Alginate/Chitosan-Based Hydrogel Film Containing α-Mangostin for Recurrent Aphthous Stomatitis Therapy in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Alg/Chi HF, α-M Alg/Chi HF, and α-M/EtOH Alg/Chi HF
2.2.2. Determination of Thickness and Weight
2.2.3. pH Measurement
2.2.4. Swelling Ratio
2.2.5. In Vitro Mucoadhesive Time
2.2.6. X-ray Diffraction (XRD) Analysis
2.2.7. Scanning Electron Microscopy (SEM) Analysis
2.2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.9. In Vitro Drug Release
- Q = Drug Release against Time (%)
- K = Value of Kinetic Constant
2.2.10. In Vitro Cell Viability
2.2.11. In Vivo Ulceration Study
2.2.12. Histopathological Analysis
2.2.13. Data Analysis
3. Results
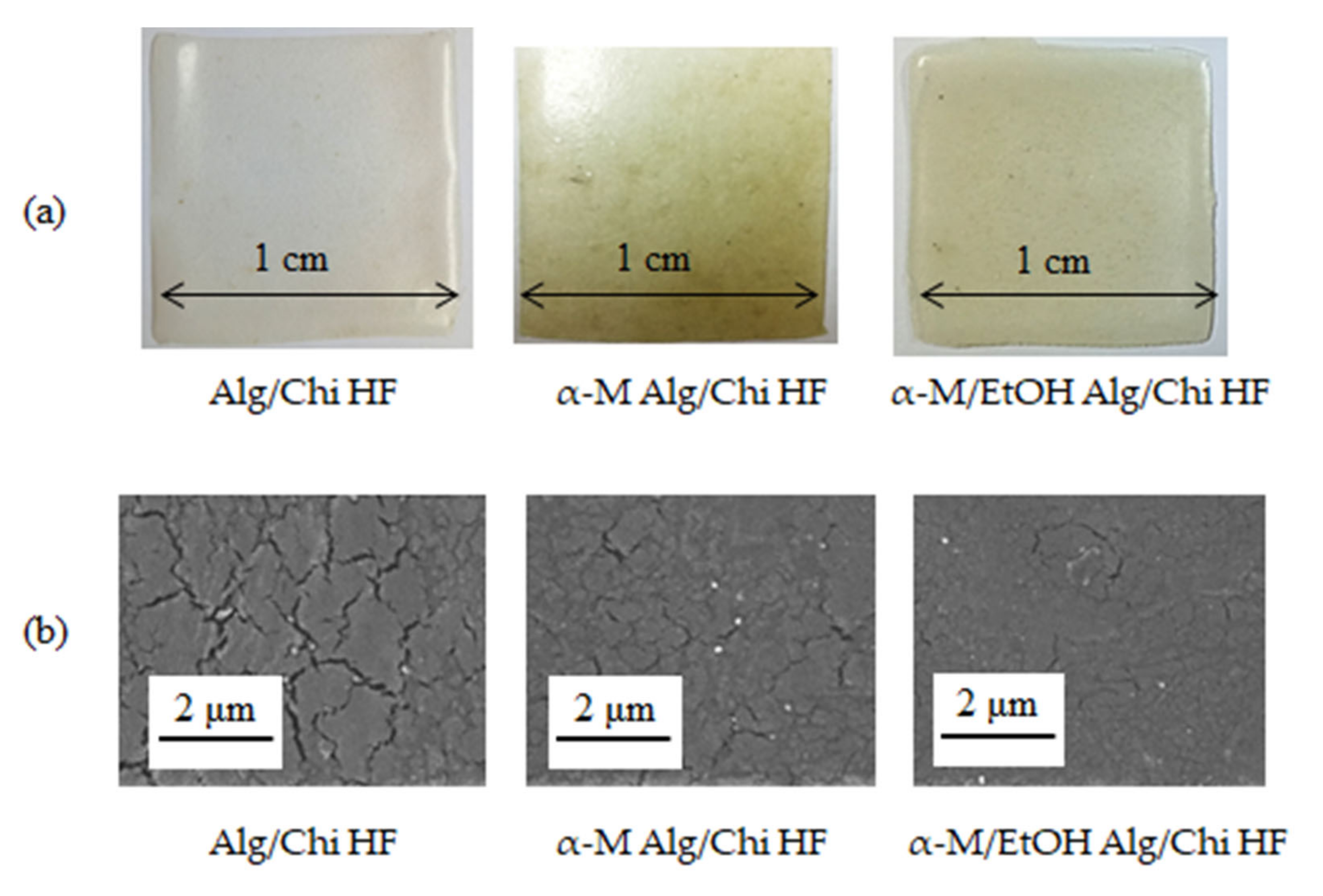
3.1. Preparation of Alg/Chi HF, α-M Alg/Chi HF, and α-M/EtOH Alg/Chi HF
3.2. Physicochemical Properties of Alg/Chi HF, α-M Alg/Chi HF, and α-M/EtOH Alg/Chi HF
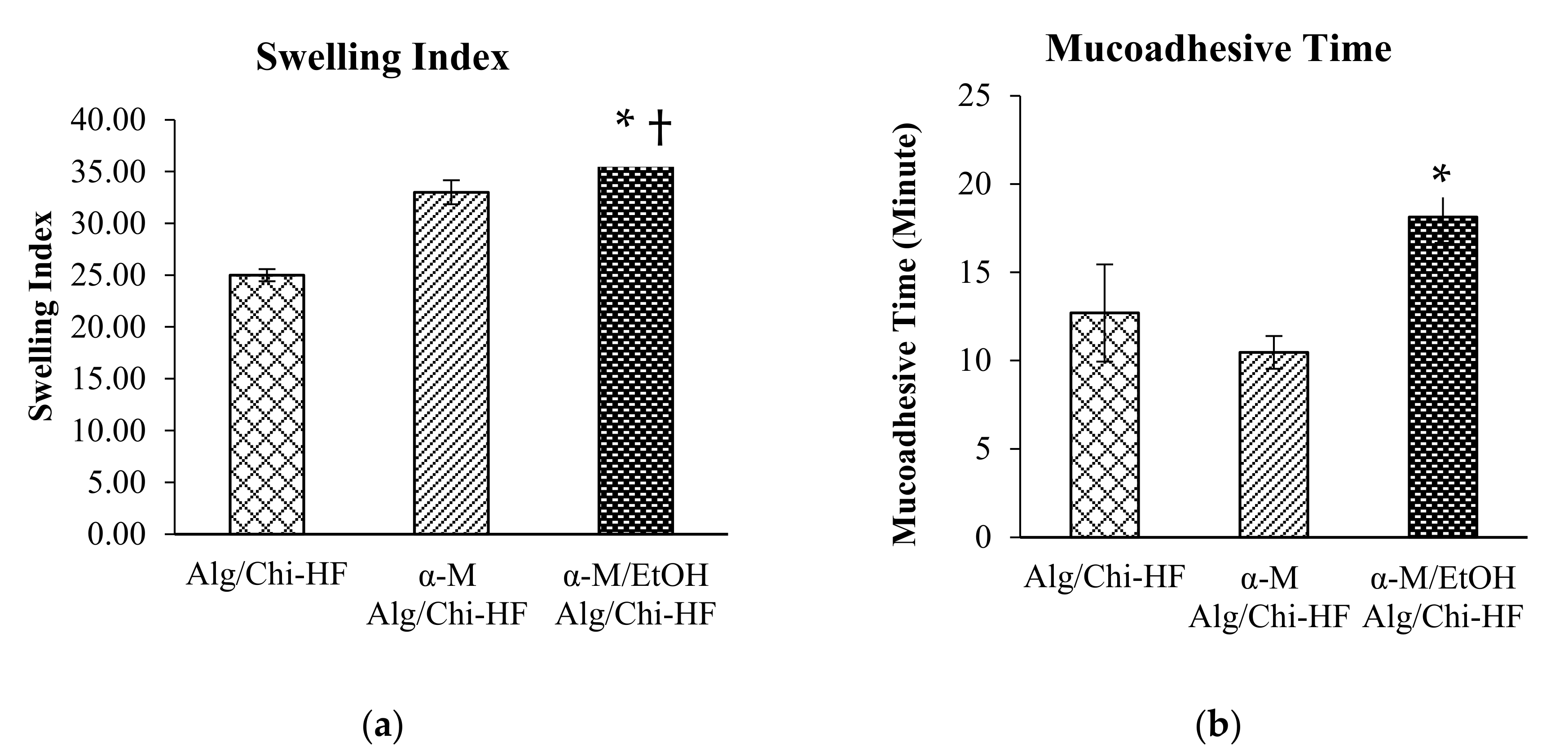
3.3. Swelling Ratio and Mucoadhesive Time
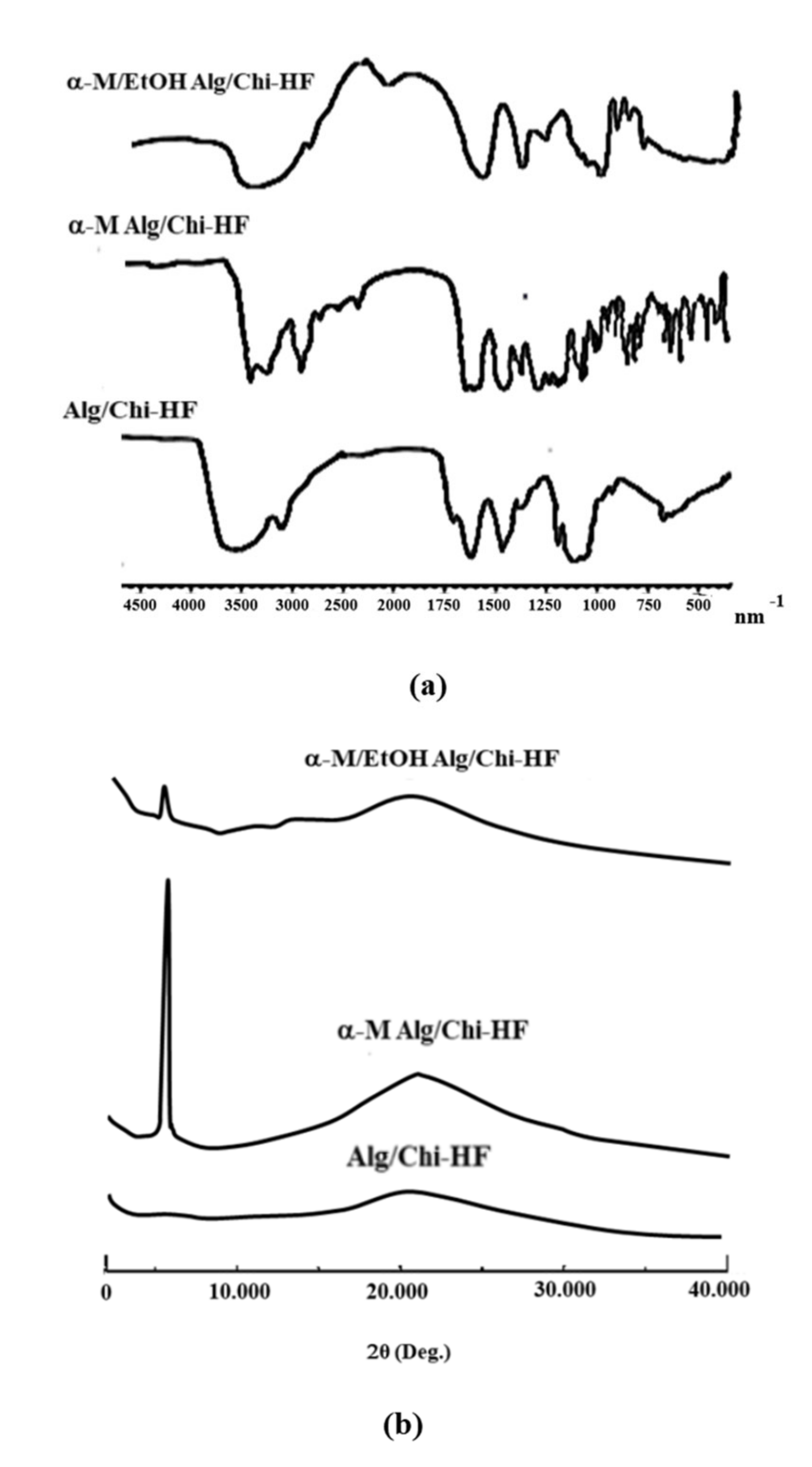
3.4. Characterization of Alg/Chi HF, α-M Alg/Chi HF, and α-M/EtOH Alg/Chi HF
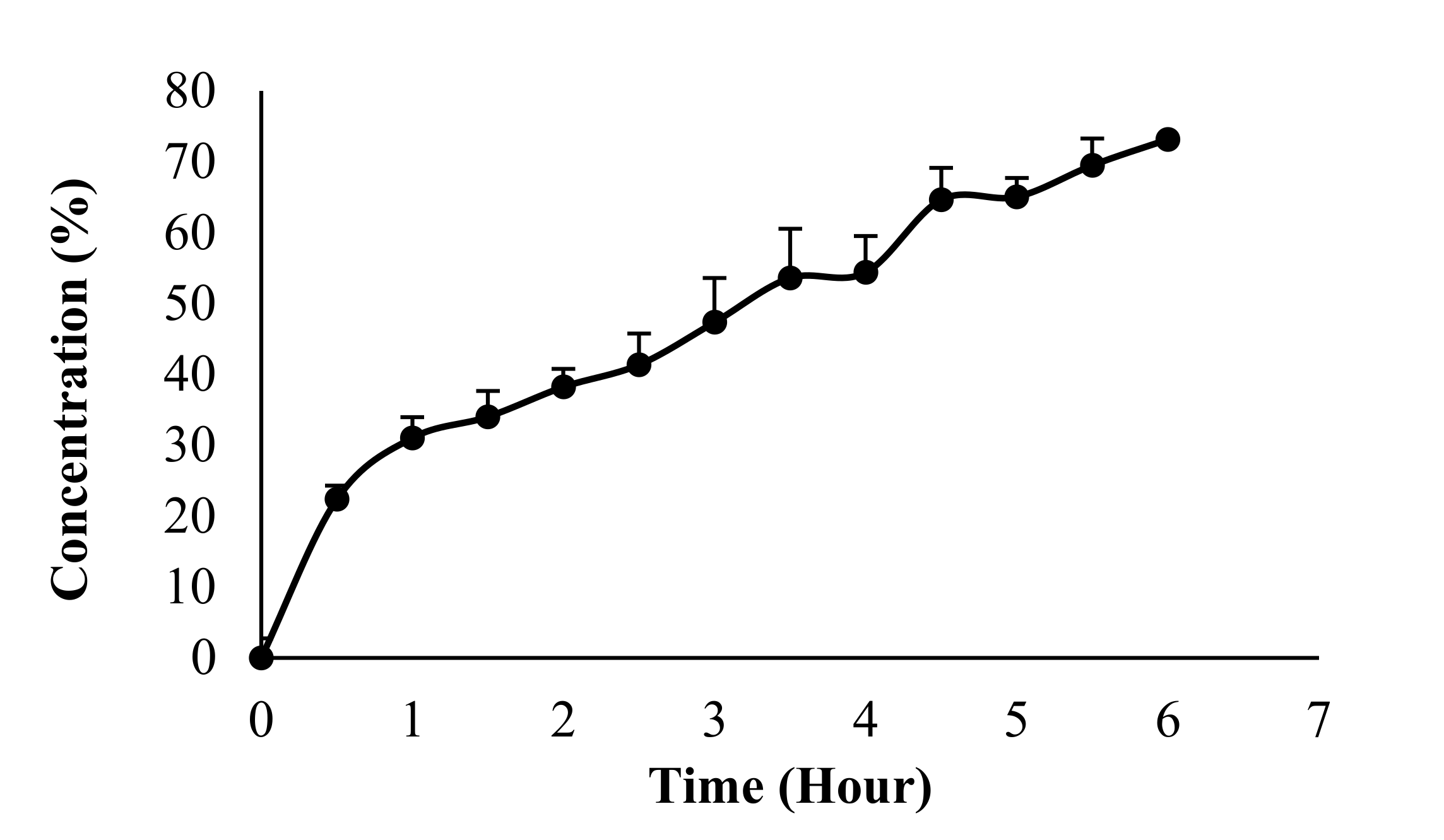
3.5. In Vitro Drug Release
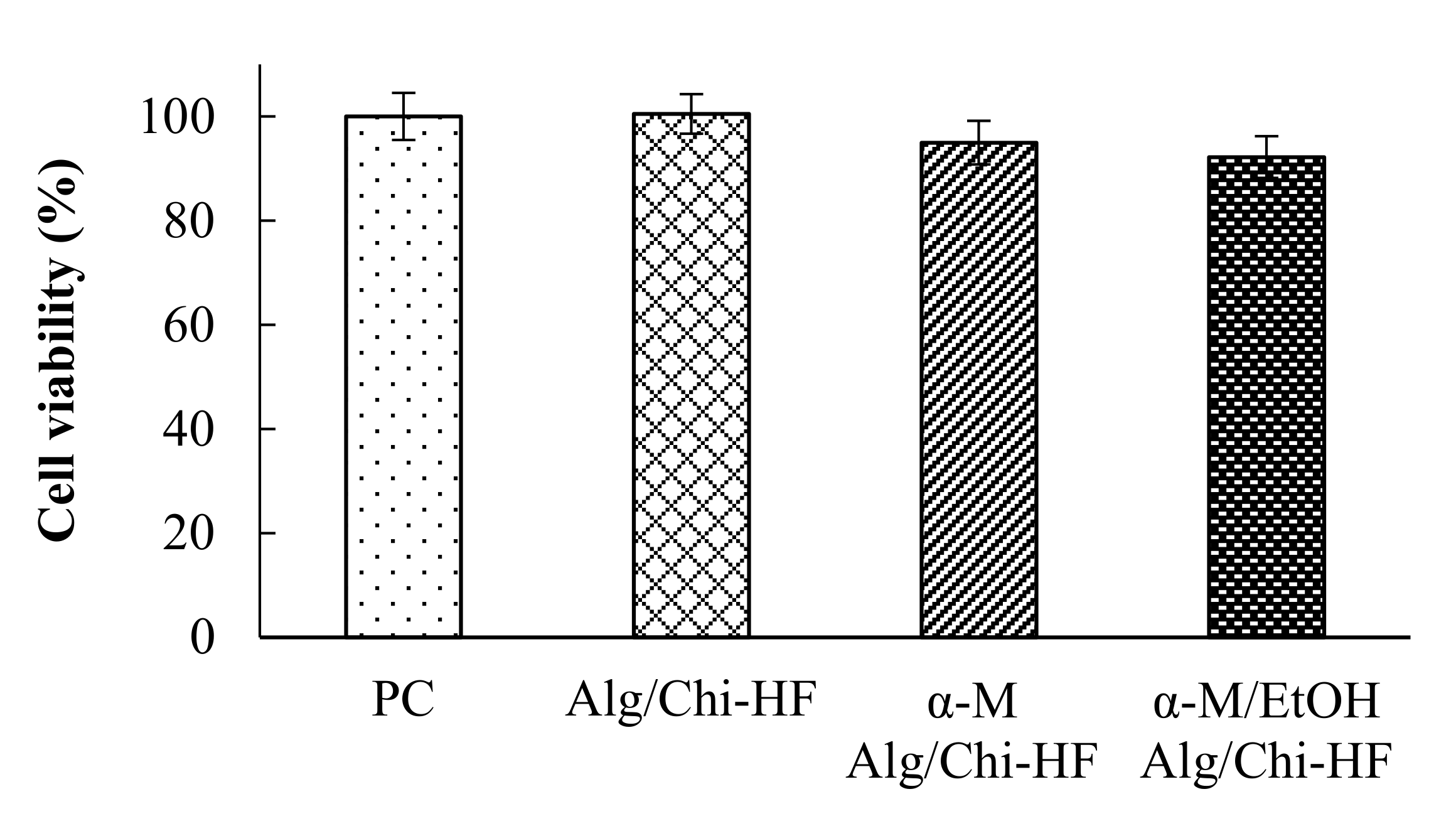
3.6. In Vitro Viability Study
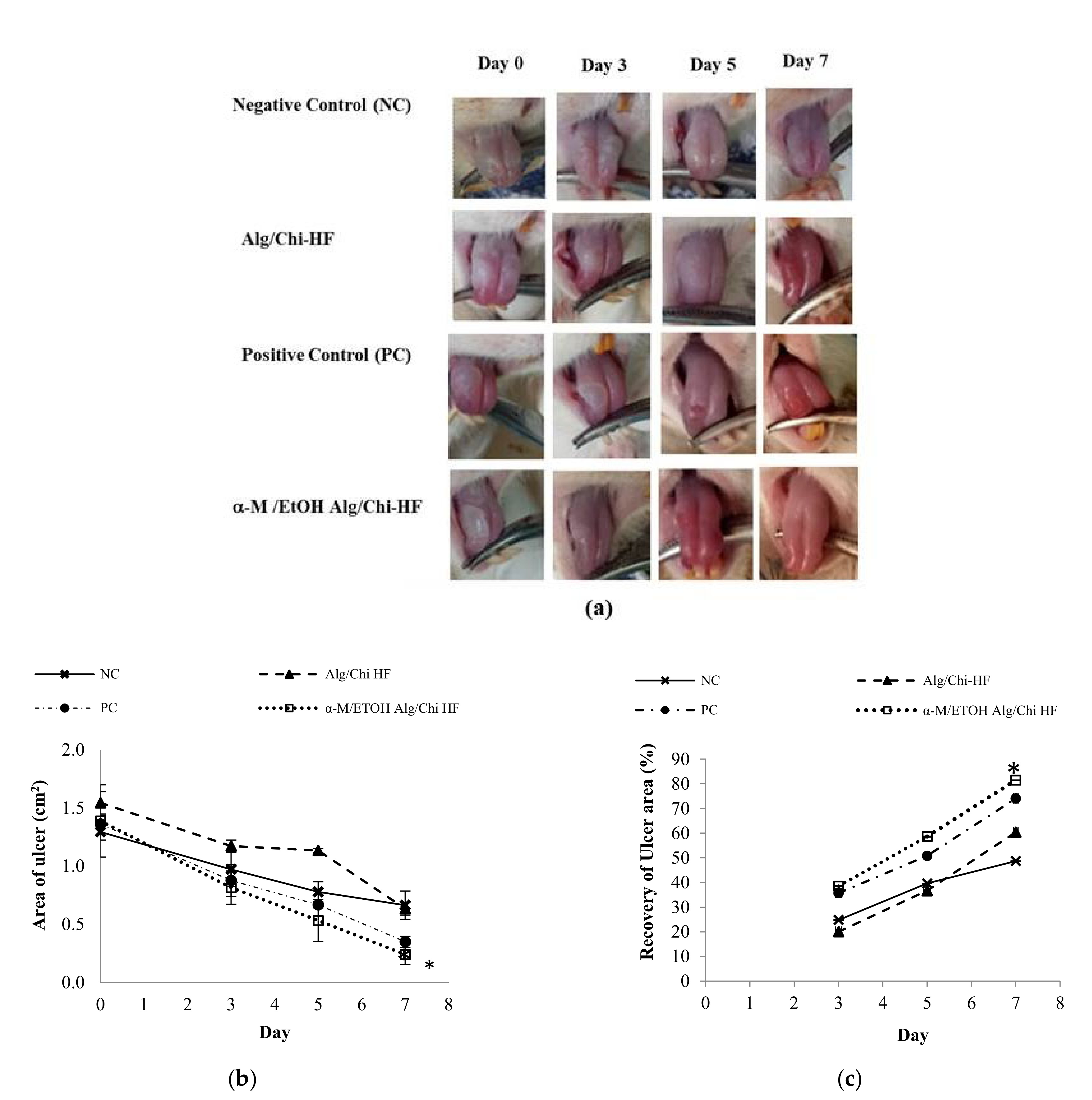
3.7. In Vivo Wound Healing Study
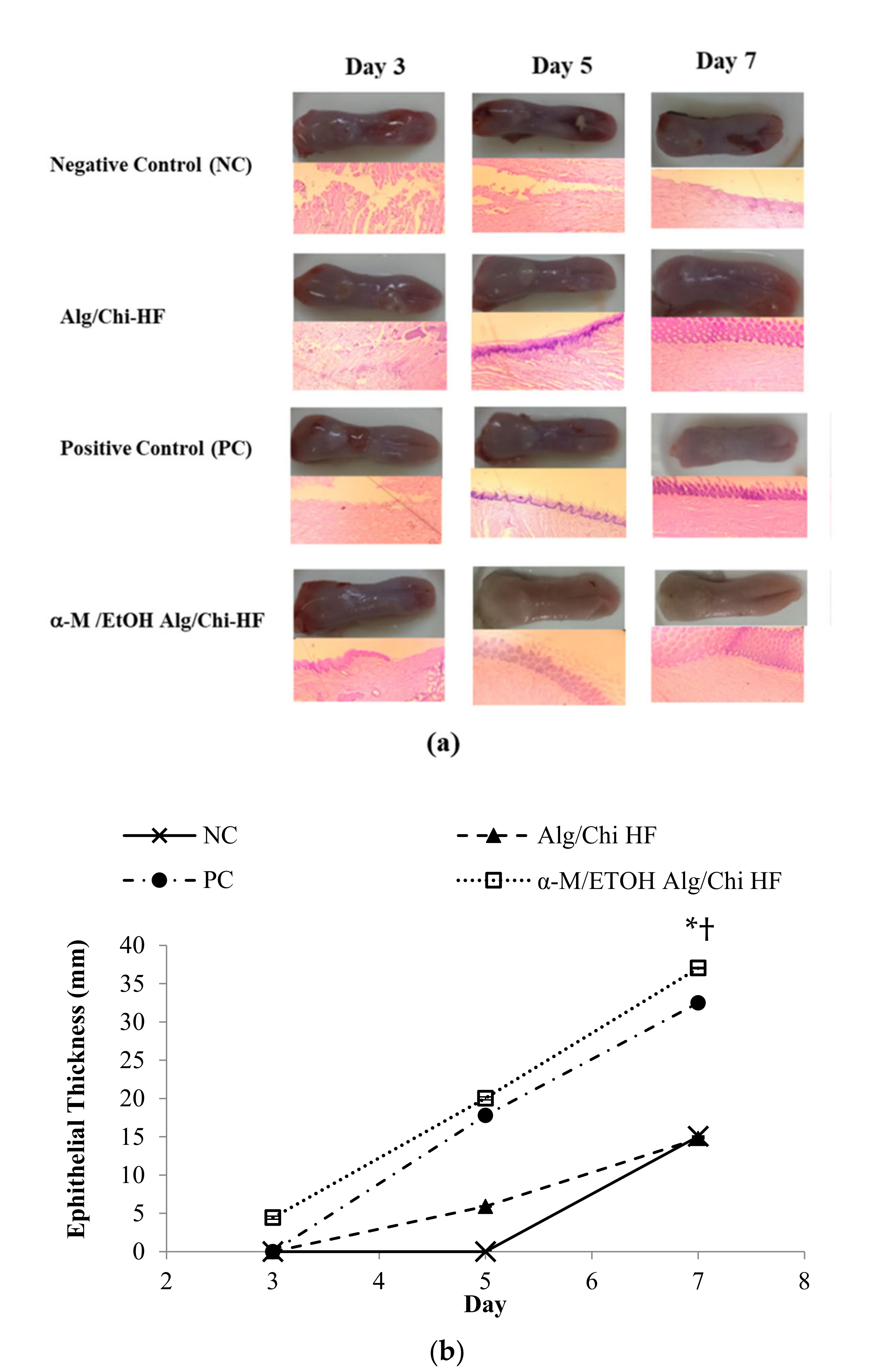
3.8. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alg | Alginate |
| α-M | Alpha mangostin |
| Chi | Chitosan |
| COX | Cyclooxigenase |
| EtOH | Ethanol |
| FTIR | Fourier transform infrared spectroscopy |
| HF | Hydrogel film |
| IL | Interleukin |
