Non-Invasive Physical Plasma for Preventing Radiation Dermatitis in Breast Cancer: A First-In-Human Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Radiation Protocol
2.3. Standard Skin Care
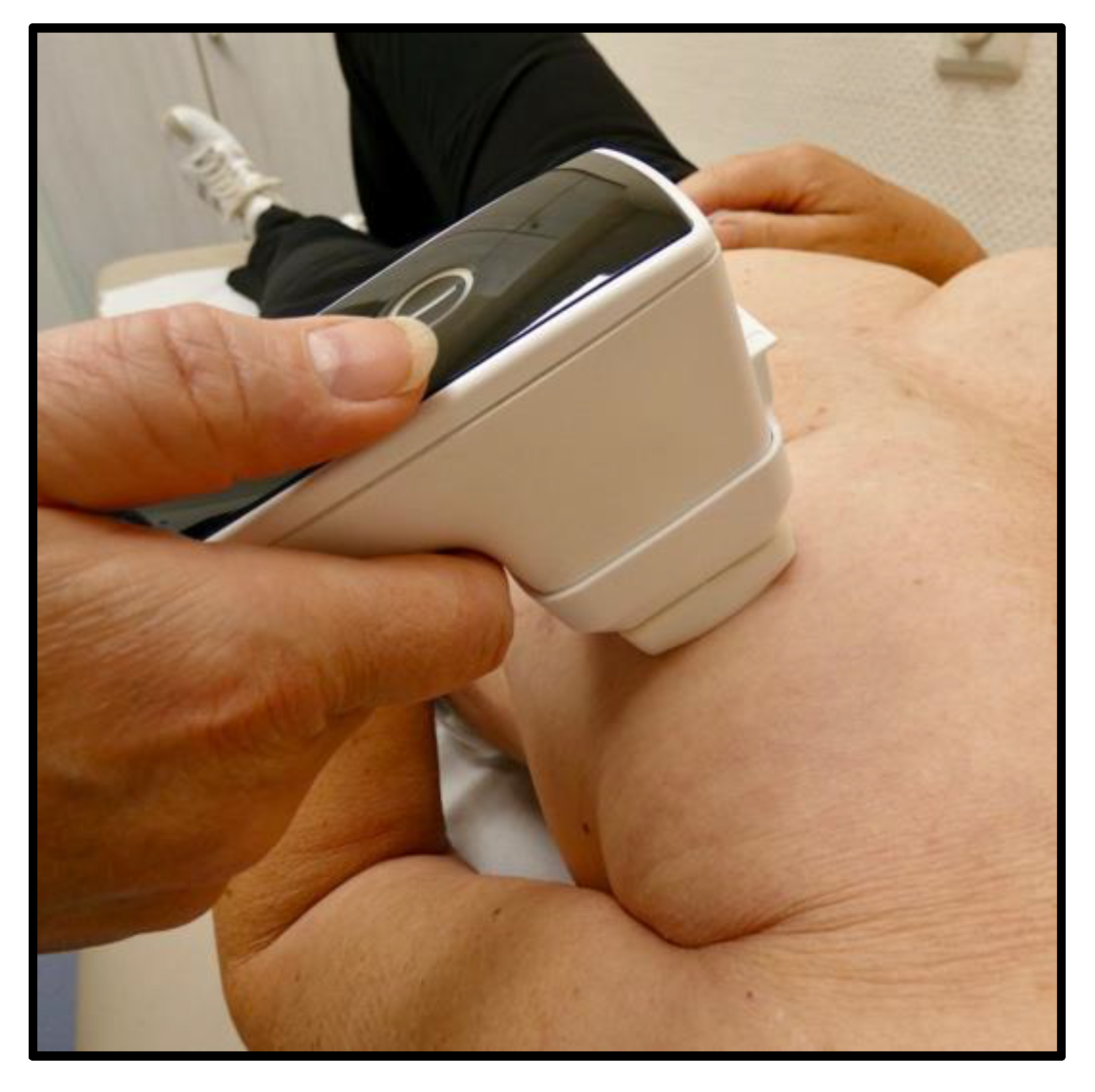
2.4. NIPP Protocol
2.5. Patient Evaluation
2.6. Feasibility
3. Results
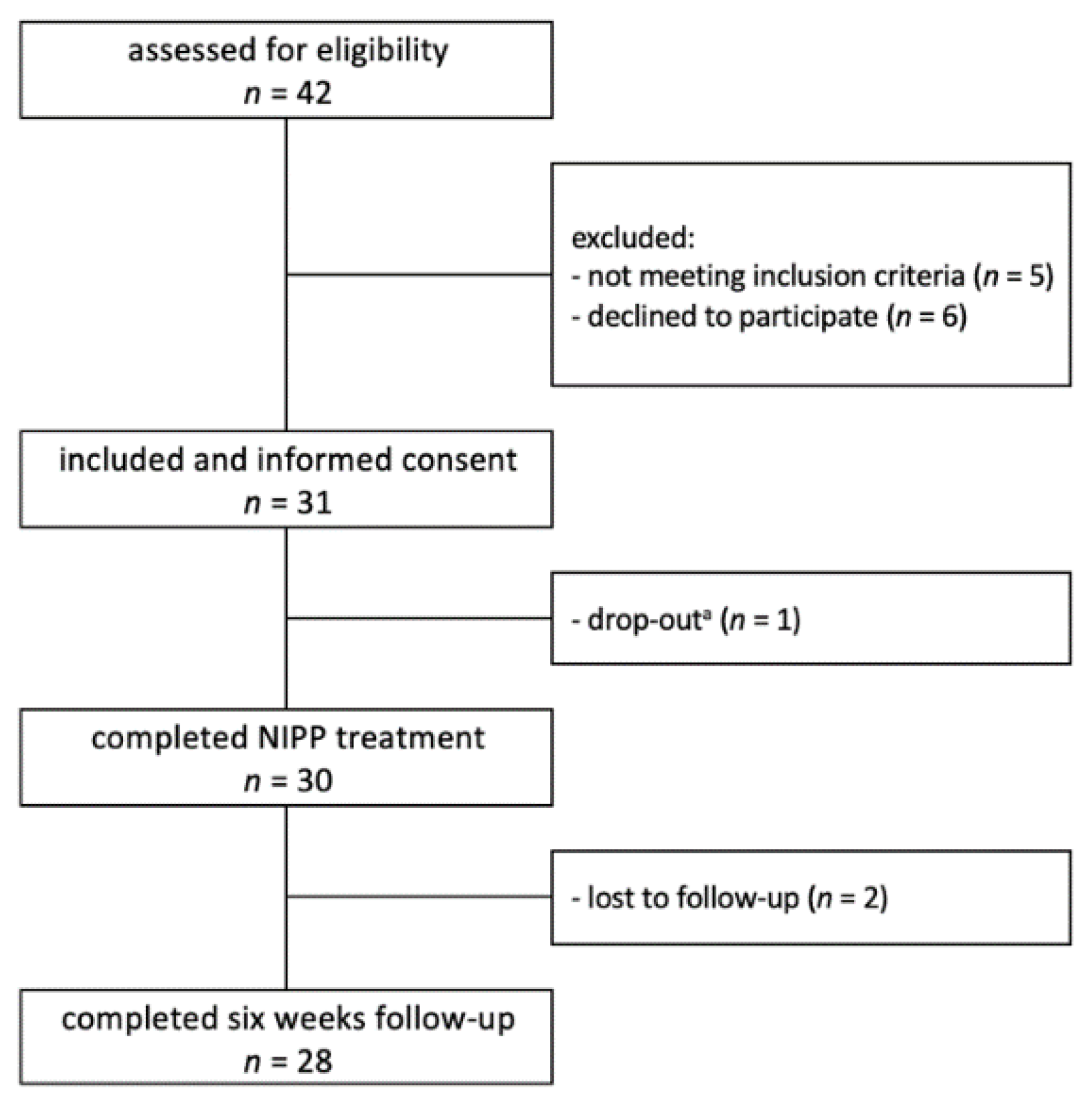
3.1. Patient Characteristics
3.2. NIPP Application Regimens
3.3. Severe Adverse Reactions
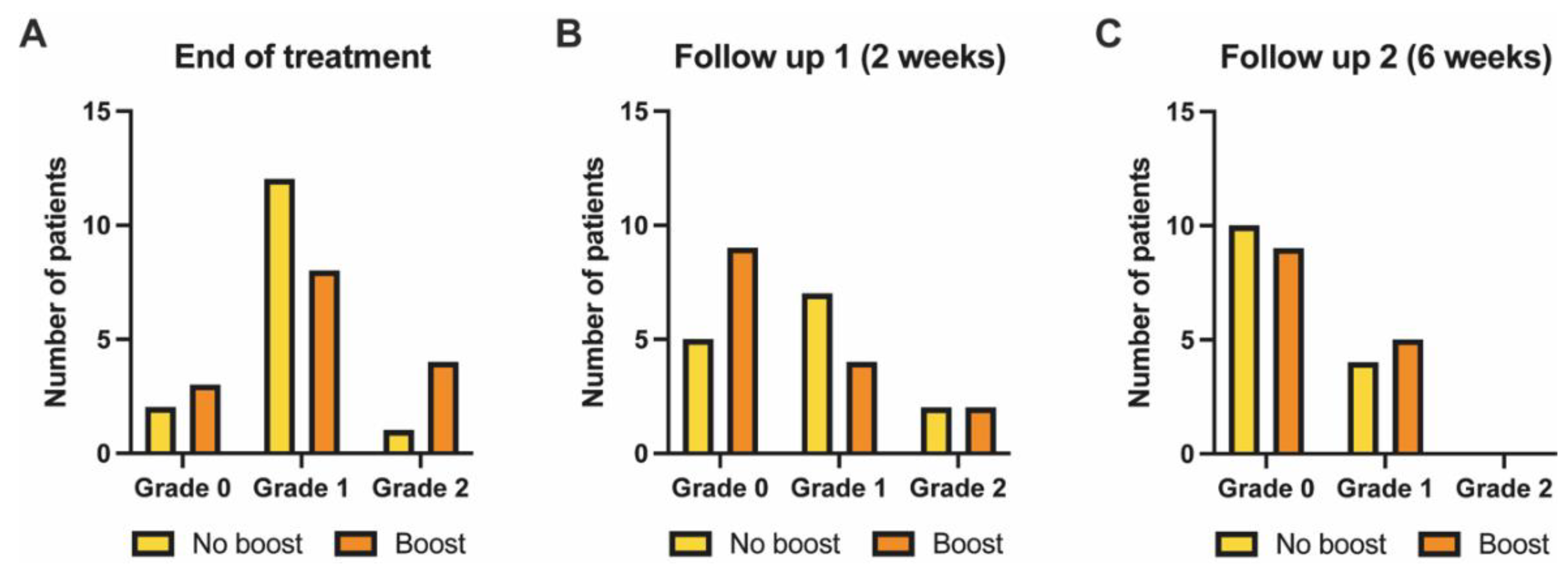
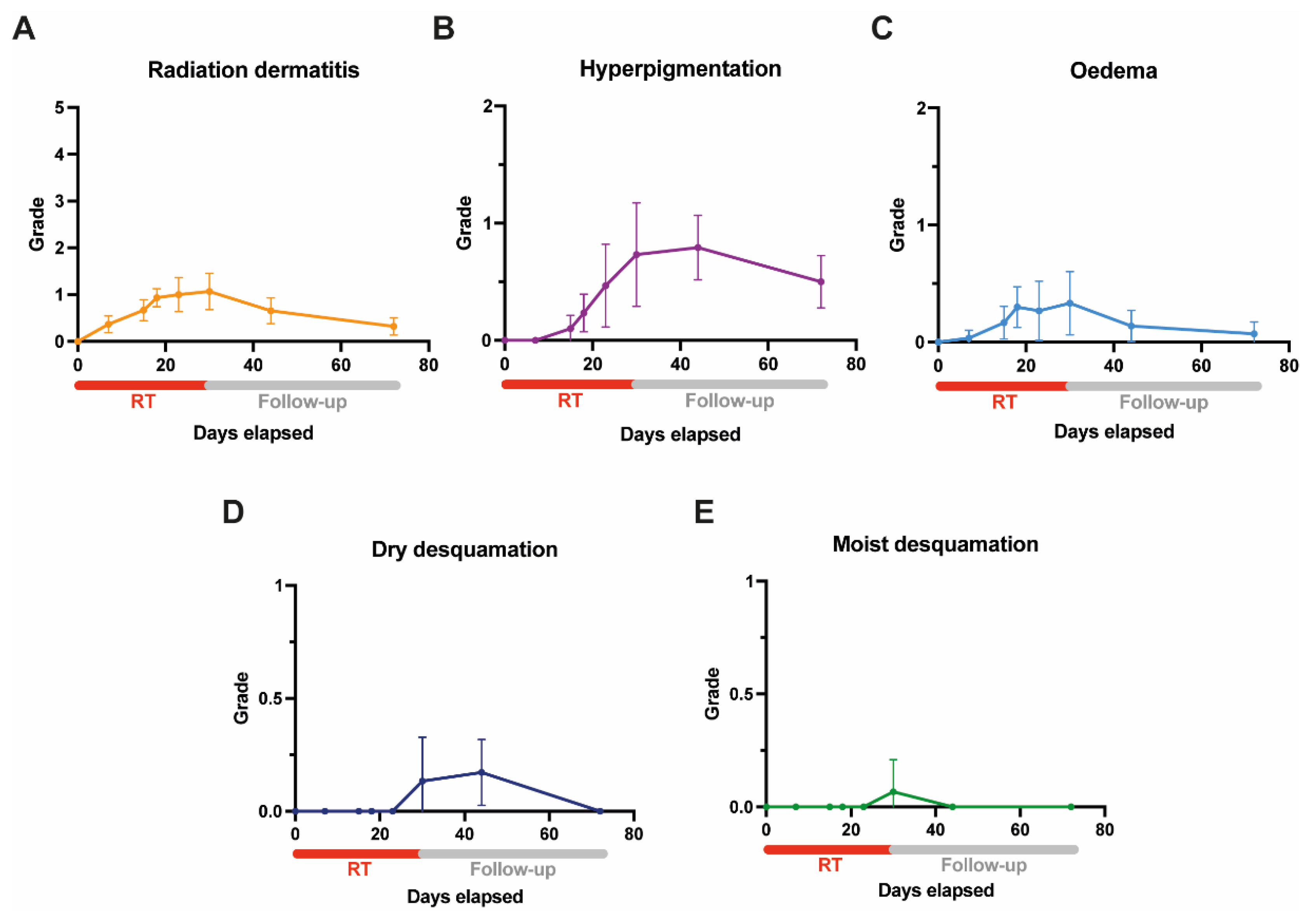
3.4. Acute Skin Toxicity as Assessed by Clinicians (Clinician-Reported Outcome)
3.5. Acute Skin Toxicity and Treatment Experience as Assessed by Patients (Patient-Reported Outcome)
3.6. Handling and Practicality
3.7. Cost Estimation
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Hennequin, C.; Barillot, I.; Azria, D.; Belkacémi, Y.; Bollet, M.; Chauvet, B.; Cowen, D.; Cutuli, B.; Fourquet, A.; Hannoun-Levi, J.M.; et al. Radiotherapy of breast cancer. Cancer Radiother. 