Development and Evaluation of a Peptide Heterodimeric Tracer Targeting CXCR4 and Integrin αvβ3 for Pancreatic Cancer Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Synthesis of NOTA-yG5 and NOTA-yG5-RGD
2.3. Radiolabeling
2.4. Stability and Octanol/Water Partition Coefficient (logP)
2.5. Cell Culture
2.6. Western Blot Assay
2.7. In Vitro Cell Assays
2.8. Animal Models
2.9. Small Animal PET/CT Static Scan
2.10. Biodistribution Studies
2.11. Immunohistochemical Analysis
2.12. Statistical Analysis
3. Results
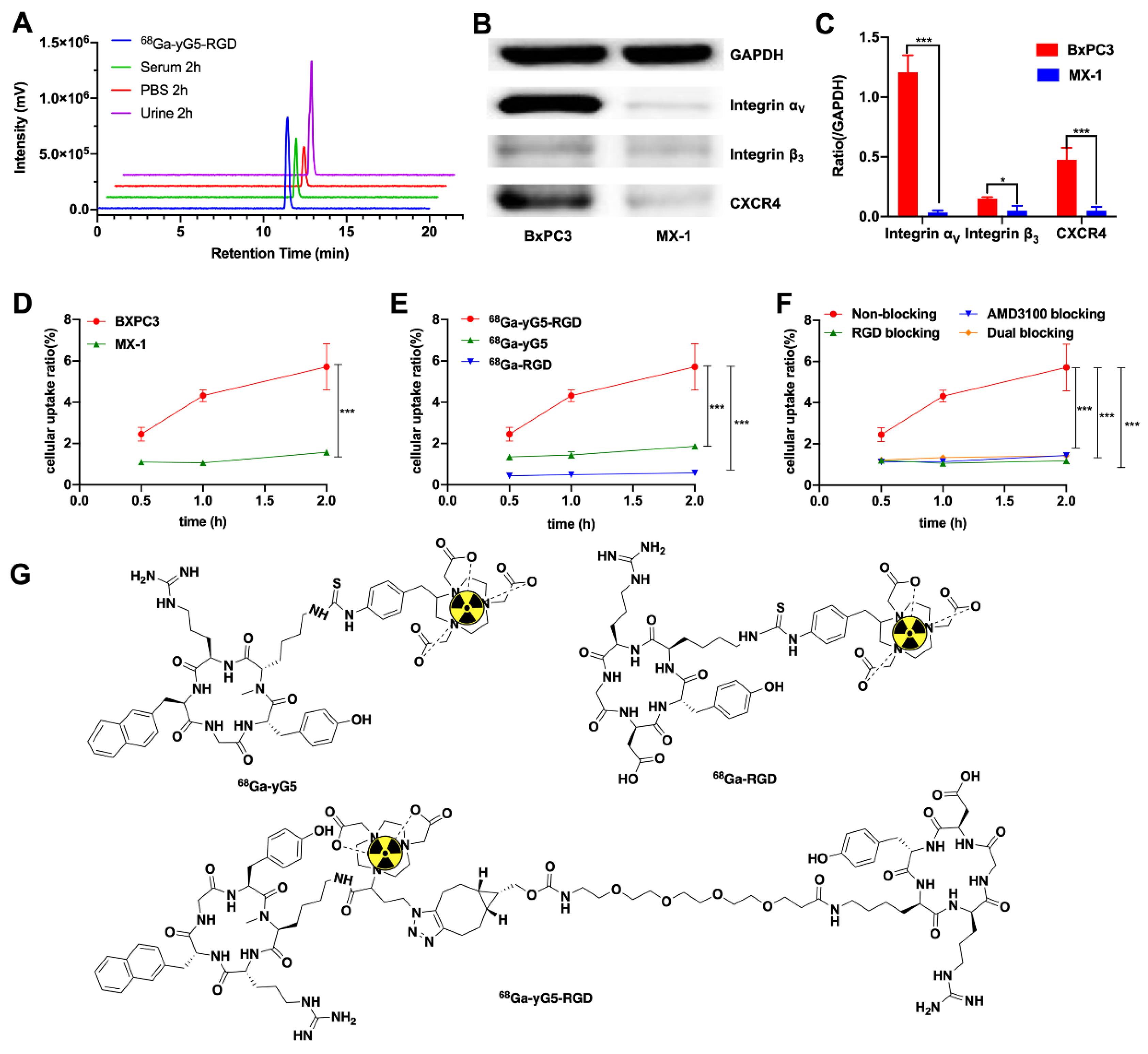
3.1. Chemical and Radiochemical Characterization
3.2. Stability and log P of 68Ga-yG5-RGD
3.3. Western Blot Analysis
3.4. In Vitro Cell Assays
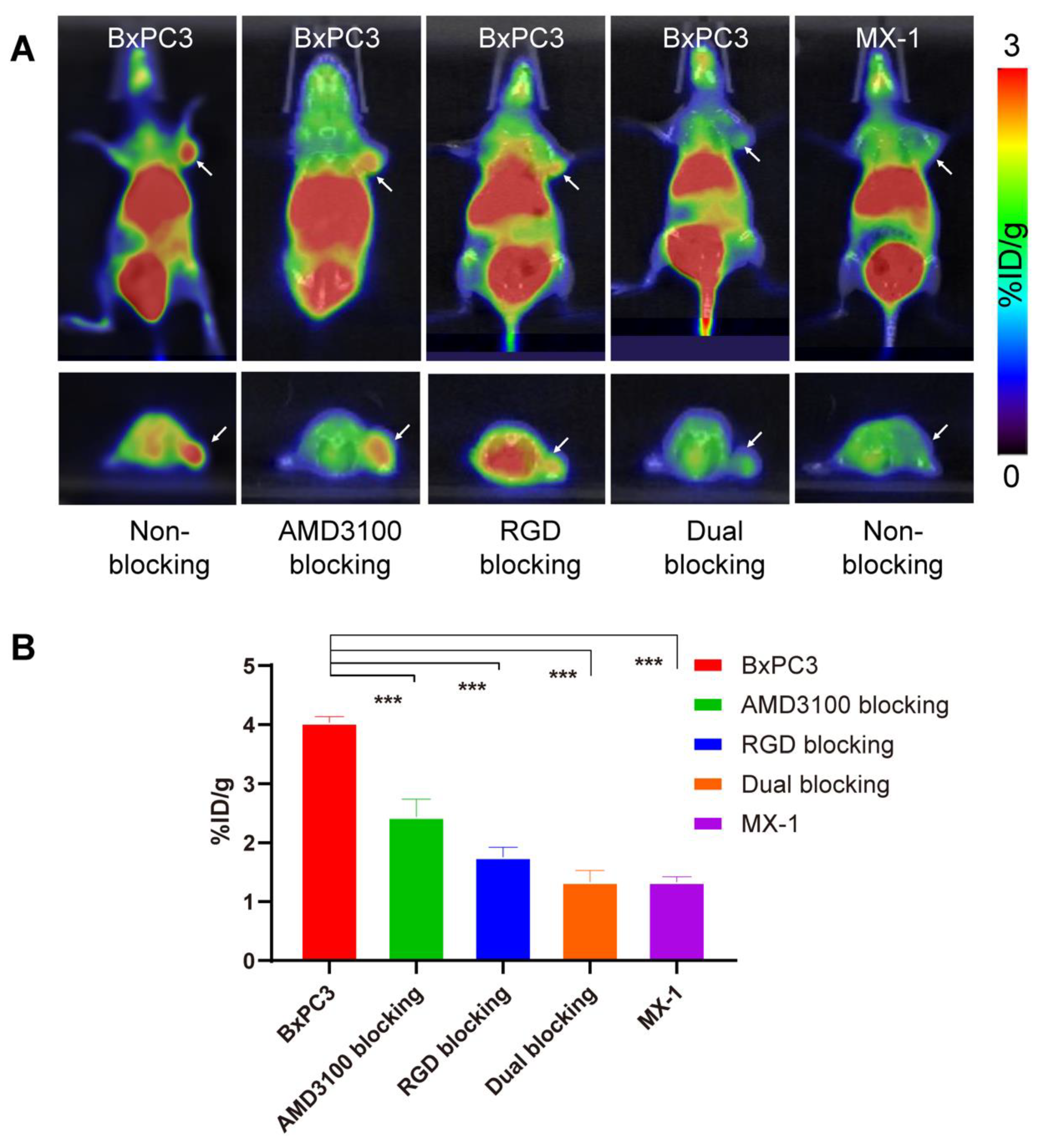
3.5. Small Animal PET/CT Scans
3.6. Biodistribution Study
3.7. PET Imaging of Pancreatic Cancer PDX Model
3.8. Immunohistochemical Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcea, G.; Dennison, A.R.; Pattenden, C.J.; Neal, C.P.; Sutton, C.D.; Berry, D.P. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP 2008, 9, 99–132. [Google Scholar] [PubMed]
- Verbeke, C.S. Resection margins and R1 rates in pancreatic cancer are we there yet? Histopathology 2008, 52, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Rana, V.; Janjan, N.A.; Varadhachary, G.R.; Abbruzzese, J.L.; Das, P.; Delclos, M.E.; Gould, M.S.; Evans, D.B.; Wolff, R.A.; et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer 2007, 110, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Mosley, J.D.; Feng, Q.; Wells, Q.S.; Van Driest, S.L.; Shaffer, C.M.; Edwards, T.L.; Bastarache, L.; Wei, W.Q.; Davis, L.K.; McCarty, C.A.; et al. A study paradigm integrating prospective epidemiologic cohorts and electronic health records to identify disease biomarkers. Nat. Commun. 2018, 9, 3522. [Google Scholar] [CrossRef]
- Balkwill, F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin. Cancer Biol. 2004, 14, 171–179. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, H.; Liu, T.; Shu, S.; Jiang, H.; Cheng, S.; Yuan, Y.; Yang, W.; Wang, L. BRCA2 dysfunction promotes malignant transformation of pancreatic intraepithelial neoplasia. Anticancer Agents Med. Chem. 2013, 13, 261–269. [Google Scholar] [CrossRef]
