Cordycepin Attenuates Testosterone-Induced Benign Prostatic Hyperplasia in Rats via Modulation of AMPK and AKT Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Toxicity Study
2.4. Experimental Design
2.5. Prostate Index and Weight
2.6. Histopathological Examination
2.7. Oxidative Status Markers Assessment
2.8. Immunohistochemical Analyses
2.9. Analysis of mRNA Expression of Bax and Bcl2
2.10. Protein Expression of Bax, Bcl2, Total & Phosphor-AKT, and Total and Phosphor-AMPK by Western Blot
2.11. Statistical Analysis
3. Results
3.1. Acute Toxicity Assessment
3.2. Prostate Weights and Indices
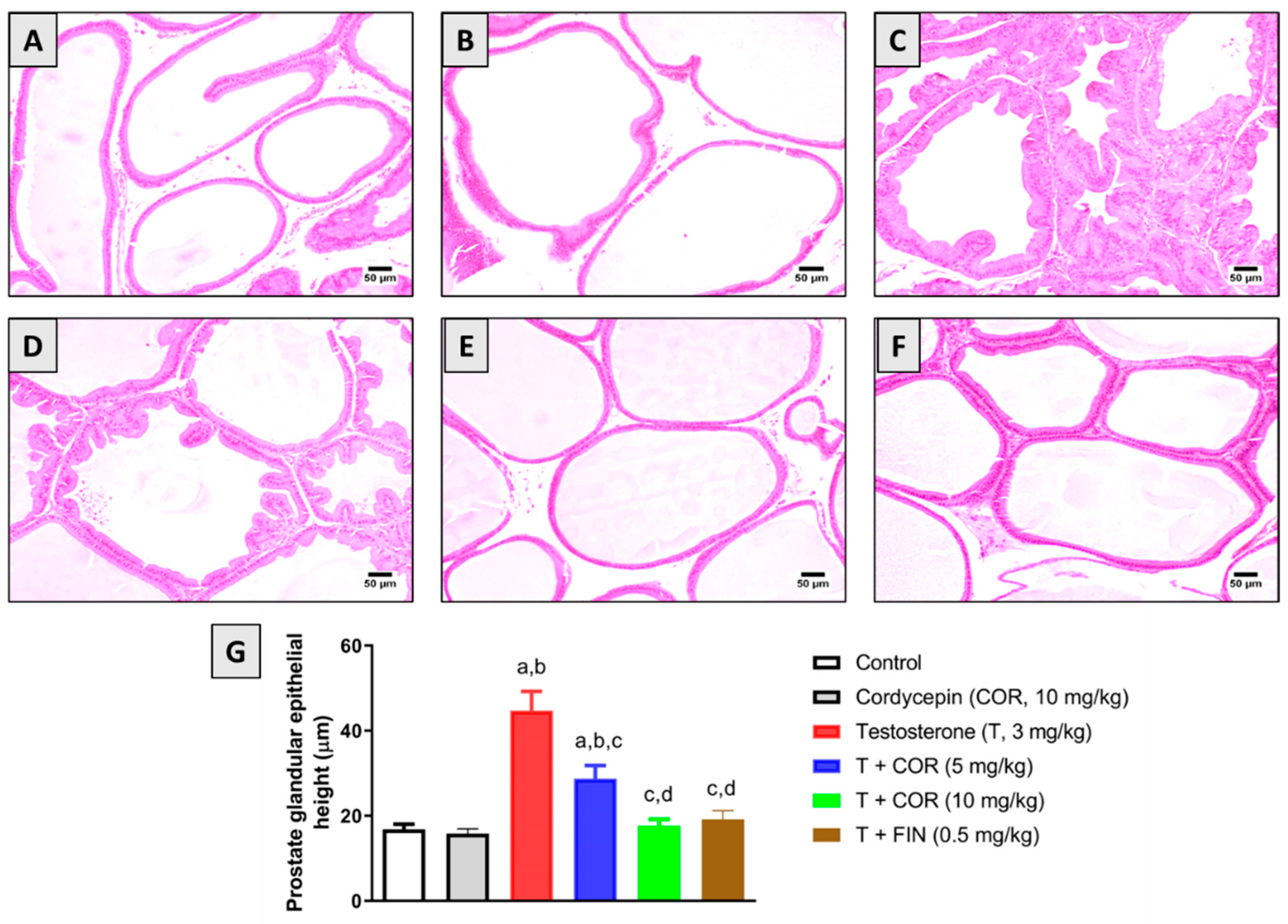
3.3. Histopathological Examination
3.4. Proliferation Markers
3.5. Oxidative Stress Markers
3.6. Inflammatory Markers
3.7. Expression of Bax and Bcl2
3.8. Expression of p-AKT and p-AMPK
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Role of inflammation in benign prostatic hyperplasia. Rev. Urol. 2011, 13, 147. [Google Scholar]
- Patel, N.D.; Parsons, J.K. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J. Urol. 2014, 30, 170. [Google Scholar]
- Bellinger, A.S.; Elliott, S.P.; Yang, L.; Wei, J.T.; Saigal, C.S.; Smith, A.; Wilt, T.J.; Strope, S.A.; Project, T.U.D.I.A. Changes in initial expenditures for benign prostatic hyperplasia evaluation in the Medicare population: A comparison to overall Medicare inflation. J. Urol. 2012, 187, 1739–1746. [Google Scholar]
- Ventura, S.; Oliver, V.L.; White, C.W.; Xie, J.H.; Haynes, J.M.; Exintaris, B. Novel drug targets for the pharmacotherapy of benign prostatic hyperplasia (BPH). Br. J. Pharmacol. 2011, 163, 891–907. [Google Scholar]
- Devlin, C.M.; Simms, M.S.; Maitland, N.J. Benign prostatic hyperplasia–what do we know? BJU Int. 2021, 127, 389–399. [Google Scholar]
- Bushman, W.A.; Jerde, T.J. The role of prostate inflammation and fibrosis in lower urinary tract symptoms. Am. J. Physiol. Physiol. 2016, 311, F817–F821. [Google Scholar]
- Minciullo, P.L.; Inferrera, A.; Navarra, M.; Calapai, G.; Magno, C.; Gangemi, S. Oxidative stress in benign prostatic hyperplasia: A systematic review. Urol. Int. 2015, 94, 249–254. [Google Scholar]
- Eid, B.G.; Abdel-Naim, A.B. Piceatannol attenuates testosterone-induced benign prostatic hyperplasia in rats by modulation of Nrf2/HO-1/NFκB axis. Front. Pharmacol. 2020, 11, 614897. [Google Scholar]
- Kim, J.H.; Baek, M.J.; Sun, H.Y.; Lee, B.; Li, S.; Khandwala, Y.; Del Giudice, F.; Chung, B.I. Efficacy and safety of 5 alpha-reductase inhibitor monotherapy in patients with benign prostatic hyperplasia: A meta-analysis. PLoS ONE 2018, 13, e0203479. [Google Scholar]
- Csikós, E.; Horváth, A.; Ács, K.; Papp, N.; Balázs, V.L.; Dolenc, M.S.; Kenda, M.; Glavač, N.K.; Nagy, M.; Protti, M.; et al. Treatment of benign prostatic hyperplasia by natural drugs. Molecules 2021, 26, 7141. [Google Scholar]