2016, 20, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Shaitelman, S.F.; Schlembach, P.J.; Arzu, I.; Ballo, M.; Bloom, E.S.; Buchholz, D.; Chronowski, G.M.; Dvorak, T.; Grade, E.; Hoffman, K.E.; et al. Acute and short-term toxic effects of conventionally fractionated vs. hypofractionated whole-breast radiation: A randomized clinical trial. JAMA Oncol. 2015, 1, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Haruna, F.; Lipsett, A.; Marignol, L. Topical management of acute radiation dermatitis in breast cancer patients: A systematic review and meta-analysis. Anticancer Res. 2017, 37, 5343–5353. [Google Scholar] [CrossRef]
- Chan, R.J.; Larsen, E.; Chan, P. Re-examining the evidence in radiation dermatitis management literature: An overview and a critical appraisal of systematic reviews. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e357–e362. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, L.C.; Koch, D.; Schmeel, F.C.; Röhner, F.; Schoroth, F.; Bücheler, B.M.; Mahlmann, B.; Leitzen, C.; Schüller, H.; Tschirner, S.; et al. Acute radiation-induced skin toxicity in hypofractionated vs. conventional whole-breast irradiation: An objective, randomized multicenter assessment using spectrophotometry. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 146, 172–179. [Google Scholar] [CrossRef]
