Impact of Sex on Proper Use of Inhaler Devices in Asthma and COPD: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Eligibility
2.2. Study Selection
2.3. Data Extraction
2.4. Endpoint
2.5. Data Synthesis and Analysis
2.6. Quality of Studies and Risk Bias
2.7. Software and Statistical Significance
3. Results
3.1. Study Characteristics
3.2. Pairwise Meta-Analysis
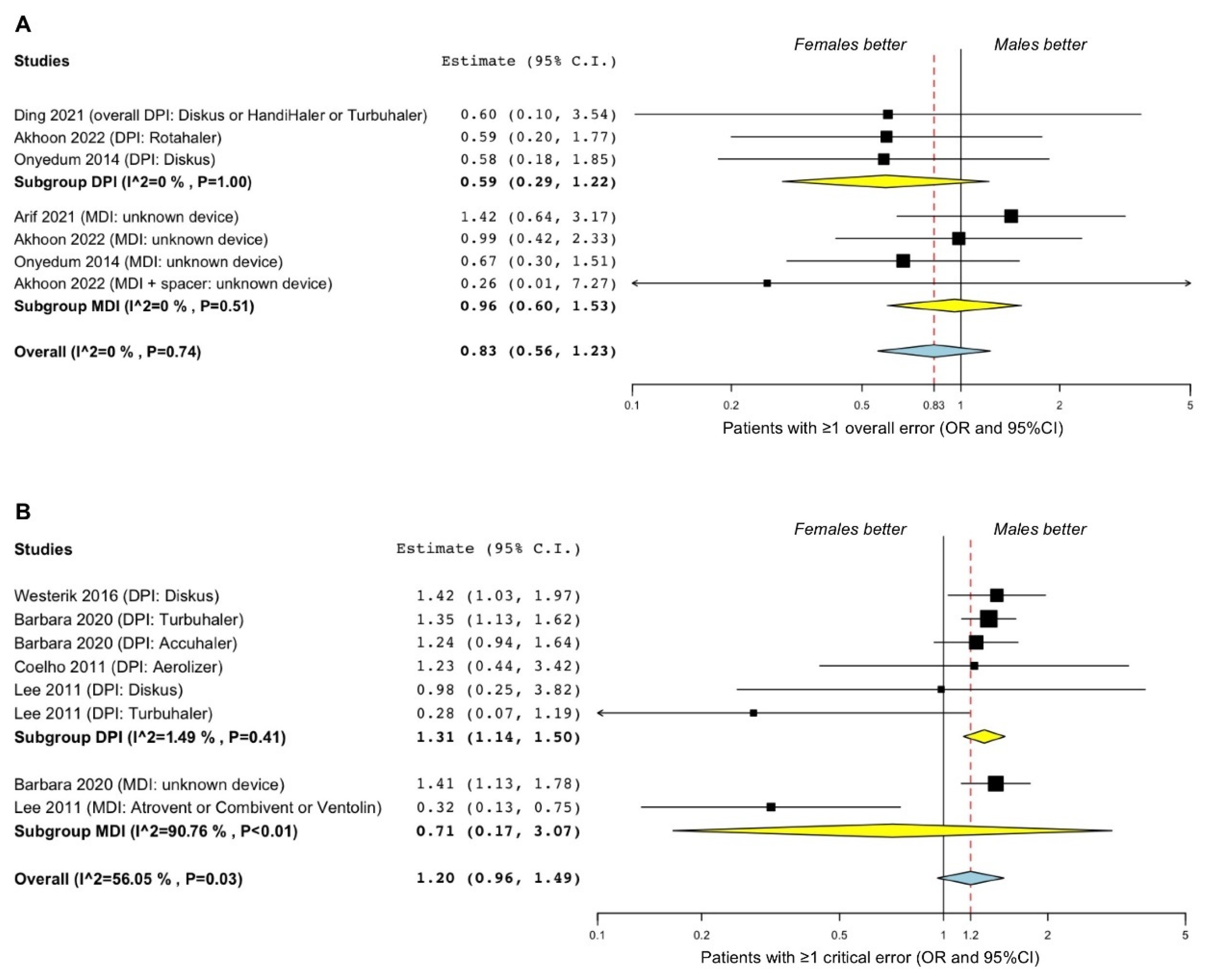
3.2.1. Asthma
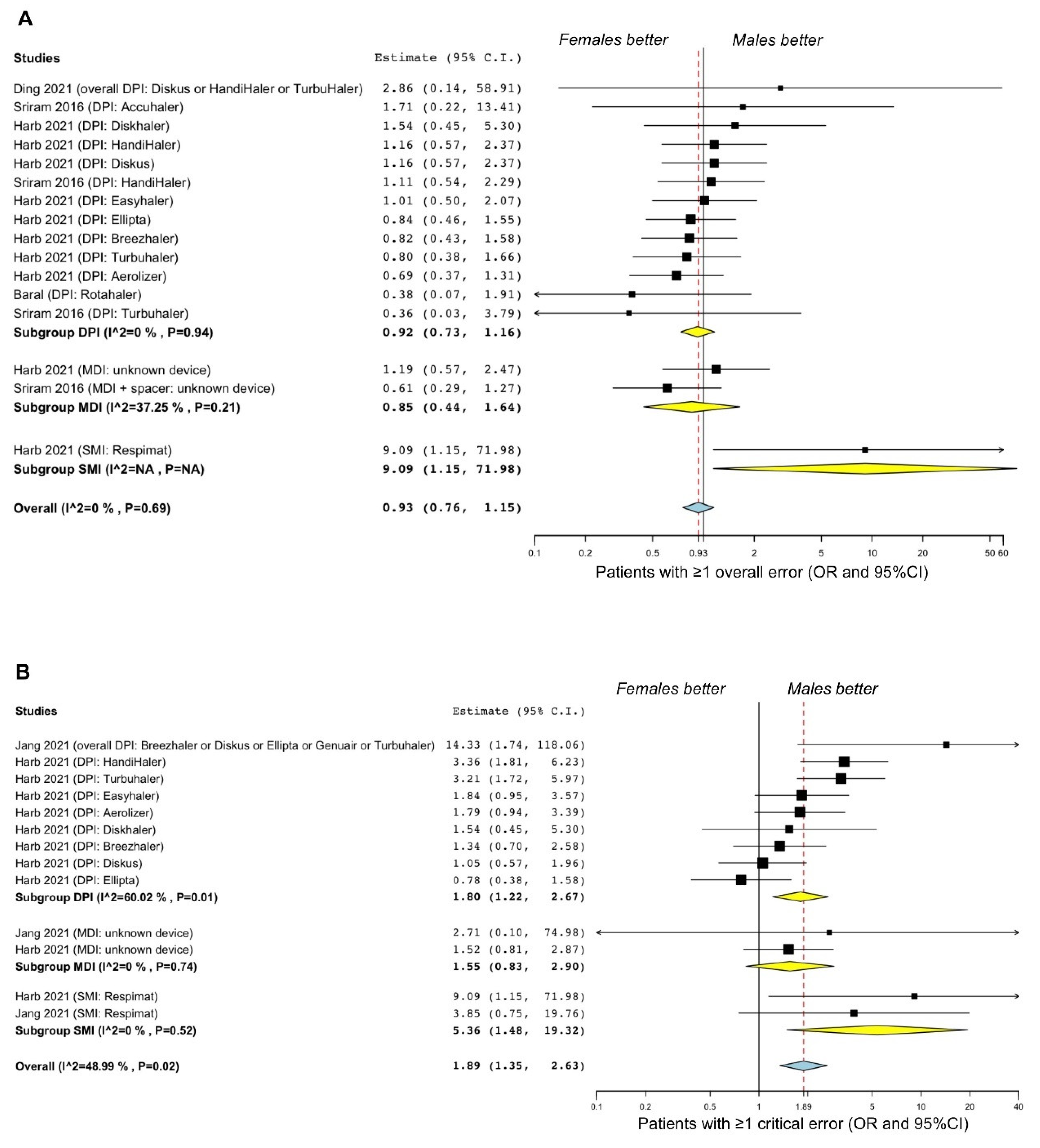
3.2.2. COPD
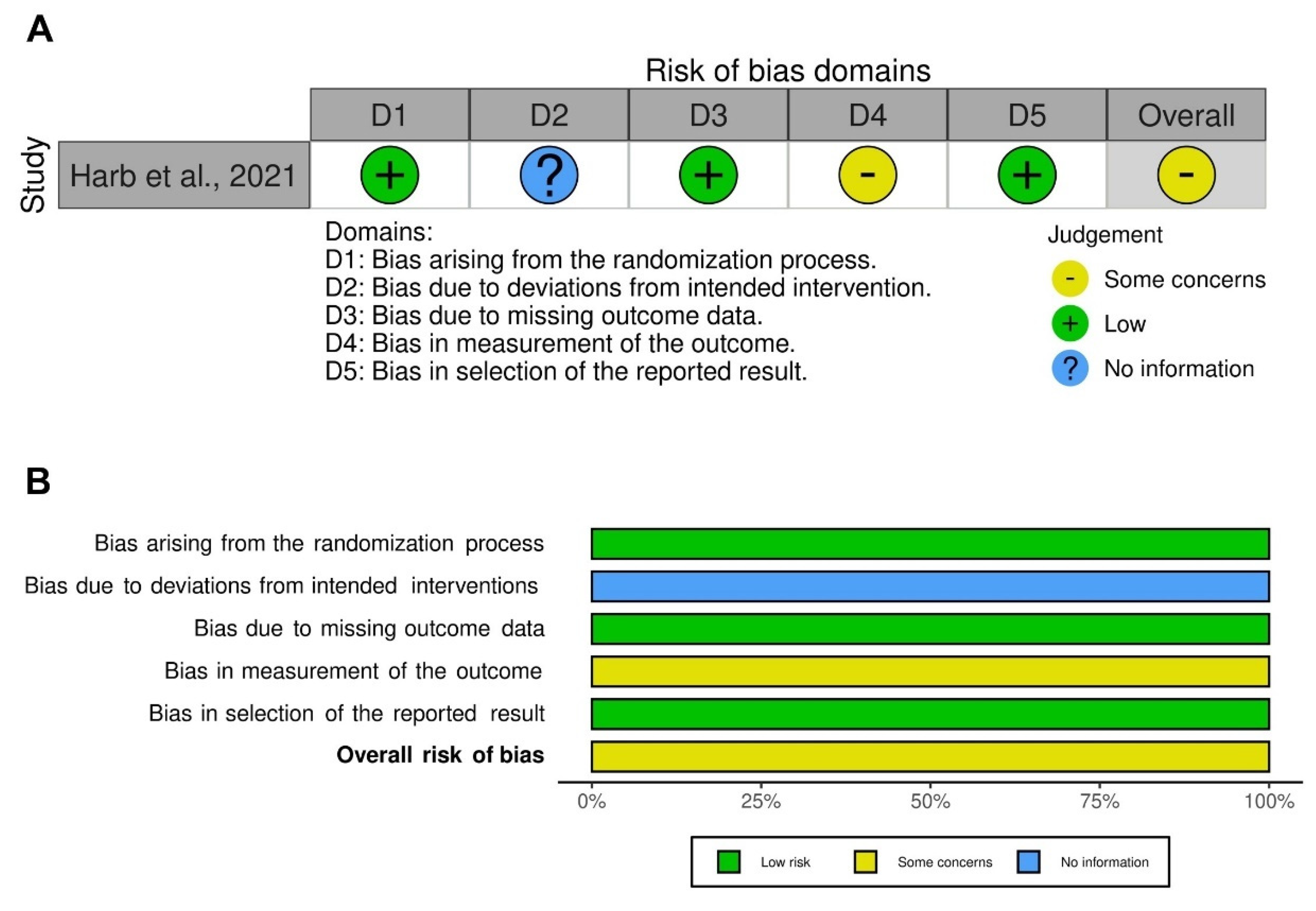
3.3. Risk of Bias and Quality of Evidence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GINA. 2021 GINA Main Report|Global Initiative for Asthma. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf (accessed on 22 July 2021).