- Tan, L.; Song, X.; Ren, Y.; Wang, M.; Guo, C.; Guo, D.; Gu, Y.; Li, Y.; Cao, Z.; Deng, Y. Anti-inflammatory effects of cordycepin: A review. Phyther. Res. 2021, 35, 1284–1297. [Google Scholar]
- Cunningham, K.G.; Manson, W.; Spring, F.S.; Hutchinson, S.A. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949. [Google Scholar]
- Jeong, J.-W.; Jin, C.-Y.; Park, C.; Hong, S.H.; Kim, G.-Y.; Jeong, Y.K.; Lee, J.-D.; Yoo, Y.H.; Choi, Y.H. Induction of apoptosis by cordycepin via reactive oxygen species generation in human leukemia cells. Toxicol. Vitr. 2011, 25, 817–824. [Google Scholar]
- Nakamura, K.; Shinozuka, K.; Yoshikawa, N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J. Pharmacol. Sci. 2015, 127, 53–56. [Google Scholar]
- Ramesh, T.; Yoo, S.-K.; Kim, S.-W.; Hwang, S.-Y.; Sohn, S.-H.; Kim, I.-W.; Kim, S.-K. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp. Gerontol. 2012, 47, 979–987. [Google Scholar]
- Cao, H.-L.; Liu, Z.-J.; Chang, Z. Cordycepin induces apoptosis in human bladder cancer cells via activation of A3 adenosine receptors. Tumor Biol. 2017, 39, 1010428317706915. [Google Scholar]
- Nakamura, K.; Yoshikawa, N.; Yamaguchi, Y.U.; Kagota, S.; Shinozuka, K.; Kunitomo, M. Antitumor effect of cordycepin (3′-deoxyadenosine) on mouse melanoma and lung carcinoma cells involves adenosine A3 receptor stimulation. Anticancer Res. 2006, 26, 43–47. [Google Scholar]
- Fishman, P.; Bar-Yehuda, S.; Ardon, E.; Rath-Wolfson, L.; Barrer, F.; Ochaion, A.; Madi, L. Targeting the A3 adenosine receptor for cancer therapy: Inhibition of prostate carcinoma cell growth by A3AR agonist. Anticancer Res. 2003, 23, 2077–2084. [Google Scholar]
- Preston, A.; Lau, W.A.K.; Pennefather, J.N.; Ventura, S. Effects of adenine nucleosides and nucleotides on neuromuscular transmission to the prostatic stroma of the rat. Br. J. Pharmacol. 2000, 131, 1073. [Google Scholar]
- Preston, A.; Frydenberg, M.; Haynes, J.M. A1 and A2A adenosine receptor modulation of α1-adrenoceptor-mediated contractility in human cultured prostatic stromal cells. Br. J. Pharmacol. 2004, 141, 302–310. [Google Scholar]
- Sirwi, A.; Shaik, R.A.; Alamoudi, A.J.; Eid, B.G.; Kammoun, A.K.; Ibrahim, S.R.; Mohamed, G.A.; Abdallah, H.M.; Abdel-Naim, A.B. Mokko lactone attenuates doxorubicin-induced hepatotoxicity in rats: Emphasis on Sirt-1/FOXO1/NF-κB axis. Nutrients 2021, 13, 4142. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Donnell, R.F. Benign prostate hyperplasia: A review of the year’s progress from bench to clinic. Curr. Opin. Urol. 2011, 21, 22–26. [Google Scholar]
- Pawlicki, B.; Zieliński, H.; Dabrowski, M. Role of apoptosis and chronic prostatitis in the pathogenesis of benign prostatic hyperplasia. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2004, 17, 307–310. [Google Scholar]