- Finkelstein, S.; Kanee, L.; Behroozian, T.; Wolf, J.R.; van den Hurk, C.; Chow, E.; Bonomo, P. Comparison of clinical practice guidelines on radiation dermatitis: A narrative review. Support. Care Cancer 2022, 30, 4663–4674. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.; Poorun, D.; Liu, D.; Lu, X.; He, M.; Duan, X.; Chen, H. Medical applications of nonthermal atmospheric pressure plasma in dermatology. JDDG J. Dtsch. Dermatol. Ges. 2018, 16, 7–13. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.-C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of cold atmospheric plasma therapy vs. standard therapy placebo on wound healing in patients with diabetic foot ulcers: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Kletschkus, K.; Haralambiev, L.; Mustea, A.; Bekeschus, S.; Stope, M.B. Review of innovative physical therapy methods: Introduction to the principles of cold physical plasma. In Vivo 2020, 34, 3103–3107. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2018, 27, 79501. [Google Scholar] [CrossRef]
- Gelbrich, N.; Stope, M.B.; Burchardt, M. Cold atmospheric plasma for the treatment of urological tumors. Urologe A 2019, 58, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Boekema, B.; Stoop, M.; Vlig, M.; van Liempt, J.; Sobota, A.; Ulrich, M.; Middelkoop, E. Antibacterial and safety tests of a flexible cold atmospheric plasma device for the stimulation of wound healing. Appl. Microbiol. Biotechnol. 2021, 105, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Assadian, O.; Ousey, K.J.; Daeschlein, G.; Kramer, A.; Parker, C.; Tanner, J.; Leaper, D.J. Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds and wound size reduction: A systematic review and meta-analysis. Int. Wound J. 2019, 16, 103–111. [Google Scholar] [CrossRef]
- Kluge, S.; Bekeschus, S.; Bender, C.; Benkhai, H.; Sckell, A.; Below, H.; Stope, M.B.; Kramer, A. Investigating the Mutagenicity of a Cold Argon-Plasma Jet in an HET-MN Model. PLoS ONE 2016, 11, e0160667. [Google Scholar] [CrossRef]