- Asthma. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 22 February 2022).
- GOLD. Global Strategy for Prevention, Diagnosis and Management of COPD: 2022 Report. 2022. Available online: https://goldcopd.org/2022-gold-reports-2/ (accessed on 14 March 2022).
- Roche, N.; Plaza, V.; Backer, V.; van der Palen, J.; Cerveri, I.; Gonzalez, C.; Safioti, G.; Scheepstra, I.; Patino, O.; Singh, D. Asthma Control and COPD Symptom Burden in Patients Using Fixed-Dose Combination Inhalers (SPRINT Study). NPJ Prim. Care Respir. Med. 2020, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Melani, A.S.; Bonavia, M.; Cilenti, V.; Cinti, C.; Lodi, M.; Martucci, P.; Serra, M.; Scichilone, N.; Sestini, P.; Aliani, M.; et al. Inhaler Mishandling Remains Common in Real Life and Is Associated with Reduced Disease Control. Respir. Med. 2011, 105, 930–938. [Google Scholar] [CrossRef]
- Lindgren, S.; Bake, B.; Larsson, S. Clinical Consequences of Inadequate Inhalation Technique in Asthma Therapy. Eur. J. Respir. Dis. 1987, 70, 93–98. [Google Scholar] [PubMed]
- Virchow, J.C.; Crompton, G.K.; Dal Negro, R.; Pedersen, S.; Magnan, A.; Seidenberg, J.; Barnes, P.J. Importance of Inhaler Devices in the Management of Airway Disease. Respir. Med. 2008, 102, 10–19. [Google Scholar] [CrossRef]
- Vanoverschelde, A.; Van Der Wel, P.; Lahousse, L. Poor Inhalation Technique Is a Major Determinant of Acute Exacerbations. Eur. Respir. J. 2019, 54 (Suppl. 63), PA4236. [Google Scholar] [CrossRef]
- Biddiscombe, M.F.; Usmani, O.S. Is There Room for Further Innovation in Inhaled Therapy for Airways Disease? Breathe 2018, 14, 216–224. [Google Scholar] [CrossRef]
- Chrystyn, H.; Van Der Palen, J.; Sharma, R.; Barnes, N.; Delafont, B.; Mahajan, A.; Thomas, M. Device Errors in Asthma and COPD: Systematic Literature Review and Meta-Analysis. NPJ Prim. Care Respir. Med. 2017, 27, 22. [Google Scholar] [CrossRef]
- Mäkelä, M.J.; Backer, V.; Hedegaard, M.; Larsson, K. Adherence to Inhaled Therapies, Health Outcomes and Costs in Patients with Asthma and COPD. Respir. Med. 2013, 107, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Gich, I.; Pedersen, S. Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time? Chest 2016, 150, 394–406. [Google Scholar] [CrossRef]
- Navaie, M.; Dembek, C.; Cho-Reyes, S.; Yeh, K.; Celli, B.R. Device Use Errors with Soft Mist Inhalers: A Global Systematic Literature Review and Meta-Analysis. Chronic Respir. Dis. 2020, 17, 1479973119901234. [Google Scholar] [CrossRef]
- Ocakli, B.; Ozmen, I.; Tuncay, E.A.; Gungor, S.; Ozalp, A.; Yasin, Y.; Adiguzel, N.; Gungor, G.; Karakurt, Z. Influence of Gender on Inhaler Technique. Respir. Care 2020, 65, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Usmani, O.S.; Lavorini, F.; Marshall, J.; Dunlop, W.C.N.; Heron, L.; Farrington, E.; Dekhuijzen, R. Critical Inhaler Errors in Asthma and COPD: A Systematic Review of Impact on Health Outcomes. Respir. Res. 2018, 19, 10. [Google Scholar] [CrossRef]
- Pothirat, C.; Chaiwong, W.; Phetsuk, N.; Pisalthanapuna, S.; Chetsadaphan, N.; Choomuang, W. Evaluating Inhaler Use Technique in COPD Patients. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1291–1298. [Google Scholar] [CrossRef]
- Goodman, D.E.; Israel, E.; Rosenberg, M.; Johnston, R.; Weiss, S.T.; Drazen, J.M. The Influence of Age, Diagnosis, and Gender on Proper Use of Metered-Dose Inhalers. Am. J. Respir. Crit. Care Med. 1994, 150, 1256–1261. [Google Scholar] [CrossRef]
- Duarte-De-Araújo, A.; Teixeira, P.; Hespanhol, V.; Correia-De-Sousa, J. COPD: Misuse of Inhaler Devices in Clinical Practice. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1209. [Google Scholar] [CrossRef]