- Aaron, L.; Franco, O.E.; Hayward, S.W. Review of prostate anatomy and embryology and the etiology of benign prostatic hyperplasia. Urol. Clin. 2016, 43, 279–288. [Google Scholar]
- Udensi, U.K.; Tchounwou, P.B. Oxidative stress in prostate hyperplasia and carcinogenesis. J. Exp. Clin. Cancer Res. 2016, 35, 1–19. [Google Scholar]
- Silva, J.; Silva, C.M.; Cruz, F. Current medical treatment of lower urinary tract symptoms/BPH: Do we have a standard? Curr. Opin. Urol. 2014, 24, 21–28. [Google Scholar]
- Traish, A.M.; Hassani, J.; Guay, A.T.; Zitzmann, M.; Hansen, M.L. Adverse side effects of 5α-reductase inhibitors therapy: Persistent diminished libido and erectile dysfunction and depression in a subset of patients. J. Sex. Med. 2011, 8, 872–884. [Google Scholar]
- Nickel, J.C.; Sander, S.; Moon, T.D. A meta-analysis of the vascular-related safety profile and efficacy of α-adrenergic blockers for symptoms related to benign prostatic hyperplasia. Int. J. Clin. Pract. 2008, 62, 1547–1559. [Google Scholar]
- Quy, T.N.; Xuan, T.D.; Andriana, Y.; Tran, H.-D.; Khanh, T.D.; Teschke, R. Cordycepin isolated from Cordyceps militaris: Its newly discovered herbicidal property and potential plant-based novel alternative to glyphosate. Molecules 2019, 24, 2901. [Google Scholar]
- Leu, S.-F.; Poon, S.L.; Pao, H.-Y.; Huang, B.-M. The in vivo and in vitro stimulatory effects of cordycepin on mouse leydig cell steroidogenesis. Biosci. Biotechnol. Biochem. 2011, 75, 723–731. [Google Scholar]
- Jeong, J.-W.; Jin, C.-Y.; Kim, G.-Y.; Lee, J.-D.; Park, C.; Kim, G.-D.; Kim, W.-J.; Jung, W.-K.; Kil Seo, S.; Choi, I.-W.; et al. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 2010, 10, 1580–1586. [Google Scholar]
- Baik, J.-S.; Mun, S.-W.; Kim, K.-S.; Park, S.-J.; Yoon, H.-K.; Kim, D.-H.; Park, M.-K.; Kim, C.-H.; Lee, Y.-C. Apoptotic effects of cordycepin through the extrinsic pathway and p38 MAPK activation in human glioblastoma U87MG cells. J. Microbiol. Biotechnol. 2016, 26, 309–314. [Google Scholar]
- Pan, B.-S.; Wang, Y.-K.; Lai, M.-S.; Mu, Y.-F.; Huang, B.-M. Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis by regulating p38 MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 2015, 5, 13372. [Google Scholar]
- Kusama, K.; Miyagawa, M.; Ota, K.; Kuwabara, N.; Saeki, K.; Ohnishi, Y.; Kumaki, Y.; Aizawa, T.; Nakasone, T.; Okamatsu, S.; et al. Cordyceps militaris fruit body extract decreases testosterone catabolism and testosterone-stimulated prostate hypertrophy. Nutrients 2020, 13, 50. [Google Scholar]
- Yoon, S.Y.; Park, S.J.; Park, Y.J. The anticancer properties of cordycepin and their underlying mechanisms. Int. J. Mol. Sci. 2018, 19, 3027. [Google Scholar]
