Micromolding of Amphotericin-B-Loaded Methoxyethylene–Maleic Anhydride Copolymer Microneedles
Abstract
1. Introduction
2. Materials and Methods
2.1. Master Structure Fabrication
2.2. Microneedle Fabrication
2.3. Variable Pressure Scanning Electron Microscopy
2.4. Mechanical tests
2.4.1. Nanoindentation
2.4.2. Compressive Loading of Microneedles
2.5. 3D Laser Scanning Confocal Microscopy
2.6. XPS
2.7. Fourier Transform Infrared Spectroscopy
2.8. Raman Spectroscopy
2.9. High Performance Liquid Chromatography
2.10. Franz Diffusion Test
2.11. Antifungal Testing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, P.R.; Narayan, R.J.; Polsky, R. Microneedle-based sensors for medical diagnosis. J. Mater. Chem. B 2016, 4, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Narayan, R.J. Applications of Microneedle Technology to Transdermal Drug Delivery. In Toxicology of the Skin; CRC Press: Boca Raton, FL, USA, 2010; pp. 315–330. [Google Scholar]
- Machekposhti, S.; Soltani, M.; Najafizadeh, P.; Ebrahimi, S.; Chen, P. Biocompatible polymer microneedle for topical/dermal delivery of tranexamic acid. J. Control. Release 2017, 261, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ezrahi, S.; Aserin, A.; Garti, N. Basic principles of drug delivery systems–the case of paclitaxel. Adv. Colloid Interface Sci. 2019, 263, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Fiegel, J.; Krauland, E.; Hanes, J. New polymeric carriers for controlled drug delivery following inhalation or injection. Biomaterials 2002, 23, 4425–4433. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Schmelz, M.; Schmid, R.; Handwerker, H.O.; Torebjörk, H.E. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 2000, 123, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Salburgo, F.; Garcia, S.; Lagier, A.; Estève, D.; Lavieille, J.-P.; Montava, M. Histological identification of nasopharyngeal mechanoreceptors. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 4127–4133. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Ovsianikov, A.; Monteiro-Riviere, N.A.; Lusk, J.; Morel, P.; Minghetti, P.; Lenardi, C.; Chichkov, B.N.; Narayan, R.J. Fabrication of polymer microneedles using a two-photon polymerization and micromolding process. J. Diabetes Sci. Technol. 