| PBS | Phosphate-buffered saline |
| PC | Positive control |
| NC | Negative control |
| NFkB | Nuclear factor kappa Beta |
| NO | Nitric oxide |
| RAS | Recurrent aphthous stomatitis |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TNF-α | Tumor mecrosis factor-alpha |
| XRD | X-ray diffraction |
References
- Groeger, S.; Meyle, J. Oral mucosal epithelial cells. Front. Immunol. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Edgar, N.R.; Saleh, D.; Miller, R.A. Recurrent aphthous stomatitis: A review. J. Clin. Aesthet. Dermatol. 2017, 10, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Belenguer-Guallar, I.; Jiménez-Soriano, Y.; Claramunt-Lozano, A. Treatment of recurrent aphthous stomatitis. A literature review. J. Clin. Exp. Dent. 2014, 6, e168–e174. [Google Scholar] [CrossRef] [PubMed]
- Tarakji, B.; Gazal, G.; Al-Maweri, S.A.; Azzeghaiby, S.N.; Alaizari, N. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J. Int. Oral Health 2015, 7, 74. [Google Scholar] [PubMed]
- Colley, H.E.; Said, Z.; Santocildes-Romero, M.E.; Baker, S.R.; D’Apice, K.; Hansen, J.; Madsen, L.S.; Thornhill, M.H.; Hatton, P.V.; Murdoch, C. Pre-clinical evaluation of novel mucoadhesive bilayer patches for local delivery of clobetasol-17-propionate to the oral mucosa. Biomaterials 2018, 178, 134–146. [Google Scholar] [CrossRef]
- Marzaimi, I.N.; Aizat, W.M. Current Review on Mangosteen Usages in Antiinflammation and Other Related Disorders, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128138205. [Google Scholar]
