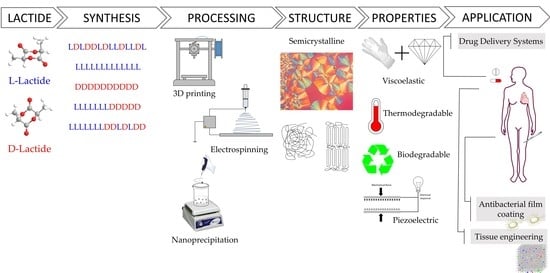
Polylactide Perspectives in Biomedicine: From Novel Synthesis to the Application Performance
Abstract
:1. Introduction
2. Poly-(lactic Acid) (PLA)
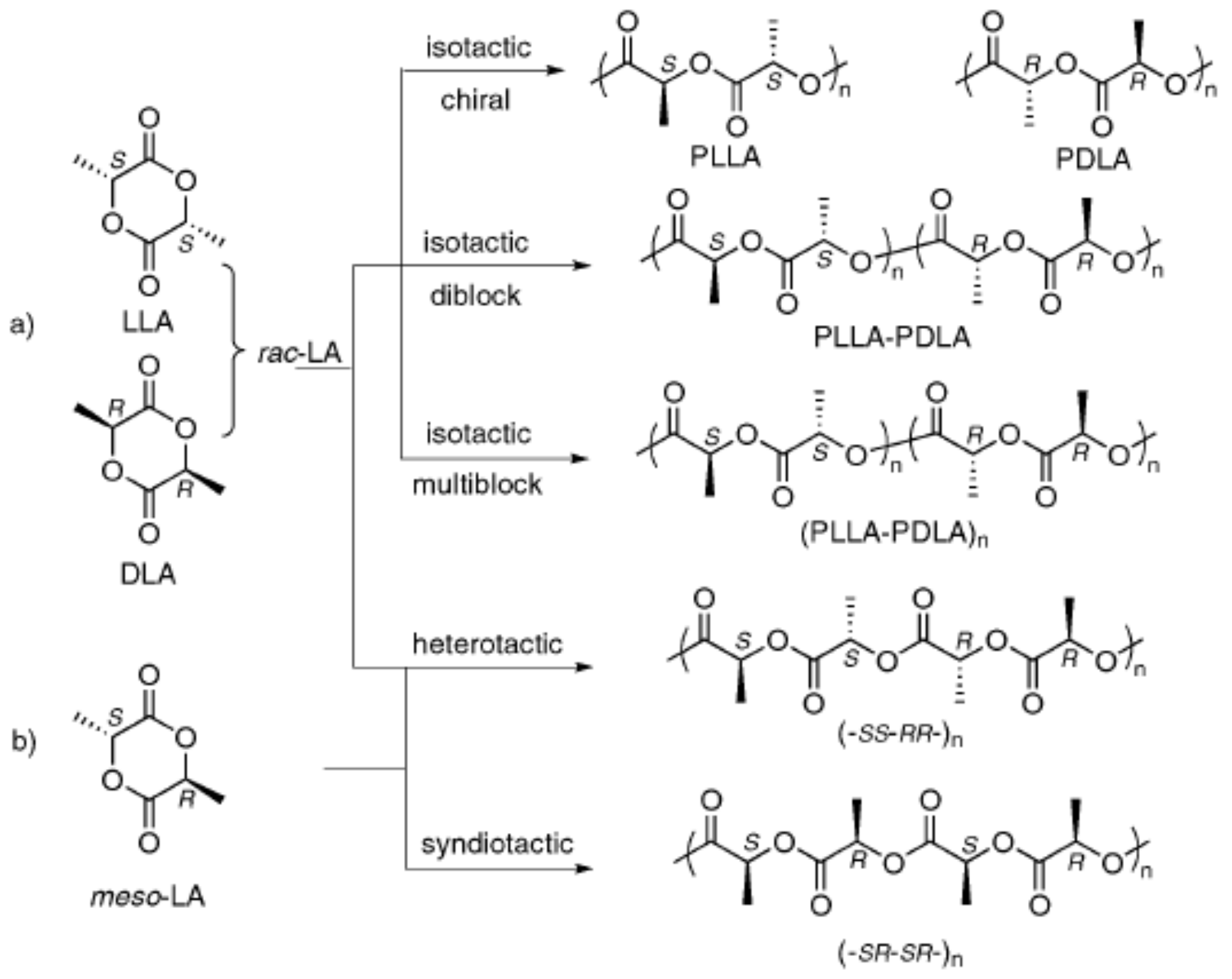
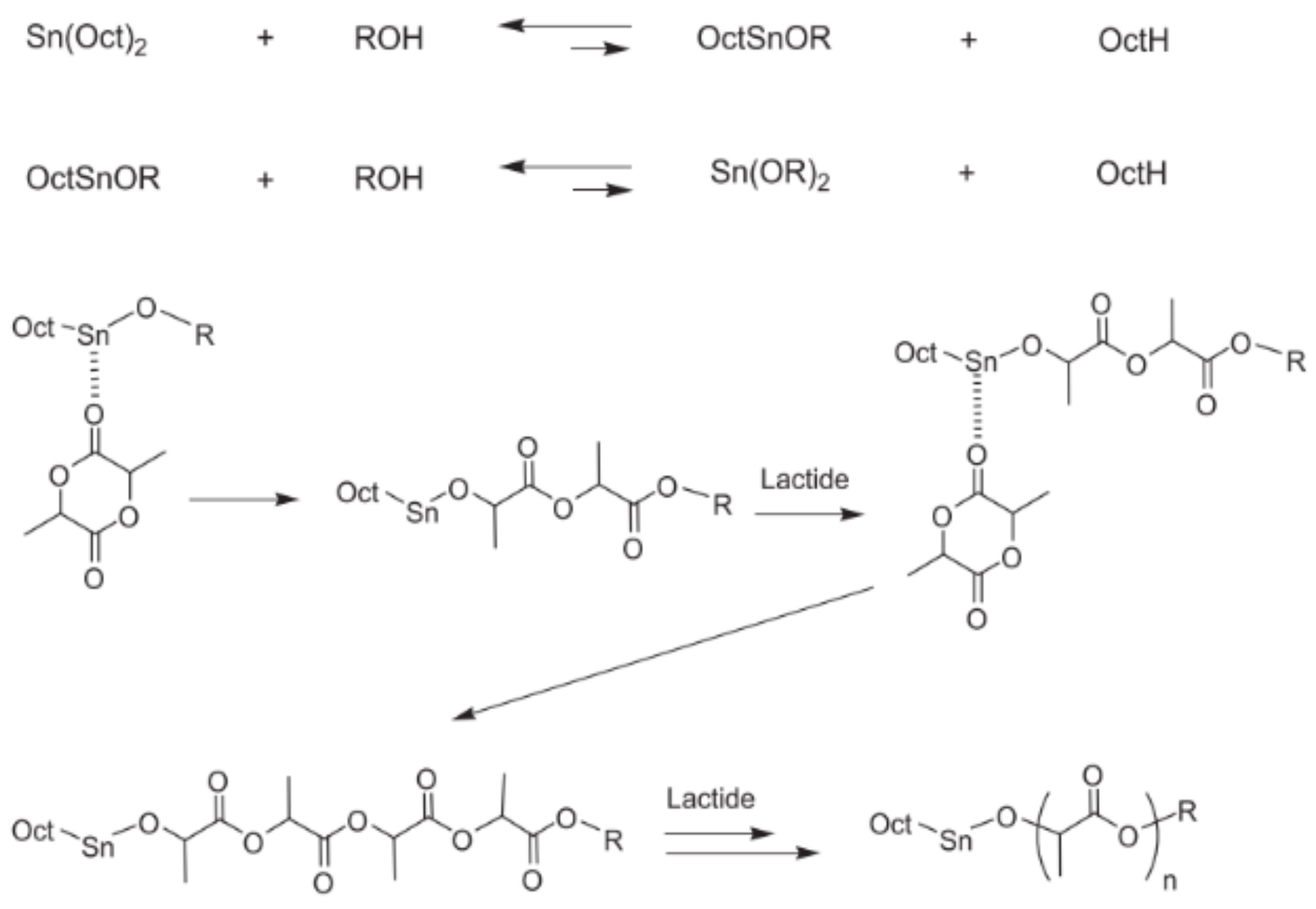
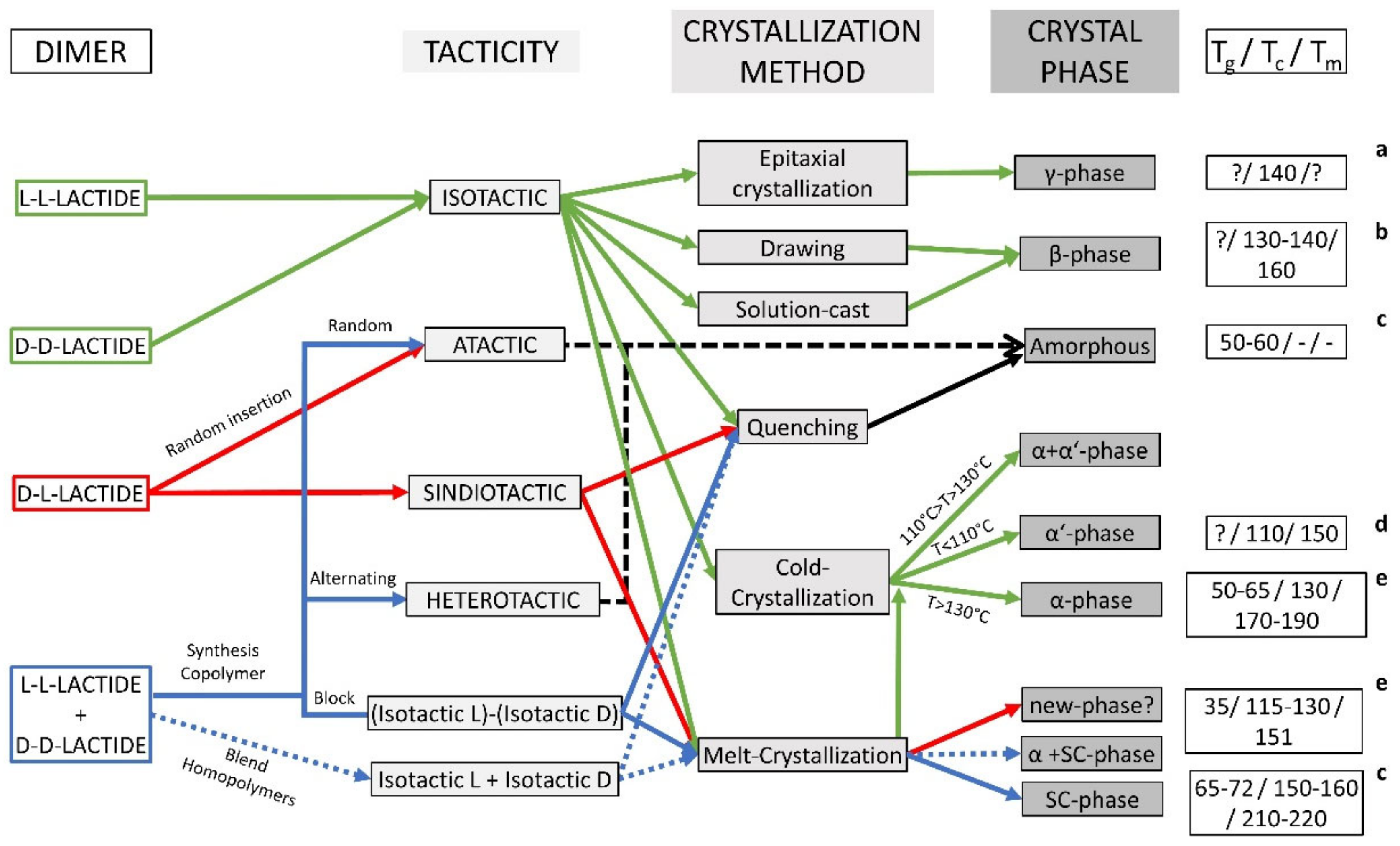
3. Synthesis of PLA
4. PLA Processing
5. PLA Properties
5.1. Mechanical Properties
5.2. Degradation
- Polymer factors
- Media factors
6. Medical Applications
6.1. Pharmaceutical Applications
6.1.1. Nanoparticles
6.1.2. Hydrogels
6.1.3. Pharmaceuticals Design
6.1.4. Chiral Drugs
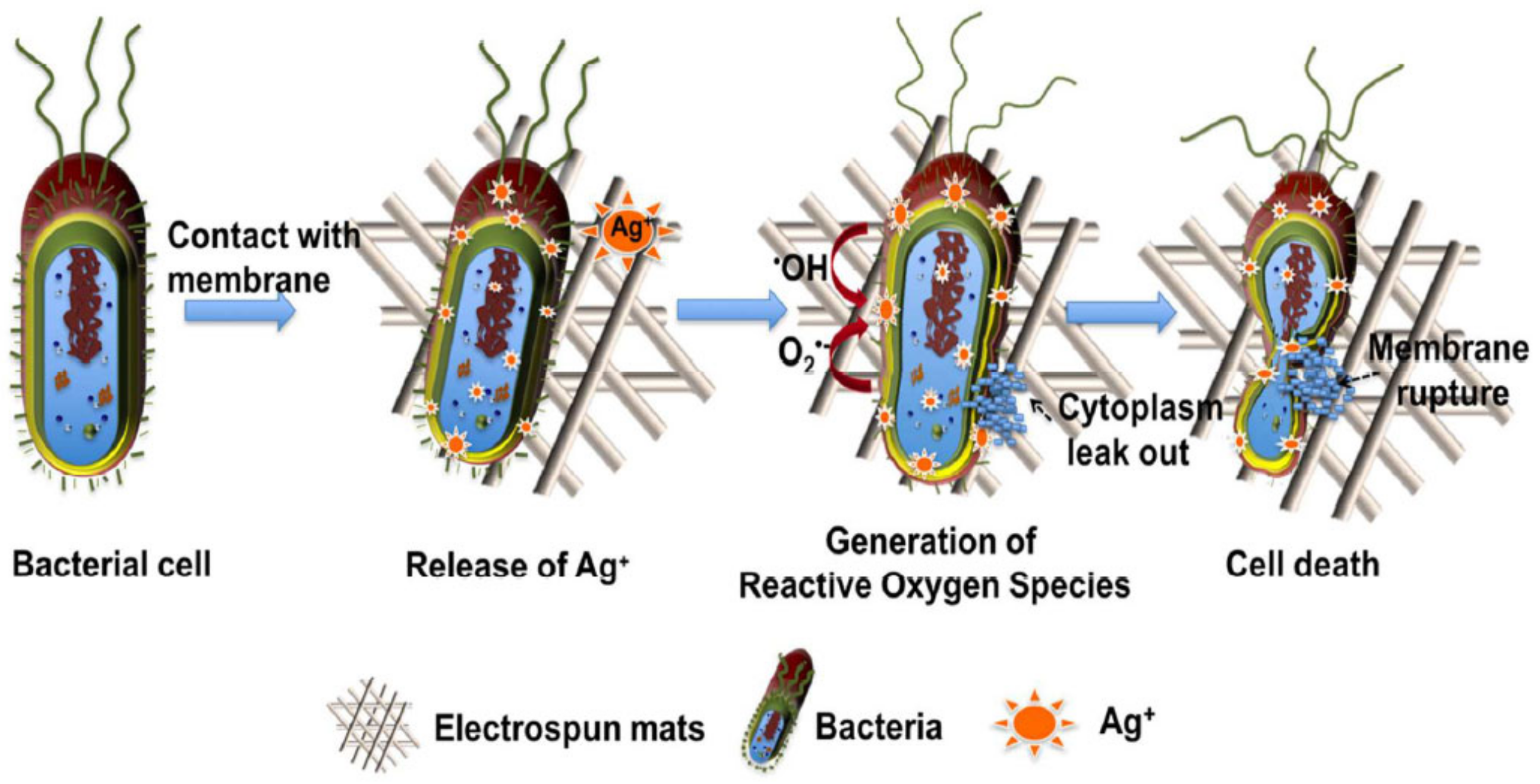
6.1.5. Antibacterial Applications
6.1.6. Polymer Therapeutics
6.2. Biomedical Applications
6.2.1. Tissue Engineering and Scaffolds
Bone Regeneration
Tendon Regeneration
Nerve Regeneration
Cartilage Regeneration
Stents for Cardiac Regeneration
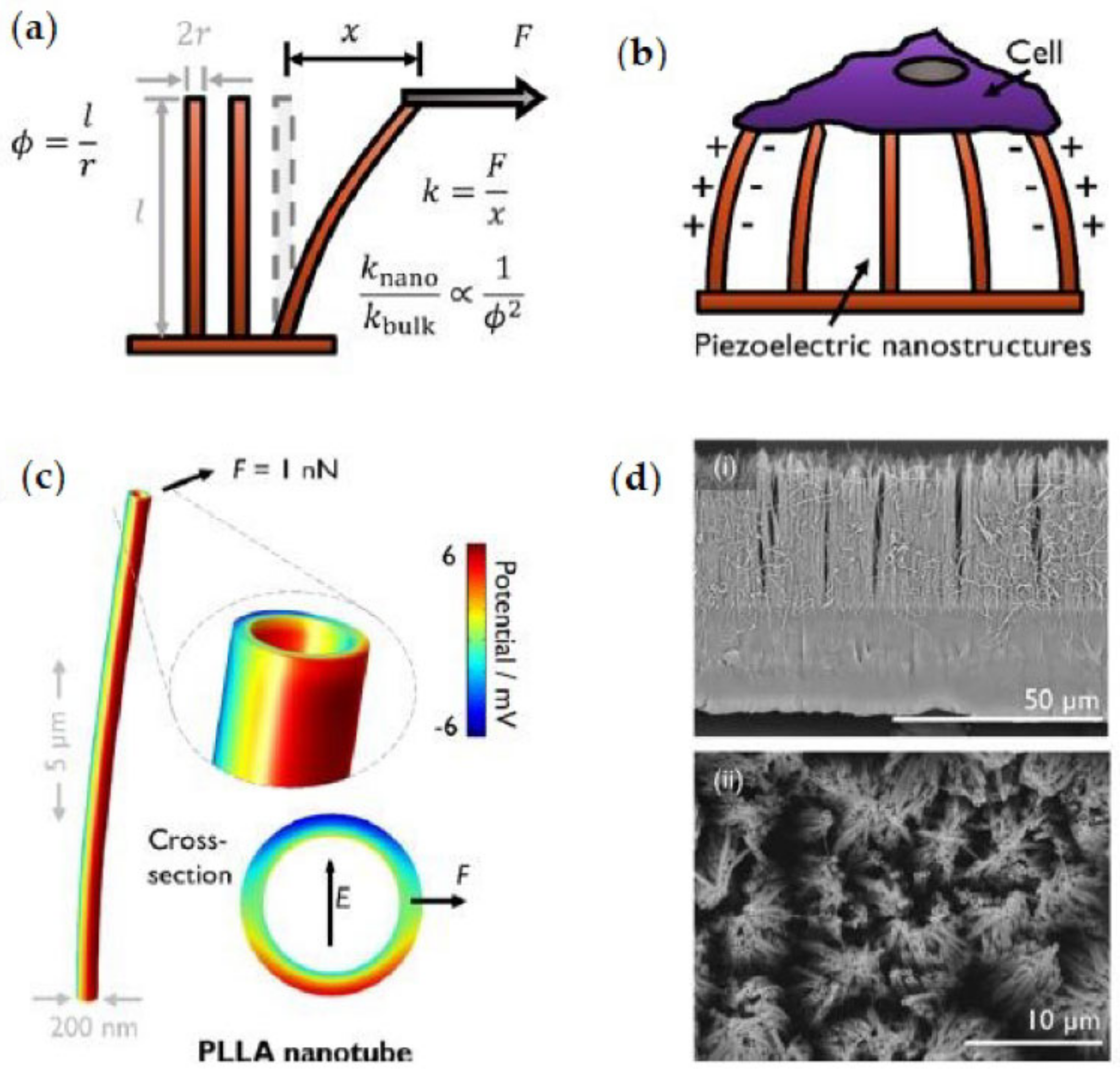
6.2.2. Piezoelectric Activity
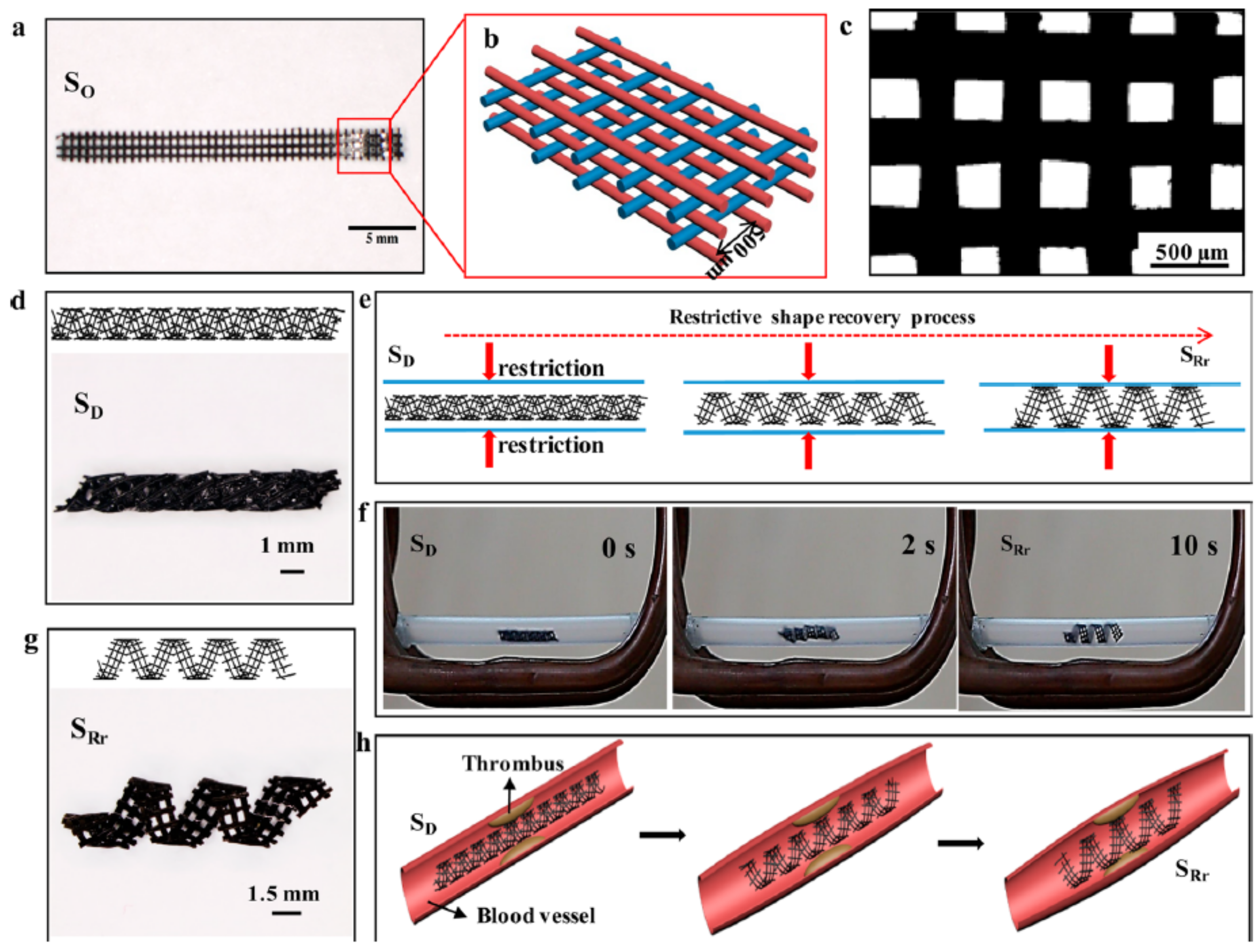
6.2.3. Shape Memory Polymers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 3DP | Three-dimensional printing |
| DDS | Drug delivery system |
| D-LA | D-Lactide |
| FDA | Food and Drug Administration |
| HC | Homocrystals |
| HMw | High molecular weight |
| i-PP | Isotactic polypropylene |
| kh | Hydrolysis constant |
| kH | Degradation constant catalysed by protons |
| ko | Degradation constant in water |
| kOH | Degradation constant catalysed by hydroxyl ions |
| LA | Lactide |
| L-LA | L-Lactide |
| Mw | Molecular weight |
| PCL | Poly(e-caprolactone) |
| PCM | Polymer of chiral acetylenic |
| PDLA | Poly(D-lactide) |
| PE | Polyethylene |
| PEG | Polyethylene glycol |
| PET | Polyethylene terephthalate |
| PEVA | Poly(ethylene-co-vinyl acetate) |
| PGA | Polyglycolic acid |
| PHA | Polyhydroxhyalkanoate |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic) acid |
| PLLA | Poly(L-lactide) |
| PMMA | Poly(methyl methacrylate) |
| PP | Polypropylene |
| PS | Polystytene |
| Rac-LA | Racemic-lactide |
| ROP | Ring-opening polymerisation |
| SC | Stereocomplex |
| SMP | Shape memory polymer |
| Tc | Crystallisation temperature |
| TE | Tissue engineering |
| Tg | Glass transition temperature |
| Th | Hydrolytic degradation temperature |
| Tm | Melting temperature |
| Xc | Crystallinity |
References
- Zhang, X.; Williams, D. Definitions of Biomaterials for the Twenty-First Century; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Barbucci, R. (Ed.) Integrated Biomaterials Science; Kluwer Academic Publishers: Norwell, MA, USA, 2002. [Google Scholar]
- Nazeer, N.; Ahmed, M. Polymers in Medicine; Narain, R., Ed.; Elsevier Inc.: Edmonton, AB, Canada, 2020; ISBN 9780128168066. [Google Scholar]
- Francis PJ, J. Biomedical Applications of Polymers—An Overview. Curr. Trends Biomed. Eng. Biosci. 2018, 15, 44–45. [Google Scholar] [CrossRef]
