Functionalized Self-Assembled Monolayers: Versatile Strategies to Combat Bacterial Biofilm Formation
Abstract
:1. Introduction
2. Biofilm Formation
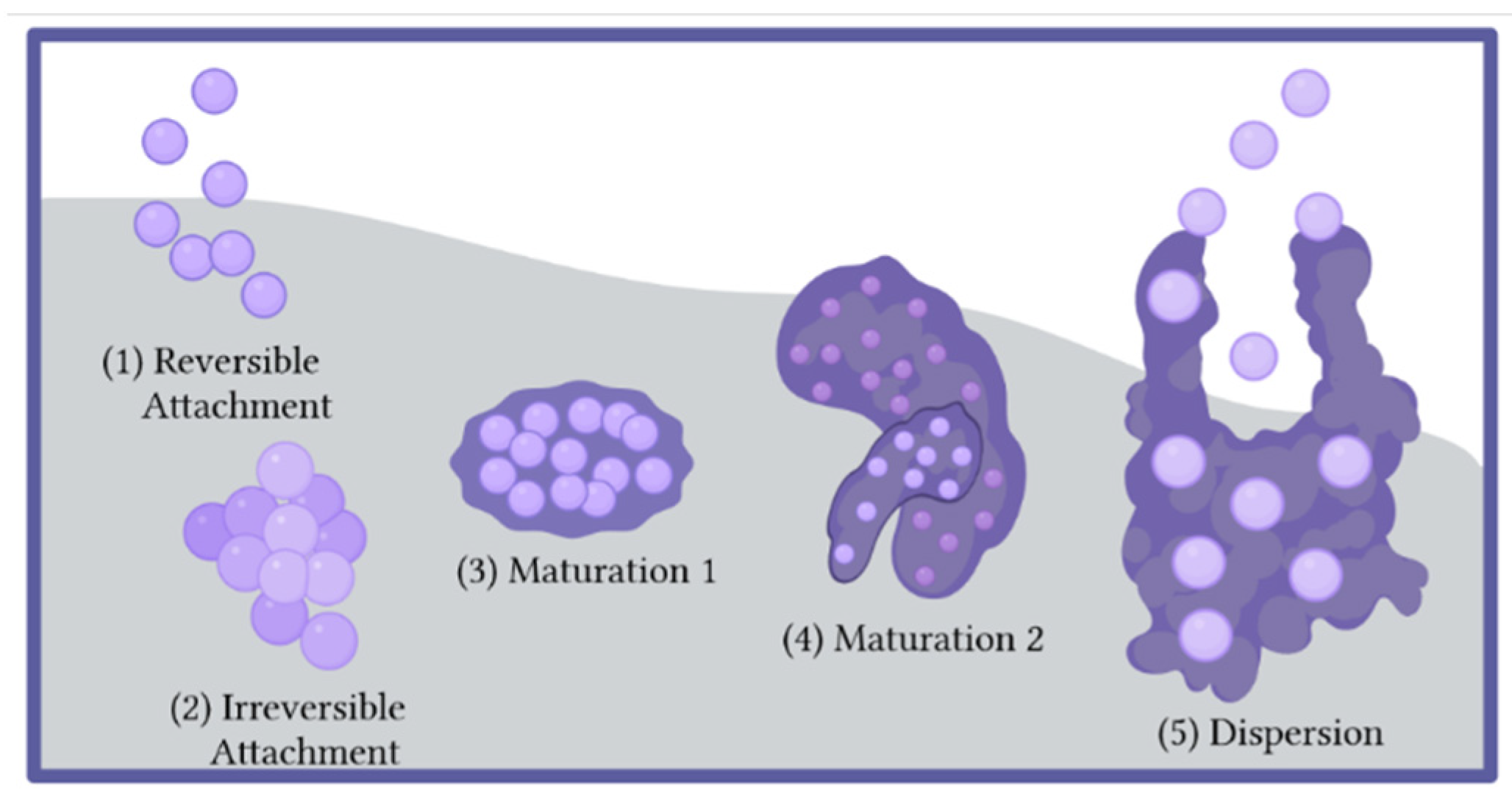
2.1. The Biofilm Lifecycle
2.2. Device Related Infections
2.3. Treatment Options for Biofilm-Based Infections
2.4. Characterization Methods for Biofilms
3. Self-Assembled Monolayers
3.1. Definition and Structure of Self-Assembled Monolayers
3.2. Surface Characterization Methods for SAMs
4. Preventative SAM Strategies for Biofilm Inhibition
4.1. Prevention of Bacterial Adhesion by Increasing Hydration of the Surface
4.2. Prevention of Biofilm Formation by Interruption of Quorum Sensing
5. Bactericidal SAM Strategies
5.1. Quaternary Ammonium SAMs
5.2. Small Molecule Antibiotic- and Antiseptic-Terminated SAMs
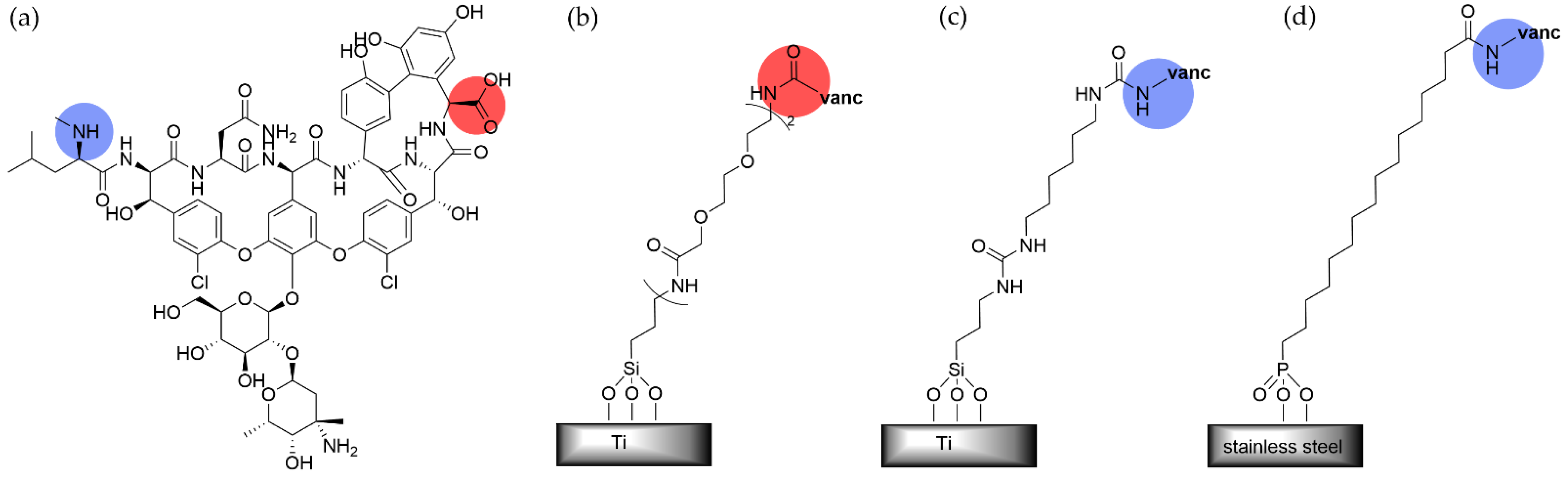
5.3. SAMs Promoting Release of Anti-Microbial Agents
5.4. Anti-Microbial Peptides Grafted to SAMs
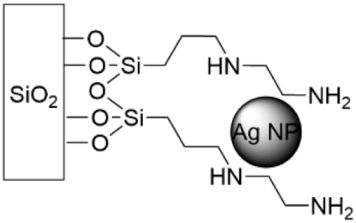
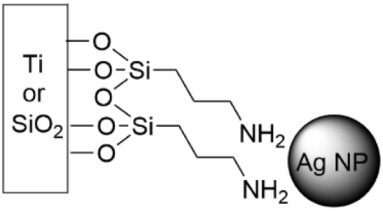
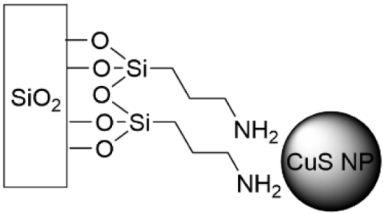
5.5. SAMs Tethering Metal Cations and Nanoparticles
5.6. Bactericidal Carbohydrates Grafted through SAMs
6. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. iScience 2021, 24, 102304. [Google Scholar] [CrossRef]
- Ghosh, S.; Bornman, C.; Zafer, M.M. Antimicrobial Resistance Threats in the emerging COVID-19 pandemic: Where do we stand? J. Infect. Public Health 2021, 14, 555–560. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Yang, Y.; Cai, P.; Cao, J.; Cai, X.; Zhang, Y. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: A retrospective analysis. Antimicrob. Resist. Infect. Control 2020, 9, 153. [Google Scholar] [CrossRef]
- Nori, P.; Szymczak, W.; Puius, Y.; Sharma, A.; Cowman, K.; Gialanella, P.; Fleischner, Z.; Corpuz, M.; Torres-Isasiga, J.; Bartash, R.; et al. Emerging Co-Pathogens: New Delhi Metallo-beta-lactamase producing Enterobacterales Infections in New York City COVID-19 Patients. Int. J. Antimicrob. Agents 2020, 56, 106179. [Google Scholar] [CrossRef]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [Green Version]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Chapman, R.G.; Liang, M.N.; Meluleni, G.; Pier, G.; Ingber, D.E.; Whitesides, G.M. Self-Assembled Monolayers That Resist the Adsorption of Proteins and the Adhesion of Bacterial and Mammalian Cells. Langmuir 2001, 17, 6336–6343. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Song, B.; Zhang, E.; Han, X.; Zhu, H.; Shi, Y.; Cao, Z. Engineering and Application Perspectives on Designing an Antimicrobial Surface. ACS Appl. Mater. Interfaces 2020, 12, 21330–21341. [Google Scholar] [CrossRef]
- Uneputty, A.; Dávila-Lezama, A.; Garibo, D.; Oknianska, A.; Bogdanchikova, N.; Hernández-Sánchez, J.F.; Susarrey-Arce, A. Strategies applied to modify structured and smooth surfaces: A step closer to reduce bacterial adhesion and biofilm formation. Colloid Interface Sci. Commun. 2022, 46, 100560. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, C.; Huskens, J. Reactive self-assembled monolayers: From surface functionalization to gradient formation. Mater. Horiz. 2014, 1, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Mi, L.; Jiang, S. Integrated Antimicrobial and Nonfouling Zwitterionic Polymers. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-fouling Coatings of Poly(dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riga, E.K.; Vöhringer, M.; Widyaya, V.T.; Lienkamp, K. Polymer-Based Surfaces Designed to Reduce Biofilm Formation: From Antimicrobial Polymers to Strategies for Long-Term Applications. Macromol. Rapid Commun. 2017, 38, 1700216. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caiazza, N.C.; O’Toole, G.A. SadB is required for the transition from reversible to irreversible attachment during biofilm formation by Pseudomonas aeruginosa PA14. J. Bacteriol. 2004, 186, 4476–4485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinsa, S.M.; Espinosa-Urgel, M.; Ramos, J.L.; O’Toole, G.A. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol. Microbiol. 2003, 49, 905–918. [Google Scholar] [CrossRef]
- Wi, Y.M.; Patel, R. Understanding Biofilms and Novel Approaches to the Diagnosis, Prevention, and Treatment of Medical Device-Associated Infections. Infect. Dis. Clin. North Am. 2018, 32, 915–929. [Google Scholar] [CrossRef]
- Floyd, K.A.; Eberly, A.R.; Hadjifrangiskou, M. 3-Adhesion of bacteria to surfaces and biofilm formation on medical devices. In Biofilms and Implantable Medical Devices; Deng, Y., Lv, W., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 47–95. ISBN 978-0-08-100382-4. [Google Scholar]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, C.; Schweitzer, O.; Gerke, C.; Vanittanakom, N.; Mack, D.; Gotz, F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 1996, 20, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef] [PubMed]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureusbiofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, A.M.S.; Ferreira, F.A.; Beltrame, C.O.; Cortes, M.F. The role of biofilms in persistent infections and factors involved in ica-independent biofilm development and gene regulation in Staphylococcus aureus. Crit. Rev. Microbiol. 2017, 43, 602–620. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.; Kolter, R. Why are bacteria refractory to antimicrobials? Curr. Opin. Microbiol. 2002, 5, 472–477. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Marques, C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, S.N.; Chung, M.C.; van Hoek, M.L. Burkholderia Diffusible Signal Factor Signals to Francisella novicida To Disperse Biofilm and Increase Siderophore Production. Appl. Env. Microbiol. 2015, 81, 7057–7066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, C.N.; Davies, D.G.; Sauer, K. Control of Biofilms with the Fatty Acid Signaling Molecule cis-2-Decenoic Acid. Pharmaceuticals 2015, 8, 816–835. [Google Scholar] [CrossRef]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef]