- Lee, H.H.; Park, C.; Jeong, J.-W.; Kim, M.J.; Seo, M.J.; Kang, B.W.; Park, J.U.; Kim, G.-Y.; Choi, B.T.; Choi, Y.H.; et al. Apoptosis induction of human prostate carcinoma cells by cordycepin through reactive oxygen species-mediated mitochondrial death pathway. Int. J. Oncol. 2013, 42, 1036–1044. [Google Scholar]
- Vital, P.; Castro, P.; Ittmann, M. Oxidative stress promotes benign prostatic hyperplasia. Prostate 2016, 76, 58–67. [Google Scholar]
- Wang, F.; Yin, P.; Lu, Y.; Zhou, Z.; Jiang, C.; Liu, Y.; Yu, X. Cordycepin prevents oxidative stress-induced inhibition of osteogenesis. Oncotarget 2015, 6, 35496. [Google Scholar]
- Park, J.M.; Lee, J.S.; Lee, K.R.; Ha, S.-J.; Hong, E.K. Cordyceps militaris extract protects human dermal fibroblasts against oxidative stress-induced apoptosis and premature senescence. Nutrients 2014, 6, 3711–3726. [Google Scholar]
- Han, F.; Dou, M.; Wang, Y.; Xu, C.; Li, Y.; Ding, X.; Xue, W.; Zheng, J.; Tian, P.; Ding, C. Cordycepin protects renal ischemia/reperfusion injury through regulating inflammation, apoptosis, and oxidative stress. Acta Biochim. Biophys. Sin. 2020, 52, 125–132. [Google Scholar]
- Ammar, A.E.; Esmat, A.; Hassona, M.D.H.; Tadros, M.G.; Abdel-Naim, A.B.; Guns, E.S.T. The effect of pomegranate fruit extract on testosterone-induced BPH in rats. Prostate 2015, 75, 679–692. [Google Scholar]
- Atawia, R.T.; Tadros, M.G.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Role of the phytoestrogenic, pro-apoptotic and anti-oxidative properties of silymarin in inhibiting experimental benign prostatic hyperplasia in rats. Toxicol. Lett. 2013, 219, 160–169. [Google Scholar]
- Wu, X.; Gu, Y.; Li, L. The anti-hyperplasia, anti-oxidative and anti-inflammatory properties of Qing Ye Dan and swertiamarin in testosterone-induced benign prostatic hyperplasia in rats. Toxicol. Lett. 2017, 265, 9–16. [Google Scholar]
- Rastrelli, G.; Vignozzi, L.; Corona, G.; Maggi, M. Testosterone and benign prostatic hyperplasia. Sex. Med. Rev. 2019, 7, 259–271. [Google Scholar]
- Choi, Y.H.; Kim, G.-Y.; Lee, H.H. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des. Devel. Ther. 2014, 8, 1941. [Google Scholar]
- Ashraf, S.; Radhi, M.; Gowler, P.; Burston, J.J.; Gandhi, R.D.; Thorn, G.; Piccinini, A.M.; Walsh, D.; Chapman, V.; De Moor, C.H. The polyadenylation inhibitor cordycepin reduces pain, inflammation and joint pathology in rodent models of osteoarthritis. Sci. Rep. 2019, 9, 4696. [Google Scholar]
- Hwang, I.-H.; Oh, S.Y.; Jang, H.-J.; Jo, E.; Joo, J.C.; Lee, K.-B.; Yoo, H.-S.; Lee, M.Y.; Park, S.J.; Jang, I.-S. Cordycepin promotes apoptosis in renal carcinoma cells by activating the MKK7-JNK signaling pathway through inhibition of c-FLIPL expression. PLoS ONE 2017, 12, e0186489. [Google Scholar]
