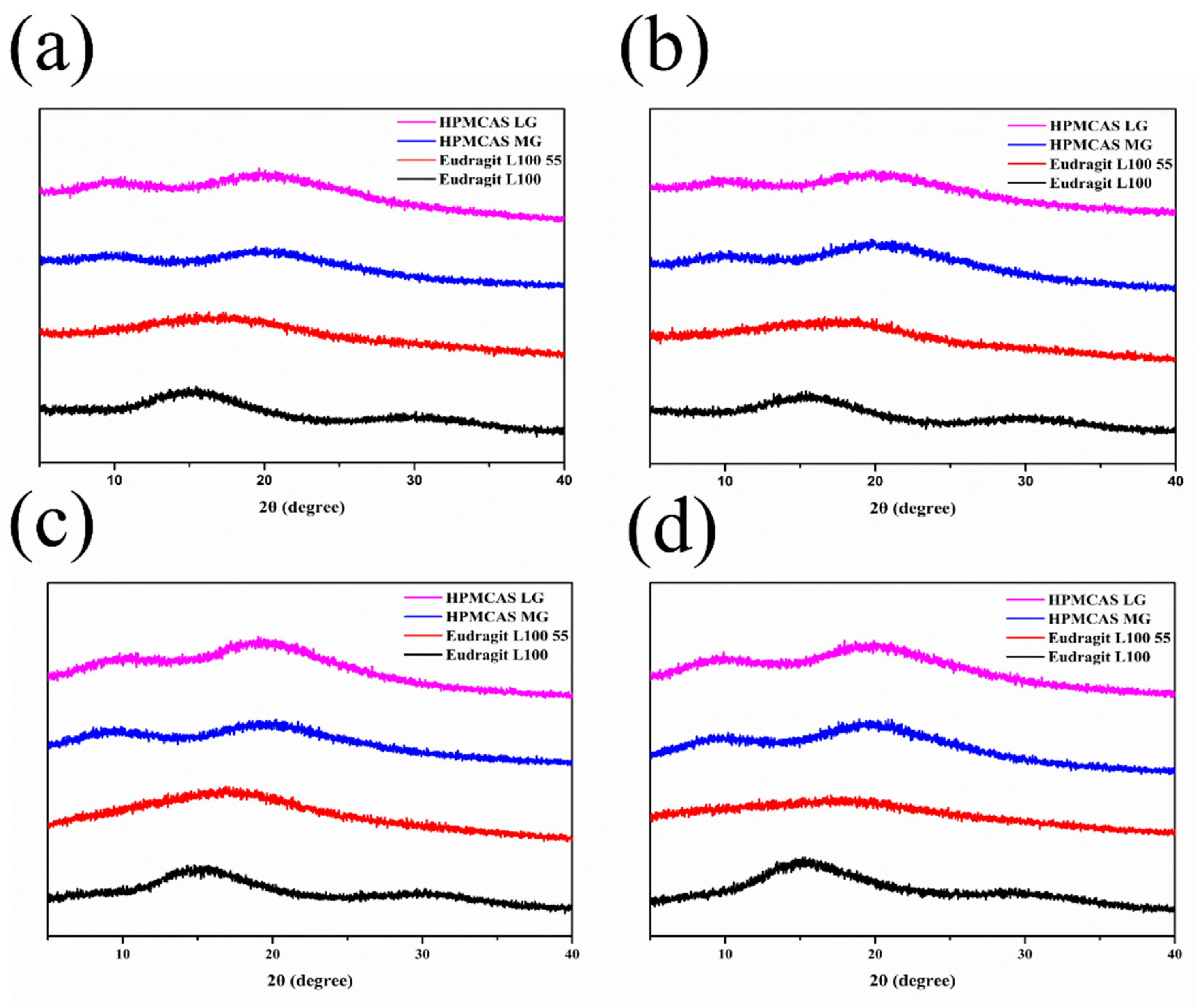
3.5.2. PXRD
3.5.3. DSC
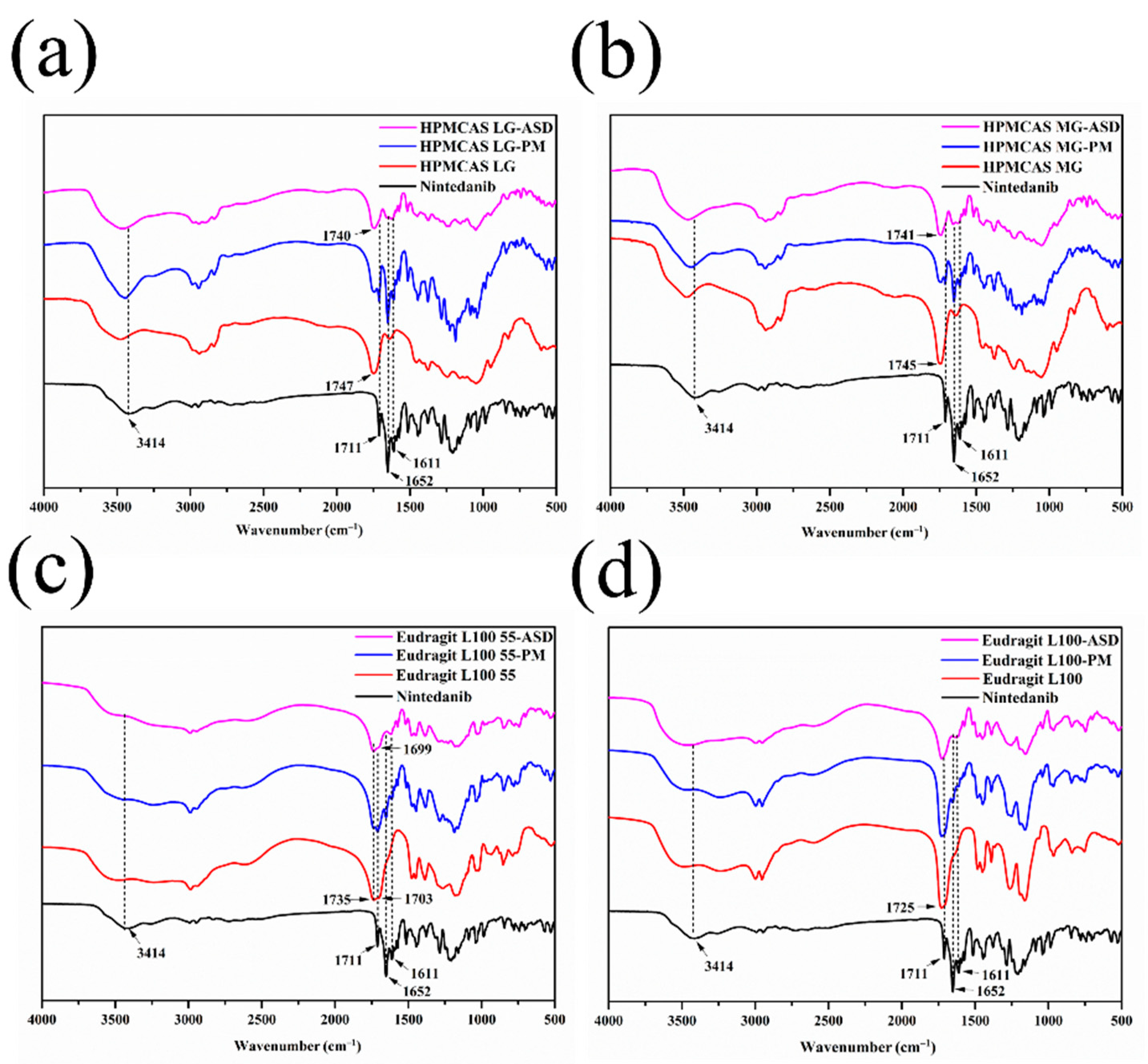
3.5.4. FT–IR
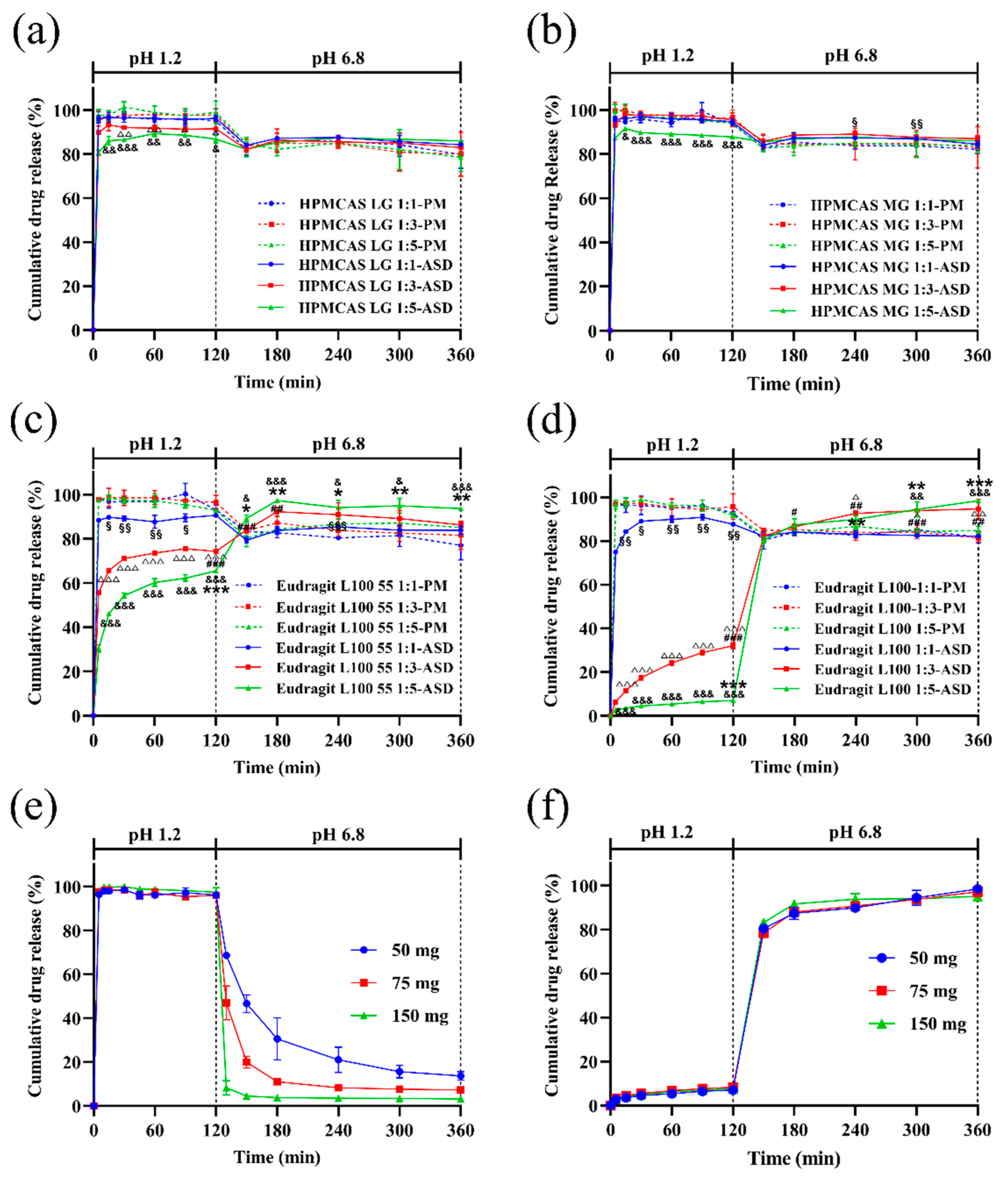
3.6. In Vitro Dissolution with pH Shift
3.7. Stability under High Temperatures and High Humidity
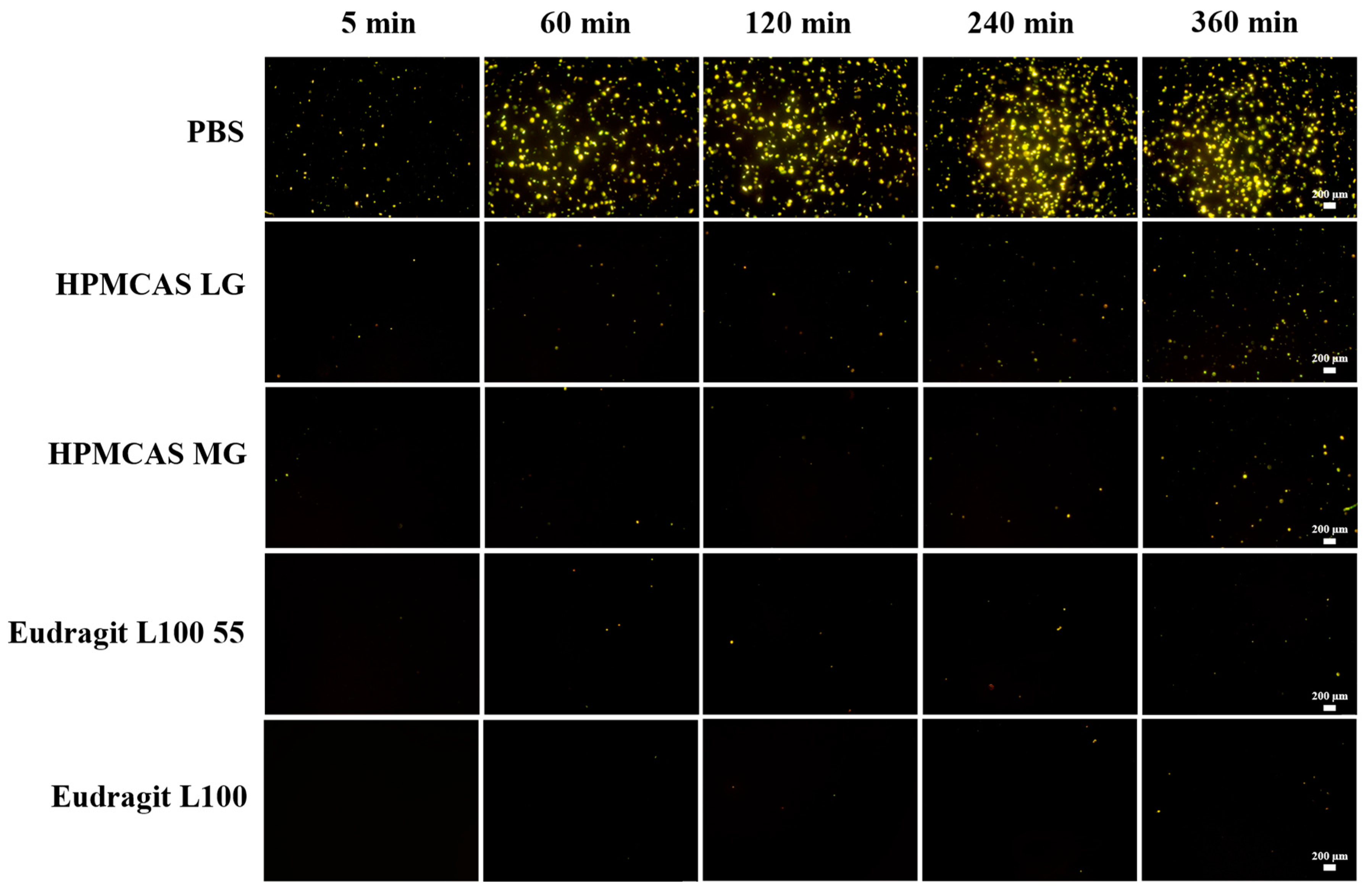
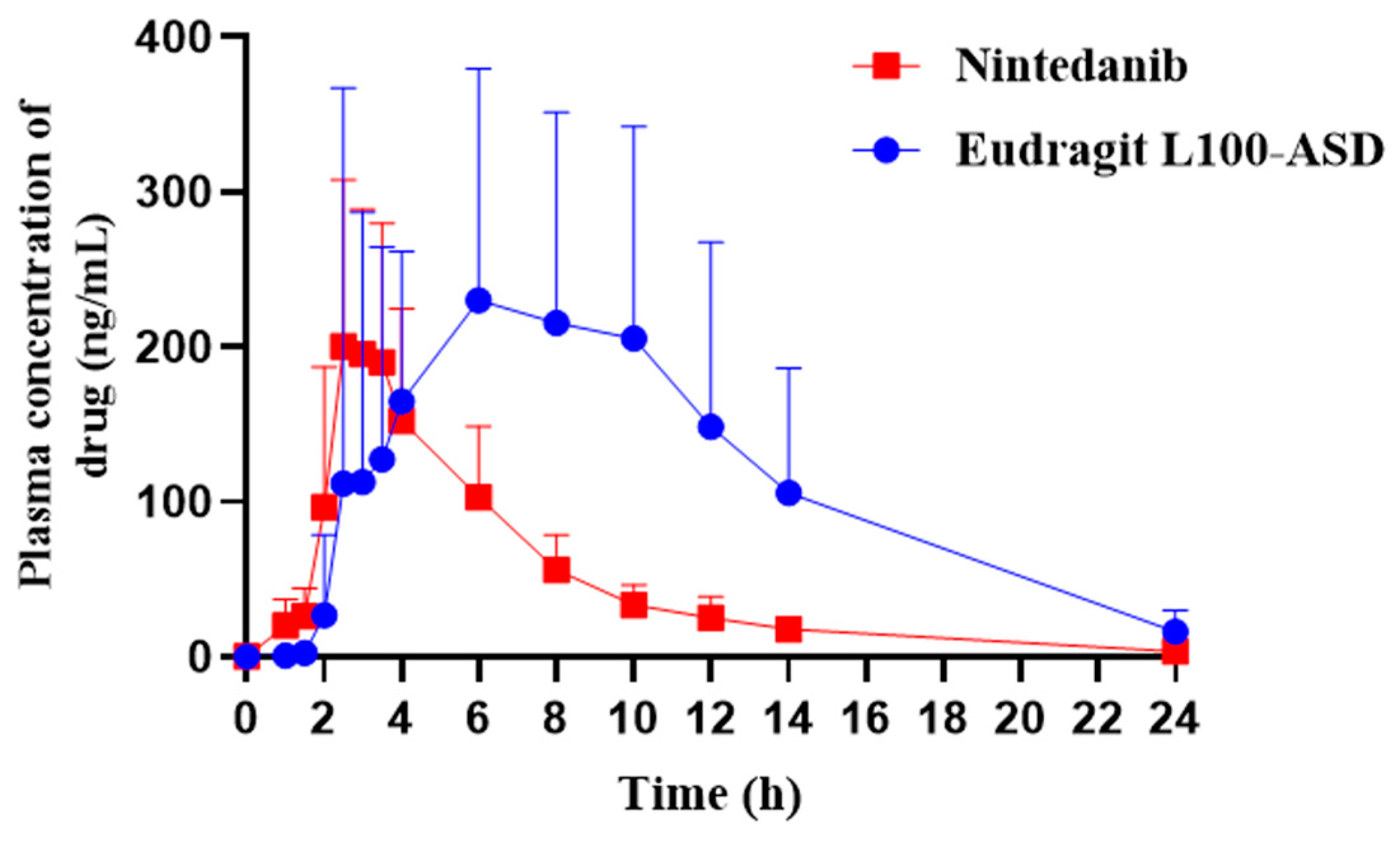
3.8. In Vivo Pharmacokinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wind, S.; Schmid, U.; Freiwald, M.; Marzin, K.; Lotz, R.; Ebner, T.; Stopfer, P.; Dallinger, C. Clinical pharmacokinetics and pharmacodynamics of nintedanib. Clin. Pharmacokinet. 2019, 58, 1131–1147. [Google Scholar] [CrossRef]
- Awasthi, N.; Hinz, S.; Brekken, R.A.; Schwarz, M.A.; Schwarz, R.E. Nintedanib, a triple angiokinase inhibitor, enhances cytotoxic therapy response in pancreatic cancer. Cancer Lett. 2015, 358, 59–66. [Google Scholar] [CrossRef]
- Manzo, A.; Carillio, G.; Montanino, A.; Costanzo, R.; Sandomenico, C.; Rocco, G.; Morabito, A. Focus on Nintedanib in NSCLC and other tumors. Front. Med. 2016, 3, 68. [Google Scholar] [CrossRef][Green Version]
- Falcomatà, C.; Bärthel, S.; Widholz, S.A.; Schneeweis, C.; Montero, J.J.; Toska, A.; Mir, J.; Kaltenbacher, T.; Heetmeyer, J.; Swietlik, J.J.; et al. Selective multi–kinase inhibition sensitizes mesenchymal pancreatic cancer to immune checkpoint blockade by remodeling the tumor microenvironment. Nat. Cancer 2022, 3, 318–336. [Google Scholar] [CrossRef]
- Liu, H.; Mei, J.; Xu, Y.; Tang, L.; Chen, D.; Zhu, Y.; Huang, S.; Webster, T.J.; Ding, H. Improving the oral absorption of nintedanib by a self–microemulsion drug delivery system: Preparation and in vitro/in vivo evaluation. Int. J. Nanomed. 2019, 14, 8739–8751. [Google Scholar] [CrossRef]
- Luedtke, D.; Marzin, K.; Jungnik, A.; von Wangenheim, U.; Dallinger, C. Effects of ketoconazole and rifampicin on the pharmacokinetics of nintedanib in healthy subjects. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 533–541. [Google Scholar] [CrossRef]
- FDA Center for Drug Evaulation and Research. Nintedanib Clinical Pharmocology and Biopharmoceutics Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205832Orig1s000ClinPharmR.pdf (accessed on 5 February 2014).