- Gray, S.L.; Williams, D.M.; Pulliam, C.C.; Sirgo, M.A.; Bishop, A.L.; Donohue, J.F. Characteristics Predicting Incorrect Metered-Dose Inhaler Technique in Older Subjects. Arch. Intern. Med. 1996, 156, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Chafin, C.C.; Tolley, E.A.; George, C.M.; Demirkan, K.; Kuhl, D.A.; Pugazhenthi, M.; Self, T.H. Gender Differences in Metered-Dose Inhaler-Spacer Device Technique. Pharmacotherapy 2000, 20, 1324–1327. [Google Scholar] [CrossRef]
- Arora, P.; Kumar, L.; Vohra, V.; Sarin, R.; Jaiswal, A.; Puri, M.M.; Rathee, D.; Chakraborty, P. Evaluating the Technique of Using Inhalation Device in COPD and Bronchial Asthma Patients. Respir. Med. 2014, 108, 992–998. [Google Scholar] [CrossRef]
- Giraud, V.; Roche, N. Misuse of Corticosteroid Metered-Dose Inhaler Is Associated with Decreased Asthma Stability. Eur. Respir. J. 2002, 19, 246–251. [Google Scholar] [CrossRef]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and Gender in Asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Puxeddu, E.; Rogliani, P. Gender-Related Responsiveness to the Pharmacological Treatment of COPD: A First Step Towards the Personalized Medicine. eBioMedicine 2017, 19, 14–15. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and Gender: Modifiers of Health, Disease, and Medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO Framework to Improve Searching PubMed for Clinical Questions. BMC Med. Inf. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Gleeson, P.K.; Feldman, S.; Apter, A.J. Controller Inhalers: Overview of Devices, Instructions for Use, Errors, and Interventions to Improve Technique. J. Allergy Clin. Immunol. Pr. 2020, 8, 2234–2242. [Google Scholar] [CrossRef]
- Biddiscombe, M.F.; Usmani, O.S. Delivery and Adherence with Inhaled Therapy in Asthma. Minerva Med. 2021, 112, 564–572. [Google Scholar] [CrossRef]
- Harb, H.S.; Ibrahim Laz, N.; Rabea, H.; Abdelrahim, M.E.A. Determinants of Incorrect Inhaler Technique in Chronic Obstructive Pulmonary Disease Patients. Int. J. Clin. Pr. 2021, 75, e14073. [Google Scholar] [CrossRef]
- Understanding Observational Studies. Drug Ther. Bull. 2016, 54, 105–108. [CrossRef]
- Stevens, N.; Dixon, J.; Lederhilger, S.; Mannerstråle, F.; Cheng, J.; Buckner, C.; Horst, S.; Limouzin, K.; Hoe, L.; Lyapustina, S.; et al. The IPAC-RS Inhaler Common Use Errors Matrix; IPAC-RS: Palm Desert, CA, USA, 2020. [Google Scholar]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; Updated July 2019; Cochrane: London, England, 2019; pp. 205–228. Available online: www.Training.Cochrane.Org/Handbook (accessed on 14 March 2022).
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute: Adelaide, Australia, 2020. [Google Scholar]
- Pedder, H.; Sarri, G.; Keeney, E.; Nunes, V.; Dias, S. Data Extraction for Complex Meta-Analysis (DECiMAL) Guide. Syst. Rev. 2016, 5, 212. [Google Scholar] [CrossRef] [PubMed]
- Gianinazzi, M.E.; Rueegg, C.S.; Zimmerman, K.; Kuehni, C.E.; Michel, G.; Swiss Paediatric Oncology Group. Intra-Rater and Inter-Rater Reliability of a Medical Record Abstraction Study on Transition of Care after Childhood Cancer. PLoS ONE 2015, 10, e0124290. [Google Scholar] [CrossRef][Green Version]
- Borenstein, M. Introduction to Meta-Analysis; John Wiley & Sons: Chichester, UK, 2009; Volume XXVIII, p. 421. [Google Scholar]
- DeCoster, J. Meta-Analysis Notes; University of Alabama: Tuscaloosa, AL, USA, 2004. [Google Scholar]
- Turner, J.R.; Durham, T.A. Meta-methodology: Conducting and Reporting Meta-analyses. J. Clin. Hypertens. 2014, 16, 91–93. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Page, C.; Jardim, J.; Chuchalin, A.G.; Rogliani, P.; Matera, M.G. Influence of N-Acetylcysteine on Chronic Bronchitis or COPD Exacerbations: A Meta-Analysis. Eur. Respir. Rev. 2015, 24, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. 9.5.2 Identifying and Measuring Heterogeneity. Available online: https://Handbook-5-1.Cochrane.Org/Chapter_9/9_5_2_identifying_and_measuring_heterogeneity.Htm (accessed on 14 March 2022).