- Rahman, I. Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseases. BMB Rep. 2003, 36, 95–109. [Google Scholar]
- Hoffmann, A.; Natoli, G.; Ghosh, G. Transcriptional regulation via the NF-κB signaling module. Oncogene 2006, 25, 6706–6716. [Google Scholar]
- Elsharkawy, A.M.; Mann, D.A. Nuclear factor-κB and the hepatic inflammation-fibrosis-cancer axis. Hepatology 2007, 46, 590–597. [Google Scholar]
- Chughtai, B.; Forde, J.C.; Thomas, D.D.M.; Laor, L.; Hossack, T.; Woo, H.H.; Te, A.E.; Kaplan, S.A. Benign prostatic hyperplasia. Nat. Rev. Dis. Prim. 2016, 2, 16031. [Google Scholar]
- Lee, H.H.; Kim, S.O.; Kim, G.-Y.; Moon, S.-K.; Kim, W.-J.; Jeong, Y.K.; Yoo, Y.H.; Choi, Y.H. Involvement of autophagy in cordycepin-induced apoptosis in human prostate carcinoma LNCaP cells. Environ. Toxicol. Pharmacol. 2014, 38, 239–250. [Google Scholar]
- Chang, M.-M.; Hong, S.-Y.; Yang, S.-H.; Wu, C.-C.; Wang, C.-Y.; Huang, B.-M. Anti-cancer effect of cordycepin on FGF9-induced testicular tumorigenesis. Int. J. Mol. Sci. 2020, 21, 8336. [Google Scholar]
- Yoshikawa, N.; Yamada, S.; Takeuchi, C.; Kagota, S.; Shinozuka, K.; Kunitomo, M.; Nakamura, K. Cordycepin (3′-deoxyadenosine) inhibits the growth of B16-BL6 mouse melanoma cells through the stimulation of adenosine A3 receptor followed by glycogen synthase kinase-3β activation and cyclin D1 suppression. Naunyn. Schmiedebergs. Arch. Pharmacol. 2008, 377, 591–595. [Google Scholar]
- Jang, H.J.; Yang, K.E.; Hwang, I.H.; Huh, Y.H.; Kim, D.J.; Yoo, H.S.; Park, S.J.; Jang, I.S. Cordycepin inhibits human ovarian cancer by inducing autophagy and apoptosis through Dickkopf-related protein 1/β-catenin signaling. Am. J. Transl. Res. 2019, 11, 6890. [Google Scholar]
- Lee, J.-D.; Jeong, J.-W.; Jin, C.-Y.; Park, C.; Han, M.H.; Kim, G.-Y.; Moon, S.-K.; Gil Kim, C.; Jeong, Y.K.; Kim, W.-J.; et al. Inhibition of migration and invasion of LNCaP human prostate carcinoma cells by cordycepin through inactivation of Akt. Int. J. Oncol. 2012, 40, 1697–1704. [Google Scholar]
- Li, H.-B.; Chen, J.-K.; Su, Z.-X.; Jin, Q.-L.; Deng, L.-W.; Huang, G.; Shen, J.-N. Cordycepin augments the chemosensitivity of osteosarcoma to cisplatin by activating AMPK and suppressing the AKT signaling pathway. Cancer Cell Int. 2021, 21, 706. [Google Scholar]
- Liao, X.-Z.; Gao, Y.; Zhao, H.-W.; Zhou, M.; Chen, D.-L.; Tao, L.-T.; Guo, W.; Sun, L.-L.; Gu, C.-Y.; Chen, H.-R.; et al. Cordycepin reverses cisplatin resistance in non-small cell lung cancer by activating AMPK and inhibiting AKT signaling pathway. Front. Cell Dev. Biol. 2021, 8, 609285. [Google Scholar]
- Gao, Y.; Chen, D.-L.; Zhou, M.; Zheng, Z.-S.; He, M.-F.; Huang, S.; Liao, X.-Z.; Zhang, J.-X. Cordycepin enhances the chemosensitivity of esophageal cancer cells to cisplatin by inducing the activation of AMPK and suppressing the AKT signaling pathway. Cell Death Dis. 2020, 11, 866. [Google Scholar]