- Trautner, B.W.; Darouiche, R.O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 2004, 32, 177–183. [Google Scholar] [CrossRef]
- Getzlaf, M.A.; Lewallen, E.A.; Kremers, H.M.; Jones, D.L.; Bonin, C.A.; Dudakovic, A.; Thaler, R.; Cohen, R.C.; Lewallen, D.G.; van Wijnen, A.J. Multi-disciplinary antimicrobial strategies for improving orthopaedic implants to prevent prosthetic joint infections in hip and knee. J. Orthop. Res. 2016, 34, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akgun, D.; Perka, C.; Trampuz, A.; Renz, N. Outcome of hip and knee periprosthetic joint infections caused by pathogens resistant to biofilm-active antibiotics: Results from a prospective cohort study. Arch. Orthop. Trauma Surg. 2018, 138, 635–642. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Centre. Evidence Review for Ultra-Clean Air: Joint Replacement (Primary): Hip, Knee and Shoulder; Evidence Review I; NICE Evidence Reviews Collection: London, UK, 2020; ISBN 978-1-4731-3722-6.
- Cano, E.J.; Caflisch, K.M.; Bollyky, P.L.; Van Belleghem, J.D.; Patel, R.; Fackler, J.; Brownstein, M.J.; Horne, B.; Biswas, B.; Henry, M.; et al. Phage Therapy for Limb-threatening Prosthetic Knee Klebsiella pneumoniae Infection: Case Report and In Vitro Characterization of Anti-biofilm Activity. Clin. Infect. Dis. 2021, 73, e144–e151. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.B.; Araujo, R.O.; Salles, M.J. Non-elective and revision arthroplasty are independently associated with hip and knee prosthetic joint infection caused by Acinetobacter baumannii: A Brazilian single center observational cohort study of 98 patients. BMC Musculoskelet Disord. 2021, 22, 511. [Google Scholar] [CrossRef] [PubMed]
- Caldara, M.; Belgiovine, C.; Secchi, E.; Rusconi, R. Environmental, Microbiological, and Immunological Features of Bacterial Biofilms Associated with Implanted Medical Devices. Clin. Microbiol. Rev. 2022, 35, e0022120. [Google Scholar] [CrossRef] [PubMed]
- Lubbert, C.; Wendt, K.; Feisthammel, J.; Moter, A.; Lippmann, N.; Busch, T.; Mossner, J.; Hoffmeister, A.; Rodloff, A.C. Epidemiology and Resistance Patterns of Bacterial and Fungal Colonization of Biliary Plastic Stents: A Prospective Cohort Study. PLoS ONE 2016, 11, e0155479. [Google Scholar] [CrossRef]
- Khoddami, S.; Chew, B.H.; Lange, D. Problems and solutions of stent biofilm and encrustations: A review of literature. Turk. J. Urol. 2020, 46, S11–S18. [Google Scholar] [CrossRef]
- Gheorghe, D.C.; Ilie, A.; Niculescu, A.G.; Grumezescu, A.M. Preventing Biofilm Formation and Development on Ear, Nose and Throat Medical Devices. Biomedicines 2021, 9, 1025. [Google Scholar] [CrossRef]
- Olsen, L.B.; Larsen, S.; Wanscher, J.H.; Faber, C.E.; Jeppesen, J. Postoperative infections following cochlear implant surgery. Acta Otolaryngol. 2018, 138, 956–960. [Google Scholar] [CrossRef]
- Virden, C.P.; Dobke, M.K.; Stein, P.; Parsons, C.L.; Frank, D.H. Subclinical infection of the silicone breast implant surface as a possible cause of capsular contracture. Aesthetic Plast. Surg. 1992, 16, 173–179. [Google Scholar] [CrossRef]
- Pajkos, A.; Deva, A.K.; Vickery, K.; Cope, C.; Chang, L.; Cossart, Y.E. Detection of subclinical infection in significant breast implant capsules. Plast. Reconstr. Surg. 2003, 111, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Heine, N.; Eisenmann-Klein, M.; Prantl, L. Bacterial colonization is of major relevance for high-grade capsular contracture after augmentation mammaplasty. Ann. Plast. Surg. 2007, 59, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Washer, L.L.; Gutowski, K. Breast implant infections. Infect. Clin. North Am. 2012, 26, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Macadam, S.A.; Mehling, B.M.; Fanning, A.; Dufton, J.A.; Kowalewska-Grochowska, K.T.; Lennox, P.; Anzarut, A.; Rodrigues, M. Nontuberculous mycobacterial breast implant infections. Plast. Reconstr. Surg. 2007, 119, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Virden, C.P.; Dobke, M.K.; Paul, S.; Lowell Parsons, C.; Frank, D.H. Subclinical Infection of the Silicone Breast Implant Surface as a Possible Cause of Capsular Contracture. Aesthetic Plast. Surg. 2020, 44, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Koves, B.; Magyar, A.; Tenke, P. Spectrum and antibiotic resistance of catheter-associated urinary tract infections. GMS Infect. Dis. 2017, 5, Doc06. [Google Scholar] [CrossRef] [PubMed]
- Cortese, Y.J.; Wagner, V.E.; Tierney, M.; Devine, D.; Fogarty, A. Review of Catheter-Associated Urinary Tract Infections and In Vitro Urinary Tract Models. J. Healthc. Eng. 2018, 2018, 2986742. [Google Scholar] [CrossRef] [Green Version]
- Yousif, A.; Jamal, M.A.; Raad, I. Biofilm-based central line-associated bloodstream infections. Adv. Exp. Med. Biol. 2015, 830, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Gominet, M.; Compain, F.; Beloin, C.; Lebeaux, D. Central venous catheters and biofilms: Where do we stand in 2017? APMIS 2017, 125, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Raghavendran, K.; Mylotte, J.M.; Scannapieco, F.A. Nursing home-associated pneumonia, hospital-acquired pneumonia and ventilator-associated pneumonia: The contribution of dental biofilms and periodontal inflammation. Periodontology 2000 2007, 44, 164–177. [Google Scholar] [CrossRef]