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and shiny web app for visualizing risk-of-bias assessments. Res. Syn. Meth. 2020, 1–7. [Google Scholar] [CrossRef]
- Page, M.J.; Higgins, J.P.; Sterne, J.A. Chapter 13: Assessing risk of bias due to missing results in a synthesis. Section 13.3.5.2 Funnel plots. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019); Cochrane: London, UK, 2019. [Google Scholar]
- Calzetta, L.; Rogliani, P.; Matera, M.G.; Cazzola, M. A Systematic Review with Meta-Analysis of Dual Bronchodilation with LAMA/LABA for the Treatment of Stable COPD. Chest 2016, 149, 1181–1196. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE Guidelines: 1. Introduction-GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Akhoon, N.; Brashier, D.B.S. A Study to Monitor Errors in Use of Inhalation Devices in Patients of Mild-to-Moderate Bronchial Asthma in a Tertiary Care Hospital in Eastern India. Perspect. Clin. Res. 2022, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Arif, N.B.M.; Lee, P.Y.; Cheong, A.T.; Ananthan, R.N.A. Factors Associated with Improper Metered-Dose Inhaler Technique among Adults with Asthma in a Primary Care Clinic in Malaysia. Malays. Fam. Physician 2021, 16, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Chang, Y.S.; Kim, C.W.; Kim, T.B.; Kim, S.H.; Kwon, Y.E.; Lee, J.M.; Lee, S.K.; Jeong, J.W.; Park, J.W.; et al. Skills in Handling Turbuhaler, Diskus, and Pressurized Metered-Dose Inhaler in Korean Asthmatic Patients. Allergy Asthma Immunol. Res. 2011, 3, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zhang, W.; Wang, Z.; Bai, C.; He, Q.; Dong, Y.; Feng, X.; Zhang, J.; Gao, S. Prevalence and Associated Factors of Suboptimal Daily Peak Inspiratory Flow and Technique Misuse of Dry Powder Inhalers in Outpatients with Stable Chronic Airway Diseases. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 1913–1924. [Google Scholar] [CrossRef]
- Jang, J.G.; Chung, J.H.; Shin, K.C.; Jin, H.J.; Lee, K.H.; Ahn, J.H. Comparative Study of Inhaler Device Handling Technique and Risk Factors for Critical Inhaler Errors in Korean COPD Patients. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 1051–1059. [Google Scholar] [CrossRef]
- Barbara, S.A.; Kritikos, V.; Price, D.B.; Bosnic-Anticevich, S. Identifying Patients at Risk of Poor Asthma Outcomes Associated with Making Inhaler Technique Errors. J. Asthma 2021, 58, 967–978. [Google Scholar] [CrossRef]
- Baral, M.A. Knowledge and Practice of Dry Powder Inhalation among Patients with Chronic Obstructive Pulmonary Disease in a Regional Hospital, Nepal. Int. J. Gen. Med. 2019, 12, 31. [Google Scholar] [CrossRef]
- Sriram, K.B.; Percival, M. Suboptimal Inhaler Medication Adherence and Incorrect Technique Are Common among Chronic Obstructive Pulmonary Disease Patients. Chronic Respir. Dis. 2016, 13, 13–22. [Google Scholar] [CrossRef]
- Westerik, J.A.M.; Carter, V.; Chrystyn, H.; Burden, A.; Thompson, S.L.; Ryan, D.; Gruffydd-Jones, K.; Haughney, J.; Roche, N.; Lavorini, F.; et al. Characteristics of Patients Making Serious Inhaler Errors with a Dry Powder Inhaler and Association with Asthma-Related Events in a Primary Care Setting. J. Asthma 2016, 53, 321–329. [Google Scholar] [CrossRef]
- Onyedum, C.; Desalu, O.; Nwosu, N.; Chukwuka, C.; Ukwaja, K.; Ezeudo, C. Evaluation of Inhaler Techniques among Asthma Patients Seen in Nigeria: An Observational Cross Sectional Study. Ann. Med. Health Sci. Res. 2014, 4, 67. [Google Scholar] [CrossRef]
- Coelho, A.C.C.; Souza-Machado, A.; Leite, M.; Almeida, P.; Castro, L.; Cruz, C.S.; Stelmach, R.; Cruz, Á.A. Use of Inhaler Devices and Asthma Control in Severe Asthma Patients at a Referral Center in the City of Salvador, Brazil. J. Bras. Pneumol. 2011, 37, 720–728. [Google Scholar] [CrossRef][Green Version]
- Ahn, J.H.; Chung, J.H.; Shin, K.C.; Jin, H.J.; Jang, J.G.; Lee, M.S.; Lee, K.H. The Effects of Repeated Inhaler Device Handling Education in COPD Patients: A Prospective Cohort Study. Sci. Rep. 2020, 10, 19676. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Small-Study Effects in Meta-Analysis; Springer: Cham, Switzerland, 2015; pp. 107–141. [Google Scholar] [CrossRef]
- Chorão, P.; Pereira, A.M.; Fonseca, J.A. Inhaler Devices in Asthma and COPD–An Assessment of Inhaler Technique and Patient Preferences. Respir. Med. 2014, 108, 968–975. [Google Scholar] [CrossRef]
- Melani, A.S.; Zanchetta, D.; Barbato, N.; Sestini, P.; Cinti, C.; Canessa, P.A.; Aiolfi, S.; Neri, M. Inhalation Technique and Variables Associated with Misuse of Conventional Metered-Dose Inhalers and Newer Dry Powder Inhalers in Experienced Adults. Ann. Allergy Asthma Immunol. 2004, 93, 439–446. [Google Scholar] [CrossRef]