- Bi, Y.; Li, H.; Yi, D.; Sun, Y.; Bai, Y.; Zhong, S.; Song, Y.; Zhao, G.; Chen, Y. Cordycepin augments the chemosensitivity of human glioma cells to temozolomide by activating AMPK and inhibiting the AKT signaling pathway. Mol. Pharm. 2018, 15, 4912–4925. [Google Scholar]
- Sreenivasulu, K.; Nandeesha, H.; Dorairajan, L.N.; Ganesh, R.N. Over expression of PI3K-AkT reduces apoptosis and increases prostate size in benign prostatic hyperplasia. Aging Male 2018, 23, 440–446. [Google Scholar]
- Jin, P.; Wang, Y.-H.; Peng, Y.-G.; Hu, S.; Lu, Q.; Yang, L.-Y. Effect of PI3K/AKT inhibitor on benign prostate hyperplasia and its mechanism: An experimental study. Natl. J. Androl. 2010, 16, 1068–1075. [Google Scholar]
- Caggia, S.; Libra, M.; Malaponte, G.; Cardile, V. Modulation of YY1 and p53 expression by transforming growth factor-β3 in prostate cell lines. Cytokine 2011, 56, 403–410. [Google Scholar]
- Cha, J.Y.; Wee, J.; Jung, J.; Jang, Y.; Lee, B.; Hong, G.-S.; Chang, B.C.; Choi, Y.-L.; Shin, Y.K.; Min, H.-Y.; et al. Anoctamin 1 (TMEM16A) is essential for testosterone-induced prostate hyperplasia. Proc. Natl. Acad. Sci. USA 2015, 112, 9722–9727. [Google Scholar]
- Vanella, L.; Russo, G.I.; Cimino, S.; Fragalà, E.; Favilla, V.; Volti, G.L.; Barbagallo, I.; Sorrenti, V.; Morgia, G. Correlation between lipid profile and heme oxygenase system in patients with benign prostatic hyperplasia. Urology 2014, 83, 1444.e7–1444.e13. [Google Scholar]
- Green, C.J.; Macrae, K.; Fogarty, S.; Hardie, D.G.; Sakamoto, K.; Hundal, H.S. Counter-modulation of fatty acid-induced pro-inflammatory nuclear factor κB signalling in rat skeletal muscle cells by AMP-activated protein kinase. Biochem. J. 2011, 435, 463–474. [Google Scholar]
- Lim, R.; Barker, G.; Lappas, M. Activation of AMPK in human fetal membranes alleviates infection-induced expression of pro-inflammatory and pro-labour mediators. Placenta 2015, 36, 454–462. [Google Scholar]
- Zhang, M.; Wang, S.; Pan, Z.; Ou, T.; Ma, J.; Liu, H.; Li, R.; Yang, P.; Han, W.; Guan, S.; et al. AMPK/NF-κB signaling pathway regulated by ghrelin participates in the regulation of HUVEC and THP1 Inflammation. Mol. Cell. Biochem. 2018, 437, 45–53. [Google Scholar]
- Kortam, M.A.; Alawady, A.S.; Sadik, N.A.H.; Fathy, N. Fenofibrate mitigates testosterone induced benign prostatic hyperplasia via regulation of Akt/FOXO3a pathway and modulation of apoptosis and proliferation in rats. Arch. Biochem. Biophys. 2022, 723, 109237. [Google Scholar]
- Wang, S.; Li, K.; Liu, Z.; Gui, S.; Liu, N.; Liu, X. Aerobic exercise ameliorates benign prostatic hyperplasia in obese mice through downregulating the AR/androgen/PI3K/AKT signaling pathway. Exp. Gerontol. 2021, 143, 111152. [Google Scholar]