- Gibbs, K.; Holzman, I.R. Endotracheal tube: Friend or foe? Bacteria, the endotracheal tube, and the impact of colonization and infection. Semin. Perinatol. 2012, 36, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Barat, L.; Torres, A. Biofilms in ventilator-associated pneumonia. Future Microbiol. 2016, 11, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.E.; Lopes, S.P.; Pereira, C.R.; Azevedo, N.F.; Lourenco, A.; Henriques, M.; Pereira, M.O. Polymicrobial Ventilator-Associated Pneumonia: Fighting In Vitro Candida albicans-Pseudomonas aeruginosa Biofilms with Antifungal-Antibacterial Combination Therapy. PLoS ONE 2017, 12, e0170433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juma, N.A.; Forsythe, S.J. Microbial biofilm development on neonatal enteral feeding tubes. Adv. Exp. Med. Biol. 2015, 830, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.A.; Magalhaes, M.; Desorcy-Scherer, K.; Torrez Lamberti, M.; Lorca, G.L.; Neu, J. Neonatal Feeding Tube Colonization and the Potential Effect on Infant Health: A Review. Front. Nutr. 2022, 9, 775014. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D. Microbial adhesion to silicone hydrogel lenses: A review. Eye Contact Lens 2013, 39, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Device-associated infections: A macroproblem that starts with microadherence. Clin. Infect. Dis. 2001, 33, 1567–1572. [Google Scholar] [CrossRef]
- Lazar, V.; Chifiriuc, M.C. Medical significance and new therapeutical strategies for biofilm associated infections. Roum. Arch. Microbiol. Immunol. 2010, 69, 125–138. [Google Scholar] [PubMed]
- Stoica, P.; Chifiriuc, M.C.; Rapa, M.; Lazăr, V. 1-Overview of biofilm-related problems in medical devices. In Biofilms and Implantable Medical Devices; Deng, Y., Lv, W., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 3–23. ISBN 978-0-08-100382-4. [Google Scholar]
- Mihai, M.M.; Holban, A.M.; Giurcaneanu, C.; Popa, L.G.; Oanea, R.M.; Lazar, V.; Chifiriuc, M.C.; Popa, M.; Popa, M.I. Microbial biofilms: Impact on the pathogenesis of periodontitis, cystic fibrosis, chronic wounds and medical device-related infections. Curr. Top. Med. Chem. 2015, 15, 1552–1576. [Google Scholar] [CrossRef]
- Deva, A.K.; Adams, W.P., Jr.; Vickery, K. The role of bacterial biofilms in device-associated infection. Plast. Reconstr. Surg. 2013, 132, 1319–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickery, K.; Hu, H.; Jacombs, A.S.; Bradshaw, D.A.; Deva, A.K. A review of bacterial biofilms and their role in device-associated infection. Healthc. Infect. 2013, 18, 61–66. [Google Scholar] [CrossRef]
- Blackledge, M.S.; Worthington, R.J.; Melander, C. Biologically inspired strategies for combating bacterial biofilms. Curr. Opin. Pharmacol. 2013, 13, 699–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Jayaraman, N.; Chatterji, D. Small-Molecule Inhibition of Bacterial Biofilm. ACS Omega 2020, 5, 3108–3115. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, F.; Rezaee, M.A.; Ebrahimzadeh, S.; Yousefi, L.; Nouri, R.; Kafil, H.S.; Gholizadeh, P. Novel Strategies to Combat Bacterial Biofilms. Mol. Biotechnol. 2021, 63, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Parrino, B.; Schillaci, D.; Carnevale, I.; Giovannetti, E.; Diana, P.; Cirrincione, G.; Cascioferro, S. Synthetic small molecules as anti-biofilm agents in the struggle against antibiotic resistance. Eur. J. Med. Chem. 2019, 161, 154–178. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C. Timothy Wencewicz Antibiotics: Challenges, Mechanisms, Opportunities, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved Glycopeptide Antibacterial Drugs: Mechanism of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.D.; Palmer, M. The action mechanism of daptomycin. Bioorg. Med. Chem. 2016, 24, 6253–6268. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Eyler, R.F.; Shvets, K. Clinical Pharmacology of Antibiotics. Clin. J. Am. Soc. Nephrol. 2019, 14, 1080–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.; Gould, F.K. Oral antibiotics for infective endocarditis: A clinical review. J. Antimicrob. Chemother. 2020, 75, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Gorr, S.-U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Hannig, C.; Follo, M.; Hellwig, E.; Al-Ahmad, A. Visualization of adherent micro-organisms using different techniques. J. Med. Microbiol. 2010, 59, 1–7. [Google Scholar] [CrossRef]
- Valotteau, C.; Roelants, S.L.K.V.; Dasaiyan, P.; Zibek, S.; Günther, M.; Soetaert, W.; Everaert, B.; Pradier, C.-M.; Babonneau, F.; Baccile, N.; et al. Antibacterial properties of glycosylated surfaces: Variation of the glucosidal moiety and fatty acid conformation of grafted microbial glycolipids. Mol. Syst. Des. Eng. 2020, 5, 1307–1316. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Casalini, S.; Bortolotti, C.A.; Leonardi, F.; Biscarini, F. Self-assembled monolayers in organic electronics. Chem. Soc. Rev. 2017, 46, 40–71. [Google Scholar] [CrossRef]
- Maciel, J.; Martins, M.C.L.; Barbosa, M.A. The stability of self-assembled monolayers with time and under biological conditions. J. Biomed. Mater. Res. A 2010, 94, 833–843. [Google Scholar] [CrossRef]