- Liu, H.; Du, K.; Li, D.; Du, Y.; Xi, J.; Xu, Y.; Shen, Y.; Jiang, T.; Webster, T.J. A high bioavailability and sustained–release nano–delivery system for nintedanib based on electrospray technology. Int. J. Nanomed. 2018, 13, 8379–8393. [Google Scholar] [CrossRef]
- Zhu, Y.; Fu, Y.; Zhang, A.; Wang, X.; Zhao, Z.; Zhang, Y.; Yin, T.; Gou, J.; Wang, Y.; He, H.; et al. Rod–shaped nintedanib nanocrystals improved oral bioavailability through multiple intestinal absorption pathways. Eur. J. Pharm. Sci. 2022, 168, 106047. [Google Scholar] [CrossRef]
- Konno, H.; Handa, T.; Alonzo, D.E.; Taylor, L.S. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur. J. Pharm. Biopharm. 2008, 70, 493–499. [Google Scholar] [CrossRef]
- Qian, K.J.; Stella, L.; Jones, D.S.; Andrews, G.P.; Du, H.C.; Tian, Y.W. Drug–rich phases induced by amorphous solid dispersion: Arbitrary or intentional goal in oral drug delivery? Pharmaceutics 2021, 13, 889. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.R.; Lee, P.I. Effect of extent of supersaturation on the evolution of kinetic solubility profiles. Mol. Pharm. 2017, 14, 206–220. [Google Scholar] [CrossRef]
- Lu, E.; Li, S.; Wang, Z. Biorelevant test for supersaturable formulation. Asian J. Pharm. Sci. 2017, 12, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Que, C.; Gao, Y.; Raina, S.A.; Zhang, G.G.Z.; Taylor, L.S. Paclitaxel crystal seeds with different intrinsic properties and their impact on dissolution of paclitaxel–HPMCAS amorphous solid dispersions. Cryst. Growth Des. 2018, 18, 1548–1559. [Google Scholar] [CrossRef]
- Feng, D.; Peng, T.; Huang, Z.; Singh, V.; Shi, Y.; Wen, T.; Lu, M.; Quan, G.; Pan, X.; Wu, C. Polymer⁻surfactant system based amorphous solid dispersion: Precipitation inhibition and bioavailability enhancement of itraconazole. Pharmaceutics 2018, 10, 53. [Google Scholar] [CrossRef]
- Kojima, T.; Higashi, K.; Suzuki, T.; Tomono, K.; Moribe, K.; Yamamoto, K. Stabilization of a supersaturated solution of mefenamic acid from a solid dispersion with EUDRAGIT® EPO. Pharm. Res. 2012, 29, 2777–2791. [Google Scholar] [CrossRef]
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating drug delivery systems: The answer to solubility–limited oral bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef]
- Sun, D.D.; Lee, P.I. Evolution of supersaturation of amorphous pharmaceuticals: The effect of rate of supersaturation generation. Mol. Pharm. 2013, 10, 4330–4346. [Google Scholar] [CrossRef]
- Six, K.; Daems, T.; de Hoon, J.; Van Hecken, A.; Depre, M.; Bouche, M.P.; Prinsen, P.; Verreck, G.; Peeters, J.; Brewster, M.E.; et al. Clinical study of solid dispersions of itraconazole prepared by hot–stage extrusion. Eur. J. Pharm. Sci. 2005, 24, 179–186. [Google Scholar] [CrossRef]
- Miller, D.A.; McConville, J.T.; Yang, W.; Williams, R.O., 3rd; McGinity, J.W. Hot–melt extrusion for enhanced delivery of drug particles. J. Pharm. Sci. 2007, 96, 361–376. [Google Scholar] [CrossRef]
- Miller, D.A.; DiNunzio, J.C.; Yang, W.; McGinity, J.W.; Williams, R.O., 3rd. Enhanced in vivo absorption of itraconazole via stabilization of supersaturation following acidic–to–neutral pH transition. Drug Dev. Ind. Pharm. 2008, 34, 890–902. [Google Scholar] [CrossRef]
- Guan, J.; Jin, L.; Liu, Q.; Xu, H.; Wu, H.; Zhang, X.; Mao, S. Exploration of supersaturable lacidipine ternary amorphous solid dispersion for enhanced dissolution and in vivo absorption. Eur. J. Pharm. Sci. 2019, 139, 105043. [Google Scholar] [CrossRef]