- Kulkarni, R.K.; Pani, K.C.; Neuman, C.; Leonard, F. Polylactic Acid for Surgical Implants. Arch. Surg. 1966, 93, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Polymers as Biomaterials for Tissue Engineering and Controlled Drug Delivery. Adv. Biochem. Eng. Biotechnol. 2006, 102, 47–90. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Sharma, C.P. Review Paper: Absorbable Polymeric Surgical Sutures: Chemistry, Production, Properties, Biodegradability, and Performance. J. Biomater. Appl. 2010, 25, 291–366. [Google Scholar] [CrossRef] [PubMed]
- European Comission. A European Strategy for Plastics in a Circular Economy; Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Comission: Brussels, Belgium, 2018. [Google Scholar]
- European Bioplastics. European Parliament Report Recognizes Potential Role of Bioplastics in the Circular Economy. Available online: https://www.european-bioplastics.org/european-parliament-report-recognizes-potential-role-of-bioplastics-in-the-circular-economy/ (accessed on 15 June 2022).
- Matthews, C.; Moran, F.; Jaiswal, A.K. A Review on European Union’s Strategy for Plastics in a Circular Economy and Its Impact on Food Safety. J. Clean. Prod. 2021, 283, 125263. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(Lactic Acid)—Mass Production, Processing, Industrial Applications, and End of Life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and Biotic Environmental Degradation of the Bioplastic Polymer Poly(Lactic Acid): A Review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic Acid Blends: The Future of Green, Light and Tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 12, 6147–6176. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-Lactic Acid Synthesis for Application in Biomedical Devices—A Review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Nishio, S.; Kosuga, K.; Igaki, K.; Okada, M.; Kyo, E.; Tsuji, T.; Takeuchi, E.; Inuzuka, Y.; Takeda, S.; Hata, T.; et al. Long-Term (>10 Years) Clinical Outcomes of First-in-Human Biodegradable Poly-l-Lactic Acid Coronary Stents: Igaki-Tamai Stents. Circulation 2012, 125, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Mattesini, A.; Bartolini, S.; Dini, C.S.; Valente, S.; Parodi, G.; Stolcova, M.; Meucci, F.; di Mario, C. The DESolve Novolimus Bioresorbable Scaffold: From Bench to Bedside. J. Thorac. Dis. 2017, 9, S950–S958. [Google Scholar] [CrossRef] [PubMed]
- Tenekecioglu, E.; Bourantas, C.; Abdelghani, M.; Zeng, Y.; Silva, R.C.; Tateishi, H.; Sotomi, Y.; Onuma, Y.; Yilmaz, M.; Serruys, P.W. From Drug Eluting Stents to Bioresorbable Scaffolds; To New Horizons in PCI. Expert Rev. Med. Devices 2016, 13, 271–286. [Google Scholar] [CrossRef]
- R3 Vascular Inc. Magnitude. Available online: https://www.r3vascular.com/magnitude (accessed on 15 June 2022).