- Eggers, B.; Stope, M.B.; Marciniak, J.; Götz, W.; Mustea, A.; Deschner, J.; Nokhbehsaim, M.; Kramer, F.-J. Non-Invasive Physical Plasma Generated by a Medical Argon Plasma Device Induces the Expression of Regenerative Factors in Human Gingival Keratinocytes, Fibroblasts, and Tissue Biopsies. Biomedicines 2022, 10, 889. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, T.; Manda, K.; Hildebrandt, G.; Stachs, O.; Bekeschus, S.; Vollmar, B.; Emmert, S.B.L. Kaltes Atmosphärendruckplasma zur Behandlung einer Radiodermatitis und Untersuchung objektiver Verlaufsparameter im Mausmodell; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Offersen, B.V.; Boersma, L.J.; Kirkove, C.; Hol, S.; Aznar, M.C.; Sola, A.B.; Kirova, Y.M.; Pignol, J.-P.; Remouchamps, V.; Verhoeven, K.; et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother. Oncol. 2015, 114, 3–10. [Google Scholar] [CrossRef]
- Sakiyama, Y.; Graves, D.B.; Chang, H.-W.; Shimizu, T.; Morfill, G.E. Plasma chemistry model of surface microdischarge in humid air and dynamics of reactive neutral species. J. Phys. D Appl. Phys. 2012, 45, 425201. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 n.d. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 1 October 2021).