- Ahn, J.H.; Chung, J.H.; Shin, K.C.; Choi, E.Y.; Jin, H.J.; Lee, M.S.; Nam, M.J.; Lee, K.H. Critical Inhaler Handling Error Is an Independent Risk Factor for Frequent Exacerbations of Chronic Obstructive Pulmonary Disease: Interim Results of a Single Center Prospective Study. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2767–2775. [Google Scholar] [CrossRef]
- Canonica, G.W.; Ferrando, M.; Baiardini, I.; Puggioni, F.; Racca, F.; Passalacqua, G.; Heffler, E. Asthma: Personalized and Precision Medicine. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 51–58. [Google Scholar] [CrossRef]
- Bakakos, P.; Chatziapostolou, P.; Katerelos, P.; Efstathopoulos, P.; Korkontzelou, A.; Katsaounou, P. Extrafine Beclometasone Dipropionate/Formoterol NEXThaler on Device Usability, Adherence, Asthma Control and Quality of Life. A Panhellenic Prospective, Non-Interventional Observational Study in Patients with Asthma-The NEXT-Step Study. J. Pers. Med. 2022, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F.; Bianco, A.; Blasi, F.; Braido, F.; Corsico, A.G.; Di Marco, F.; Gentile, A.; Paggiaro, P.L.; Pegoraro, V.; Pelaia, G.; et al. What Drives Inhaler Prescription for Asthma Patients? Results from a Real-Life Retrospective Analysis. Respir. Med. 2020, 166, 105937. [Google Scholar] [CrossRef]
- Molimard, M.; Colthorpe, P. Inhaler Devices for Chronic Obstructive Pulmonary Disease: Insights from Patients and Healthcare Practitioners. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 219–228. [Google Scholar] [CrossRef]
- Anderson, S.; Atkins, P.; Bäckman, P.; Cipolla, D.; Clark, A.; Daviskas, E.; Disse, B.; Entcheva-Dimitrov, P.; Fuller, R.; Gonda, I.; et al. Inhaled Medicines: Past, Present, and Future. Pharmacol. Rev. 2022, 74, 50–118. [Google Scholar] [CrossRef]
- Rogliani, P.; Calzetta, L.; Coppola, A.; Cavalli, F.; Ora, J.; Puxeddu, E.; Matera, M.G.; Cazzola, M. Optimizing Drug Delivery in COPD: The Role of Inhaler Devices. Respir. Med. 2017, 124, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Scichilone, N. Asthma Control: The Right Inhaler for the Right Patient. Adv. Ther. 2015, 32, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F.; Janson, C.; Braido, F.; Stratelis, G.; Løkke, A. What to Consider before Prescribing Inhaled Medications: A Pragmatic Approach for Evaluating the Current Inhaler Landscape. Ther. Adv. Respir. Dis. 2019, 13. [Google Scholar] [CrossRef]
- Ali, A.; Subhi, Y.; Ringsted, C.; Konge, L. Gender Differences in the Acquisition of Surgical Skills: A Systematic Review. Surg. Endosc. 2015, 29, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Braithwaite, V.A.; Healy, S.D. The Evolution of Sex Differences in Spatial Ability. Behav. Neurosci. 2003, 117, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Colice, G.L.; Hodder, R. Practical Aspects of Inhaler Use in the Management of Chronic Obstructive Pulmonary Disease in the Primary Care Setting. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Bartolo, K.; Balzan, M.; Schembri, E.L.; Asciak, R.; Mercieca Balbi, D.; Pace Bardon, M.; Montefort, S. Predictors of Correct Technique in Patients Using Pressurized Metered Dose Inhalers. BMC Pulm. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Ohar, J.A.; Ferguson, G.T.; Mahler, D.A.; Drummond, M.B.; Dhand, R.; Pleasants, R.A.; Anzueto, A.; Halpin, D.M.G.; Price, D.B.; Drescher, G.S.; et al. Measuring Peak Inspiratory Flow in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 79–92. [Google Scholar] [CrossRef]
- Altman, P.; Wehbe, L.; Dederichs, J.; Guerin, T.; Ament, B.; Moronta, M.C.; Pino, A.V.; Goyal, P. Comparison of Peak Inspiratory Flow Rate via the Breezhaler®, Ellipta® and HandiHaler® Dry Powder Inhalers in Patients with Moderate to Very Severe COPD: A Randomized Cross-over Trial. BMC Pulm. Med. 2018, 18, 100. [Google Scholar] [CrossRef]
- Haughney, J.; Lee, A.J.; McKnight, E.; Pertsovskaya, I.; O’Driscoll, M.; Usmani, O.S. Peak Inspiratory Flow Measured at Different Inhaler Resistances in Patients with Asthma. J. Allergy Clin. Immunol. Pr. 2021, 9, 890–896. [Google Scholar] [CrossRef]
- Taylor, T.E.; Holmes, M.S.; Sulaiman, I.; Costello, R.W.; Reilly, R.B. Influences of Gender and Anthropometric Features on Inspiratory Inhaler Acoustics and Peak Inspiratory Flow Rate. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Milan, Italy, 25–29 August 2015. [Google Scholar] [CrossRef]
- Dalby, R.N.; Eicher, J.; Zierenberg, B. Development of Respimat® Soft MistTM Inhaler and Its Clinical Utility in Respiratory Disorders. Med. Devices: Evid. Res. 2011, 4, 145–155. [Google Scholar] [CrossRef][Green Version]