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(Lactic Acid): A Review. J. Macromol. Sci.-Polym. Rev. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic Acid (PLA) Synthesis and Modifications: A Review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, P. Crystallization of Biodegradable and Biobased Polyesters: Polymorphism, Cocrystallization, and Structure-Property Relationship. Prog. Polym. Sci. 2020, 109, 101291. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(Lactide) Stereocomplexes: Formation, Structure, Properties, Degradation, and Applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(Lactic Acid) Stereocomplexes: A Decade of Progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(Lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties-from Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Groot, W.; van Krieken, J.; Sliekersl, O.; de Vos, S. Production and Purification of Lactic Acid and Lactide. In Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; Auras, R., Lim, L.-T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 27–41. ISBN 9780470293669. [Google Scholar]
- Song, L.; Pan, M.; Zhao, R.; Deng, J.; Wu, Y. Recent Advances, Challenges and Perspectives in Enantioselective Release. J. Control. Release 2020, 324, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.J.; Dove, A.P. Stereocontrolled Ring-Opening Polymerisation of Lactide. Chem. Soc. Rev. 2010, 39, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Garlotta, D. A Literature Review of Poly (Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. PLA Synthesis. From the Monomer to the Polymer. In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; The Royal Society of Chemistry: Cambridge, 2015; pp. 3–36. ISBN 9781849738798. [Google Scholar]
- Tsuji, H.; Ikada, Y. Crystallization from the Melt of Poly(Lactide)s with Different Optical Purities and Their Blends. Macromol. Chem. Phys. 1996, 197, 3483–3499. [Google Scholar] [CrossRef]
- Pretula, J.; Slomkowski, S.; Penczek, S. Polylactides—Methods of Synthesis and Characterization. Adv. Drug Deliv. Rev. 2016, 107, 3–16. [Google Scholar] [CrossRef]
- Tsuji, H.; Arakawa, Y. Synthesis, Properties, and Crystallization of the Alternating Stereocopolymer Poly(l-Lactic Acid-: Alt -d-Lactic Acid) [Syndiotactic Poly(Lactic Acid)] and Its Blend with Isotactic Poly(Lactic Acid). Polym. Chem. 2018, 9, 2446–2457. [Google Scholar] [CrossRef]
- Szwarc, M. © 1956 Nature Publishing. Nature 1956, 178, 1168–1169. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Influence of A′-/α-Crystal Polymorphism on Properties of Poly(l-Lactic Acid). Polym. Int. 2019, 68, 320–334. [Google Scholar] [CrossRef]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of Melting of A′- and α-Crystals of Poly(L-Lactic Acid). Eur. Polym. J. 2015, 70, 215–220. [Google Scholar] [CrossRef]
- Takahashi, K.; Sawai, D.; Yokoyama, T.; Kanamoto, T.; Hyon, S.H. Crystal Transformation from the α- to the β-Form upon Tensile Drawing of Poly(L-Lactic Acid). Polymer 2004, 45, 4969–4976. [Google Scholar] [CrossRef]
- Sawai, D.; Yokoyama, T.; Kanamoto, T.; Sungil, M.; Hyon, S.H.; Myasnikova, L.P. Crystal Transformation and Development of Tensile Properties upon Drawing of Poly(L-Lactic Acid) by Solid-State Coextrusion: Effects of Molecular Weight. Macromol. Symp. 2006, 242, 93–103. [Google Scholar] [CrossRef]
- Xue, J.; Yan, L.; Tian, X.; Huang, D.; Redyy, N.; Yang, Y. Chemical Structure of Poly(Lactic Acid). In Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; Auras, R., Lim, L.-T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Lee, S.J.; Arun, A.P.; Kim, K.J. Piezoelectric Properties of Electrospun Poly(l-Lactic Acid) Nanofiber Web. Mater. Lett. 2015, 148, 58–62. [Google Scholar] [CrossRef]
- Cartier, L.; Okihara, T.; Ikada, Y.; Tsuji, H.; Puiggali, J.; Lotz, B. Epitaxial Crystallization and Crystalline Polymorphism of Polylactides. Polymer 2000, 41, 8909–8919. [Google Scholar] [CrossRef]
- Zhang, J.; Sato, H.; Tsuji, H.; Noda, I.; Ozaki, Y. Differences in the CH3⋯O=C Interactions among Poly(L-Lactide), Poly(L-Lactide)/Poly(D-Lactide) Stereocomplex, and Poly(3-Hydroxybutyrate) Studied by Infrared Spectroscopy. J. Mol. Struct. 2005, 735–736, 249–257. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.-H. Stereocomplex Formation between Enantiomeric Poly(Lactides). Am. Chem. Soc. 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Bertin, A. Emergence of Polymer Stereocomplexes for Biomedical Applications. Macromol. Chem. Phys. 2012, 213, 2329–2352. [Google Scholar] [CrossRef]
- Pan, P.; Han, L.; Bao, J.; Xie, Q.; Shan, G.; Bao, Y. Competitive Stereocomplexation, Homocrystallization, and Polymorphic Crystalline Transition in Poly(L-Lactic Acid)/Poly(D-Lactic Acid) Racemic Blends: Molecular Weight Effects. J. Phys. Chem. B 2015, 119, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Ikada, Y. Stereocomplex Formation between Enantiomeric Poly(Lactic Acid)s. 9. Stereocomplexation from the Melt. Macromolecules 1993, 26, 6918–6926. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Investigation of Phase Transitional Behavior of Poly(L-Lactide)/ Poly(D-Lactide) Blend Used to Prepare the Highly-Oriented Stereocomplex. Macromolecules 2007, 40, 1049–1054. [Google Scholar] [CrossRef]
- D’Auria, I.; D’Alterio, M.C.; Tedesco, C.; Pellecchia, C. Tailor-Made Block Copolymers of l-, d- and: Rac -Lactides and ϵ-Caprolactone via One-Pot Sequential Ring Opening Polymerization by Pyridylamidozinc(Ii) Catalysts. RSC Adv. 2019, 9, 32771–32779. [Google Scholar] [CrossRef]
- Rosen, T.; Goldberg, I.; Venditto, V.; Kol, M. Tailor-Made Stereoblock Copolymers of Poly(Lactic Acid) by a Truly Living Polymerization Catalyst. J. Am. Chem. Soc. 2016, 138, 12041–12044. [Google Scholar] [CrossRef]
- Puthumana, M.; Santhana Gopala Krishnan, P.; Nayak, S.K. Chemical Modifications of PLA through Copolymerization. Int. J. Polym. Anal. Charact. 2020, 25, 634–648. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(Lactic Acid) Blends in Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Wang, H.; Yin, G.; Dong, Z. Fabrication and Properties of the Electrospun Polylactide/Silk Fibroin-Gelatin Composite Tubular Scaffold. Biomacromolecules 2009, 10, 2240–2244. [Google Scholar] [CrossRef]
- Moya-Lopez, C.; Valcarcel, J.; Vázquez, J.A.; Bourson, P.; Chapron, D.; Solano, E.; Piñeiro, M.M.; Hermida-Merino, C.; Hermida-Merino, D. Biocompatibility Enhancement of PLA by the Generation of Bionanocomposites with Fish Collagen Derivatives. Emergent Mater. 2022, 5, 695–702. [Google Scholar] [CrossRef]
- Jin, S.; Sun, F.; Zou, Q.; Huang, J.; Zuo, Y.; Li, Y.; Wang, S.; Cheng, L.; Man, Y.; Yang, F.; et al. Fish Collagen and Hydroxyapatite Reinforced Poly(Lactide- Co-Glycolide) Fibrous Membrane for Guided Bone Regeneration. Biomacromolecules 2019, 20, 2058–2067. [Google Scholar] [CrossRef]
- Naffakh, M.; Rica, P.; Moya-Lopez, C.; Castro-osma, J.A.; Alonso-moreno, C.; Moreno, D.A. The Effect of WS 2 Nanosheets on the Non- I Sothermal Cold- and Melt-Crystallization Kinetics of Poly(L-Lactic Acid) Nanocomposites. Polymers 2021, 13, 2214. [Google Scholar] [CrossRef] [PubMed]
- Naffakh, M.; Marco, C.; Ellis, G. Non-Isothermal Cold-Crystallization Behavior and Kinetics of Poly(L-Lactic Acid)/WS2 Inorganic Nanotube Nanocomposites. Polymers 2015, 7, 2175–2189. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M.; Marco, C. Polymer Blend Nanocomposites Based on Poly(l-Lactic Acid), Polypropylene and WS2 Inorganic Nanotubes. RSC Adv. 2016, 6, 40033–40044. [Google Scholar] [CrossRef]
- Sanivada, U.K.; Mármol, G.; Brito, F.P.; Fangueiro, R. PLA composites reinforced with flax and jute fibers—A review of recent trends, processing parameters and mechanical properties. Polymers 2020, 12, 2373. [Google Scholar] [CrossRef] [PubMed]
- Brounstein, Z.; Yeager, C.M.; Labouriau, A. Development of Antimicrobial PLA Composites for Fused Filament Fabrication. Polymers 2021, 13, 580. [Google Scholar] [CrossRef]
- Eltouby, P.; Shyha, I.; Li, C.; Khaliq, J. Factors Affecting the Piezoelectric Performance of Ceramic-Polymer Composites: A Comprehensive Review. Ceram. Int. 2021, 47, 17813–17825. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Polyesters from Dilactones. In Handbook of Ring-Opening; Dubois, P., Coulembier, O., Raquez, J.-M., Eds.; WILEY-VCH Verlag GmbH & C: Weinheim, Germany, 2009; pp. 255–286. ISBN 9783527317103. [Google Scholar]
- Stjerndahl, A.; Wistrand, A.F.; Albertsson, A.C. Industrial Utilization of Tin-Initiated Resorbable Polymers: Synthesis on a Large Scale with a Low Amount of Initiator Residue. Biomacromolecules 2007, 8, 937–940. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Alonso-Moreno, C.; Lara-Sánchez, A.; Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Rodríguez, A.M. Catalytic Behaviour in the Ring-Opening Polymerisation of Organoaluminiums Supported by Bulky Heteroscorpionate Ligands. Dalt. Trans. 2015, 44, 12388–12400. [Google Scholar] [CrossRef]
- Otero, A.; Lara-Sánchez, A.; Fernández-Baeza, J.; Alonso-Moreno, C.; Castro-Osma, J.A.; Márquez-Segovia, I.; Sánchez-Barba, L.F.; Rodríguez, A.M.; Garcia-Martinez, J.C. Neutral and Cationic Aluminum Complexes Supported by Acetamidate and Thioacetamidate Heteroscorpionate Ligands as Initiators for Ring-Opening Polymerization of Cyclic Esters. Organometallics 2011, 30, 1507–1522. [Google Scholar] [CrossRef]
- Yui, N.; Dijkstra, P.J.; Feijen, J. Stereo Block Copolymers of L- and D-Lactides. Die Makromol. Chemie 1990, 191, 481–488. [Google Scholar] [CrossRef]
- Thomas, C.M. Stereocontrolled Ring-Opening Polymerization of Cyclic Esters: Synthesis of New Polyester Microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Moya-Lopez, C.; Bravo, I.; Castro-Osma, J.A.; Chapron, D.; Bourson, P.; Vagner, C.; Cochez, M.; Leon, N.; Alonso-Moreno, C.; Hermida-Merino, D. Synthesis of High Molecular Weight Stereo-Di-Block Copolymers Driven by a Co-Initiator Free Catalyst. Polymers 2022, 14, 232. [Google Scholar] [CrossRef]
- Rosen, T.; Goldberg, I.; Navarra, W.; Venditto, V.; Kol, M. Divergent [{ONNN}Mg–Cl] Complexes in Highly Active and Living Lactide Polymerization. Chem. Sci. 2017, 8, 5476–5481. [Google Scholar] [CrossRef]
- Lotz, B.A. Single Crystals of the Frustrated β-Phase and Genesis of the Disordered A′-Phase of Poly(l -Lactic Acid). ACS Macro Lett. 2015, 4, 602–605. [Google Scholar] [CrossRef]
- Auras, R.; Lim, L.-T.; Selke, S.E.M.; Tsuji, H. Poly(Lactic Acid). Synthesis, Structures, Properties, Processing and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 9780470293669. [Google Scholar]
- Francis, L.F. Melt Processes. In Materials Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 105–249. ISBN 9780123851321. [Google Scholar]
- Le Marec, P.E.; Ferry, L.; Quantin, J.C.; Bénézet, J.C.; Bonfils, F.; Guilbert, S.; Bergeret, A. Influence of Melt Processing Conditions on Poly(Lactic Acid) Degradation: Molar Mass Distribution and Crystallization. Polym. Degrad. Stab. 2014, 110, 353–363. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing Technologies for Poly(Lactic Acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Shen, C. Crystallization Behavior of Poly(Lactic Acid) and Its Blends. Polym. Cryst. 2021, 4, 2–5. [Google Scholar] [CrossRef]
- Tábi, T.; Hajba, S.; Kovács, J.G. Effect of Crystalline Forms (A′ and α) of Poly(Lactic Acid) on Its Mechanical, Thermo-Mechanical, Heat Deflection Temperature and Creep Properties. Eur. Polym. J. 2016, 82, 232–243. [Google Scholar] [CrossRef]
- Takasaki, M.; Ito, H.; Kikutani, T. Development of Stereocomplex Crystal of Polylactide in High-Speed Melt Spinning and Subsequent Drawing and Annealing Processes. J. Macromol. Sci.-Phys. 2003, 42, 403–420. [Google Scholar] [CrossRef]
- Im, S.H.; Im, D.H.; Park, S.J.; Chung, J.J.; Jung, Y.; Kim, S.H. Stereocomplex Polylactide for Drug Delivery and Biomedical Applications: A Review. Molecules 2021, 26, 2846. [Google Scholar] [CrossRef]
- Elbadawi, M.; Muñiz Castro, B.; Gavins, F.K.H.; Ong, J.J.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, A. M3DISEEN: A Novel Machine Learning Approach for Predicting the 3D Printability of Medicines. Int. J. Pharm. 2020, 590, 119837. [Google Scholar] [CrossRef] [PubMed]
- Durga Prasad Reddy, R.; Sharma, V. Additive Manufacturing in Drug Delivery Applications: A Review. Int. J. Pharm. 2020, 589, 119820. [Google Scholar] [CrossRef] [PubMed]
- Organovo. Available online: https://organovo.com/ (accessed on 15 June 2022).