- Wathoni, N.; Yuniarsih, N.; Cahyanto, A.; Muhctaridi, M. α-mangostin hydrogel film based chitosan-alginate for recurrent aphthous stomatitis. Appl. Sci. 2019, 9, 5235. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Enhancement of curcumin wound healing ability by complexation with 2-hydroxypropyl-γ-cyclodextrin in sacran hydrogel film. Int. J. Biol. Macromol. 2017, 98, 268–276. [Google Scholar] [CrossRef]
- Wathoni, N.; Hasanah, A.N.; Mohammed, A.F.A.; Pratiwi, E.D.; Mahmudah, R. Accelerated wound healing ability of sacran hydrogel film by keratinocyte growth factor in alloxan-induced diabetic mice. Int. J. Appl. Pharm. 2018, 10, 57–61. [Google Scholar] [CrossRef][Green Version]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Enhancing effect of γ-cyclodextrin on wound dressing properties of sacran hydrogel film. Int. J. Biol. Macromol. 2017, 94, 181–186. [Google Scholar] [CrossRef]
- Salehi, S.; Boddohi, S. New formulation and approach for mucoadhesive buccal film of rizatriptan benzoate. Prog. Biomater. 2017, 6, 175–187. [Google Scholar] [CrossRef]
- Wathoni, N.; Sari, D.P.; Suharyani, I.; Motoyama, K.; Mohammed, A.F.A.; Cahyanto, A.; Abdassah, M.; Muchtaridi, M. Enhancement of α-mangostin wound healing ability by complexation with 2-hydroxypropyl-β-cyclodextrin in hydrogel formulation. Pharmaceuticals 2020, 13, 290. [Google Scholar] [CrossRef] [PubMed]
- Hindi, S.S.Z. Microcrystalline Cellulose: The Inexhaustible Treasure for Pharmaceutical Industry. Nanosci. Nanotechnol. Res. 2017, 4, 17–24. [Google Scholar] [CrossRef]
- Kumria, R.; Al-Dhubiab, B.E.; Shah, J.; Nair, A.B. Formulation and Evaluation of Chitosan-Based Buccal Bioadhesive Films of Zolmitriptan. J. Pharm. Innov. 2018, 13, 133–143. [Google Scholar] [CrossRef]