- Mosli, H.H.; Esmat, A.; Atawia, R.T.; Shoieb, S.M.; Mosli, H.A.; Abdel-Naim, A.B. Metformin attenuates testosterone-induced prostatic hyperplasia in rats: A pharmacological perspective. Sci. Rep. 2015, 5, 15639. [Google Scholar]





| Final Body Weight (g) | Prostate Weight (mg) | Prostate Index (×103) | |
|---|---|---|---|
| Control | 245 ± 20.2 | 281 ± 30.1 | 1.15 ± 0.16 |
| Cordycepin (COR 10 mg/kg) | 250 ± 21.2 | 305 ± 32.5 | 1.23 ± 0.19 |
| Testosterone (T; 3 mg/kg) | 272 ± 30.8 | 782 a,b ± 81.4 | 2.91 a,b ± 0.48 |
| T + COR (5 mg/kg) | 260 ± 22.8 | 530 a,b,c ± 58.3 | 2.05 a,b,c ± 0.26 |
| T + COR (10 mg/kg) | 255 ± 26.2 | 428 a,b,c,d ± 46.7 | 1.70 a,c ± 0.31 |
| T + FIN (0.5 mg/kg) | 251 ± 27.2 | 371 a,c,d ± 32.5 | 1.50 c,d ± 0.23 |
| MDA (nmol/mg Protein) | GSH (mmol/mg Protein) | SOD (U/mg Protein) | |
|---|---|---|---|
| Control | 18.77 ± 2.10 | 89.00 ± 9.73 | 8.3 ± 0.91 |
| Cordycepin (COR 10 mg/kg) | 17.11 ± 1.86 | 97.41 ± 10.2 | 8.8 ± 0.98 |
| Testosterone (T; 3 mg/kg) | 65.10 a,b ± 7.53 | 32.17 a,b ± 3.41 | 2.37 a,b ± 0.30 |
| T + COR (5 mg/kg) | 43.61 a,b,c ± 4.51 | 66.31 a,b,c ± 7.05 | 6.53 a,c,b ± 0.68 |
| T + COR (10 mg/kg) | 37.71 a,b,c ± 4.05 | 75.52 b,c ± 6.34 | 6.62 a,b,c ± 0.72 |
| T + FIN (0.5 mg/kg) | 27.52 a,b,c,d,e ± 2.3 | 80.45 b,c,d ± 8.7 | 8.6 c,d,e ± 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamoudi, A.J.; Alessi, S.A.; Rizg, W.Y.; Jali, A.M.; Safhi, A.Y.; Sabei, F.Y.; Alshehri, S.; Hosny, K.M.; Abdel-Naim, A.B. Cordycepin Attenuates Testosterone-Induced Benign Prostatic Hyperplasia in Rats via Modulation of AMPK and AKT Activation. Pharmaceutics 2022, 14, 1652. https://doi.org/10.3390/pharmaceutics14081652
Alamoudi AJ, Alessi SA, Rizg WY, Jali AM, Safhi AY, Sabei FY, Alshehri S, Hosny KM, Abdel-Naim AB. Cordycepin Attenuates Testosterone-Induced Benign Prostatic Hyperplasia in Rats via Modulation of AMPK and AKT Activation. Pharmaceutics. 2022; 14(8):1652. https://doi.org/10.3390/pharmaceutics14081652
Chicago/Turabian StyleAlamoudi, Abdulmohsin J., Sami A. Alessi, Waleed Y. Rizg, Abdulmajeed M. Jali, Awaji Y. Safhi, Fahad Y. Sabei, Sameer Alshehri, Khaled M. Hosny, and Ashraf B. Abdel-Naim. 2022. "Cordycepin Attenuates Testosterone-Induced Benign Prostatic Hyperplasia in Rats via Modulation of AMPK and AKT Activation" Pharmaceutics 14, no. 8: 1652. https://doi.org/10.3390/pharmaceutics14081652
APA StyleAlamoudi, A. J., Alessi, S. A., Rizg, W. Y., Jali, A. M., Safhi, A. Y., Sabei, F. Y., Alshehri, S., Hosny, K. M., & Abdel-Naim, A. B. (2022). Cordycepin Attenuates Testosterone-Induced Benign Prostatic Hyperplasia in Rats via Modulation of AMPK and AKT Activation. Pharmaceutics, 14(8), 1652. https://doi.org/10.3390/pharmaceutics14081652