- Srinivas, V.; van Hooy-Corstjens, C.S.J.; Rastogi, S.; Harings, J.A.W. Promotion of Molecular Diffusion and/or Crystallization in Fused Deposition Modeled Poly(Lactide) Welds. Polymer 2020, 202, 122637. [Google Scholar] [CrossRef]
- Bedell, M.L.; Navara, A.M.; Du, Y.; Zhang, S.; Mikos, A.G. Polymeric Systems for Bioprinting. Chem. Rev. 2020, 120, 10744–10792. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, G.; Zhu, P.; Gao, C. Recent Progress on 3D-Printed Polylactic Acid and Its Applications in Bone Repair. Adv. Eng. Mater. 2020, 22, 1901065. [Google Scholar] [CrossRef]
- Cheng, C.H.; Shie, M.Y.; Lai, Y.H.; Foo, N.P.; Lee, M.J.; Yao, C.H. Fabrication of 3d Printed Poly(Lactic Acid)/Polycaprolactone Scaffolds Using Tgf-Β1 for Promoting Bone Regeneration. Polymers 2021, 13, 3731. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Gerke, R.; Götz, H.; Stein, S.; Rommens, P.M. A New Bone Substitute Developed from 3D-Prints of Polylactide (PLA) Loaded with Collagen i: An in Vitro Study. Int. J. Mol. Sci. 2017, 18, 2569. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Han, D.; Li, Q. Three Dimensional Printed Macroporous Polylactic Acid/Hydroxyapatite Composite Scaffolds for Promoting Bone Formation in a Critical-Size Rat Calvarial Defect Model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef]
- Sahvieh, S.; Oryan, A.; Hassanajili, S.; Kamali, A. Role of Bone 1stem Cell–Seeded 3D Polylactic Acid/Polycaprolactone/Hydroxyapatite Scaffold on a Critical-Sized Radial Bone Defect in Rat. Cell Tissue Res. 2021, 383, 735–750. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Q.; Wang, M. Cryogenic 3D Printing for Producing Hierarchical Porous and RhBMP-2-Loaded Ca-P/PLLA Nanocomposite Scaffolds for Bone Tissue Engineering. Biofabrication 2017, 9, 025031. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, L.K.; Huebner, P.; Fisher, M.B.; Spang, J.T.; Starly, B.; Shirwaiker, R.A. 3D-Bioprinting of Polylactic Acid (PLA) Nanofiber-Alginate Hydrogel Bioink Containing Human Adipose-Derived Stem Cells. ACS Biomater. Sci. Eng. 2016, 2, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Diez-Escudero, A.; Andersson, B.; Persson, C.; Hailer, N.P. Hexagonal Pore Geometry and the Presence of Hydroxyapatite Enhance Deposition of Mineralized Bone Matrix on Additively Manufactured Polylactic Acid Scaffolds. Mater. Sci. Eng. C 2021, 125, 112091. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Costa, C.M.; Gómez Ribelles, J.L.; Lanceros-Méndez, S. Tailoring the Morphology and Crystallinity of Poly(L-Lactide Acid) Electrospun Membranes. Sci. Technol. Adv. Mater. 2011, 12, 015001. [Google Scholar] [CrossRef]
- Gómez-Pachón, E.Y.; Sánchez-Arévalo, F.M.; Sabina, F.J.; Maciel-Cerda, A.; Campos, R.M.; Batina, N.; Morales-Reyes, I.; Vera-Graziano, R. Characterisation and Modelling of the Elastic Properties of Poly(Lactic Acid) Nanofibre Scaffolds. J. Mater. Sci. 2013, 48, 8308–8319. [Google Scholar] [CrossRef]
- Echeverría, C.; Limón, I.; Muñoz-Bonilla, A.; Fernández-García, M.; López, D. Development of Highly Crystalline Polylactic Acid with β-Crystalline Phase from the Induced Alignment of Electrospun Fibers. Polymers 2021, 13, 2860. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric Polymers as Biomaterials for Tissue Engineering Applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, P.; Shi, X.; Zhang, G.; Wang, C. Multilevel Structural Stereocomplex Polylactic Acid/Collagen Membranes by Pattern Electrospinning for Tissue Engineering. Polymer 2018, 156, 250–260. [Google Scholar] [CrossRef]
- Fundador, N.G.V.; Takemura, A.; Iwata, T. Structural Properties and Enzymatic Degradation Behavior of PLLA and Stereocomplexed PLA Nanofibers. Macromol. Mater. Eng. 2010, 295, 865–871. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Y.; Shao, J.; Hou, H. The Crystallization Behavior of Poly(l-Lactic Acid)/Poly(d-Lactic Acid) Electrospun Fibers: Effect of Distance of Isomeric Polymers. Ind. Eng. Chem. Res. 2020, 59, 8480–8491. [Google Scholar] [CrossRef]
- Nagahama, K.; Nishimura, Y.; Ohya, Y.; Ouchi, T. Impacts of Stereoregularity and Stereocomplex Formation on Physicochemical, Protein Adsorption and Cell Adhesion Behaviors of Star-Shaped 8-Arms Poly(Ethylene Glycol)-Poly(Lactide) Block Copolymer Films. Polymer 2007, 48, 2649–2658. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.S.; Bhaduri, S. Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics 2019, 11, 5. [Google Scholar] [CrossRef]
- Siqueira, L.; Passador, F.R.; Costa, M.M.; Lobo, A.O.; Sousa, E. Influence of the Addition of β-TCP on the Morphology, Thermal Properties and Cell Viability of Poly (Lactic Acid) Fibers Obtained by Electrospinning. Mater. Sci. Eng. C 2015, 52, 135–143. [Google Scholar] [CrossRef]
- Holderegger, C.; Schmidlin, P.R.; Weber, F.E.; Mohn, D. Preclinical in Vivo Performance of Novel Biodegradable, Electrospun Poly(Lactic Acid) and Poly(Lactic-Co-Glycolic Acid) Nanocomposites: A Review. Materials 2015, 8, 4912–4931. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Zhu, X.; Yu, G.; Zhou, S.; Weng, J. Investigation of Drug Release and Matrix Degradation of Electrospun Poly(DL-Lactide) Fibers with Paracetanol Inoculation. Biomacromolecules 2006, 7, 1623–1629. [Google Scholar] [CrossRef]
- Shahverdi, S.; Hajimiri, M.; Esfandiari, M.A.; Larijani, B.; Atyabi, F.; Rajabiani, A.; Dehpour, A.R.; Gharehaghaji, A.A.; Dinarvand, R. Fabrication and Structure Analysis of Poly(Lactide-Co-Glycolic Acid)/Silk Fibroin Hybrid Scaffold for Wound Dressing Applications. Int. J. Pharm. 2014, 473, 345–355. [Google Scholar] [CrossRef]
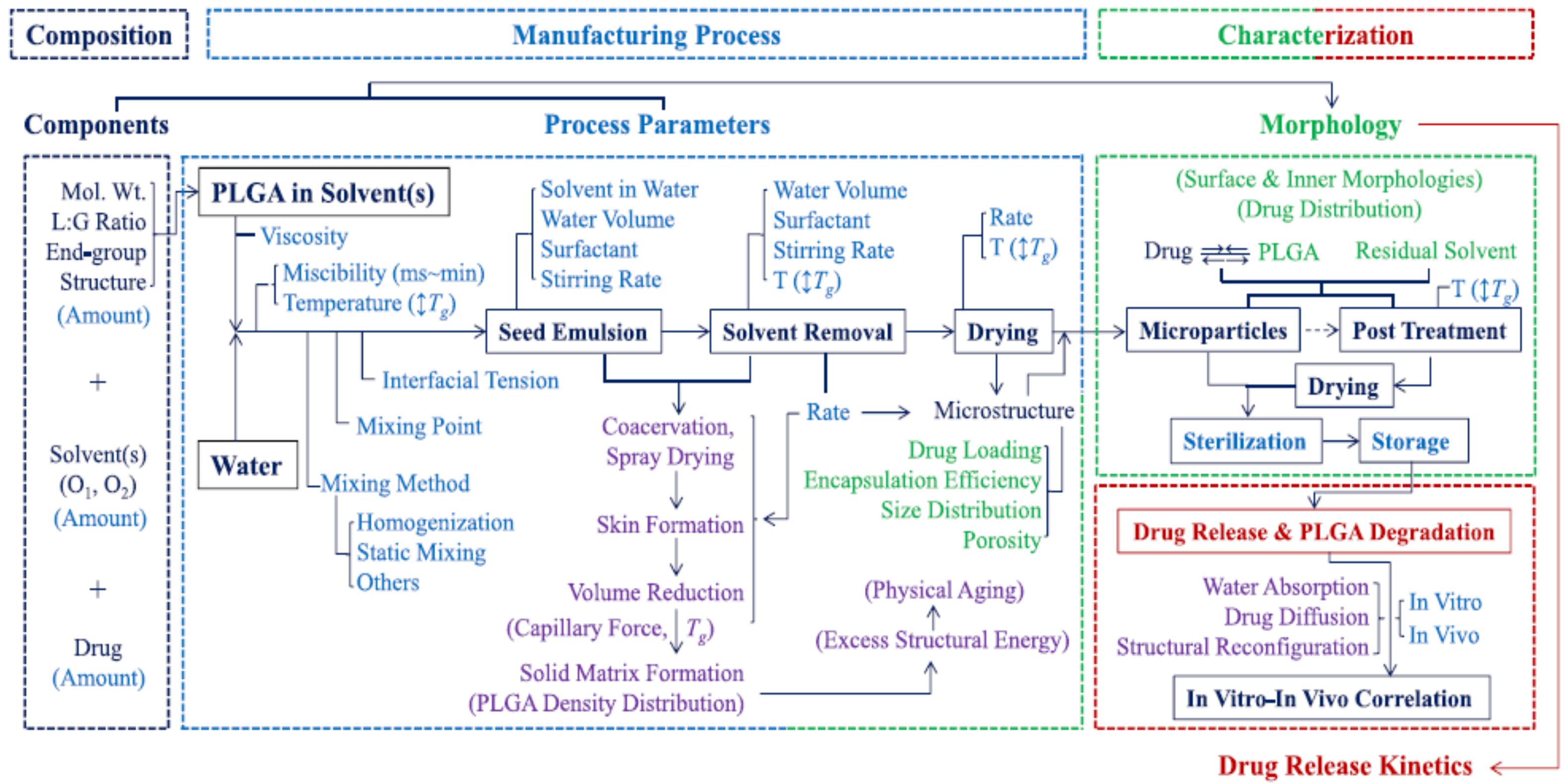
- Park, K.; Otte, A.; Sharifi, F.; Garner, J.; Skidmore, S.; Park, H.; Jhon, Y.K.; Qin, B.; Wang, Y. Formulation Composition, Manufacturing Process, and Characterization of Poly(Lactide-Co-Glycolide) Microparticles. J. Control. Release 2021, 329, 1150–1161. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Y.; Hou, S.; Xu, F.; Zhao, R.; He, J.; Cai, Z.; Li, Y.; Chen, Q. Dual Agents Loaded PLGA Nanoparticles: Systematic Study of Particle Size and Drug Entrapment Efficiency. Eur. J. Pharm. Biopharm. 2008, 69, 445–453. [Google Scholar] [CrossRef]
- Slimane, M.; Gaye, I.; Ghoul, M.; Chebil, L. Mesoscale Modeling and Experimental Study of Quercetin Organization as Nanoparticles in the Poly-Lactic- Co-Glycolic Acid/Water System under Different Conditions. Ind. Eng. Chem. Res. 2020, 59, 4809–4816. [Google Scholar] [CrossRef]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Haloperidol-Loaded PLGA Nanoparticles: Systematic Study of Particle Size and Drug Content. Int. J. Pharm. 2007, 336, 367–375. [Google Scholar] [CrossRef]
- Ahlin Grabnar, P.; Kristl, J. The Manufacturing Techniques of Drug-Loaded Polymeric Nanoparticles from Preformed Polymers. J. Microencapsul. 2011, 28, 323–335. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive Hydrogels in Biomedical Applications A Seven-Year Update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef]
- Yin, X.; Hewitt, D.R.O.; Quah, S.P.; Zheng, B.; Mattei, G.S.; Khalifah, P.G.; Grubbs, R.B.; Bhatia, S.R. Impact of Stereochemistry on Rheology and Nanostructure of PLA-PEO-PLA Triblocks: Stiff Gels at Intermediate l/d-Lactide Ratios. Soft Matter 2018, 14, 7255–7263. [Google Scholar] [CrossRef]
- Yin, X.; Hewitt, D.R.O.; Preston, A.N.; Heroux, L.A.; Agamalian, M.M.; Quah, S.P.; Zheng, B.; Smith, A.J.; Laughlin, S.T.; Grubbs, R.B.; et al. Hierarchical Assembly in PLA-PEO-PLA Hydrogels with Crystalline Domains and Effect of Block Stereochemistry. Colloids Surf. B Biointerfaces 2019, 180, 102–109. [Google Scholar] [CrossRef]
- Moya-Lopez, C.; Juan, A.; Donizeti, M.; Valcarcel, J.; Vazquez, J.A.; Solano, E.; Chapron, D.; Bourson, P.; Bravo, I.; Alonso-Moreno, C.; et al. Multifunctional PLA/Gelatin Bionanocomposites for Tailored Drug Delivery Systems. Pharmaceutics 2022, 14, 1138. [Google Scholar] [CrossRef]
- Wang, X.; Ronsin, O.; Gravez, B.; Farman, N.; Baumberger, T.; Jaisser, F.; Coradin, T.; Hélary, C. Nanostructured Dense Collagen-Polyester Composite Hydrogels as Amphiphilic Platforms for Drug Delivery. Adv. Sci. 2021, 8, 2004213. [Google Scholar] [CrossRef]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef]
- Van De Velde, K.; Kiekens, P. Biopolymers: Overview of Several Properties and Consequences on Their Applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of Poly(Lactic Acid): Characterization of Chemical Structure, Thermal Stability and Mechanical Properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Perego, G.; Cella, G.D.; Bastioli, C. Effect of Molecular Weight and Crystallinity on Poly(Lactic Acid) Mechanical Properties. J. Appl. Polym. Sci. 1996, 59, 37–43. [Google Scholar] [CrossRef]
- Li, G.; Yang, B.; Han, W.; Li, H.; Kang, Z.; Lin, J. Tailoring the Thermal and Mechanical Properties of Injection-Molded Poly (Lactic Acid) Parts through Annealing. J. Appl. Polym. Sci. 2021, 138, 49648. [Google Scholar] [CrossRef]
- Cocca, M.; Di Lorenzo, M.L.; Malinconico, M.; Frezza, V. Influence of Crystal Polymorphism on Mechanical and Barrier Properties of Poly(l-Lactic Acid). Eur. Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; He, Y.; Dong, Z.; Zhang, X.; Chen, X.; Liu, T. Effect of Poly(Lactic Acid) Crystallization on Its Mechanical and Heat Resistance Performances. Polymer 2021, 212, 123280. [Google Scholar] [CrossRef]
- Tsuji, H. Properties and Morphologies of Poly(L-Lactide): 1. Annealing Condition Effects on Properties and Morphologies of Poly(L-Lactide). Polymer 1995, 36, 2709–2716. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex Formation between Enantiomeric Poly(Lactic Acid)s. XI. Mechanical Properties and Morphology of Solution-Cast Films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
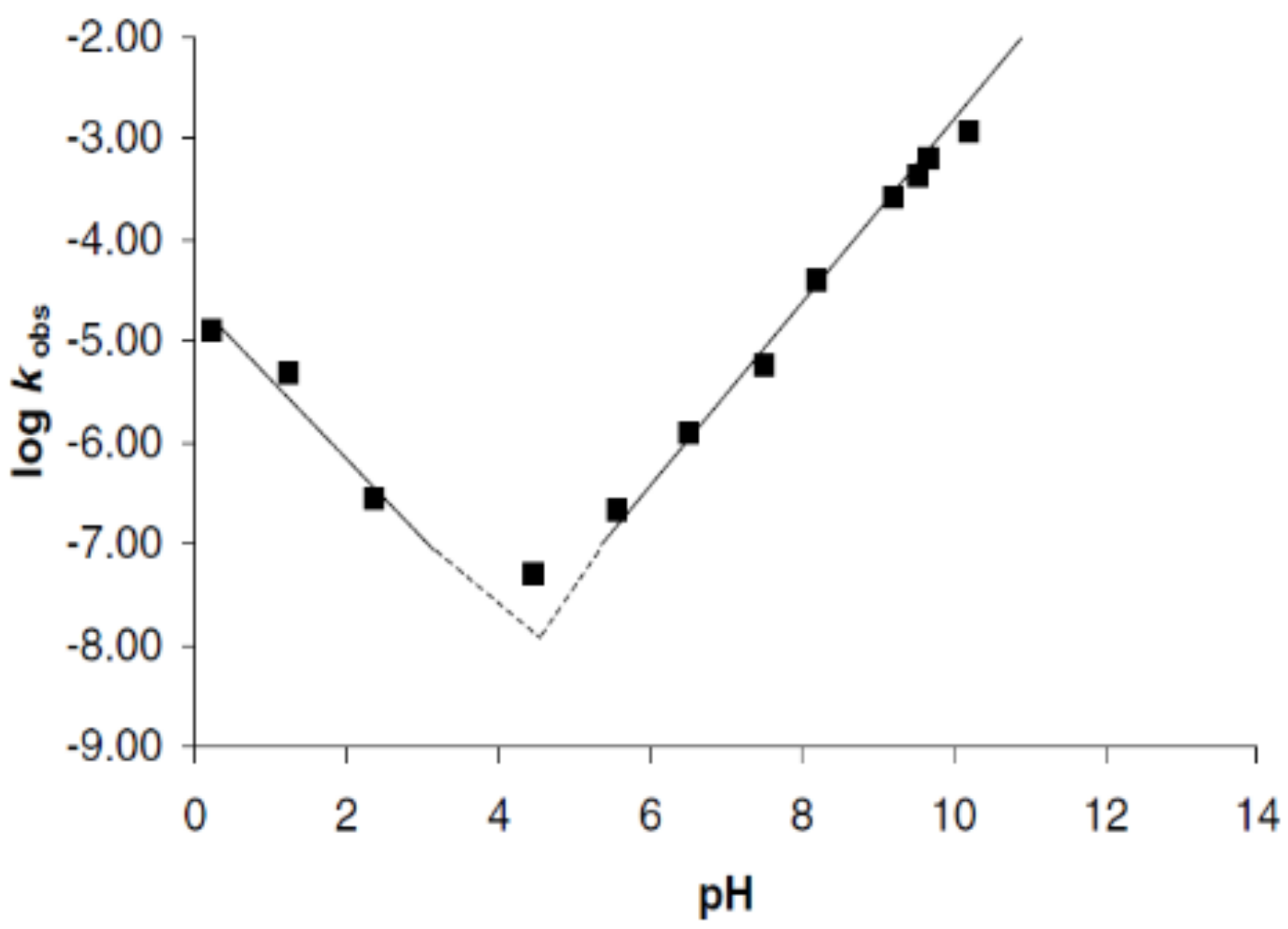
- Tsuji, H. Hydrolytic Degradation. In Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 345–382. ISBN 9780470293669. [Google Scholar]
- Sin, L.T.; Tueen, B.S. Degradation and Stability of Poly(Lactic Acid); Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128144725. [Google Scholar]
- Siparsky, G.L.; Voorhees, K.J.; Miao, F. Hydrolysis of Polylactic Acid (PLA) and Polycaprolactone (PCL) in Aqueous Acetonitrile Solutions: Autocatalysis. J. Environ. Polym. Degrad. 1998, 6, 31–41. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Hydrolysis and Biodegradation of Poly(Lactic Acid). In Advances in Polymer Science; Springer: New York, NY, USA, 2018; Volume 279, pp. 119–151. [Google Scholar]
- Celikkaya, E.; Denkbas, E.B.; Piskin, E. Poly(DL-Lactide)/Poly(Ehylene Glycol) Copolymer Particles. Preparation and Characterization. J. Appl. Polym. Sci. 1996, 61, 1439–1446. [Google Scholar] [CrossRef]
- Saha, S.K.; Tsuji, H. Effects of Molecular Weight and Small Amounts of D-Lactide Units on Hydrolytic Degradation of Poly(l-Lactic Acid)S. Polym. Degrad. Stab. 2006, 91, 1665–1673. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and Morphology of Poly(L-Lactide). III. Effects of Initial Crystallinity on Long-Term in Vitro Hydrolysis of High Molecular Weight Poly(L-Lactide) Film in Phosphate-Buffered Solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Tsuji, H. In Vitro Hydrolysis of Blends from Enantiomeric Poly(Lactide)s. Part 4: Well-Homo-Crystallized Blend and Nonblended Films. Polymer 2000, 41, 3621–3630. [Google Scholar] [CrossRef]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic Degradation of Devices Based on Poly(Dl-Lactic Acid) Size-Dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef]
- De Jong, S.J.; Arias, E.R.; Rijkers, D.T.S.; Van Nostrum, C.F.; Kettenes-Van Den Bosch, J.J.; Hennink, W.E. New Insights into the Hydrolytic Degradation of Poly(Lactic Acid): Participation of the Alcohol Terminus. Polymer 2001, 42, 2795–2802. [Google Scholar] [CrossRef]
- Schliecker, G.; Schmidt, C.; Fuchs, S.; Kissel, T. Characterization of a Homologous Series of D,L-Lactic Acid Oligomers; a Mechanistic Study on the Degradation Kinetics in Vitro. Biomaterials 2003, 24, 3835–3844. [Google Scholar] [CrossRef]
- Shih, C. Controlled Release Chain-End Scission in Acid Catalyzed Hydrolysis of Poly(D, L-Lactide) in Solution. J. Control. Release 1995, 34, 9–15. [Google Scholar] [CrossRef]
- Hayman, D.; Bergerson, C.; Miller, S.; Moreno, M.; Moore, J.E. The Effect of Static and Dynamic Loading on Degradation of PLLA Stent Fibers. J. Biomech. Eng. 2014, 136, 081006. [Google Scholar] [CrossRef]
- Blasi, P. Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid)-Based Microparticles: An Overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef]
- Valantin, M.A.; Aubron-Olivier, C.; Ghosn, J.; Laglenne, E.; Pauchard, M.; Schoen, H.; Bousquet, R.; Katz, P.; Costagliola, D.; Katlama, C. Polylactic Acid Implants (New-Fill)® to Correct Facial Lipoatrophy in HIV-Infected Patients: Results of the Open-Label Study VEGA. Aids 2003, 17, 2471–2477. [Google Scholar] [CrossRef]
- Fitzgerald, R.; Bass, L.M.; Goldberg, D.J.; Graivier, M.H.; Lorenc, Z.P. Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA). Aesthetic Surg. J. 2018, 38, S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- De Mattos, A.C.; Altmeyer, C.; Toyomi, T.; Maissar, N.; Mara, R. Polymeric Nanoparticles for Oral Delivery of 5- Fluorouracil: Formulation Optimization, Cytotoxicity Assay and Pre-Clinical Pharmacokinetics Study. Eur. J. Pharm. Sci. 2016, 84, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Estupiñán, Ó.; Niza, E.; Bravo, I.; Rey, V.; Tornín, J.; Gallego, B.; Casares, P.C.; Moris, F.; Ocaña, A.; Lorenzo, V.B.; et al. Mithramycin Delivery Systems to Develop Effective Therapies in Sarcomas. J. Nanobiotechnol. 2021, 19, 267. [Google Scholar] [CrossRef] [PubMed]
- Campos de Mello, C.G.; Branquinho, T.; Oliveira, T.; Milagre, M. Efficacy of Lychnopholide Polymeric Nanocapsules after Oral and Intravenous Administration in Murine Experimental Chagas Disease. Antimicrob. Agents Chemother. 2016, 60, 5215–5222. [Google Scholar] [CrossRef]
- Zhang, C.; Wan, X.; Zheng, X.; Shao, X.; Liu, Q.; Zhang, Q.; Qian, Y. Dual-Functional Nanoparticles Targeting Amyloid Plaques in the Brains of Alzheimer’s Disease Mice. Biomaterials 2014, 35, 456–465. [Google Scholar] [CrossRef]
- Sun, S.; Liang, N.; Piao, H.; Yamamoto, H.; Kawashima, Y.; Cui, F. Insulin-S.O (Sodium Oleate) Complex-Loaded PLGA Nanoparticles: Formulation, Characterization and in Vivo Evaluation. J. Microencapsul. 2010, 27, 471–478. [Google Scholar] [CrossRef]
- Cimas, F.J.; Niza, E.; Juan, A.; Noblejas-López, M.D.M.; Bravo, I.; Lara-Sanchez, A.; Alonso-Moreno, C.; Ocaña, A. Controlled Delivery of Bet-Protacs: In Vitro Evaluation of MZ1-Loaded Polymeric Antibody Conjugated Nanoparticles in Breast Cancer. Pharmaceutics 2020, 12, 986. [Google Scholar] [CrossRef]
- Xia, H.; Gao, X.; Gu, G.; Liu, Z.; Hu, Q.; Tu, Y.; Song, Q.; Yao, L.; Pang, Z.; Jiang, X.; et al. Penetratin-Functionalized PEG-PLA Nanoparticles for Brain Drug Delivery. Int. J. Pharm. 2012, 436, 840–850. [Google Scholar] [CrossRef]
- Hu, Q.; Gao, X.; Gu, G.; Kang, T.; Tu, Y.; Liu, Z.; Song, Q.; Yao, L.; Pang, Z.; Jiang, X.; et al. Glioma Therapy Using Tumor Homing and Penetrating Peptide-Functionalized PEG-PLA Nanoparticles Loaded with Paclitaxel. Biomaterials 2013, 34, 5640–5650. [Google Scholar] [CrossRef]
- Thomsen, T.; Reissmann, R.; Kaba, E.; Engelhardt, B.; Klok, H. Covalent and Noncovalent Conjugation of Degradable Polymer Nanoparticles to T Lymphocytes. Biomacromolecules 2021, 22, 3416–3430. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Otte, A.; Sharifi, F.; Garner, J.; Skidmore, S.; Park, H.; Jhon, Y.K.; Qin, B.; Wang, Y. Potential Roles of the Glass Transition Temperature of PLGA Microparticles in Drug Release Kinetics. Mol. Pharm. 2021, 18, 18–32. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Oliveira, J.W.; da Silva, M.F.A.; Damasceno, I.Z.; Rocha, H.A.O.; da Silva Júnior, A.A.; Silva, M.S. In Vitro Validation of Antiparasitic Activity of PLA-Nanoparticles of Sodium Diethyldithiocarbamate against Trypanosoma Cruzi. Pharmaceutics 2022, 14, 497. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kunduru, K.R.; Basu, A.; Mizrahi, B.; Domb, A.J.; Khan, W. Injectable Formulations of Poly(Lactic Acid) and Its Copolymers in Clinical Use. Adv. Drug Deliv. Rev. 2016, 107, 213–227. [Google Scholar] [CrossRef]