- Noble-Adams, R. Radiation-induced skin reactions 3: Evaluating the RISRAS. Br. J. Nurs. 1999, 8, 1305–1312. [Google Scholar] [CrossRef]
- Yee, C.; Wang, K.; Asthana, R.; Drost, L.; Lam, H.; Lee, J.; Vesprini, D.; Leung, E.; DeAngelis, C.; Chow, E. Radiation-induced Skin Toxicity in Breast Cancer Patients: A Systematic Review of Randomized Trials. Clin. Breast Cancer 2018, 18, e825–e840. [Google Scholar] [CrossRef]
- Behroozian, T.; Milton, L.; Zhang, L.; Lou, J.; Karam, I.; Lam, E.; Wong, G.; Szumacher, E.; Chow, E. How do patient-reported outcomes compare with clinician assessments? A prospective study of radiation dermatitis in breast cancer. Radiother. Oncol. 2021, 159, 98–105. [Google Scholar] [CrossRef]
- Behroozian, T.; Milton, L.T.; Shear, N.H.; McKenzie, E.; Razvi, Y.; Karam, I.; Pon, K.; Lam, H.; Lam, E.; Chow, E. Radiation dermatitis assessment tools used in breast cancer: A systematic review of measurement properties. Support. Care Cancer 2021, 29, 2265–2278. [Google Scholar] [CrossRef] [PubMed]
- Jagsi, R.; Griffith, K.A.; Vicini, F.; Boike, T.; Dominello, M.; Gustafson, G.; Hayman, J.A.; Moran, J.M.; Radawski, J.D.; Walker, E.; et al. Identifying patients whose symptoms are under-recognized during treatment with breast radiotherapy. JAMA Oncol. 2022, 8, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A. Free Radicals in Biology: Oxidative Stress and the Effects of Ionizing Radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Jablonowski, L.; Kocher, T.; Schindler, A.; Müller, K.; Dombrowski, F.; Von Woedtke, T.; Arnold, T.; Lehmann, A.; Rupf, S.; Evert, M.; et al. Side effects by oral application of atmospheric pressure plasma on the mucosa in mice. PLoS ONE 2019, 14, e0215099. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Kramer, A.; Metelmann, H.-R.; Adler, F.; von Woedtke, T.; Niessner, F.; Weltmann, K.-D.; Wende, K. High throughput image cytometry micronucleus assay to investigate the presence or absence of mutagenic effects of cold physical plasma. Environ. Mol. Mutagen. 2018, 59, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Woedtke, T.; von Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. Int. J. Mol. Sci. 2017, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Jagsi, R.; Griffith, K.A.; Boike, T.P.; Walker, E.M.; Nurushev, T.; Grills, I.S.; Moran, J.M.; Feng, M.; Hayman, J.; Pierce, L.J. Differences in the Acute Toxic Effects of Breast Radiotherapy by Fractionation Schedule: Comparative analysis of physician-assessed and patient-reported outcomes in a large multicenter cohort. JAMA Oncol. 2015, 1, 918–930. [Google Scholar] [CrossRef]
- Böhner, A.M.C.; Koch, D.; Schmeel, F.C.; Röhner, F.; Schoroth, F.; Sarria, G.R.; Abramian, A.-V.; Baumert, B.G.; Giordano, F.A.; Schmeel, L.C. Objective Evaluation of Risk Factors for Radiation Dermatitis in Whole-Breast Irradiation Using the Spectrophotometric L*a*b Color-Space. Cancers 2020, 12, 2444. [Google Scholar] [CrossRef]