- Popp, F.C.; Popp, M.C.; Zhao, Y.; Betzler, C.; Kropf, S.; Garlipp, B.; Benckert, C.; Kalinski, T.; Lippert, H.; Bruns, C.J. Protocol of the PANCALYZE trial: A multicenter, prospective study investigating the tumor biomarkers CXCR4, SMAD4, SOX9 and IFIT3 in patients with resected pancreatic adenocarcinoma to predict the pattern of recurrence of the disease. BMC Cancer 2017, 17, 229. [Google Scholar] [CrossRef]
- Woods, A.J.; White, D.P.; Caswell, P.T.; Norman, J.C. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004, 23, 2531–2543. [Google Scholar] [CrossRef]
- Lakshmikanthan, S.; Sobczak, M.; Chun, C.; Henschel, A.; Dargatz, J.; Ramchandran, R.; Chrzanowska-Wodnicka, M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin αvβ3. Blood 2011, 118, 2015–2026. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Thakar, D.; Dalonneau, F.; Migliorini, E.; Lortat-Jacob, H.; Boturyn, D.; Albiges-Rizo, C.; Coche-Guerente, L.; Picart, C.; Richter, R.P. Binding of the chemokine CXCL12α to its natural extracellular matrix ligand heparan sulfate enables myoblast adhesion and facilitates cell motility. Biomaterials 2017, 123, 24–38. [Google Scholar] [CrossRef]
- Grande, F.; Giancotti, G.; Ioele, G.; Occhiuzzi, M.A.; Garofalo, A. An update on small molecules targeting CXCR4 as starting points for the development of anti-cancer therapeutics. Eur. J. Med. Chem. 2017, 139, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Xu, S.; Grande, F.; Garofalo, A.; Neamati, N. Small molecule inhibitors of CXCR4. Theranostics 2013, 3, 47–75. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, M.R.; Mulvey, T.; Belanger, B.; Chen, S.; Pan, C.; Chong, C.; Cao, F.; Niekro, W.; Kempe, T.; Henning, K.A.; et al. BMS-936564/MDX-1338: A fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin. Cancer Res. 2013, 19, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Peled, A. CXCR4 antagonists: Targeting the microenvironment in leukemia and other cancers. Leukemia 2009, 23, 43–52. [Google Scholar] [CrossRef]
- Weiss, I.D.; Jacobson, O. Molecular imaging of chemokine receptor CXCR4. Theranostics 2013, 3, 76–84. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F. Development of RGD-based radiotracers for tumor imaging and therapy: Translating from bench to bedside. Curr. Mol. Med. 2013, 13, 1487–1505. [Google Scholar] [CrossRef]