- Basu, A.; Kunduru, K.R.; Doppalapudi, S.; Domb, A.J.; Khan, W. Poly(Lactic Acid) Based Hydrogels. Adv. Drug Deliv. Rev. 2016, 107, 192–205. [Google Scholar] [CrossRef]
- Sokolsky-Papkov, M.; Domb, A.J. Stereoisomeric Effect on in Vitro Drug Release from Injectable Poly(Lactic Acid Co Castor Oil) Polyesters. Polym. Adv. Technol. 2008, 19, 671–679. [Google Scholar] [CrossRef]
- Le, T.M.D.; Nguyen, V.V.L.; Trinh, T.A.; Pham, N.S.; Lee, D.S.; Huynh, D.P. Sulfonamide Functionalized Amino Acid-Based PH- and Temperature-Sensitive Biodegradable Injectable Hydrogels: Synthesis, Physicochemical Characterization and in Vivo Degradation Kinetics. J. Appl. Polym. Sci. 2021, 138, 50488. [Google Scholar] [CrossRef]
- Mao, H.; Shan, G.; Bao, Y.; Wu, Z.L.; Pan, P. Thermoresponsive Physical Hydrogels of Poly(Lactic Acid)/Poly(Ethylene Glycol) Stereoblock Copolymers Tuned by Stereostructure and Hydrophobic Block Sequence. Soft Matter 2016, 12, 4628–4637. [Google Scholar] [CrossRef]
- Arun, Y.; Ghosh, R.; Domb, A.J. Biodegradable Hydrophobic Injectable Polymers for Drug Delivery and Regenerative Medicine. Adv. Funct. Mater. 2021, 31, 2010284. [Google Scholar] [CrossRef]
- Steinman, N.Y.; Domb, A.J. Instantaneous Degelling Thermoresponsive Hydrogel. Gels 2021, 7, 169. [Google Scholar] [CrossRef]
- Kim, T.; Suh, J.; Kim, W.J. Polymeric Aggregate-Embodied Hybrid Nitric-Oxide-Scavenging and Sequential Drug-Releasing Hydrogel for Combinatorial Treatment of Rheumatoid Arthritis. Adv. Mater. 2021, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; Polini, A.; Petre, D.G.; Zhang, Y.; Leeuwenburgh, S.C.G. Fiber-Reinforced Colloidal Gels as Injectable and Moldable Biomaterials for Regenerative Medicine. Mater. Sci. Eng. C 2018, 92, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.K.; Sohn, Y.S.; Jeong, B. Stereoisomeric Effect on Reverse Thermal Gelation of Poly(Ethylene Glycol)/Poly(Lactide) Multiblock Copolymer. Macromolecules 2007, 40, 5111–5115. [Google Scholar] [CrossRef]
- Sanabria-DeLong, N.; Agrawal, S.K.; Bhatia, S.R.; Tew, G.N. Controlling Hydrogel Properties by Crystallization of Hydrophobic Domains. Macromolecules 2006, 39, 1308–1310. [Google Scholar] [CrossRef]
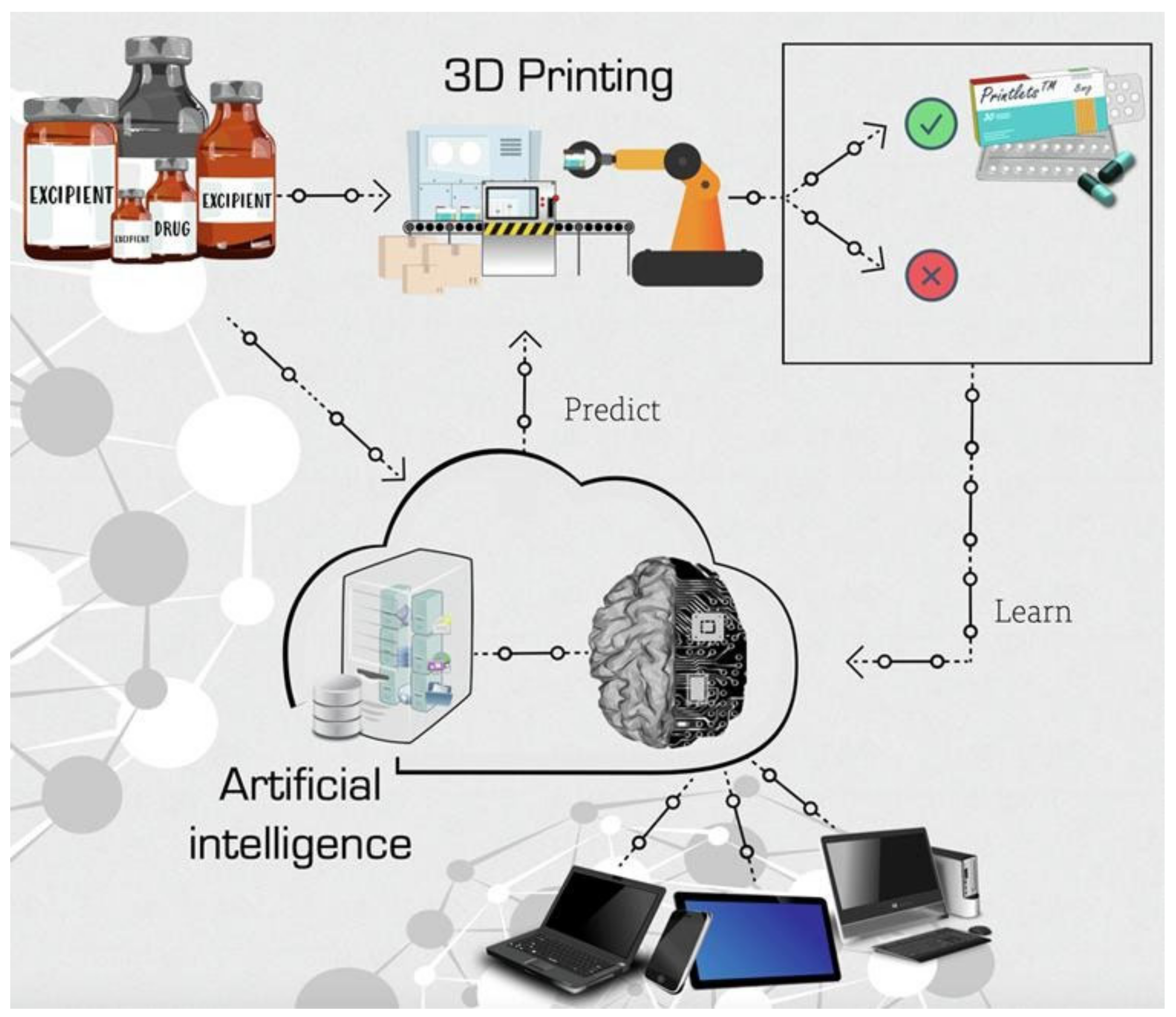
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D Printing: An Appealing Route for Customized Drug Delivery Systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef] [PubMed]
- NHS. England Improving Outcomes through Personalised Medicine; NHS England: London, UK, 2016; pp. 1–18. [Google Scholar]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D Printed Pharmaceuticals: From Hype to Real-World Clinical Applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.K.; Lorber, B.; Reitsamer, H.; Khinast, J. 3D Printing of Oral Drugs: A New Reality or Hype? Expert Opin. Drug Deliv. 2018, 15, 1–4. [Google Scholar] [CrossRef]
- Aprecia Pharmaceuticals. Available online: https://www.spritam.com/ (accessed on 15 June 2022).
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D Printing of Five-in-One Dose Combination Polypill with Defined Immediate and Sustained Release Profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D Printed Oral Solid Dosage Forms Containing Hydrochlorothiazide for Controlled Drug Delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Development of a Biodegradable Subcutaneous Implant for Prolonged Drug Delivery Using 3D Printing. Pharmaceutics 2020, 12, 105. [Google Scholar] [CrossRef] [PubMed]
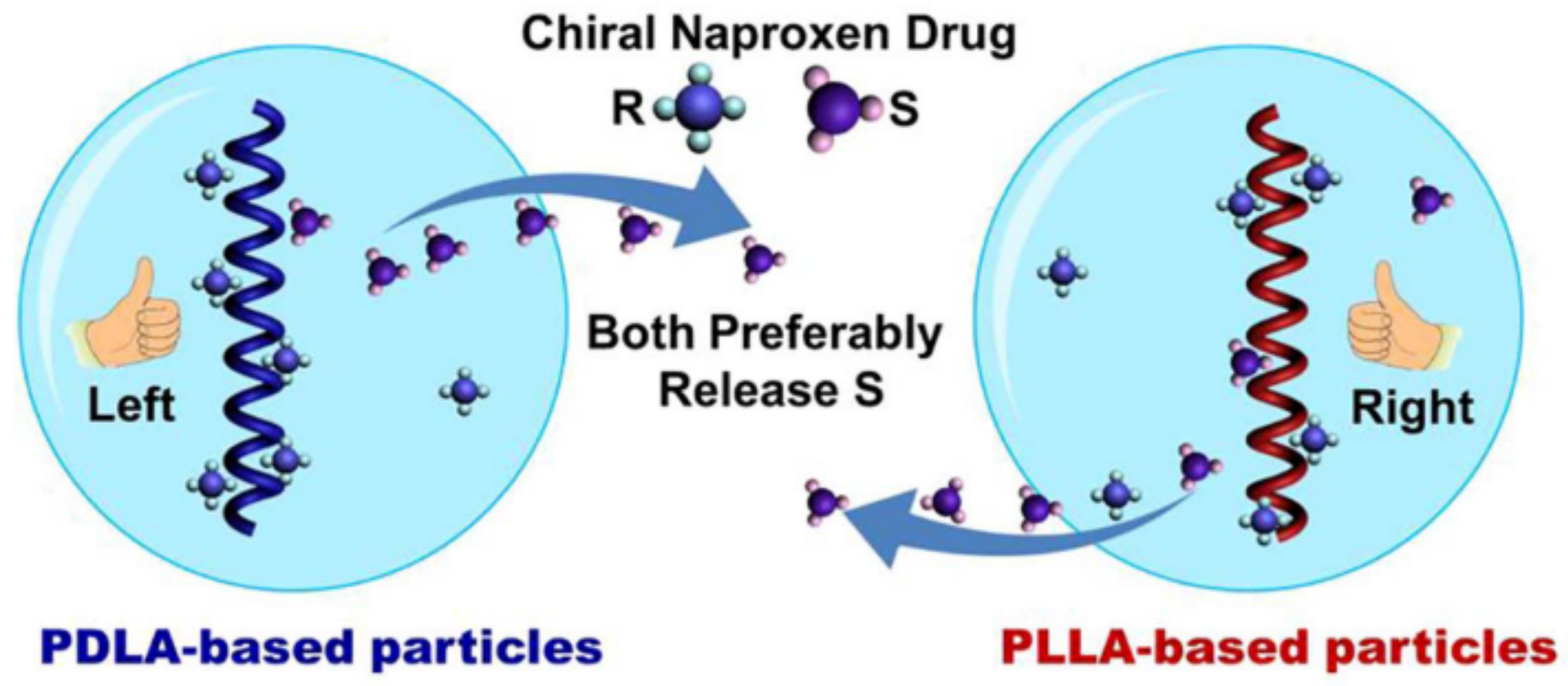
- Liang, J.; Yang, B.; Deng, J. Polylactide-Based Chiral Particles with Enantio-Differentiating Release Ability. Chem. Eng. J. 2018, 344, 262–269. [Google Scholar] [CrossRef]
- Liang, J.; Deng, J. Chiral Particles Consisting of Helical Polylactide and Helical Substituted Polyacetylene: Preparation and Synergistic Effects in Enantio-Differentiating Release. Macromolecules 2018, 51, 4003–4011. [Google Scholar] [CrossRef]
- Slager, J.; Domb, A.J. Biopolymer Stereocomplexes. Adv. Drug Deliv. Rev. 2003, 55, 549–583. [Google Scholar] [CrossRef]
- Slager, J.; Domb, A.J. Stereocomplexes Based on Poly(Lactic Acid) and Insulin: Formulation and Release Studies. Biomaterials 2002, 23, 4389–4396. [Google Scholar] [CrossRef]
- Bishara, A.; Domb, A.J. PLA Stereocomplexes for Controlled Release of Somatostatin Analogue. J. Control. Release 2005, 107, 474–483. [Google Scholar] [CrossRef]
- Fraschini, C.; Jalabert, M.; Prud’homme, R.E. Physical Characterization of Blends of Poly(D-Lactide) and LHRH (A Leuprolide Decapeptide Analog). Biomacromolecules 2005, 6, 3112–3118. [Google Scholar] [CrossRef]
- Slager, J.; Domb, A.J. Hetero-Stereocomplexes of D-Poly(Lactic Acid) and the LHRH Analogue Leuprolide. Application in Controlled Release. Eur. J. Pharm. Biopharm. 2004, 58, 461–469. [Google Scholar] [CrossRef]
- Zhang, J.; Beshra, A.; Domb, A.J.; Ozaki, Y. D-Poly(Lactide) and LHRH Decapeptide Stereointeractions Investigated by Vibrational Spectroscopy. Eur. Polym. J. 2007, 43, 3016–3027. [Google Scholar] [CrossRef]
- Senapati, S.; Upadhyaya, A.; Dhruw, S.; Giri, D.; Maiti, P. Controlled DNA Delivery Using Poly(Lactide) Nanoparticles and Understanding the Binding Interactions. J. Phys. Chem. B 2021, 125, 10009–10017. [Google Scholar] [CrossRef]
- Prabha, S.; Labhasetwar, V. Critical Determinants in PLGA/PLA Nanoparticle-Mediated Gene Expression. Pharm. Res. 2004, 21, 354–364. [Google Scholar] [CrossRef]
- Mansoor, F.; Earley, B.; Cassidy, J.P.; Markey, B.; Doherty, S.; Welsh, M.D. Comparing the Immune Response to a Novel Intranasal Nanoparticle PLGA Vaccine and a Commercial BPI3V Vaccine in Dairy Calves. BMC Vet. Res. 2015, 11, 220. [Google Scholar] [CrossRef]
- Zare, S.; Kabiri, M.; Amini, Y.; Najafi, A.; Mohammadpour, F.; Ayati, S.H.; Nikpoor, A.R.; Tafaghodi, M. Immunological Assessment of Chitosan or Trimethyl Chitosan-Coated PLGA Nanospheres Containing Fusion Antigen as the Novel Vaccine Candidates Against Tuberculosis. AAPS PharmSciTech 2022, 23, 15. [Google Scholar] [CrossRef]
- Olmos, D.; González-benito, J. Polymeric Materials with Antibacterial Activity: A Review. Polymers 2021, 13, 613. [Google Scholar] [CrossRef]
- Fortunati, E.; Rinaldi, S.; Peltzer, M.; Bloise, N.; Visai, L.; Armentano, I.; Jiménez, A.; Latterini, L.; Kenny, J.M. Nano-Biocomposite Films with Modified Cellulose Nanocrystals and Synthesized Silver Nanoparticles. Carbohydr. Polym. 2014, 101, 1122–1133. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional Bionanocomposite Films of Poly(Lactic Acid), Cellulose Nanocrystals and Silver Nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- González, E.A.S.; Olmos, D.; Lorente, M.Á.; Vélaz, I.; González-Benito, J. Preparation and Characterization of Polymer Composite Materials Based on PLA/TiO2 for Antibacterial Packaging. Polymers 2018, 10, 1365. [Google Scholar] [CrossRef]
- Salmieri, S.; Islam, F.; Khan, R.A.; Hossain, F.M.; Ibrahim, H.M.M.; Miao, C.; Hamad, W.Y.; Lacroix, M. Antimicrobial Nanocomposite Films Made of Poly(Lactic Acid)–Cellulose Nanocrystals (PLA–CNC) in Food Applications—Part B: Effect of Oregano Essential Oil Release on the Inactivation of Listeria Monocytogenes in Mixed Vegetables. Cellulose 2014, 21, 4271–4285. [Google Scholar] [CrossRef]
- PLACTIVE AN1 Copper3D. Available online: https://filament2print.com/es/pla-especial/994-plactive-copper3d-antibacteriano.html (accessed on 15 June 2022).