- Ossama, M.; Lamie, C.; Tarek, M.; Wagdy, H.A.; Attia, D.A.; Elmazar, M.M. Management of recurrent aphthous ulcers exploiting polymer-based Muco-adhesive sponges: In-vitro and in-vivo evaluation. Drug Deliv. 2021, 28, 87–99. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Physically crosslinked-sacran hydrogel films for wound dressing application. Int. J. Biol. Macromol. 2016, 89, 465–470. [Google Scholar] [CrossRef]
- Suharyani, I.; Muchtaridi, M.; Mohammed, A.F.; Elamin, K.; Wathoni, N.; Abdassah, M. α-Mangostin/γ-Cyclodextrin Inclusion Complex: Formation and Thermodynamic Studyand Thermodynamic Study. Polymers 2021, 13, 2890. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Puteri, A.; Milanda, T.; Musfiroh, I. Validation analysis methods of α-mangostin, γ-mangostin and gartanin mixture in Mangosteen (Garcinia mangostana L.) fruit rind extract from west java with HPLC. J. Appl. Pharm. Sci. 2017, 7, 125–130. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Karavana, S.Y.; Gökçe, E.H.; Rençber, S.; Özbal, S.; Pekçetin, Ç.; Güneri, P.; Ertan, G. A new approach to the treatment of recurrent aphthous stomatitis with bioadhesive gels containing cyclosporine A solid lipid nanoparticles: In vivo/in vitro examinations. Int. J. Nanomedicine 2012, 7, 5693–5704. [Google Scholar] [CrossRef]
- Majumder, R.; Adhikari, L.; Dhara, M.; Sahu, J. Evaluation of anti-inflammatory, analgesic and TNF-α inhibition (upon RAW 264.7 cell line) followed by the selection of extract (leaf and stem) with respect to potency to introduce anti-oral-ulcer model obtained from Olax psittacorum (Lam.) Vahl in additi. J. Ethnopharmacol. 2020, 263, 113146. [Google Scholar] [CrossRef]