- Liu, N.; Wan, Q.; Cheng, Z.; Chen, Y. Radionuclide-Labeled Peptides for Imaging and Treatment of CXCR4-Overexpressing Malignant Tumors. Curr. Top. Med. Chem. 2019, 19, 17–32. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Zarebkohan, A.; Salehi, R.; Rahimi, F.; Torchilin, V.P.; Hamblin, M.R.; Seifalian, A. An update on dual targeting strategy for cancer treatment. J. Control. Release 2022, 349, 67–96. [Google Scholar] [CrossRef]
- Xu, L.; Josan, J.S.; Vagner, J.; Caplan, M.R.; Hruby, V.J.; Mash, E.A.; Lynch, R.M.; Morse, D.L.; Gillies, R.J. Heterobivalent ligands target cell-surface receptor combinations in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 21295–21300. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, X. Peptide heterodimers for molecular imaging. Amino Acids 2011, 41, 1081–1892. [Google Scholar] [CrossRef] [PubMed]
- Judmann, B.; Braun, D.; Wängler, B.; Schirrmacher, R.; Fricker, G.; Wängler, C. Current state of radiolabeled heterobivalent peptidic ligands in tumor imaging and therapy. Pharmaceuticals 2020, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Xiang, G.; Ma, X.; Hui, W.; Ouyang, Q.; Sun, L.; Ding, J.; Sheng, J.; Zeng, D. Universal Molecular Scaffold for Facile Construction of Multivalent and Multimodal Imaging Probes. Bioconjugate Chem. 2016, 27, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mao, F.; Niu, G.; Peng, L.; Lang, L.; Li, F.; Ying, H.; Wu, H.; Pan, B.; Zhu, Z.; et al. 68Ga-BBN-RGD PET/CT for GRPR and Integrin αvβ3 Imaging in Patients with Breast Cancer. Theranostics 2018, 8, 1121–1130. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, G.; Lang, L.; Li, F.; Fan, X.; Yan, X.; Yao, S.; Yan, W.; Huo, L.; Chen, L.; et al. Clinical Translation of a Dual Integrin αvβ3- and Gastrin-Releasing Peptide Receptor-Targeting PET Radiotracer, 68Ga-BBN-RGD. J. Nucl. Med. 2017, 58, 228–234. [Google Scholar] [CrossRef]
- Gai, Y.; Jiang, Y.; Long, Y.; Sun, L.; Liu, Q.; Qin, C.; Zhang, Y.; Zeng, D.; Lan, X. Evaluation of an Integrin αvβ3 and Aminopeptidase N Dual-Receptor Targeting Tracer for Breast Cancer Imaging. Mol. Pharm. 2020, 17, 349–358. [Google Scholar] [CrossRef]