- PLActive Red. Available online: https://www.3djake.es/copper-3d/plactive-red (accessed on 15 June 2022).
- Zuniga, J.M. 3D Printed Antibacterial Prostheses. Appl. Sci. 2018, 8, 1651. [Google Scholar] [CrossRef]
- Alam, F.; Shukla, V.R.; Varadarajan, K.M.; Kumar, S. Microarchitected 3D Printed Polylactic Acid (PLA) Nanocomposite Scaffolds for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2020, 103, 103576. [Google Scholar] [CrossRef]
- Liu, C.; Shen, J.; Yeung, K.W.K.; Tjong, S.C. Development and Antibacterial Performance of Novel Polylactic Acid-Graphene Oxide-Silver Nanoparticle Hybrid Nanocomposite Mats Prepared by Electrospinning. ACS Biomater. Sci. Eng. 2017, 3, 471–486. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Villani, F.; Rizzo, A.; Caputo, I.; Paolella, G.; Vigliotta, G. Antibacterial Al-Doped ZnO Coatings on PLA Films. J. Mater. Sci. 2020, 55, 4830–4847. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, J.; Qin, C.; Xu, A.; Zhang, Z.; Lin, S.; Ren, X.; Zhang, P. Spin-Coating Synthesis and Characterization of Zn-Doped Hydroxyapatite/Polylactic Acid Composite Coatings. Surf. Coatings Technol. 2016, 307, 461–469. [Google Scholar] [CrossRef]
- Vicent, M.J.; Duncan, R. Polymer Conjugates: Nanosized Medicines for Treating Cancer. Trends Biotechnol. 2006, 24, 39–47. [Google Scholar] [CrossRef]
- Duncan, R.; Vicent, M.J. Polymer Therapeutics-Prospects for 21st Century: The End of the Beginning. Adv. Drug Deliv. Rev. 2013, 65, 60–70. [Google Scholar] [CrossRef]
- Tong, R.; Cheng, J. Drug-Initiated, Controlled Ring-Opening Polymerization for the Synthesis of Polymer−Drug Conjugates. Macromolecules 2012, 45, 2225–2232. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.; Law, W.; Mok, J.; Zou, J.; Prasad, P.N.; Cheng, C. Well-Defined Degradable Brush Polymer−Drug Conjugates for Sustained Delivery of Paclitaxel. Mol. Pharm. 2013, 10, 867–874. [Google Scholar] [CrossRef]
- Danafar, H.; Rostamizadeh, K.; Davaran, S.; Hamidi, M. Drug-Conjugated PLA–PEG–PLA Copolymers: A Novel Approach for Controlled Delivery of Hydrophilic Drugs by Micelle Formation. Pharm. Dev. Technol. 2017, 22, 947–957. [Google Scholar] [CrossRef]
- Sanchez-Bodon, J.; Ruiz-rubio, L.; Hernaez-Laviña, E.; Vilas-vilela, J.L.; Moreno-Benitez, M.I. Poly(L-Lactide)-Based Anti-Inflammatory Responsive Surfaces for Surgical Implants. Polymers 2021, 13, 34. [Google Scholar] [CrossRef]
- Plichta, A.; Kowalczyk, S.; Kamiński, K.; Wasyaeczko, M.; Wieckowski, S.; Oledzka, E.; Naecz-Jawecki, G.; Zgadzaj, A.; Sobczak, M. ATRP of Methacrylic Derivative of Camptothecin Initiated with PLA toward Three-Arm Star Block Copolymer Conjugates with Favorable Drug Release. Macromolecules 2017, 50, 6439–6450. [Google Scholar] [CrossRef]
- Litowczenko, J.; Woźniak-Budych, M.J.; Staszak, K.; Wieszczycka, K.; Jurga, S.; Tylkowski, B. Milestones and Current Achievements in Development of Multifunctional Bioscaffolds for Medical Application. Bioact. Mater. 2021, 6, 2412–2438. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive Polymeric Scaffolds for Tissue Engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, K.; Kitakami, E.; Kobayashi, S.; Hoshiba, T.; Fukushima, K. Design of Biocompatible and Biodegradable Polymers Based on Intermediate Water Concept. Polym. J. 2015, 47, 114–121. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Ju, H.W.; Moon, B.M.; Lee, O.J. Hybrid Scaffolds Based on PLGA and Silk for Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2016, 10, 209–221. [Google Scholar] [CrossRef]
- Cai, J.; Peng, X.; Nelson, K.D.; Eberhart, R.; Smith, G.M. Permeable Guidance Channels Containing Microfilament Scaffolds Enhance Axon Growth and Maturation. J. Biomed. Mater. Res.-Part A 2005, 75, 374–386. [Google Scholar] [CrossRef]
- Deng, D.; Wang, W.; Wang, B.; Zhang, P.; Zhou, G.; Zhang, W.J.; Cao, Y.; Liu, W. Repair of Achilles Tendon Defect with Autologous ASCs Engineered Tendon in a Rabbit Model. Biomaterials 2014, 35, 8801–8809. [Google Scholar] [CrossRef]
- Ngo, H.X.; Bai, Y.; Sha, J.; Ishizuka, S.; Toda, E.; Osako, R.; Kato, A.; Morioka, R.; Ramanathan, M.; Tatsumi, H.; et al. A Narrative Review of U-HA/PLLA, a Bioactive Resorbable Reconstruction Material: Applications in Oral and Maxillofacial Surgery. Materials 2022, 15, 150. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Katase, N.; Shibata, A.; Takahashi, Y.; Furuki, Y. Clinical Evaluation of an Unsintered Hydroxyapatite/Poly-l-Lactide Osteoconductive Composite Device for the Internal Fixation of Maxillofacial Fractures. J. Craniofac. Surg. 2016, 27, 1391–1397. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, J.H. Clinical Courses and Degradation Patterns of Absorbable Plates in Facial Bone Fracture Patients. Arch. Craniofacial Surg. 2019, 20, 297–303. [Google Scholar] [CrossRef]
- El Khatib, M.; Mauro, A.; Di Mattia, M.; Wyrwa, R.; Schweder, M.; Ancora, M.; Lazzaro, F.; Berardinelli, P.; Valbonetti, L.; Di Giacinto, O.; et al. Electrospun PLGA Fiber Diameter and Alignment of Tendon Biomimetic Fleece Potentiate Tenogenic Differentiation and Immunomodulatory Function of Amniotic Epithelial Stem Cells. Cells 2020, 9, 1207. [Google Scholar] [CrossRef]
- Russo, V.; El Khatib, M.; di Marcantonio, L.; Ancora, M.; Wyrwa, R.; Mauro, A.; Walter, T.; Weisser, J.; Citeroni, M.R.; Lazzaro, F.; et al. Tendon Biomimetic Electrospun PLGA Fleeces Induce an Early Epithelial-Mesenchymal Transition and Tenogenic Differentiation on Amniotic Epithelial Stem Cells. Cells 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Ciardulli, M.C.; Marino, L.; Lovecchio, J.; Giordano, E.; Forsyth, N.R.; Selleri, C.; Maffulli, N.; Porta, G. Della Tendon and Cytokine Marker Expression by Human Bone Marrow Mesenchymal Stem Cells in a Hyaluronate/Poly-Lactic-Co-Glycolic Acid (PLGA)/Fibrin Three-Dimensional (3D) Scaffold. Cells 2020, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Toh, S.L.; Goh, J.C.H. A BFGF-Releasing Silk/PLGA-Based Biohybrid Scaffold for Ligament/Tendon Tissue Engineering Using Mesenchymal Progenitor Cells. Biomaterials 2010, 31, 2990–2998. [Google Scholar] [CrossRef]
- Ouyang, H.W.; Goh, J.C.H.; Thambyah, A.; Teoh, S.H.; Lee, E.H. Knitted Poly-Lactide-Co-Glycolide Scaffold Loaded with Bone Marrow Stromal Cells in Repair and Regeneration of Rabbit Achilles Tendon. Tissue Eng. 2003, 9, 431–439. [Google Scholar] [CrossRef]
- Wang, C.; Feng, N.; Chang, F.; Wang, J.; Yuan, B.; Cheng, Y.; Liu, H.; Yu, J.; Zou, J.; Ding, J.; et al. Injectable Cholesterol-Enhanced Stereocomplex Polylactide Thermogel Loading Chondrocytes for Optimized Cartilage Regeneration. Adv. Healthc. Mater. 2019, 8, 1900312. [Google Scholar] [CrossRef]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone Fracture Healing in Mechanobiological Modeling: A Review of Principles and Methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials Evolution of Bone Plates for Internal Fixation of Bone Fractures: A Review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Felfel, R.M.; Ahmed, I.; Parsons, A.J.; Rudd, C.D. Bioresorbable Composite Screws Manufactured via Forging Process: Pull-out, Shear, Flexural and Degradation Characteristics. J. Mech. Behav. Biomed. Mater. 2013, 18, 108–122. [Google Scholar] [CrossRef]
- Zan, J.; Qian, G.; Deng, F.; Zhang, J.; Zeng, Z.; Peng, S.; Shuai, C. Dilemma and Breakthrough of Biodegradable Poly-l-Lactic Acid in Bone Tissue Repair. J. Mater. Res. Technol. 2022, 17, 2369–2387. [Google Scholar] [CrossRef]
- Waris, E.; Konttinen, Y.T.; Ashammakhi, N.; Suuronen, R.; Santavirta, S. Bioabsorbable Fixation Devices in Trauma and Bone Surgery: Current Clinical Standing. Expert Rev. Med. Devices 2004, 1, 229–240. [Google Scholar] [CrossRef]
- Ngo, H.X.; Dong, Q.N.; Bai, Y.; Sha, J.; Ishizuka, S.; Okui, T.; Sukegawa, S.; Kanno, T. Bone Regeneration Capacity of Newly Developed Uncalcined/Unsintered Hydroxyapatite and Poly-l-Lactide-Co-Glycolide Sheet in Maxillofacial Surgery: An in Vivo Study. Nanomaterials 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Aihemaiti, P.; Jiang, H.; Aiyiti, W.; Kasimu, A. Optimization of 3D Printing Parameters of Biodegradable Polylactic Acid/Hydroxyapatite Composite Bone Plates. Int. J. Bioprint. 2022, 8, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef] [PubMed]
- Lampe, K.J.; Namba, R.M.; Silverman, T.R.; Bjugstad, K.B. Impact of Lactic Acid on Cell Proliferation and Free Radical Induced Cell Death in Monolayer Cultures of Neural Precursor Cells. Biotechnol. Bioeng. 2009, 15, 1214–1223. [Google Scholar] [CrossRef]
- Tuan, R.S.; Chen, A.F.; Klatt, B.A. Cartilage Regeneration. J. Am. Acad. Orthop. Surg. 2013, 21, 303–311. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite Design of Injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef]
- Yu, F.; Li, M.; Yuan, Z.; Rao, F.; Fang, X.; Jiang, B.; Wen, Y.; Zhang, P. Mechanism Research on a Bioactive Resveratrol– PLA–Gelatin Porous Nano-Scaffold in Promoting the Repair of Cartilage Defect. Int. J. Nanomed. 2018, 13, 7845–7858. [Google Scholar] [CrossRef]
- Abbott FDA Approves Abbott’s AbsorbTM Bioresorbable Stent, the Only Fully Dissolving Heart Stent. 2016. Available online: https://abbott.mediaroom.com/2016-07-05-FDA-approves-Abbotts-Absorb-bioresorbable-stent-the-only-fully-dissolving-heart-stent (accessed on 15 June 2022).