- Alexander, J.; Edwards, R.A.; Savoldelli, A.; Manca, L.; Grugni, R.; Emir, B.; Whalen, E.; Watt, S.; Brodsky, M.; Parsons, B. Integrating Data from Randomized Controlled Trials and Observational Studies to Predict the Response to Pregabalin in Patients with Painful Diabetic Peripheral Neuropathy. BMC Med. Res. Methodol. 2017, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Arditi, C.; Burnand, B.; Peytremann-Bridevaux, I. Adding Non-Randomised Studies to a Cochrane Review Brings Complementary Information for Healthcare Stakeholders: An Augmented Systematic Review and Meta-Analysis. BMC Health Serv. Res. 2016, 16, 598. [Google Scholar] [CrossRef]
- Norris, S.; Atkins, D.; Bruening, W.; Fox, S.; Johnson, E.; Kane, R.; Morton, S.C.; Oremus, M.; Ospina, M.; Randhawa, G.; et al. Selecting Observational Studies for Comparing Medical Interventions. In Methods Guide for Effectiveness and Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. [Google Scholar]
- Shrier, I.; Boivin, J.F.; Steele, R.J.; Platt, R.W.; Furlan, A.; Kakuma, R.; Brophy, J.; Rossignol, M. Should Meta-Analyses of Interventions Include Observational Studies in Addition to Randomized Controlled Trials? A Critical Examination of Underlying Principles. Am. J. Epidemiol. 2007, 166, 1203–1209. [Google Scholar] [CrossRef]
- Gershon, A.S.; Jafarzadeh, S.R.; Wilson, K.C.; Walkey, A.J. Clinical Knowledge from Observational Studies: Everything You Wanted to Know but Were Afraid to Ask. Am. J. Respir. Crit. Care Med. 2018, 198, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Aiello, M.; Frizzelli, A.; Bertorelli, G.; Rogliani, P.; Chetta, A. Oral Corticosteroids Dependence and Biologic Drugs in Severe Asthma: Myths or Facts? A Systematic Review of Real-World Evidence. Int. J. Mol. Sci. 2021, 22, 7132. [Google Scholar] [CrossRef]

| Study, Year, and Reference | Study Characteristics | Observation Duration (Months) | Number of Analyzed Patients | Data Reported in the Primary Publication | Inhaler Device (Brand) | Patients’ Diagnosis (Setting) | Age (Years) | Male (%) | Post Bronchodilator FEV1 (% Predicted) | Post Bronchodilator FEV1/FVC | AECOPD in the Previous Year (Ratio) | JBI Checklist Tool | Evaluated Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akhoon et al., 2022 [51] | Single-center, observational, cross-sectional study | 12.0 | 207 | Number of patients making ≥1 overall error in inhaler technique | DPI (Rotahaler), pMDI (NA), and pMDI + spacer (NA) | Mild to moderate asthma (outpatient) | 39.0 | 54.6 | NA | NA | NA | Moderate bias | Patients making ≥1 overall error in inhaler technique |
| Arif et al., 2021 [52] | Single-center, observational, cross-sectional study | 3.0 | 146 | Crude OR with 95%CI for the association between sex and improper inhaler technique | MDI (NA) | Asthma (outpatient) | 38.5 | 32.2 | NA | NA | NA | Moderate bias | Patients making ≥1 overall error in inhaler technique |
| Ding et al., 2021 [54] | Single-center, observational, cross-sectional study | 9.0 | 52 (COPD); 22 (asthma) | Number of patients making ≥1 overall error in inhaler technique | DPI (Diskus, HandiHaler, Turbuhaler) | Stable asthma, COPD (outpatient) | 68.0 (COPD); 58.0 (asthma) | 94.2 (COPD); 36.4 (asthma) | 57.6 (COPD); 86.5 (asthma) | NA | NA | Moderate bias | Patients making ≥1 overall error in inhaler technique |
| Harb et al., 2021 [31] | Single-center, non-drug interventional, randomized, open-label, crossover study | NA | 180 | Number of patients making ≥1 overall error and ≥1 critical error in inhaler technique | DPI (Aerolizer, Breezhaler, Diskhaler, Diskus, Easyhaler, Ellipta, Handihaler, Turbuhaler), non-breath-actuated pMDI, and SMI (Respimat) | COPD (inpatient) | 61.7 | 57.2 | NA | NA | NA | NA * | Patients making ≥1 overall error and ≥1 critical error in inhaler technique |
| Jang et al., 2021 [55] | Single-center, observational, prospective, cross-sectional study (secondary analysis of a previous cohort study [62]) | 22.0 | 261 | Number of patients making ≥1 critical error in inhaler technique | DPI (Breezhaler, Diskus, Ellipta, Genuair, Turbuhaler), pMDI, and SMI (Respimat) | COPD (outpatient) | 69.8 | 93.5 | 63.5 | 0.59 | 24.9% of patients with frequent AECOPD | Moderate bias | Patients making ≥1 critical error in inhaler technique |
| Barbara et al., 2020 [56] | Multicenter, observational, retrospective cross-sectional study using data from the iHARP database | 42.0 | 4,134 | Number of patients making ≥1 critical error in inhaler technique | DPI (Accuhaler, Turbuhaler) and MDI (NA) | Asthma (primary care practice) | 50.0 | 39.0 | NA | NA | NA | Moderate bias | Patients making ≥1 critical error in inhaler technique |
| Baral et al., 2019 [57] | Single-center, observational, cross-sectional study | 1.0 | 204 | Number of patients making ≥1 overall error in inhaler technique | DPI (Rotahaler) | COPD (outpatient and inpatient) | 67.2 | 46.1 | <80.0 | <0.7 | NA | Moderate bias | Patients making ≥1 overall error in inhaler technique |