- Wahyuni, I.S. Anti-Inflammatory Activity and Wound Healing Effect of Kaempferia galanga L. Rhizome on the Chemical-Induced Oral Mucosal Ulcer in Wistar Rats. J. Inflamm. Res. 2022, 15, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Rusdiana, T.; Hasanah, A.N.; Muhtadi, A.; Pratiwi, E.D.; Mahmudah, R.; Mohammed, A.F.A.; Okajima, M.; Kaneko, T.; Arima, H. Sacran Hydrogel Film Containing Keratinocyte Growth Factor Accelerates Wound Healing by Stimulating Fibroblast Migration and Re-epithelization. Chem. Pharm. Bull. 2019, 67, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.P.S.; Verma, S.; Singh, U.; Agarwal, N. Acute primary herpetic gingivostomatitis. BMJ Case Rep. 2013, 2013, bcr2013200074. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, H.; Safi, Y.; Baharvand, M.; Rahmani, S. Diagnostic Features of Common Oral Ulcerative Lesions: An Updated Decision Tree. Int. J. Dent. 2016, 2016, 7278925. [Google Scholar] [CrossRef]
- Jacobsen, J.; Meng-Lund, E.; Muff-Westergaard, C.; Sander, C.; Madelung, P. A mechanistic based approach for enhancing buccal mucoadhesion of chitosan. Int. J. Pharm. 2014, 461, 280–285. [Google Scholar] [CrossRef]
- Rungnim, C.; Phunpee, S.; Kunaseth, M.; Namuangruk, S.; Rungsardthong, K.; Rungrotmongkol, T.; Ruktanonchai, U. Co-solvation effect on the binding mode of the α-mangostin/β-cyclodextrin inclusion complex. Beilstein J. Org. Chem. 2015, 11, 2306–2317. [Google Scholar] [CrossRef]