- Ellis, S.G.; Kereiakes, D.J.; Metzger, D.C.; Caputo, R.P.; Rizik, D.G.; Teirstein, P.S.; Litt, M.R.; Kini, A.; Kabour, A.; Marx, S.O.; et al. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N. Engl. J. Med. 2015, 373, 1905–1915. [Google Scholar] [CrossRef]
- Sorrentino, S.; Giustino, G.; Mehran, R.; Kini, A.S.; Sharma, S.K.; Faggioni, M.; Farhan, S.; Vogel, B.; Indolfi, C.; Dangas, G.D. Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents. J. Am. Coll. Cardiol. 2017, 69, 3055–3066. [Google Scholar] [CrossRef]
- Yamaji, K.; Ueki, Y.; Souteyrand, G.; Daemen, J.; Wiebe, J.; Nef, H.; Adriaenssens, T.; Loh, J.P.; Lattuca, B.; Wykrzykowska, J.J.; et al. Mechanisms of Very Late Bioresorbable Scaffold Thrombosis: The INVEST Registry. J. Am. Coll. Cardiol. 2017, 70, 2330–2344. [Google Scholar] [CrossRef]
- Kraak, R.P.; Kajita, A.H.; Garcia-Garcia, H.M.; Henriques, J.P.S.; Piek, J.J.; Arkenbout, E.K.; van der Schaaf, R.J.; Tijssen, J.G.P.; de Winter, R.J.; Wykrzykowska, J.J. Scaffold Thrombosis Following Implantation of the ABSORB BVS in Routine Clinical Practice: Insight into Possible Mechanisms from Optical Coherence Tomography. Catheter. Cardiovasc. Interv. 2018, 92, E106–E114. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Jinnouchi, H.; Torii, S.; Virmani, R.; Finn, A.V. Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View. Bioengineering 2018, 5, 71. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, J.; Liu, M.; Tian, Y.; Cheng, J.; Liu, W.; Ni, Z. Influence of Parameters on Mechanical Properties of Poly (L-Lactic Acid) Helical Stents. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2022, 110, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Onuma, Y.; Brugaletta, S.; Garcia-Garcia, H.M.; Backx, B.; Iñiguez, A.; Jensen, L.O.; Cequier-Fillat, À.; Pilgrim, T.; Christiansen, E.H.; et al. Arterial Healing Following Primary PCI Using the Absorb Everolimus-Eluting Bioresorbable Vascular Scaffold (Absorb BVS) versus the Durable Polymer Everolimus-Eluting Metallic Stent (XIENCE) in Patients with Acute ST-Elevation Myocardial Infarction: Ration. EuroIntervention 2016, 12, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhao, C.; Zhang, J.; Ren, K. Study of Field-Induced Chain Conformation Transformation in Poly(L-Lactic Acid) Based Piezoelectric Film by Infrared Spectroscopy. J. Appl. Phys. 2016, 120, 164101. [Google Scholar] [CrossRef]
- Yoshida, T.; Imoto, K.; Tahara, K.; Naka, K.; Uehara, Y.; Kataoka, S.; Date, M.; Fukada, E.; Tajitsu, Y. Piezoelectricity of Poly(L-Lactic Acid) Composite Film with Stereocomplex of Poly(L-Lactide) and Poly(D-Lactide). Jpn. J. Appl. Phys. 2010, 49, 9MC11. [Google Scholar] [CrossRef]
- Ando, M.; Kawamura, H.; Kageyama, K.; Tajitsu, Y. Film Sensor Device Fabricated by a Piezoelectric Poly(L-Lactic Acid) Film. Jpn. J. Appl. Phys. 2012, 51, 9LD14. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive Polymers for Tissue Regeneration: Developments and Perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef]
- Smith, M.; Calahorra, Y.; Jing, Q.; Kar-Narayan, S. Direct Observation of Shear Piezoelectricity in Poly-l-Lactic Acid Nanowires. APL Mater. 2017, 5, 74105. [Google Scholar] [CrossRef]
- Smith, M.; Chalklen, T.; Lindackers, C.; Calahorra, Y.; Howe, C.; Tamboli, A.; Bax, D.V.; Barrett, D.J.; Cameron, R.E.; Best, S.M.; et al. Poly-l-Lactic Acid Nanotubes as Soft Piezoelectric Interfaces for Biology: Controlling Cell Attachment via Polymer Crystallinity. ACS Appl. Bio Mater. 2020, 3, 2140–2149. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the Future: Recent Advances of 3D Printing in Drug Delivery and Healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, Q.; Yao, Y.; Liu, L.; Liu, Y.; Leng, J. Direct-Write Fabrication of 4D Active Shape-Changing Structures Based on a Shape Memory Polymer and Its Nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, Z.; Liu, L.; Wang, W.; Leng, J.; Liu, Y. Porous Bone Tissue Scaffold Concept Based on Shape Memory PLA/Fe3O4. Compos. Sci. Technol. 2021, 203, 108563. [Google Scholar] [CrossRef]
| Material | Processing Technique | Polymer Structure | Molecular Weight (kDa) | Material Characterisation 1 | Studies 2 | Ref. |
|---|---|---|---|---|---|---|
| Nanoparticles | Double emulsion-solvent evaporation | PLLA-PEG | 85–160 | Size, PDI, EE (%) | In vitro/in vivo | [149] |
| PLLA | ||||||
| Nanoprecipitation-solvent displacement | Rac-PLA | - | Size, PDI, Z-potential, LE (%), EE (%), Morphology (SEM) | In vitro/in vivo/clinical | [150] | |
| Interfacial deposition-solvent displacement | Rac-PLA-Peg | 18–28 | Size, PDI, Z-potential | In vivo | [151] | |
| Emulsion-solvent evaporation | Maleimide-PEG-PLA (no PLA specification) | PEG3-PLA70 | Size, Z-potential, Morphology (TEM) | In vivo | [152] | |
| Methoxy-PEG-PLA (no PLA specification) | PEG3-PLA50 | |||||
| Emulsion-solvent evaporation | PLGA (75L:25G) | 15 | CE (%) complexation efficiency, Z-potential, morphology (SEM) | In vivo | [153] | |
| Nanoprecipitation-solvent displacement | Trastuzumab-PEIcoating-Rac-PLA | 22 | LE (%), EE (%), Morphology (TEM) | In vitro | [154] | |
| Emulsion-solvent evaporation | Maleimide-PEG-PLA (no PLA specification) | PEG3.4–PLA34 | Size, PDI, Z-potential, Morphology (TEM) | In vitro/In vivo | [155] | |
| Methoxy-PEG-PLA (no PLA specification) | PEG3.4–PLA30 | |||||
| Emulsion-solvent evaporation | Maleimide-PEG-PLA (no PLA specification) | PEG3.4–PLA34 | Size, PDI, Z-potential, EE (%), LC (%), Morphology (TEM) | In vitro/In vivo | [156] | |
| Methoxy-PEG-PLA (no PLA specification) | PEG3.4–PLA30 | |||||
| Nanoprecipitation-solvent displacement | Rac-PLA-OH | 10.5 | Size, Z-potential, Morphology (TEM) | In vitro | [157] | |
| Rac-PLA-PEG-NH2 | PLA4.5–PEG3.5–NH2 | |||||
| Nanoprecipitation-solvent evaporation | PLA | - | Size, Z-potential, pH, EE (%), LE (%), Morphology (AFM, SEM), Molecular structure (FTIR) | In vitro | [159] |
| Material | Processing Technique | Polymer Structure | Molecular Weight (kDa) | Material Characterisation 1 | Studies 2 | Ref. |
|---|---|---|---|---|---|---|
| Hydrogels | Temperature increase of solutions (RT → 37 °C) | PLLA-Castor Oil | 4.8 | Viscosity, specific optical rotation, Tg, shear stress | - | [162] |
| Rac-PLA-Castor Oil | 4.6 | |||||
| Temperature increase of solutions (RT → 37 °C) | PLLA | 1.5 | Sol-gel transition, micelles size, circular dichroism, morphology (TEM), nanostructure (XRD) | - | [169] | |
| Rac-PLA | 1.5 | |||||
| PLLA-PEG | 14.4 | |||||
| Rac-PLA-PEG | 14.4 | |||||
| Hydrogelation by concentration | PLLA-PEG-PLLA | 11.5–15.5 | Storage modulus, nanostructure (WAXD) | - | [170] | |
| Rac-PLA-PEG-Rac-PLA | ||||||
| Temperature increase of solutions (RT → 37 °C) | OS-rac-PLA-PEG-rac-PLA-OS (olygomer serin) | ~3 | Sol-gel phase transition (depending on PEG Mw) | In vitro/In vivo | [163] | |
| “Click” reaction | DA-NOCCL + N3 + rac-PLA-PEG-N3 (NP) | ~6 | Aggregates size, morphology (Crio-SEM and TEM), mechanical properties | In vitro/In vivo | [167] |
| Material | Processing Technique | Polymer Structure | Molecular Weight (kDa) | Material Characterisation 1 | Studies 2 | Ref. |
|---|---|---|---|---|---|---|
| Polymer therapeutics | ROP mediated by Paclitaxel | PEG-RacPLA-Ptxl | ~30 | Structure (NMR), Mw (GPC) | - | [206] |
| ROP mediated by Docetaxel | PEG-RacPLA-Dtxl | |||||
| Azide-alkine click reaction | Ptxl (23%)-RacPLA-PEG (25%) | ~10–15 | Size (DLS), structure (NMR), morphology (TEM), molecular weight (GPC) | In vitro | [207] | |
| Conjugation + micelles formulation | RacPLA-PEG-RacPLA-Lisinorpil | ~10 | Size (DLS), morphology (AFM) | In vitro drug release | [208] | |
| Hot press film | PLLA-indomethacin | - | Conjugation (fluorescence, XPS), contact angle | - | [209] | |
| ROP | (PLLA)3-Camptothecin [3-armed] | ~30 | Molecular weight (GPC), structure (NMR), morphology (AFM), thermal properties (TGA, DSC) | In vitro drug release and enzymatic degradation | [210] |
| Material | Processing Technique | Polymer Structure | Molecular Weight (kDa) | Material Characterisation 1 | Studies 2 | Ref. |
|---|---|---|---|---|---|---|
| Scaffolds | Freeze-drying, salt-leaching (3D scaffold) | PLGA (75L:25G) + Silk + HA(Hidroxiapatite) | 90–126 | Structure (FTIR), degradation temperature (TGA), morphology (SEM), swelling (%), water uptake (%), mechanical properties | In vitro/In vivo | [214] |
| Wet-spinning (microfilament) | P(L-co-rac-LA) (75:25) | 200 | Morphology (SEM) | In vivo | [215] | |
| Bought (kinnet filmanets) | PGA/PLA (2:1) | - | Morphology (SEM and TEM), biomechanical properties | In vitro/In vivo | [216] | |
| Bought (forged composite sheets) | PLLA-PGA (88:12) + HA | - | Morphology (SEM) | In vivo | [217] | |
| PLLA + HA | ||||||
| Bought (OSTEOTRANS MX) | PLLA + HA | - | Molecular weight, crystallinity, morphology (SEM) (after surgery) | Clinical | [218] | |
| Bought (plates) | PLLA/PGA | - | - | Clinical | [219] | |
| PLLA/HA | ||||||
| Electrospinning | PLGA (85/15) | 285 | Morphology (SEM), mechanical properties, structure (FTIR) | In vitro | [220] | |
| Electrospinning | PLGA (85/15) | 285 | Morphology (SEM), mechanical properties, structure (FTIR) | In vitro | [221] | |
| Supercritical emulsion extraction | PLGA carriers + fibrin hydrogel | 38–54 | Size, morphology (SEM) | In vitro | [222] | |
| Electrospinning | PLGA + silk | - | Morphology (SEM), mechanical properties | In vitro | [223] | |
| Knitted fibres | PLGA (10/90) | - | Mechanical properties | In vivo | [224] | |
| Micelles in an aqueous solution (hydrogel) | Four-armed PEG-(PLLA)4 | PEG10-PLLA1 | Size, morphology (TEM), nanostructure (FTIR, XRD), thermal properties (DSC), mechanical properties | In vitro | [225] | |
| Four-armed PEG-(PDLA)4 | PEG10-PDLA1 | |||||
| Four-armed PEG-(PLLA-Cho)4 | PEG10-PLLA1-Chol | |||||
| Four-armed PEG-(PDLA-Chol)4 | PEG10-PDLA1-Chol | |||||
| Hydrogel by solution | PDLA-PLLA-PEG-PLLA-PDLA | ~20 | Specific optical rotation, gel-sol transition, physical gelation, nanostructure (WAXS), microstructure (SAXS) | In vitro drug release | [164] |
| Material | Processing Technique | Polymer Structure | Molecular Weight (kDa) | Material Characterisation 1 | Studies 2 | Ref. |
|---|---|---|---|---|---|---|
| Stents | Bought (REMEDY) 3 | Rac-PLA | - | - | Clinical | [16] |
| Desolve Cx 3 | PLLA-Novolimus | - | In vitro/in vivo degradation (MW lost) | Clinical | [17] | |
| Mirage 3 | PLLA (4% D-LA)-sirolimus | - | - | Clinical | [18] | |
| ABSORB V G2 3 | PLLA | - | - | Clinical trial (RENASCENT III) | [245] | |
| MAGNITUDE 3 | PLLA-sirolimus | - | - | Clinical | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya-Lopez, C.; González-Fuentes, J.; Bravo, I.; Chapron, D.; Bourson, P.; Alonso-Moreno, C.; Hermida-Merino, D. Polylactide Perspectives in Biomedicine: From Novel Synthesis to the Application Performance. Pharmaceutics 2022, 14, 1673. https://doi.org/10.3390/pharmaceutics14081673
Moya-Lopez C, González-Fuentes J, Bravo I, Chapron D, Bourson P, Alonso-Moreno C, Hermida-Merino D. Polylactide Perspectives in Biomedicine: From Novel Synthesis to the Application Performance. Pharmaceutics. 2022; 14(8):1673. https://doi.org/10.3390/pharmaceutics14081673
Chicago/Turabian StyleMoya-Lopez, Carmen, Joaquín González-Fuentes, Iván Bravo, David Chapron, Patrice Bourson, Carlos Alonso-Moreno, and Daniel Hermida-Merino. 2022. "Polylactide Perspectives in Biomedicine: From Novel Synthesis to the Application Performance" Pharmaceutics 14, no. 8: 1673. https://doi.org/10.3390/pharmaceutics14081673
APA StyleMoya-Lopez, C., González-Fuentes, J., Bravo, I., Chapron, D., Bourson, P., Alonso-Moreno, C., & Hermida-Merino, D. (2022). Polylactide Perspectives in Biomedicine: From Novel Synthesis to the Application Performance. Pharmaceutics, 14(8), 1673. https://doi.org/10.3390/pharmaceutics14081673