- Mani, G.; Johnson, D.M.; Marton, D.; Dougherty, V.L.; Feldman, M.D.; Patel, D.; Ayon, A.A.; Agrawal, C.M. Stability of Self-Assembled Monolayers on Titanium and Gold. Langmuir 2008, 24, 6774–6784. [Google Scholar] [CrossRef] [PubMed]
- Chandekar, A.; Sengupta, S.K.; Whitten, J.E. Thermal stability of thiol and silane monolayers: A comparative study. Appl. Surf. Sci. 2010, 256, 2742–2749. [Google Scholar] [CrossRef]
- Srisombat, L.; Jamison, A.C.; Lee, T.R. Stability: A key issue for self-assembled monolayers on gold as thin-film coatings and nanoparticle protectants. Colloids Surf. A: Physicochem. Eng. Asp. 2011, 390, 1–19. [Google Scholar] [CrossRef]
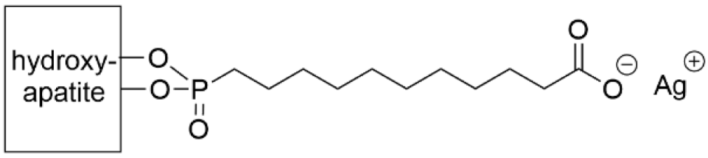
- Torres, N.; Oh, S.; Appleford, M.; Dean, D.D.; Jorgensen, J.H.; Ong, J.L.; Agrawal, C.M.; Mani, G. Stability of antibacterial self-assembled monolayers on hydroxyapatite. Acta Biomater. 2010, 6, 3242–3255. [Google Scholar] [CrossRef] [PubMed]
- Seitz, O.; Fernandes, P.G.; Tian, R.; Karnik, N.; Wen, H.-C.; Stiegler, H.; Chapman, R.A.; Vogel, E.M.; Chabal, Y.J. Control and stability of self-assembled monolayers under biosensing conditions. J. Mater. Chem. 2011, 21, 4384–4392. [Google Scholar] [CrossRef]
- Min, H.; Girard-Lauriault, P.-L.; Gross, T.; Lippitz, A.; Dietrich, P.; Unger, W.E.S. Ambient-ageing processes in amine self-assembled monolayers on microarray slides as studied by ToF-SIMS with principal component analysis, XPS, and NEXAFS spectroscopy. Anal. Bioanal. Chem. 2012, 403, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.J.; Fischer, D.A.; Lenhart, J.L. Thermal and Mechanical Aging of Self-Assembled Monolayers as Studied by Near Edge X-ray Absorption Fine Structure. Langmuir 2011, 27, 12423–12433. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Nieman, T.A. Ch. 21 Surface Characterization by Spectroscopy and Microscopy. In Principles of Instrumental Analysis; Harcourt Brace College Publishers: Philadelphia, PA, USA, 1998. [Google Scholar]
- Skoog, D.A.; Holler, F.J.; Nieman, T.A. Ch. 17 Applications of Infrared Spectroscopy. In Principles of Instrumental Analysis; Harcourt Brace College Publishers: Philadelphia, PA, USA, 1998. [Google Scholar]
- Sharma, P.K.; Rao, K.H. Analysis of different approaches for evaluation of surface energy of microbial cells by contact angle goniometry. Adv. Colloid Interface Sci. 2002, 98, 341–463. [Google Scholar] [CrossRef]
- Lamour, G.; Hamraoui, A.; Buvailo, A.; Xing, Y.; Keuleyan, S.; Prakash, V.; Eftekhari-Bafrooei, A.; Borguet, E. Contact Angle Measurements Using a Simplified Experimental Setup. J. Chem. Educ. 2010, 87, 1403–1407. [Google Scholar] [CrossRef]
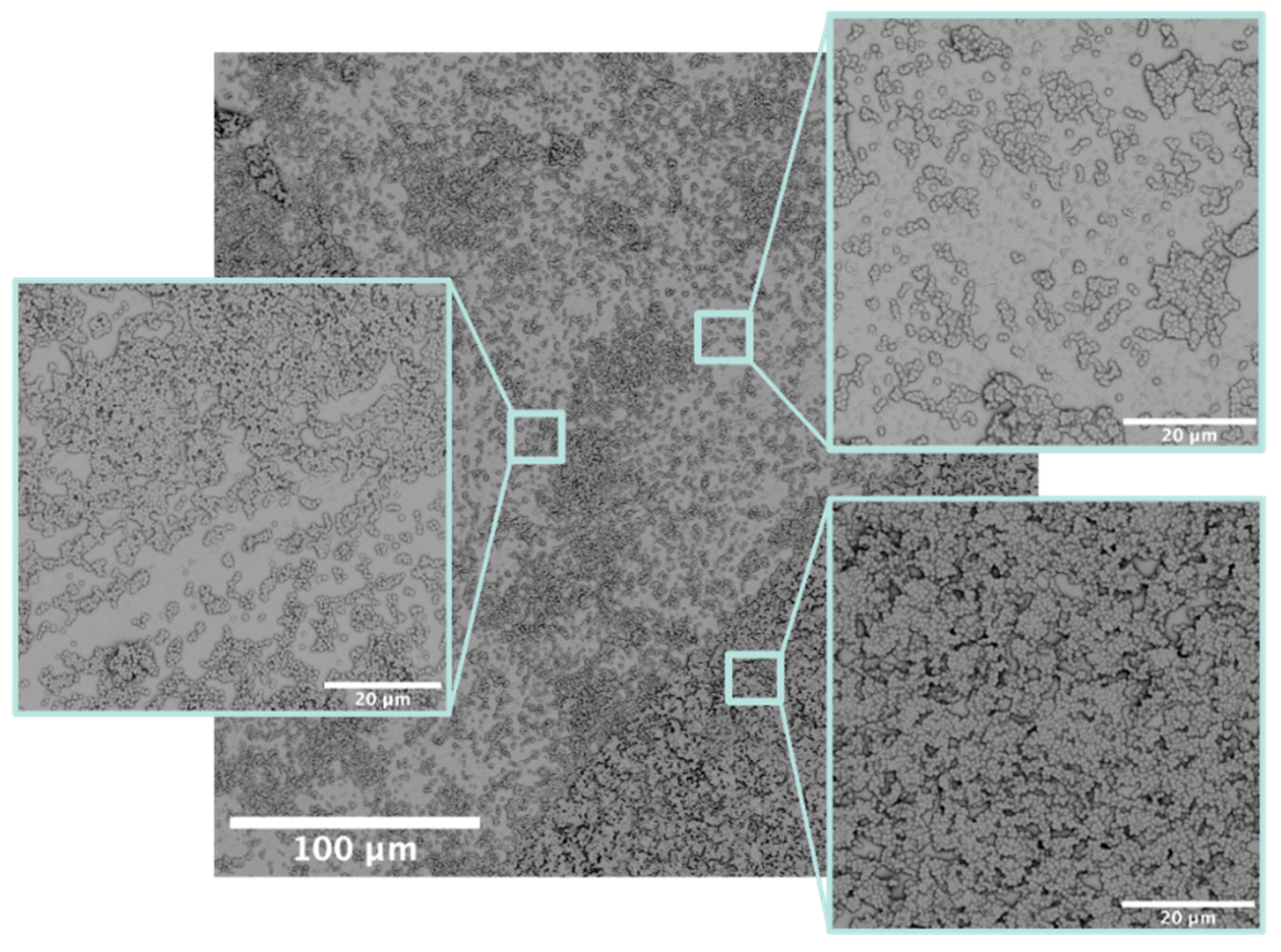
- Ploux, L.; Beckendorff, S.; Nardin, M.; Neunlist, S. Quantitative and morphological analysis of biofilm formation on self-assembled monolayers. Colloids Surf. B Biointerfaces 2007, 57, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Burton, E.A.; Simon, K.A.; Blodgett, D.; Luk, Y.-Y.; Ren, D. Inhibition of Escherichia coli Biofilm Formation by Self-Assembled Monolayers of Functional Alkanethiols on Gold. Appl. Environ. Microbiol. 2007, 73, 4300–4307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Cheng, L.; Hong, S.; Yang, C.; Lin, Y. Preparation of anti-fouling silicone elastomers by covalent immobilization of carboxybetaine. RSC Adv. 2015, 5, 88456–88463. [Google Scholar] [CrossRef]
- Ista, L.K.; Fan, H.; Baca, O.; López, G.P. Attachment of bacteria to model solid surfaces: Oligo(ethylene glycol) surfaces inhibit bacterial attachment. FEMS Microbiol. Lett. 1996, 142, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Metallo, S.J.; Choi, I.S.; Wu, H.; Liang, M.N.; Whitesides, G.M. Arrays of Self-Assembled Monolayers for Studying Inhibition of Bacterial Adhesion. Anal. Chem. 2002, 74, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Burton, E.A.; Wu, R.L.; Luk, Y.-Y.; Ren, D. Prolonged control of patterned biofilm formation by bio-inert surface chemistry. Chem. Commun. 2009, 1207–1209. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Prashar, D.; Luk, Y.-Y. Anti-Fouling Chemistry of Chiral Monolayers: Enhancing Biofilm Resistance on Racemic Surface. Langmuir 2011, 27, 6124–6131. [Google Scholar] [CrossRef]
- Perl, A.; Reinhoudt, D.N.; Huskens, J. Microcontact Printing: Limitations and Achievements. Adv. Mater. 2009, 21, 2257–2268. [Google Scholar] [CrossRef]
- Burton, E.A.; Simon, K.A.; Hou, S.; Ren, D.; Luk, Y.-Y. Molecular Gradients of Bioinertness Reveal a Mechanistic Difference between Mammalian Cell Adhesion and Bacterial Biofilm Formation. Langmuir 2009, 25, 1547–1553. [Google Scholar] [CrossRef]
- Gadenne, V.; Lebrun, L.; Jouenne, T.; Thebault, P. Antiadhesive activity of ulvan polysaccharides covalently immobilized onto titanium surface. Colloids Surf. B Biointerfaces 2013, 112, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Gadenne, V.; Lebrun, L.; Jouenne, T.; Thebault, P. Role of molecular properties of ulvans on their ability to elaborate antiadhesive surfaces. J. Biomed. Mater. Res. A 2015, 103, 1021–1028. [Google Scholar] [CrossRef]
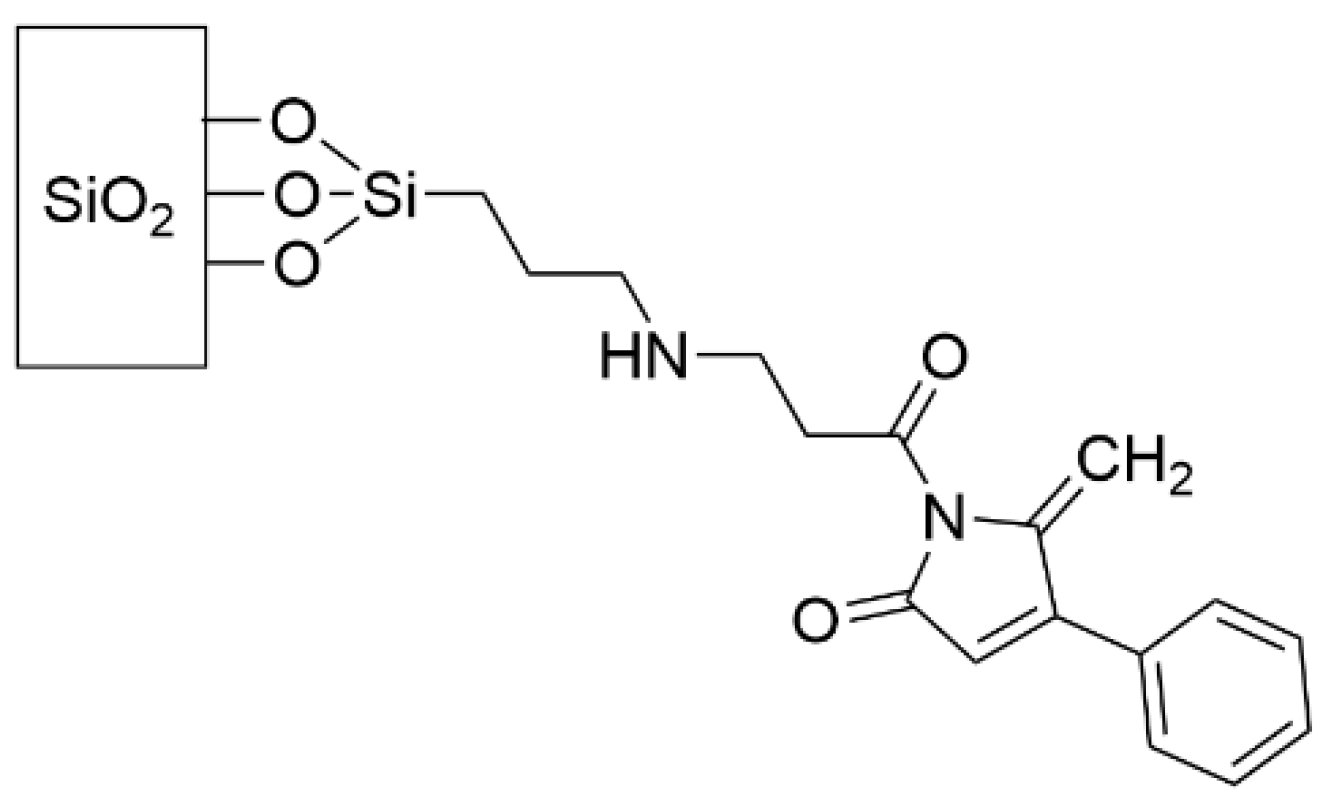