- Lundmark, F.; Abouzayed, A.; Mitran, B.; Rinne, S.S.; Varasteh, Z.; Larhed, M.; Tolmachev, V.; Rosenström, U.; Orlova, A. Heterodimeric Radiotracer Targeting PSMA and GRPR for Imaging of Prostate Cancer-Optimization of the Affinity towards PSMA by Linker Modification in Murine Model. Pharmaceutics 2020, 12, 614. [Google Scholar] [CrossRef]
- Yang, J.; Guo, H.; Gallazzi, F.; Berwick, M.; Padilla, R.S.; Miao, Y. Evaluation of a novel Arg-Gly-Asp-conjugated α-melanocyte stimulating hormone hybrid peptide for potential melanoma therapy. Bioconjugate Chem. 2009, 20, 1634–1642. [Google Scholar] [CrossRef]
- Capello, A.; Krenning, E.P.; Bernard, B.F.; Breeman, W.A.; Erion, J.L.; de Jong, M. Anticancer activity of targeted proapoptotic peptides. J. Nucl. Med. 2006, 47, 122–129. [Google Scholar]
- Vall-Sagarra, A.; Litau, S.; Decristoforo, C.; Wängler, B.; Schirrmacher, R.; Fricker, G.; Wängler, C. Design, Synthesis, In Vitro, and Initial In Vivo Evaluation of Heterobivalent Peptidic Ligands Targeting Both NPY (Y1)-and GRP-Receptors—An Improvement for Breast Cancer Imaging? Pharmaceuticals 2018, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.; Fiedler, L.; Wängler, B.; Bartenstein, P.; Schirrmacher, R.; Wängler, C. Design, synthesis and in vitro evaluation of heterobivalent peptidic radioligands targeting both GRP-and VPAC1-Receptors concomitantly overexpressed on various malignancies–Is the concept feasible? Eur. J. Med. Chem. 2018, 155, 84–95. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Sun, L.; Lan, X.; Zeng, D.; Cai, W. Dual-Targeted Molecular Imaging of Cancer. J. Nucl. Med. 2018, 59, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gai, Y.; Li, Z.; Li, H.; Li, J.; Muschler, J.; Kang, R.; Tang, D.; Zeng, D. Heterodimeric RGD-NGR PET Tracer for the Early Detection of Pancreatic Cancer. Mol. Imaging Biol. 2022, 24, 580–589. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, H.; Jin, Y.; Wang, Z.; Huang, W.; Qiu, J.; Kang, F.; Wang, K.; Zhao, X.; Tian, J. A novel plectin/integrin-targeted bispecific molecular probe for magnetic resonance/near-infrared imaging of pancreatic cancer. Biomaterials 2018, 183, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Lan, X.; Liu, Q.; Jiang, Y.; Li, H. Molecular Tracer Targeting CXCR4 and Usage. CN Patent ZL 2020 1 0833363.9, 14 January 2022. [Google Scholar]