2009, 3, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Ovsianikov, A.; Chichkov, B.N.; Doraiswamy, A.; Narayan, R.J. Two-photon polymerization of microneedles for transdermal drug delivery. Expert Opin. Drug Deliv. 2010, 7, 513–533. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Camović, M.; Biščević, A.; Brčić, I.; Borčak, K.; Bušatlić, S.; Ćenanović, N.; Dedović, A.; Mulalić, A.; Osmanlić, M.; Sirbubalo, M. Coated 3d printed PLA microneedles as transdermal drug delivery systems. In Proceedings of the 9th International Conference on Medical and Biological Engineering, Tokyo, Japan, 28–30 March 2019; pp. 735–742. [Google Scholar]
- Gao, Y.; Hou, M.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. PEGDA/PVP microneedles with tailorable matrix constitutions for controllable transdermal drug delivery. Macromol. Mater. Eng. 2018, 303, 1800233. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Banga, A.K. Delivery of methotrexate and characterization of skin treated by fabricated PLGA microneedles and fractional ablative laser. Pharm. Res. 2018, 35, 68. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Choi, S.-O.; Kamath, R.; Yoon, Y.-K.; Allen, M.G.; Prausnitz, M.R. Polymer particle-based micromolding to fabricate novel microstructures. Biomed. Microdevices 2007, 9, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-H.; Ha, S.K.; Choi, I.; Kim, K.S.; Park, J.; Choi, N.; Kim, B.; Sung, J.H. Fabrication of degradable carboxymethyl cellulose (CMC) microneedle with laser writing and replica molding process for enhancement of transdermal drug delivery. Biotechnol. Bioprocess Eng. 2016, 21, 110–118. [Google Scholar] [CrossRef]
- Boehm, R.D.; Miller, P.R.; Singh, R.; Shah, A.; Stafslien, S.; Daniels, J.; Narayan, R.J. Indirect rapid prototyping of antibacterial acid anhydride copolymer microneedles. Biofabrication 2012, 4, 011002. [Google Scholar] [CrossRef] [PubMed]