- Ho, K.K.K.; Cole, N.; Chen, R.; Willcox, M.D.P.; Rice, S.A.; Kumar, N. Characterisation and in vitro activities of surface attached dihydropyrrol-2-ones against Gram-negative and Gram-positive bacteria. Biofouling 2010, 26, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.K.; Chen, R.; Willcox, M.D.P.; Rice, S.A.; Cole, N.; Iskander, G.; Kumar, N. Quorum sensing inhibitory activities of surface immobilized antibacterial dihydropyrrolones via click chemistry. Biomaterials 2014, 35, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- Taunk, A.; Chen, R.; Iskander, G.; Ho, K.K.K.; Almohaywi, B.; Black, D.S.; Willcox, M.D.P.; Kumar, N. The Role of Orientation of Surface Bound Dihydropyrrol-2-ones (DHP) on Biological Activity. Molecules 2019, 24, 2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taunk, A.; Kit Ho, K.K.; Iskander, G.; Willcox, M.D.; Kumar, N. Surface Immobilization of Antibacterial Quorum Sensing Inhibitors by Photochemical Activation. J. Biotechnol. Biomater. 2016, 6, 3. [Google Scholar] [CrossRef]
- Vogel, J.; Wakker-Havinga, M.; Setroikromo, R.; Quax, W.J. Immobilized Acylase PvdQ Reduces Pseudomonas aeruginosa Biofilm Formation on PDMS Silicone. Front. Chem. 2020, 8, 54. [Google Scholar] [CrossRef]
- Gottenbos, B.; van der Mei, H.C.; Klatter, F.; Nieuwenhuis, P.; Busscher, H.J. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 2002, 23, 1417–1423. [Google Scholar] [CrossRef]
- Nikawa, H.; Ishida, K.; Hamada, T.; Satoda, T.; Murayama, T.; Takemoto, T.; Tamamoto, M.; Tajima, H.; Shimoe, S.; Fujimoto, H.; et al. Immobilization of Octadecyl Ammonium Chloride on the Surface of Titanium and Its Effect on Microbial Colonization In Vitro. Dent. Mater. J. 2005, 24, 570–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thebault, P.; Taffin de Givenchy, E.; Levy, R.; Vandenberghe, Y.; Guittard, F.; Géribaldi, S. Contact-active microbicidal gold surfaces using immobilization of quaternary ammonium thiol derivatives. Eur. J. Med. Chem. 2009, 44, 4227–4234. [Google Scholar] [CrossRef]
- Celesti, C.; Gervasi, T.; Cicero, N.; Giofrè, S.V.; Espro, C.; Piperopoulos, E.; Gabriele, B.; Mancuso, R.; Lo Vecchio, G.; Iannazzo, D. Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties. Materials 2022, 15, 3283. [Google Scholar] [CrossRef] [PubMed]
- Antoci, V., Jr.; King, S.B.; Jose, B.; Parvizi, J.; Zeiger, A.R.; Wickstrom, E.; Freeman, T.A.; Composto, R.J.; Ducheyne, P.; Shapiro, I.M.; et al. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J. Orthop. Res. 2007, 25, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Antoci, V.J.; Adams, C.S.; Parvizi, J.; Ducheyne, P.; Shapiro, I.M.; Hickok, N.J. Covalently Attached Vancomycin Provides a Nanoscale Antibacterial Surface. Clin. Orthop. Relat. Res. 2007, 461, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Antoci, V.; Adams, C.S.; Parvizi, J.; Davidson, H.M.; Composto, R.J.; Freeman, T.A.; Wickstrom, E.; Ducheyne, P.; Jungkind, D.; Shapiro, I.M.; et al. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials 2008, 29, 4684–4690. [Google Scholar] [CrossRef] [Green Version]
- Rottman, M.; Goldberg, J.; Hacking, S.A. Titanium-Tethered Vancomycin Prevents Resistance to Rifampicin in Staphylococcus aureus in vitro. PLoS ONE 2012, 7, e52883. [Google Scholar] [CrossRef]
- Antoci, V.J.; Adams, C.S.; Hickok, N.J.; Shapiro, I.M.; Parvizi, J. Vancomycin Bound to Ti Rods Reduces Periprosthetic Infection: Preliminary Study. Clin. Orthop. Relat. Res. 2007, 461, 88–95. [Google Scholar] [CrossRef]
- Stewart, S.; Barr, S.; Engiles, J.; Hickok, N.J.; Shapiro, I.M.; Richardson, D.W.; Parvizi, J.; Schaer, T.P. Vancomycin-Modified Implant Surface Inhibits Biofilm Formation and Supports Bone-Healing in an Infected Osteotomy Model in Sheep: A Proof-of-Concept Study. JBJS 2012, 94, 1406–1415. [Google Scholar] [CrossRef] [Green Version]
- Kucharíková, S.; Gerits, E.; De Brucker, K.; Braem, A.; Ceh, K.; Majdič, G.; Španič, T.; Pogorevc, E.; Verstraeten, N.; Tournu, H.; et al. Covalent immobilization of antimicrobial agents on titanium prevents Staphylococcus aureus and Candida albicans colonization and biofilm formation. J. Antimicrob. Chemother. 2016, 71, 936–945. [Google Scholar] [CrossRef] [Green Version]
- Kruszewski, K.M.; Nistico, L.; Longwell, M.J.; Hynes, M.J.; Maurer, J.A.; Hall-Stoodley, L.; Gawalt, E.S. Reducing Staphylococcus aureus biofilm formation on stainless steel 316L using functionalized self-assembled monolayers. Mater. Sci. Eng. C 2013, 33, 2059–2069. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-P.; Jing, R.-Y.; Wickstrom, E. Covalent Attachment of Daptomycin to Ti6Al4V Alloy Surfaces by a Thioether Linkage to Inhibit Colonization by Staphylococcus aureus. ACS Omega 2017, 2, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Davidson, H.; Poon, M.; Saunders, R.; Shapiro, I.M.; Hickok, N.J.; Adams, C.S. Tetracycline tethered to titanium inhibits colonization by Gram-negative bacteria. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1381–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yang, Y.; Li, W.; Wu, Z.; Li, J.; Xu, K.; Zhang, W.; Zheng, X.; Chen, J. Study of the Relationship Between Chlorhexidine-Grafted Amount and Biological Performances of Micro/Nanoporous Titanium Surfaces. ACS Omega 2019, 4, 18370–18380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reger, N.A.; Meng, W.S.; Gawalt, E.S. Antimicrobial Activity of Nitric Oxide-Releasing Ti-6Al-4V Metal Oxide. J. Funct. Biomater. 2017, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorzabal-Bellido, I.; Diaz-Fernandez, Y.A.; Susarrey-Arce, A.; Skelton, A.A.; McBride, F.; Beckett, A.J.; Prior, I.A.; Raval, R. Exploiting Covalent, H-Bonding, and π–π Interactions to Design Antibacterial PDMS Interfaces That Load and Release Salicylic Acid. ACS Appl. Bio Mater. 2019, 2, 4801–4811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef] [PubMed]