- Sun, M.; Wu, C.; Fu, Q.; Di, D.; Kuang, X.; Wang, C.; He, Z.; Wang, J.; Sun, J. Solvent–shift strategy to identify suitable polymers to inhibit humidity–induced solid–state crystallization of lacidipine amorphous solid dispersions. Int. J. Pharm. 2016, 503, 238–246. [Google Scholar] [CrossRef]
- Pinto, J.M.O.; Leão, A.F.; Riekes, M.K.; França, M.T.; Stulzer, H.K. HPMCAS as an effective precipitation inhibitor in amorphous solid dispersions of the poorly soluble drug candesartan cilexetil. Carbohydr. Polym. 2018, 184, 199–206. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.Y.; Chen, Y.J.; Zhu, A.; Qian, F. Aggregation of hydroxypropyl methylcellulose acetate succinate under its dissolving pH and the impact on drug supersaturation. Mol. Pharm. 2018, 15, 4643–4653. [Google Scholar] [CrossRef]
- Kataoka, M.; Nakanishi, R.; Umesaki, M.; Kobayashi, M.; Minami, K.; Higashino, H.; Yamaguchi, S.; Yamashita, S. An enteric polymer mitigates the effects of gastric pH on oral absorption of poorly soluble weak acid drugs from supersaturable formulations: A case study with dantrolene. Eur. J. Pharm. Biopharm. 2020, 155, 29–36. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Shao, Y.; Ni, R.; Zhang, T.; Mao, S. Drug release characteristics from chitosan–alginate matrix tablets based on the theory of self–assembled film. Int. J. Pharm. 2013, 450, 197–207. [Google Scholar] [CrossRef]
- Li, Q.; Sun, M.; Li, G.; Qiu, L.; Huang, Z.; Gong, J.; Huang, J.; Li, G.; Si, L. The sub–chronic impact of mPEG(2k)–PCL(x) polymeric nanocarriers on cytochrome P450 enzymes after intravenous administration in rats. Eur. J. Pharm. Biopharm. 2019, 142, 101–113. [Google Scholar] [CrossRef]
- Zhu, W.W.; Liu, W.Y.; Li, H.J.; Xu, G.J.; Li, Q.; Huang, J.G.; Li, G.; Si, L.Q. Simultaneous Quantitation of a Novel alpha(1)/beta(1)–Blocker TJ0711 and Its Two Metabolites in Dog Plasma Using LC–MS/MS and Its Application to a Pharmacokinetic Study after Intravenous Infusion. Pharmaceutics 2019, 11, 38. [Google Scholar] [CrossRef]
- Ilevbare, G.A.; Liu, H.Y.; Edgar, K.J.; Taylor, L.S. Understanding polymer properties important for crystal growth inhibition–impact of chemically diverse polymers on solution crystal growth of ritonavir. Cryst. Growth Des. 2012, 12, 3133–3143. [Google Scholar] [CrossRef]
- Ueda, K.; Higashi, K.; Yamamoto, K.; Moribe, K. The effect of HPMCAS functional groups on drug crystallization from the supersaturated state and dissolution improvement. Int. J. Pharm. 2014, 464, 205–213. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Margulis, E.B.; Sibgatullina, L.F.; Kemenova, V.A.; Van den Mooter, G. Comparative evaluation of interpolyelectrolyte complexes of chitosan with Eudragit L100 and Eudragit L100–55 as potential carriers for oral controlled drug delivery. Eur. J. Pharm. Biopharm. 2008, 70, 215–225. [Google Scholar] [CrossRef]
- Guan, Q.; Ma, Q.; Zhao, Y.; Jiang, X.; Zhang, H.; Liu, M.; Wang, Z.; Han, J. Cellulose derivatives as effective recrystallization inhibitor for ternary ritonavir solid dispersions: In vitro–in vivo evaluation. Carbohydr. Polym. 2021, 273, 118562. [Google Scholar] [CrossRef]
- Maghsoodi, M.; Pourasghari Azar, H. The effect of some acrylic polymers on dissolution of celecoxib solid dispersion formulations. Pharm. Dev. Technol. 2021, 26, 788–796. [Google Scholar] [CrossRef]