| Sriram et al., 2016 [58] | Single-center, observational, cross-sectional study | 12.0 | 150 | Number of patients making ≥1 overall error in inhaler technique | DPI (Accuhaler, HandiHaler, Turbuhaler) and MDI + spacer (NA) | COPD (inpatient and community-based participants) | 70.3 | 52.0 | NA | NA | 1.7 | Moderate bias | Patients making ≥1 overall error in inhaler technique |
| Westerik et al., 2016 [59] | Multicenter, observational, historical, cross-sectional study using data from the iHARP database | 29.0 | 623 | Number of patients making ≥1 critical error in inhaler technique | DPI (Diskus) | Asthma (primary care practice) | 51.0 | 39.0 | NA | NA | 0.6 | Moderate bias | Patients making ≥1 critical error in inhaler technique |
| Onyedum et al., 2014 [60] | Multicenter, observational, cross-sectional study | 7.0 | 140 | Number of patients making ≥1 overall error in inhaler technique | DPI (Diskus) and pMDI (NA) | Asthma (outpatient) | 47.6 | 46.4 | NA | NA | NA | High bias | Patients making ≥1 overall error in inhaler technique |
| Coelho et al., 2011 [61] | Single-center, observational, cross-sectional study | 16.0 | 229 | Number of patients making ≥1 critical error in inhaler technique | DPI (Aerolizer) | Severe asthma (outpatient) | ≥18.0 | 21.4 | NA | NA | NA | Moderate bias | Patients making ≥1 critical error in inhaler technique |
| Lee et al., 2011 [53] | Multicenter, observational, cross-sectional study | NA | 223 | Number of patients making ≥1 critical error in inhaler technique | DPI (Turbuhaler (Pulmicort and Symbicort), Diskus (Flixotide and Seretide)), and pMDI (Ventolin, Atrovent, or Combivent) | Asthma (outpatient) | 56.7 | 50.4 | NA | NA | NA | High bias | Patients making ≥1 critical error in inhaler technique |
| Author, Year, and Reference | Critical Error Definition |
|---|---|
| Harb et al., 2021 [31] | The definition of critical error agreed with the recently published IPAC-RS critical error matrix. The critical error was equivalent to IPAC-RS maximal effect (score 10) and IPAC-RS high effect (score 7); “critical errors presented within the checklist are those exposing patients to the risk of receiving no dose or severely reduced dose”. |
| Jang et al., 2021 [55] | “Critical errors were defined as errors seriously compromising drug delivery to the lung”. |
| Barbara et al., 2020 [56] | “Inhaler technique errors associated with poor asthma outcomes were defined as errors significantly associated with uncontrolled asthma and/or an increased rate of asthma exacerbations (ie, having at least one exacerbation in the 12 months prior to review)”. |
| Westerik et al., 2016 [59] | “Serious inhaler technique errors identified by the HCPs were defined as errors potentially limiting drug uptake to the lungs, as enumerated by the iHARP steering committee before commencing the study”. |
| Coelho et al., 2011 [61] | Error in a key step that “when incorrectly performed by users, can significantly affect total deposition of the dose in the lungs”. “These steps are related to preparing the dose for total drug release and to inhaling the drug”.The following were considered key steps in the present study: “for the use of an Aerolizer DPI, placing the capsule in the appropriate chamber, pressing the lateral buttons of the inhaler, and inhaling quickly and deeply”. |
| Lee et al., 2011 [53] | Failure of any one of the key steps, including “coordinate hand movement and inhalation,” “load and prime device,” and “inhale forcefully and deeply”. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzetta, L.; Aiello, M.; Frizzelli, A.; Ritondo, B.L.; Pistocchini, E.; Rogliani, P.; Chetta, A. Impact of Sex on Proper Use of Inhaler Devices in Asthma and COPD: A Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 1565. https://doi.org/10.3390/pharmaceutics14081565
Calzetta L, Aiello M, Frizzelli A, Ritondo BL, Pistocchini E, Rogliani P, Chetta A. Impact of Sex on Proper Use of Inhaler Devices in Asthma and COPD: A Systematic Review and Meta-Analysis. Pharmaceutics. 2022; 14(8):1565. https://doi.org/10.3390/pharmaceutics14081565
Chicago/Turabian StyleCalzetta, Luigino, Marina Aiello, Annalisa Frizzelli, Beatrice Ludovica Ritondo, Elena Pistocchini, Paola Rogliani, and Alfredo Chetta. 2022. "Impact of Sex on Proper Use of Inhaler Devices in Asthma and COPD: A Systematic Review and Meta-Analysis" Pharmaceutics 14, no. 8: 1565. https://doi.org/10.3390/pharmaceutics14081565
APA StyleCalzetta, L., Aiello, M., Frizzelli, A., Ritondo, B. L., Pistocchini, E., Rogliani, P., & Chetta, A. (2022). Impact of Sex on Proper Use of Inhaler Devices in Asthma and COPD: A Systematic Review and Meta-Analysis. Pharmaceutics, 14(8), 1565. https://doi.org/10.3390/pharmaceutics14081565