- Onaizi, S.A.; Leong, S.S.J. Tethering antimicrobial peptides: Current status and potential challenges. Biotechnol. Adv. 2011, 29, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Yala, J.-F.; Thebault, P.; Héquet, A.; Humblot, V.; Pradier, C.-M.; Berjeaud, J.-M. Elaboration of antibiofilm materials by chemical grafting of an antimicrobial peptide. Appl. Microbiol. Biotechnol. 2011, 89, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Humblot, V.; Yala, J.-F.; Thebault, P.; Boukerma, K.; Héquet, A.; Berjeaud, J.-M.; Pradier, C.-M. The antibacterial activity of Magainin I immobilized onto mixed thiols Self-Assembled Monolayers. Biomaterials 2009, 30, 3503–3512. [Google Scholar] [CrossRef]
- Lombana, A.; Raja, Z.; Casale, S.; Pradier, C.-M.; Foulon, T.; Ladram, A.; Humblot, V. Temporin-SHa peptides grafted on gold surfaces display antibacterial activity. J. Pept. Sci. 2014, 20, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oger, P.-C.; Piesse, C.; Ladram, A.; Humblot, V. Engineering of Antimicrobial Surfaces by Using Temporin Analogs to Tune the Biocidal/antiadhesive Effect. Molecules 2019, 24, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Willcox, M.D.P.; Cole, N.; Ho, K.K.K.; Rasul, R.; Denman, J.A.; Kumar, N. Characterization of chemoselective surface attachment of the cationic peptide melimine and its effects on antimicrobial activity. Acta Biomater. 2012, 8, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Xiong, M.; Hu, G.; Zhan, J.; Li, T.; Wang, L.; Wang, Y. Antimicrobial Titanium Surface via Click-Immobilization of Peptide and Its in Vitro/Vivo Activity. ACS Biomater. Sci. Eng. 2019, 5, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Masurier, N.; Tissot, J.-B.; Boukhriss, D.; Jebors, S.; Pinese, C.; Verdié, P.; Amblard, M.; Mehdi, A.; Martinez, J.; Humblot, V.; et al. Site-specific grafting on titanium surfaces with hybrid temporin antibacterial peptides. J. Mater. Chem. B 2018, 6, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Willcox, M.D.P.; Ho, K.K.K.; Smyth, D.; Kumar, N. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Chen, J.; Li, T.; Hu, G.; Fang, Z.; Zhou, H.; Guo, K.; Wang, L.; Wang, Y. One-step preparation of the engineered titanium implant by rationally designed linear fusion peptides with spacer-dependent antimicrobial, anti-inflammatory and osteogenic activities. Chem. Eng. J. 2021, 424, 130380. [Google Scholar] [CrossRef]
- Chen, X.; Hirt, H.; Li, Y.; Gorr, S.-U.; Aparicio, C. Antimicrobial GL13K Peptide Coatings Killed and Ruptured the Wall of Streptococcus gordonii and Prevented Formation and Growth of Biofilms. PLoS ONE 2014, 9, e111579. [Google Scholar] [CrossRef] [Green Version]
- Pinese, C.; Jebors, S.; Echalier, C.; Licznar-Fajardo, P.; Garric, X.; Humblot, V.; Calers, C.; Martinez, J.; Mehdi, A.; Subra, G. Simple and Specific Grafting of Antibacterial Peptides on Silicone Catheters. Adv. Healthc. Mater. 2016, 5, 3067–3073. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Hafeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Maillard, J.-Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tăbăran, F.-A.; Mocan, L. Review on Silver Nanoparticles as a Novel Class of Antibacterial Solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, e1701503. [Google Scholar] [CrossRef] [PubMed]
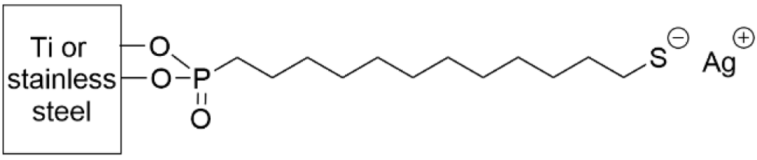
- Amalric, J.; Mutin, P.H.; Guerrero, G.; Ponche, A.; Sotto, A.; Lavigne, J.-P. Phosphonate monolayers functionalized by silver thiolate species as antibacterial nanocoatings on titanium and stainless steel. J. Mater. Chem. 2009, 19, 141–149. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Mathieu, M.; Lavigne, J.-P.; Toupet, K.; Guerrero, G.; Ponche, A.; Amalric, J.; Noël, D.; Mutin, P.H. In vitro and in vivo characterization of antibacterial activity and biocompatibility: A study on silver-containing phosphonate monolayers on titanium. Acta Biomater. 2015, 15, 266–277. [Google Scholar] [CrossRef]
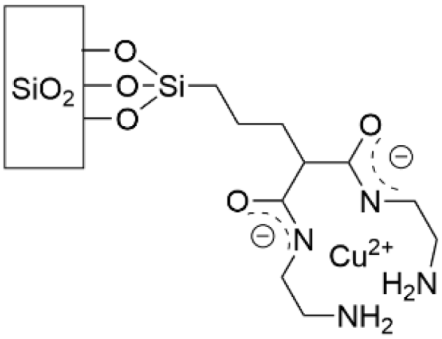
- Pallavicini, P.; Dacarro, G.; Grisoli, P.; Mangano, C.; Patrini, M.; Rigoni, F.; Sangaletti, L.; Taglietti, A. Coordination chemistry for antibacterial materials: A monolayer of a Cu2+ 2,2′-bipyridine complex grafted on a glass surface. Dalton Trans. 2013, 42, 4552–4560. [Google Scholar] [CrossRef]
- Taglietti, A.; Grisoli, P.; Dacarro, G.; Gattesco, A.; Mangano, C.; Pallavicini, P. Grafted monolayers of the neutral Cu(II) complex of a dioxo-2,3,2 ligand: Surfaces with decreased antibacterial action. New J. Chem. 2018, 42, 7595–7598. [Google Scholar] [CrossRef]
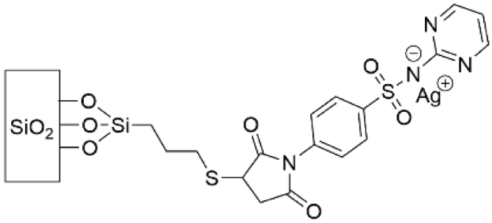
- Taglietti, A.; Dacarro, G.; Barbieri, D.; Cucca, L.; Grisoli, P.; Patrini, M.; Arciola, C.R.; Pallavicini, P. High Bactericidal Self-Assembled Nano-Monolayer of Silver Sulfadiazine on Hydroxylated Material Surfaces. Materials 2019, 12, 2761. [Google Scholar] [CrossRef] [Green Version]
- Pallavicini, P.; Dacarro, G.; Diaz-Fernandez, Y.A.; Taglietti, A. Coordination chemistry of surface-grafted ligands for antibacterial materials. Coord. Chem. Rev. 2014, 275, 37–53. [Google Scholar] [CrossRef]
- Olar, R.; Badea, M.; Chifiriuc, M.C. Metal Complexes—A Promising Approach to Target Biofilm Associated Infections. Molecules 2022, 27, 758. [Google Scholar] [CrossRef] [PubMed]
- Yliniemi, K.; Vahvaselkä, M.; Ingelgem, Y.V.; Baert, K.; Wilson, B.P.; Terryn, H.; Kontturi, K. The formation and characterisation of ultra-thin films containing Ag nanoparticles. J. Mater. Chem. 2008, 18, 199–206. [Google Scholar] [CrossRef]