- Schmeel, L.; Koch, D.; Schmeel, F.; Bücheler, B.; Leitzen, C.; Mahlmann, B.; Kunze, D.; Heimann, M.; Brüser, D.; Abramian, A.-V.; et al. Hydrofilm Polyurethane Films Reduce Radiation Dermatitis Severity in Hypofractionated Whole-Breast Irradiation: An Objective, Intra-Patient Randomized Dual-Center Assessment. Polymers 2019, 11, 2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robijns, J.; Censabella, S.; Claes, S.; Pannekoeke, L.; Bussé, L.; Colson, D.; Kaminski, I.; Lodewijckx, J.; Bulens, P.; Maes, A.; et al. Biophysical skin measurements to evaluate the effectiveness of photobiomodulation therapy in the prevention of acute radiation dermatitis in breast cancer patients. Support. Care Cancer 2019, 27, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Kost, Y.; Mieczkowska, K.; Deutsch, A.; Nazarian, R.; Muskat, A.; Hosgood, D.; Lin, J.; Shinoda, K.; Daily, J.; Kabarriti, R.; et al. Bacterial decolonization to prevent acute radiation dermatitis: A randomized controlled trial. J. Clin. Oncol. 2022, 40, LBA12003. [Google Scholar] [CrossRef]
- Hill, A.; Hanson, M.; Bogle, M.A.; Duvic, M. Severe Radiation Dermatitis is Related to Staphylococcus Aureus. Am. J. Clin. Oncol. 2004, 27, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Robijns, J.; Laubach, H.-J. Acute and chronic radiodermatitis: Clinical signs, pathophysiology, risk factors and management options. J. Egypt Women’s Dermatol. Soc. 2018, 15, 2–9. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Potential applications of nonthermal plasmas against biofilm-associated micro-organisms in vitro. J. Appl. Microbiol. 2017, 122, 1134–1148. [Google Scholar] [CrossRef]
- Daeschlein, G.; Napp, M.; Lutze, S.; Arnold, A.; von Podewils, S.; Guembel, D.; Jünger, M. Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. JDDG J. Dtsch. Dermatol. Ges. 2015, 13, 143–150. [Google Scholar] [CrossRef]
- Haertel, B.; von Woedtke, T.; Weltmann, K.-D.; Lindequist, U. Non-Thermal Atmospheric-Pressure Plasma Possible Application in Wound Healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef]
- Kisch, T.; Schleusser, S.; Helmke, A.; Mauss, K.L.; Wenzel, E.T.; Hasemann, B.; Mailaender, P.; Kraemer, R. The repetitive use of non-thermal dielectric barrier discharge plasma boosts cutaneous microcirculatory effects. Microvasc. Res. 2016, 106, 8–13. [Google Scholar] [CrossRef]
- Duchesne, C.; Banzet, S.; Lataillade, J.; Rousseau, A.; Frescaline, N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J. Pathol. 2019, 249, 368–380. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.-F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold Atmospheric Plasma (CAP) Changes Gene Expression of Key Molecules of the Wound Healing Machinery and Improves Wound Healing In Vitro and In Vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef] [Green Version]
- Marschewski, M.; Hirschberg, J.; Omairi, T.; Höfft, O.; Viöl, W.; Emmert, S.; Maus-Friedrichs, W. Electron spectroscopic analysis of the human lipid skin barrier: Cold atmospheric plasma-induced changes in lipid composition. Exp. Dermatol. 2012, 21, 921–925. [Google Scholar] [CrossRef] [PubMed]
| 1. I found the NIPP treatment to be unpleasant. |
| 2. I would recommend NIPP treatment to a friend undergoing irradiation for breast cancer. |
| 3. I would also have wanted to be treated with NIPP to potentially prevent and/or treat RD outside of this study. |
| 4. I have the impression that my symptoms have been reduced by the NIPP treatment. |
| Median Age (Range) in Years | 56 (30–83) |
|---|---|
| Sex | n (%) |
| female | 29 (96.7) |
| male | 1 (3.3) |
| Ethnicity | n (%) |
| Caucasian | 27 (90.0) |
| other | 3 (10.0) |
| Fitzpatrick skin type | n (%) |
| I | 4 (13.3) |
| II | 23 (76.7) |
| III | 3 (10.0) |
| Side | n (%) |
| left | 16 (53.3) |
| right | 14 (46.7) |
| pT-stage | n (%) |
| Tis | 6 (20) |
| T1 | 19 (63.3) |
| T2 | 4 (13.3) |
| T3 | 1 (3.3) |