- Campos, J.C.; Cunha, D.; Ferreira, D.C.; Reis, S.; Costa, P.J. Oromucosal precursors of in loco hydrogels for wound-dressing and drug delivery in oral mucositis: Retain, resist, and release. Mater. Sci. Eng. C 2021, 118, 111413. [Google Scholar] [CrossRef]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A Review of Selected Studies That Determine the Physical and Chemical Properties of Saliva in the Field of Dental Treatment. BioMed Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef]
- Hezaveh, H.; Muhamad, I.I.; Noshadi, I.; Shu Fen, L.; Ngadi, N. Swelling behaviour and controlled drug release from cross-linked -carrageenan/NaCMC hydrogel by diffusion mechanism. J. Microencapsul. 2012, 29, 368–379. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Tejamukti, E.P.; Setyaningsih, W.; Yasir, B.; Alam, G.; Rohman, A. Application of FTIR spectroscopy and HPLC combined with multivariate calibration for analysis of xanthones in mangosteen extracts. Sci. Pharm. 2020, 88, 35. [Google Scholar] [CrossRef]
- Inoue, Y.; Hirano, A.; Murata, I.; Kobata, K.; Kanamoto, I. Assessment of the Physical Properties of Inclusion Complexes of Forchlorfenuron and γ-Cyclodextrin Derivatives and Their Promotion of Plant Growth. ACS Omega 2018, 3, 13160–13169. [Google Scholar] [CrossRef] [PubMed]
- Catarina, F.; Vieirab, M.; Veiga, B.F. Physico-chemical characterization and in vitro dissolution assessment of clonazepam-cyclodextrins inclusion compounds. Eur. J. Pharm. Sci. 2002, 15, 79–88. [Google Scholar] [CrossRef]
- Higashi, K.; Ideura, S.; Waraya, H.; Moribe, K.; Yamamoto, K. Incorporation of salicylic acid molecules into the intermolecular spaces of y-cyclodextrin-polypseudorotaxane. Cryst. Growth Des. 2009, 9, 4243–4246. [Google Scholar] [CrossRef]