- Cartagena, A.F.; Esmerino, L.A.; Polak-Junior, R.; Parreiras, S.O.; Michél, M.D.; Farago, P.V.; Campanha, N.H. New denture adhesive containing miconazole nitrate polymeric microparticles: Antifungal, adhesive force and toxicity properties. Dent. Mater. 2017, 33, e53–e61. [Google Scholar] [CrossRef]
- Achila, S.; Muthu Kumar, B.; Vasanthakumar, M. Mystery of denture adhesives—A literature review. SRM J. Dent. Sci. 2011, 2, 112–117. [Google Scholar]
- Gittard, S.D.; Chen, B.; Xu, H.; Ovsianikov, A.; Chichkov, B.N.; Monteiro-Riviere, N.A.; Narayan, R.J. The effects of geometry on skin penetration and failure of polymer microneedles. J. Adhes. Sci. Technol. 2013, 27, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.P.; Koutsonanos, D.G.; del Pilar Martin, M.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.K.; Yang, K.-H.; Bryant, K.; Li, J.; Joice, A.C.; Werbovetz, K.A.; Narayan, R.J. Microneedle-based delivery of Amphotericin B for treatment of cutaneous Leishmaniasis. Biomed. Microdevices 2019, 21, 8. [Google Scholar] [CrossRef]
- Li, X.; Gao, H.; Murphy, C.J.; Caswell, K. Nanoindentation of silver nanowires. Nano Lett. 2003, 3, 1495–1498. [Google Scholar] [CrossRef]
- Skoog, S.A.; Miller, P.R.; Boehm, R.D.; Sumant, A.V.; Polsky, R.; Narayan, R.J. Nitrogen-incorporated ultrananocrystalline diamond microneedle arrays for electrochemical biosensing. Diam. Relat. Mater. 2015, 54, 39–46. [Google Scholar] [CrossRef]
- Sachan, R.; Jaipan, P.; Zhang, J.Y.; Degan, S.; Erdmann, D.; Tedesco, J.; Vanderwal, L.; Stafslien, S.J.; Negut, I.; Visan, A. Printing amphotericin B on microneedles using matrix-assisted pulsed laser evaporation. Int. J. Bioprint. 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Camden, J.P.; Dieringer, J.A.; Wang, Y.; Masiello, D.J.; Marks, L.D.; Schatz, G.C.; Van Duyne, R.P. Probing the structure of single-molecule surface-enhanced Raman scattering hot spots. J. Am. Chem. Soc. 2008, 130, 12616–12617. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, Y. Roles of interstitial fluid pH and weak organic acids in development and amelioration of insulin resistance. Biochem. Soc. Trans. 