- Liu, Z.; Yan, Y.; Chin, F.T.; Wang, F.; Chen, X. Dual integrin and gastrin-releasing peptide receptor targeted tumor imaging using 18F-labeled PEGylated RGD-bombesin heterodimer 18F-FB-PEG3-Glu-RGD-BBN. J. Med. Chem. 2009, 52, 425–432. [Google Scholar] [CrossRef]
- Wu, H.; Huang, J. PEGylated Peptide-Based Imaging Agents for Targeted Molecular Imaging. Curr. Protein Pept. Sci. 2016, 17, 582–595. [Google Scholar] [CrossRef]
- Yan, X.; Niu, G.; Wang, Z.; Yang, X.; Kiesewetter, D.O.; Jacobson, O.; Shen, B.; Chen, X. Al[18F]NOTA-T140 Peptide for Noninvasive Visualization of CXCR4 Expression. Mol. Imaging Biol. 2016, 18, 135–142. [Google Scholar] [CrossRef][Green Version]
- Clézardin, P. Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res. 2011, 13, 207. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Long, Y.; Ji, H.; Qiao, P.; Liu, Q.; Xia, X.; Qin, C.; Zhang, Y.; Lan, X.; Gai, Y. Development and Evaluation of a Peptide Heterodimeric Tracer Targeting CXCR4 and Integrin αvβ3 for Pancreatic Cancer Imaging. Pharmaceutics 2022, 14, 1791. https://doi.org/10.3390/pharmaceutics14091791
Jiang Y, Long Y, Ji H, Qiao P, Liu Q, Xia X, Qin C, Zhang Y, Lan X, Gai Y. Development and Evaluation of a Peptide Heterodimeric Tracer Targeting CXCR4 and Integrin αvβ3 for Pancreatic Cancer Imaging. Pharmaceutics. 2022; 14(9):1791. https://doi.org/10.3390/pharmaceutics14091791
Chicago/Turabian StyleJiang, Yaqun, Yu Long, Hao Ji, Pengxin Qiao, Qingyao Liu, Xiaotian Xia, Chunxia Qin, Yongxue Zhang, Xiaoli Lan, and Yongkang Gai. 2022. "Development and Evaluation of a Peptide Heterodimeric Tracer Targeting CXCR4 and Integrin αvβ3 for Pancreatic Cancer Imaging" Pharmaceutics 14, no. 9: 1791. https://doi.org/10.3390/pharmaceutics14091791
APA StyleJiang, Y., Long, Y., Ji, H., Qiao, P., Liu, Q., Xia, X., Qin, C., Zhang, Y., Lan, X., & Gai, Y. (2022). Development and Evaluation of a Peptide Heterodimeric Tracer Targeting CXCR4 and Integrin αvβ3 for Pancreatic Cancer Imaging. Pharmaceutics, 14(9), 1791. https://doi.org/10.3390/pharmaceutics14091791