- Mulia, K.; Halimah, N.; Krisanti, E. Effect of alginate composition on profile release and characteristics of chitosanalginate microparticles loaded with mangosteen extract. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2016; p. 020010. [Google Scholar]
- Basu, A.R.; Basu, S.K. Alginate-Chitosan Particulate System for Sustained Release of Nimodipine. Trop. J. Pharm. Res. 2009, 8, 433–440. [Google Scholar]
- Apriasari, M.L.; Endariantari, A.; Oktaviyanti, I.K. The effect of 25% Mauli banana stem extract gel to increase the epithel thickness of wound healing process in oral mucosa. Dent. J. 2015, 48, 150. [Google Scholar] [CrossRef][Green Version]
- Sunarjo, L.; Hendari, R.; Rimbyastuti, H. Manfaat Xanthone Terhadap Kesembuhan Ulkus Rongga Mulut Dilihat Dari Jumlah Sel Pmn Dan Fibroblast. ODONTO Dent. J. 2016, 2, 14. [Google Scholar] [CrossRef]
- Mohan, S.; Syam, S.; Abdelwahab, S.I.; Thangavel, N. An anti-inflammatory molecular mechanism of action of α-mangostin, the major xanthone from the pericarp of Garcinia mangostana: An in silico, in vitro and in vivo approach. Food Funct. 2018, 9, 3860–3871. [Google Scholar] [CrossRef]
- Kovalik, A.C.; Bisetto, P.; Pochapski, M.T.; Campagnoli, E.B.; Pilatti, G.L.; Santos, F.A. Effects of an orabase formulation with ethanolic extract of malva sylvestris L. in oral wound healing in rats. J. Med. Food 2014, 17, 618–624. [Google Scholar] [CrossRef]


| Alg/Chi HF | Alginate Sodium (g) | Chitosan (g) | Propylene Glycol (%w/v) | Glycerin (%w/v) | α-M (μg) | Ethanol (μL) | Acetic Acid (mL) | Distilled Water (mL) |