2021, 49, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Saurer, E.M.; Flessner, R.M.; Sullivan, S.P.; Prausnitz, M.R.; Lynn, D.M. Layer-by-layer assembly of DNA-and protein-containing films on microneedles for drug delivery to the skin. Biomacromolecules 2010, 11, 3136–3143. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Yoo, D.-G.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Cross-protection by co-immunization with influenza hemagglutinin DNA and inactivated virus vaccine using coated microneedles. J. Control. Release 2013, 172, 579–588. [Google Scholar] [CrossRef]
- McGrath, M.G.; Vucen, S.; Vrdoljak, A.; Kelly, A.; O’Mahony, C.; Crean, A.M.; Moore, A. Production of dissolvable microneedles using an atomised spray process: Effect of microneedle composition on skin penetration. Eur. J. Pharm. Biopharm. 2014, 86, 200–211. [Google Scholar] [CrossRef]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef]
- Yin, J.; Tang, H.; Xu, Z.; Li, N. Enhanced mechanical strength and performance of sulfonated polysulfone/Tröger’s base polymer blend ultrafiltration membrane. J. Membr. Sci. 2021, 625, 119138. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Yang, S.; Tan, S.; Li, X.; Dai, H.; Yu, X.; Zhang, X.; Weng, N.; Jian, B. Double-network hydrogel with high mechanical strength prepared from two biocompatible polymers. J. Appl. Polym. Sci. 2009, 112, 3063–3070. [Google Scholar] [CrossRef]
- Radhika, C.; Gnanavel, B. Buckling analysis of polymer microneedle for transdermal drug delivery. Mater. Today Proc. 2021, 46, 3538–3541. [Google Scholar] [CrossRef]
- Du, G.; Zhang, Z.; He, P.; Zhang, Z.; Sun, X. Determination of the mechanical properties of polymeric microneedles by micromanipulation. J. Mech. Behav. Biomed. Mater. 2021, 117, 104384. [Google Scholar] [CrossRef]
- Kochhar, J.S.; Soon, W.J.; Choi, J.; Zou, S.; Kang, L. Effect of microneedle geometry and supporting substrate on microneedle array penetration into skin. J. Pharm. Sci. 2013, 102, 4100–4108. [Google Scholar] [CrossRef]
- Schuh, C.A. Nanoindentation studies of materials. Mater. Today 2006, 9, 32–40. [Google Scholar] [CrossRef]