- Juan, L.; Zhimin, Z.; Anchun, M.; Lei, L.; Jingchao, Z. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int. J. Nanomed. 2010, 5, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallavicini, P.; Taglietti, A.; Dacarro, G.; Diaz-Fernandez, Y.A.; Galli, M.; Grisoli, P.; Patrini, M.; Santucci De Magistris, G.; Zanoni, R. Self-assembled monolayers of silver nanoparticles firmly grafted on glass surfaces: Low Ag+ release for an efficient antibacterial activity. J. Colloid Interface Sci. 2010, 350, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P.; et al. Antibiofilm activity of a monolayer of silver nanoparticles anchored to an amino-silanized glass surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Gargioni, C.; Borzenkov, M.; D’Alfonso, L.; Sperandeo, P.; Polissi, A.; Cucca, L.; Dacarro, G.; Grisoli, P.; Pallavicini, P.; D’Agostino, A.; et al. Self-Assembled Monolayers of Copper Sulfide Nanoparticles on Glass as Antibacterial Coatings. Nanomaterials 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Pallavicini, P.; Dacarro, G.; Cucca, L.; Denat, F.; Grisoli, P.; Patrini, M.; Sok, N.; Taglietti, A. A monolayer of a Cu2+-tetraazamacrocyclic complex on glass as the adhesive layer for silver nanoparticles grafting, in the preparation of surface-active antibacterial materials. New J. Chem. 2011, 35, 1198–1201. [Google Scholar] [CrossRef]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the biological windows: Current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef]
- Pallavicini, P.; Chirico, G.; Taglietti, A. Harvesting Light To Produce Heat: Photothermal Nanoparticles for Technological Applications and Biomedical Devices. Chemistry 2021, 27, 15361–15374. [Google Scholar] [CrossRef]
- Borzenkov, M.; Pallavicini, P.; Taglietti, A.; D’Alfonso, L.; Collini, M.; Chirico, G. Photothermally active nanoparticles as a promising tool for eliminating bacteria and biofilms. Beilstein J. Nanotechnol. 2020, 11, 1134–1146. [Google Scholar] [CrossRef]
- Pallavicini, P.; Donà, A.; Taglietti, A.; Minzioni, P.; Patrini, M.; Dacarro, G.; Chirico, G.; Sironi, L.; Bloise, N.; Visai, L.; et al. Self-assembled monolayers of gold nanostars: A convenient tool for near-IR photothermal biofilm eradication. Chem. Commun. 2014, 50, 1969–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dacarro, G.; Grisoli, P.; Borzenkov, M.; Milanese, C.; Fratini, E.; Ferraro, G.; Taglietti, A.; Pallavicini, P. Self-assembled monolayers of Prussian blue nanoparticles with photothermal effect. Supramol. Chem. 2017, 29, 823–833. [Google Scholar] [CrossRef]
- D’Agostino, A.; Taglietti, A.; Desando, R.; Bini, M.; Patrini, M.; Dacarro, G.; Cucca, L.; Pallavicini, P.; Grisoli, P. Bulk Surfaces Coated with Triangular Silver Nanoplates: Antibacterial Action Based on Silver Release and Photo-Thermal Effect. Nanomaterials 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Bassi, B.; Chirico, G.; Collini, M.; Dacarro, G.; Fratini, E.; Grisoli, P.; Patrini, M.; Sironi, L.; Taglietti, A.; et al. Modular approach for bimodal antibacterial surfaces combining photo-switchable activity and sustained biocidal release. Sci. Rep. 2017, 7, 5259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Q.; Shan, Y.; Lu, Y.; Xue, P.; Liu, Y.; Liu, X. Enhanced Antibacterial Activity of Poly (dimethylsiloxane) Membranes by Incorporating SiO2 Microspheres Generated Silver Nanoparticles. Nanomaterials 2019, 9, 705. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.-Y.; Lin, H.-Y.; Yang, L.-C.; Tseng, S.-C.; Sun, A.Y.; Chen, C.; Wan, D. Substrate-independent adsorption of nanoparticles as anti-biofilm coatings. Biomater. Sci. 2022, 10, 410–422. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Pandey, L.M. Design of antibiofilm surfaces by immobilization of biogenic silver nanoparticles on amine self-assembled monolayers. Mater. Lett. 2022, 311, 131574. [Google Scholar] [CrossRef]
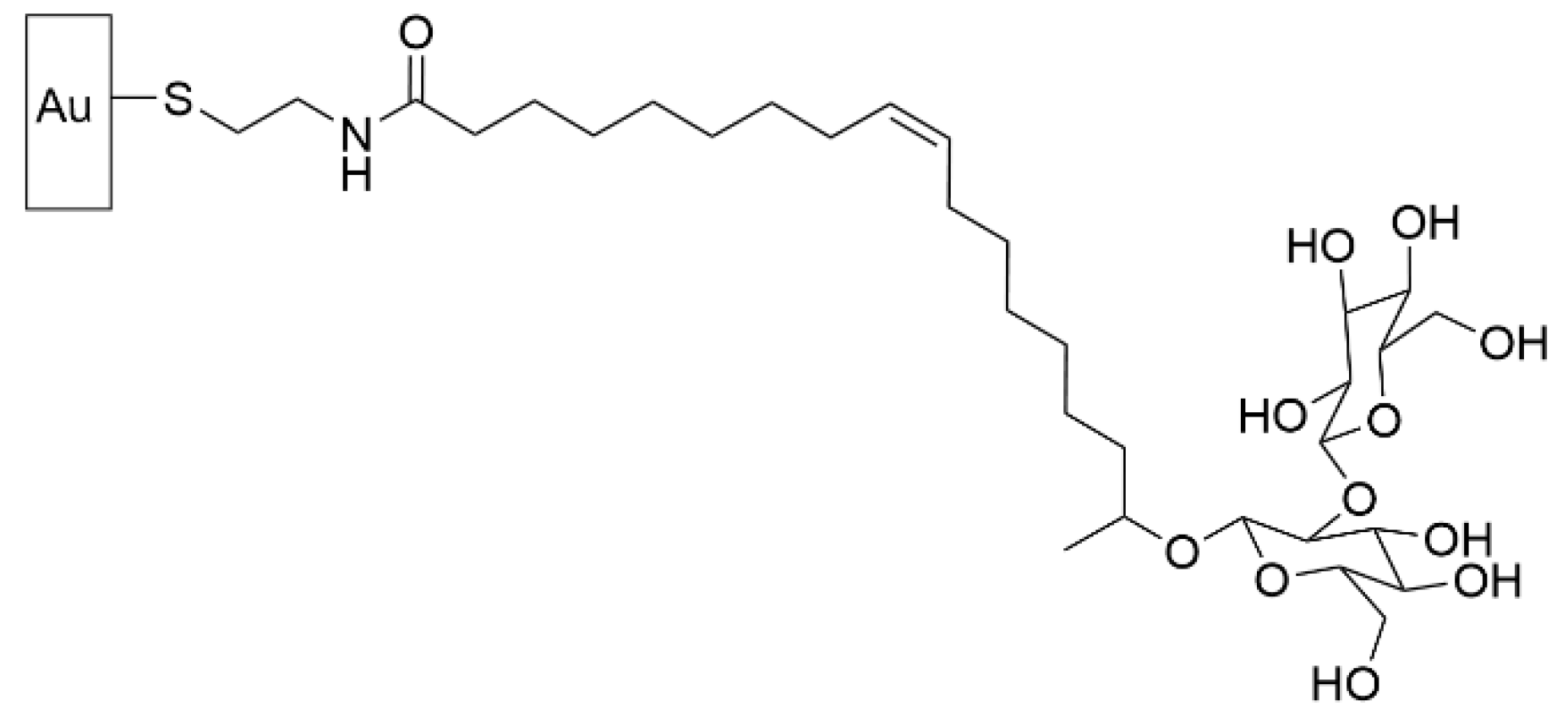
- Valotteau, C.; Calers, C.; Casale, S.; Berton, J.; Stevens, C.V.; Babonneau, F.; Pradier, C.-M.; Humblot, V.; Baccile, N. Biocidal Properties of a Glycosylated Surface: Sophorolipids on Au(111). ACS Appl. Mater. Interfaces 2015, 7, 18086–18095. [Google Scholar] [CrossRef] [Green Version]
- Valotteau, C.; Banat, I.M.; Mitchell, C.A.; Lydon, H.; Marchant, R.; Babonneau, F.; Pradier, C.-M.; Baccile, N.; Humblot, V. Antibacterial properties of sophorolipid-modified gold surfaces against Gram positive and Gram negative pathogens. Colloids Surf. B Biointerfaces 2017, 157, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Hu, K.; Xing, X.; Zhang, J.; Zhang, M.-R.; Ma, X.; Shi, R.; Zhang, L. Smart Titanium Coating Composed of Antibiotic Conjugated Peptides as an Infection-Responsive Antibacterial Agent. Macromol. Biosci. 2021, 21, 2000194. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Lee, J.; Kim, W.; Kang, H.; Bae, S.W.; Chang, R.; Kim, S.; Yeo, W.-S. On-Demand Modulation of Bacterial Cell Fates on Multifunctional Dynamic Substrates. ACS Appl. Mater. Interfaces 2018, 10, 4324–4332. [Google Scholar] [CrossRef] [PubMed]
- Sjollema, J.; Zaat, S.A.J.; Fontaine, V.; Ramstedt, M.; Luginbuehl, R.; Thevissen, K.; Li, J.; van der Mei, H.C.; Busscher, H.J. In vitro methods for the evaluation of antimicrobial surface designs. Acta Biomater. 2018, 70, 12–24. [Google Scholar] [CrossRef]
- EPA Method and Proposed Guidance to Assess the Efficacy of Antimicrobial Pesticide Products Intended to Control Public Health Biofilms. Available online: https://www.regulations.gov/docket/EPA-HQ-OPP-2016-0357 (accessed on 28 June 2022).