|---|---|---|---|---|---|---|---|---|
| Alg | 0.5 | - | - | - | - | - | - | 25 |
| Chi | - | 0.1 | - | - | - | - | 0.5 | 24.5 |
| Alg/Chi | 0.5 | 0.1 | 5 | 2 | - | - | 0.5 | 49.5 |
| α-M | 0.5 | 0.1 | 5 | 2 | 250 | - | 0.5 | 49.5 |
| α-M/EtOH | 0.5 | 0.1 | 5 | 2 | 250 | 10 | 0.5 | 49.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanda, T.; Cindana Mo’o, F.R.; Mohammed, A.F.A.; Elamin, K.M.; Wilar, G.; Suharyani, I.; Wathoni, N. Alginate/Chitosan-Based Hydrogel Film Containing α-Mangostin for Recurrent Aphthous Stomatitis Therapy in Rats. Pharmaceutics 2022, 14, 1709. https://doi.org/10.3390/pharmaceutics14081709
Milanda T, Cindana Mo’o FR, Mohammed AFA, Elamin KM, Wilar G, Suharyani I, Wathoni N. Alginate/Chitosan-Based Hydrogel Film Containing α-Mangostin for Recurrent Aphthous Stomatitis Therapy in Rats. Pharmaceutics. 2022; 14(8):1709. https://doi.org/10.3390/pharmaceutics14081709
Chicago/Turabian StyleMilanda, Tiana, Faradila Ratu Cindana Mo’o, Ahmed Fouad Abdelwahab Mohammed, Khaled M. Elamin, Gofarana Wilar, Ine Suharyani, and Nasrul Wathoni. 2022. "Alginate/Chitosan-Based Hydrogel Film Containing α-Mangostin for Recurrent Aphthous Stomatitis Therapy in Rats" Pharmaceutics 14, no. 8: 1709. https://doi.org/10.3390/pharmaceutics14081709
APA StyleMilanda, T., Cindana Mo’o, F. R., Mohammed, A. F. A., Elamin, K. M., Wilar, G., Suharyani, I., & Wathoni, N. (2022). Alginate/Chitosan-Based Hydrogel Film Containing α-Mangostin for Recurrent Aphthous Stomatitis Therapy in Rats. Pharmaceutics, 14(8), 1709. https://doi.org/10.3390/pharmaceutics14081709