- Monschke, M.; Wagner, K.G. Amorphous solid dispersions of weak bases with pH–dependent soluble polymers to overcome limited bioavailability due to gastric pH variability—An in-vitro approach. Int. J. Pharm. 2019, 564, 162–170. [Google Scholar] [CrossRef]
- Li, J.; Lee, I.W.; Shin, G.H.; Chen, X.; Park, H.J. Curcumin–Eudragit® E PO solid dispersion: A simple and potent method to solve the problems of curcumin. Eur. J. Pharm. Biopharm. 2015, 94, 322–332. [Google Scholar] [CrossRef]
- Metre, S.; Mukesh, S.; Samal, S.K.; Chand, M.; Sangamwar, A.T. Enhanced biopharmaceutical performance of rivaroxaban through polymeric amorphous solid dispersion. Mol. Pharm. 2018, 15, 652–668. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Chen, Y.; Chen, Z.; Chen, H.; Pui, Y.; Qian, F. Oral bioavailability enhancement of β-lapachone, a poorly soluble fast crystallizer, by cocrystal, amorphous solid dispersion, and crystalline solid dispersion. Eur. J. Pharm. Biopharm. 2018, 124, 73–81. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, H.K. Enhancement of solubility and dissolution of cilostazol by solid dispersion technique. Arch. Pharm. Res. 2015, 38, 1336–1344. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.S.; Cho, W.; Cha, K.H.; Park, H.J.; Park, J.; Hwang, S.J. Supersaturatable formulations for the enhanced oral absorption of sirolimus. Int. J. Pharm. 2013, 445, 108–116. [Google Scholar] [CrossRef]
- Li, H.L.; Zhang, M.M.; Xiong, L.L.; Feng, W.Y.; Williams, R.O. Bioavailability improvement of carbamazepine via oral administration of modified–release amorphous solid dispersions in rats. Pharmaceutics 2020, 12, 1023. [Google Scholar] [CrossRef] [PubMed]


| Compound | Precursor Ion > Product Ion (m/z) | DP (V) | EP (V) | CE (eV) | CXP (V) |
|---|---|---|---|---|---|
| Nintedanib | 540.3 > 113.0 | 126 | 10 | 39 | 8 |
| Carbamazepine (IS) | 236.9 > 193.9 | 161 | 10 | 31 | 16 |
| Parameters | Nintedanib | Eudragit L100–ASD |
|---|---|---|
| Tmax (h) | 2.8 ± 0.4 | 5.3 ± 2.7 * |
| Cmax (ng/mL) | 248.3 ± 70.4 | 370.0 ± 159.6 |
| AUC0–24h (ng/mL·h) | 1107.6 ± 292.3 | 2710.6 ± 1479.4 * |
| t1/2 (h) | 3.2 ± 0.8 | 3.6 ± 1.1 |
| MRT (h) | 6.3 ± 0.8 | 9.3 ± 1.4 ** |
| Frel (%) | / | 245% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Xiao, C.; Li, X.; Huang, J.; Si, L.; Sun, M. Enteric Polymer–Based Amorphous Solid Dispersions Enhance Oral Absorption of the Weakly Basic Drug Nintedanib via Stabilization of Supersaturation. Pharmaceutics 2022, 14, 1830. https://doi.org/10.3390/pharmaceutics14091830
Qin Y, Xiao C, Li X, Huang J, Si L, Sun M. Enteric Polymer–Based Amorphous Solid Dispersions Enhance Oral Absorption of the Weakly Basic Drug Nintedanib via Stabilization of Supersaturation. Pharmaceutics. 2022; 14(9):1830. https://doi.org/10.3390/pharmaceutics14091830
Chicago/Turabian StyleQin, Yuling, Chuyao Xiao, Xiaoyue Li, Jiangeng Huang, Luqin Si, and Minghui Sun. 2022. "Enteric Polymer–Based Amorphous Solid Dispersions Enhance Oral Absorption of the Weakly Basic Drug Nintedanib via Stabilization of Supersaturation" Pharmaceutics 14, no. 9: 1830. https://doi.org/10.3390/pharmaceutics14091830
APA StyleQin, Y., Xiao, C., Li, X., Huang, J., Si, L., & Sun, M. (2022). Enteric Polymer–Based Amorphous Solid Dispersions Enhance Oral Absorption of the Weakly Basic Drug Nintedanib via Stabilization of Supersaturation. Pharmaceutics, 14(9), 1830. https://doi.org/10.3390/pharmaceutics14091830