- Campos, M.D.; Zucchi, P.C.; Phung, A.; Leonard, S.N.; Hirsch, E.B. The Activity of Antimicrobial Surfaces Varies by Testing Protocol Utilized. PLoS ONE 2016, 11, e0160728. [Google Scholar] [CrossRef]
- Parker, A.E.; Hamilton, M.A.; Goeres, D.M. Reproducibility of antimicrobial test methods. Sci. Rep. 2018, 8, 12531. [Google Scholar] [CrossRef]
- Sikosana, M.K.L.N.; Ruland, A.; Werner, C.; Renner, L.D. Combining Microscopy Assays of Bacteria-Surface Interactions To Better Evaluate Antimicrobial Polymer Coatings. Appl. Environ. Microbiol. 2022, 88, e02241-21. [Google Scholar] [CrossRef] [PubMed]

| Indwelling Medical Device | Commonly Isolated Bacteria |
|---|---|
| Long-Term Devices | |
| Orthopedic implants [43,44,45,46,47,48] | K. pneumoniae |
| A. baumannii | |
| S. epidermidis | |
| S. aureus | |
| Stents [49,50] | E. coli |
| Enterobacter | |
| Klebsiella | |
| P. aeruginosa | |
| E. faecalis | |
| Streptococci | |
| S. aureus | |
| S. epidermidis | |
| Cochlear implants [51,52] | P. aeruginosa |
| S. pyogenes | |
| S. epidermidis | |
| S. aureus | |
| Breast implants [53,54,55,56,57,58] | E. coli |
| Mycobacterium | |
| S. epidermidis | |
| S. aureus | |
| Streptococci | |
| Bacillus | |
| Short-Term Devices | |
| Urinary catheter [42,59,60] | E. coli |
| P. aeruginosa | |
| K. pneumoniae | |
| A. baumannii | |
| Enterobacter | |
| S. epidermidis | |
| E. faecalis | |
| Central venous catheter [61,62] | P. aeruginosa |
| K. pneumoniae | |
| S. epidermidis | |
| S. aureus | |
| E. faecalis | |
| Endotracheal tube [63,64,65,66] | P. aeruginosa |
| K. pneumoniae | |
| Acinetobacter | |
| Enterobacter | |
| S. aureus | |
| E. faecalis | |
| Feeding tube [67,68] | Pseudomonas |
| Enterococci | |
| Bacilli | |
| Staphylococci | |
| Contact lenses [48,59,69] | E. coli |
| P. aeruginosa | |
| S. aureus | |
| SAM Precursor Molecule | Surface | Reference |
|---|---|---|
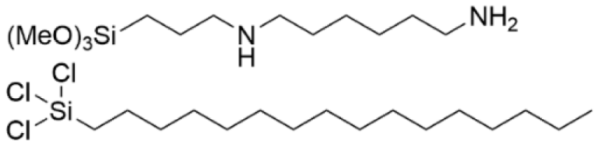 | Si | [108] |
 | Au | [109] |
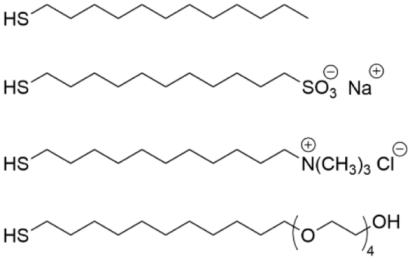 | Au | [110] |
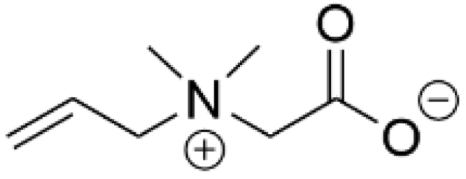 | PDMS | [111] |
| SAM Precursor Molecule/SAM Structure | Surface | Reference |
|---|---|---|
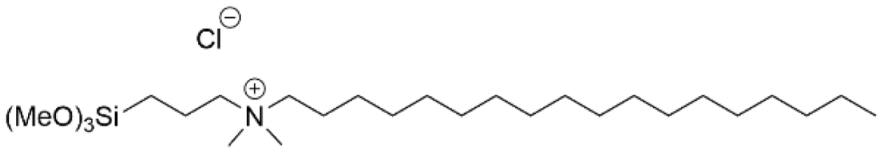 | Silicone Ti | [125] [126] |
 | Au | [127] |
 | Ti | [128] |
| SAM Structure | Reference |
|---|---|
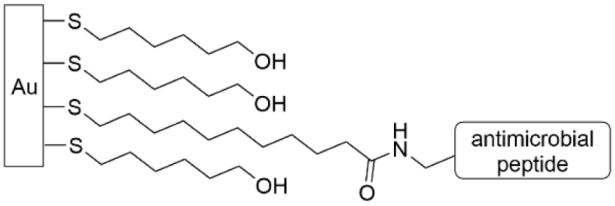 | [146,147,148] |
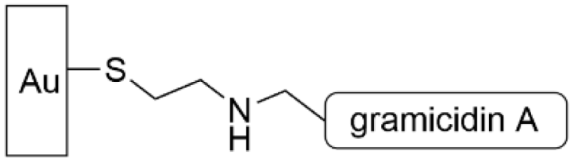 | [145] |
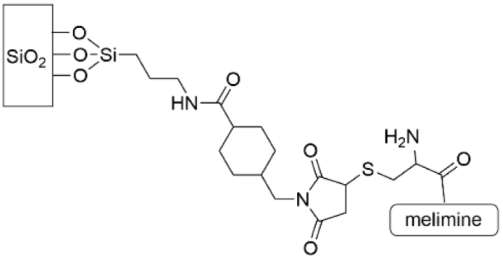 | [149] |
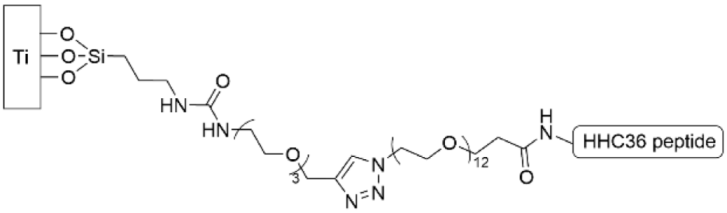 | [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundin, P.M.; Fiser, B.L.; Blackledge, M.S.; Pickett, H.L.; Copeland, A.L. Functionalized Self-Assembled Monolayers: Versatile Strategies to Combat Bacterial Biofilm Formation. Pharmaceutics 2022, 14, 1613. https://doi.org/10.3390/pharmaceutics14081613
Lundin PM, Fiser BL, Blackledge MS, Pickett HL, Copeland AL. Functionalized Self-Assembled Monolayers: Versatile Strategies to Combat Bacterial Biofilm Formation. Pharmaceutics. 2022; 14(8):1613. https://doi.org/10.3390/pharmaceutics14081613
Chicago/Turabian StyleLundin, Pamela M., Briana L. Fiser, Meghan S. Blackledge, Hannah L. Pickett, and Abigail L. Copeland. 2022. "Functionalized Self-Assembled Monolayers: Versatile Strategies to Combat Bacterial Biofilm Formation" Pharmaceutics 14, no. 8: 1613. https://doi.org/10.3390/pharmaceutics14081613
APA StyleLundin, P. M., Fiser, B. L., Blackledge, M. S., Pickett, H. L., & Copeland, A. L. (2022). Functionalized Self-Assembled Monolayers: Versatile Strategies to Combat Bacterial Biofilm Formation. Pharmaceutics, 14(8), 1613. https://doi.org/10.3390/pharmaceutics14081613