| pN-stage | n (%) |
| N0 | 25 (83.3) |
| N1 | 5 (16.7) |
| Boost | 15 (50.0) |
| Median PTV Breast (mL) | 798 (129–1771) |
| Median PTV Boost (mL) | 126 (52–307) |
| n (%) | Not at All | a Little | Quite a Bit | Very Much |
|---|---|---|---|---|
| Pain | 16 (53.3) | 12 (40) | 2 (6.6) | 0 (0) |
| Itching | 19 (63.3) | 10 (33.3) | 1 (3.3) | 0 (0) |
| Burning | 25 (83.3) | 5 (16.7) | 0 (0) | 0 (0) |
| Limitations in ADL | 23 (76.7) | 5 (16.7) | 2 (6.6) | 0 (0) |
| NIPP Treatment Frequency Per Week | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| NIPP treatment duration a | 60 s | spacers/w | 1–2 | 2–4 | 3–6 | 4–8 | 5–10 |
| cost/w | EUR 14–28 | EUR 28–56 | EUR 42–84 | EUR 56–112 | EUR 70–140 | ||
| time/w | 10–20 min | 20–40 min | 30–60 min | 40–80 min | 50–100 min | ||
| 120 s | spacers/w | 2–3 | 4–6 | 6–9 | 8–12 | 10–15 | |
| cost/w | EUR 28–42 | EUR 56–84 | EUR 84–126 | EUR 112–168 | EUR 140–210 | ||
| time/w | 20–30 min | 40–60 min | 1–1.5 h | 80–120 min | 100–150 min | ||
| 180 s | spacers/w | 3–4 | 6–8 | 9–12 | 12–16 | 15–20 | |
| cost/w | EUR 42–56 | EUR 84–112 | EUR 126–168 | EUR 168–224 | EUR 210–280 | ||
| time/w | 30–40 min | 60–80 min | 1.5–2 h | 120–160 min | 150–200 min | ||
| RD Grade b | Desquamation | PRO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | n | 0 | 1 | ≥2 | dry | moist | pain | itching | burning | ADL limitations |
| Current study (2022) | 30 | 17% | 67% | 17% | 7% | 3% | 0.53 ± 0.63 c (47%) | 0.40 ± 0.56 c (36%) | 0.17 ± 0.38 c (17%) | 0.30 ± 0.60 c (23%) |
| Schmeel et al. (2020) [6] | 70 a | 21% | 51% | 27% | 34% | 9% | 0.62 ± 0.66 c | 0.89 ± 0.93 c | 0.63 ± 0.68 c | 0.24 ± 0.66 c |
| Shaitelman et al. (2015) [3] | 138 a | 6% | 58% | 36% | N/A | N/A | 55% | 54% | N/A | N/A |
| Jagsi et al. (2015) [30] | 578 a | 6% | 67% | 27% | 19% | 7% | 72% | 37% | 16% | 7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dejonckheere, C.S.; Torres-Crigna, A.; Layer, J.P.; Layer, K.; Wiegreffe, S.; Sarria, G.R.; Scafa, D.; Koch, D.; Leitzen, C.; Köksal, M.A.; et al. Non-Invasive Physical Plasma for Preventing Radiation Dermatitis in Breast Cancer: A First-In-Human Feasibility Study. Pharmaceutics 2022, 14, 1767. https://doi.org/10.3390/pharmaceutics14091767
Dejonckheere CS, Torres-Crigna A, Layer JP, Layer K, Wiegreffe S, Sarria GR, Scafa D, Koch D, Leitzen C, Köksal MA, et al. Non-Invasive Physical Plasma for Preventing Radiation Dermatitis in Breast Cancer: A First-In-Human Feasibility Study. Pharmaceutics. 2022; 14(9):1767. https://doi.org/10.3390/pharmaceutics14091767
Chicago/Turabian StyleDejonckheere, Cas Stefaan, Adriana Torres-Crigna, Julian Philipp Layer, Katharina Layer, Shari Wiegreffe, Gustavo Renato Sarria, Davide Scafa, David Koch, Christina Leitzen, Mümtaz Ali Köksal, and et al. 2022. "Non-Invasive Physical Plasma for Preventing Radiation Dermatitis in Breast Cancer: A First-In-Human Feasibility Study" Pharmaceutics 14, no. 9: 1767. https://doi.org/10.3390/pharmaceutics14091767
APA StyleDejonckheere, C. S., Torres-Crigna, A., Layer, J. P., Layer, K., Wiegreffe, S., Sarria, G. R., Scafa, D., Koch, D., Leitzen, C., Köksal, M. A., Müdder, T., Abramian, A., Kaiser, C., Faridi, A., Stope, M. B., Mustea, A., Giordano, F. A., & Schmeel, L. C. (2022). Non-Invasive Physical Plasma for Preventing Radiation Dermatitis in Breast Cancer: A First-In-Human Feasibility Study. Pharmaceutics, 14(9), 1767. https://doi.org/10.3390/pharmaceutics14091767