- Ramesh, S.; Leen, K.H.; Kumutha, K.; Arof, A. FTIR studies of PVC/PMMA blend based polymer electrolytes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 66, 1237–1242. [Google Scholar] [CrossRef]
- Singh, P.K.; Sah, P.; Meher, J.G.; Joshi, S.; Pawar, V.K.; Raval, K.; Singh, Y.; Sharma, K.; Kumar, A.; Dube, A. Macrophage-targeted chitosan anchored PLGA nanoparticles bearing doxorubicin and amphotericin B against visceral leishmaniasis. RSC Adv. 2016, 6, 71705–71718. [Google Scholar] [CrossRef]
- Boehm, R.D.; Miller, P.R.; Schell, W.A.; Perfect, J.R.; Narayan, R.J. Inkjet printing of amphotericin B onto biodegradable microneedles using piezoelectric inkjet printing. JOM 2013, 65, 525–533. [Google Scholar] [CrossRef]
- Crine, J.-P. Silicone oil as replacement fluid for PCBs in transformers. Can. Electr. Eng. J. 1986, 11, 110–113. [Google Scholar] [CrossRef]
- Bal, S.M.; Caussin, J.; Pavel, S.; Bouwstra, J.A. In vivo assessment of safety of microneedle arrays in human skin. Eur. J. Pharm. Sci. 2008, 35, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Jang, W.S.; Lim, Y.Y.; Ahn, J.H.; Lee, S.J.; Kim, C.W.; Kim, S.E.; Kim, B.J.; Kim, M.N. Safety evaluation of stamp type digital microneedle devices in hairless mice. Ann. Dermatol. 2013, 25, 46–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vicente-Perez, E.M.; Larrañeta, E.; McCrudden, M.T.; Kissenpfennig, A.; Hegarty, S.; McCarthy, H.O.; Donnelly, R.F. Repeat application of microneedles does not alter skin appearance or barrier function and causes no measurable disturbance of serum biomarkers of infection, inflammation or immunity in mice in vivo. Eur. J. Pharm. Biopharm. 2017, 117, 400–407. [Google Scholar] [CrossRef]
- Bunow, M.R.; Levin, I.W. Vibrational Raman spectra of lipid systems containing amphotericin B. Biochim. Biophys. Acta (BBA)-Biomembr. 1977, 464, 202–216. [Google Scholar] [CrossRef]
- Miyaoka, R.; Hosokawa, M.; Ando, M.; Mori, T.; Hamaguchi, H.-O.; Takeyama, H. In situ detection of antibiotic amphotericin B produced in Streptomyces nodosus using Raman microspectroscopy. Mar. Drugs 2014, 12, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
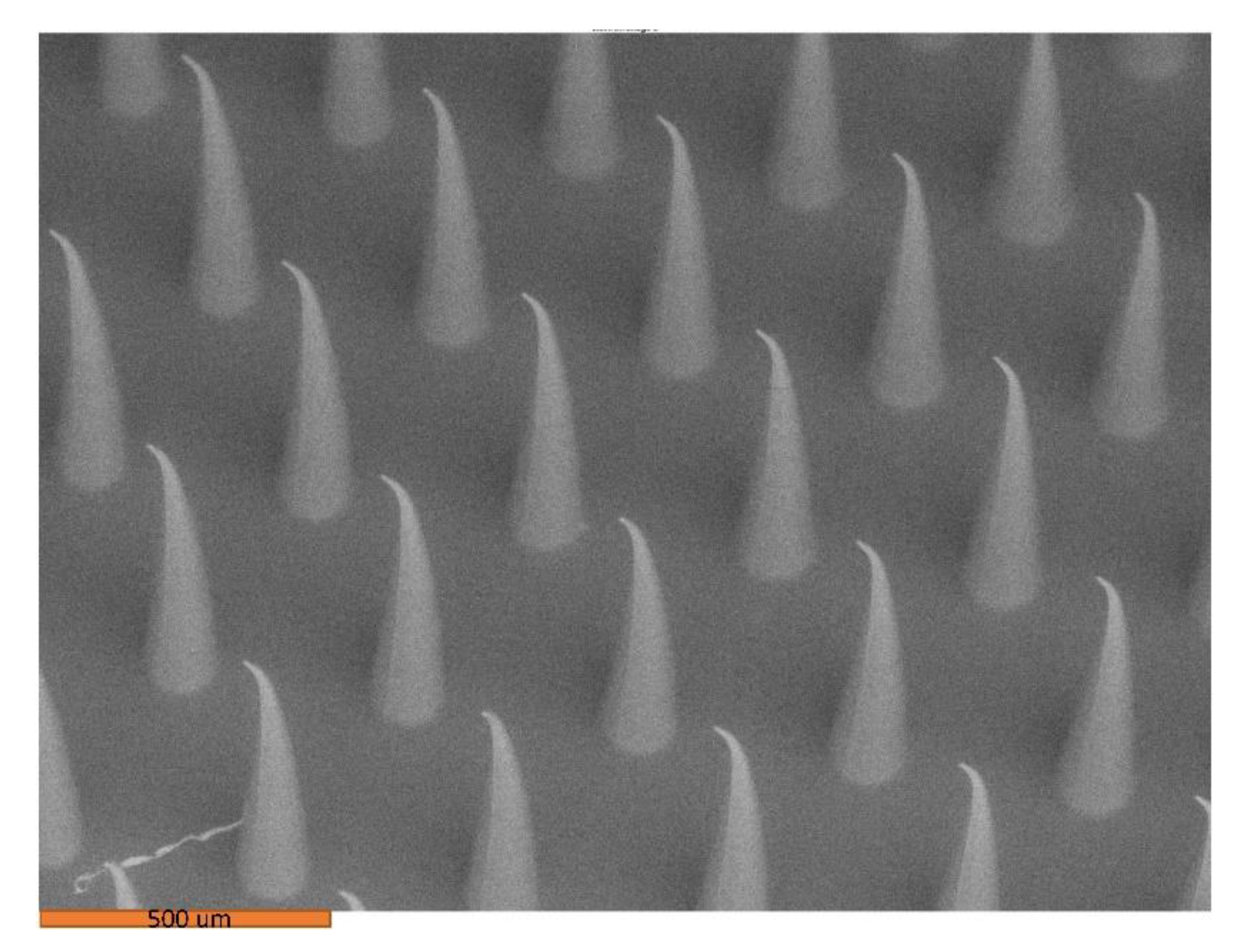

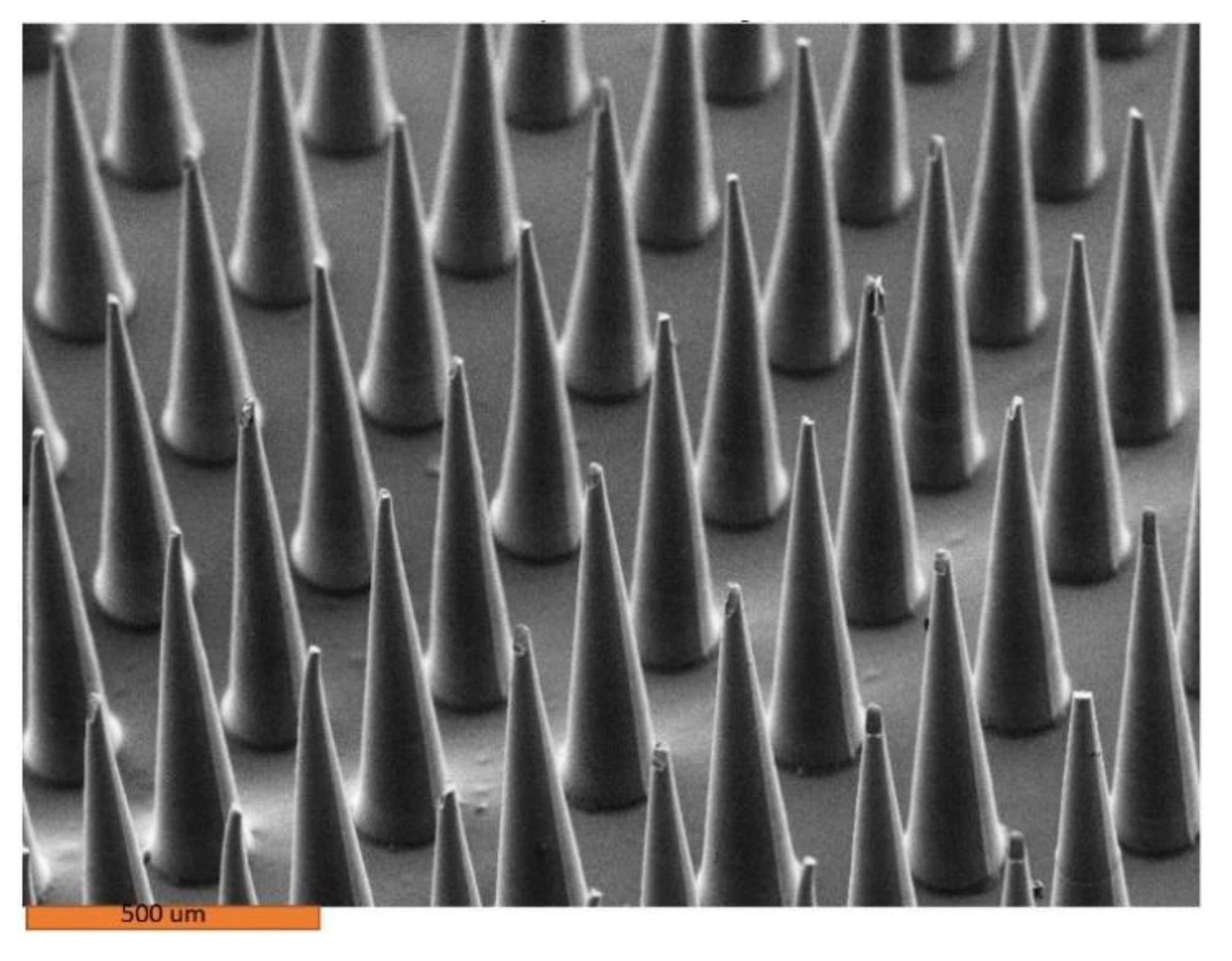


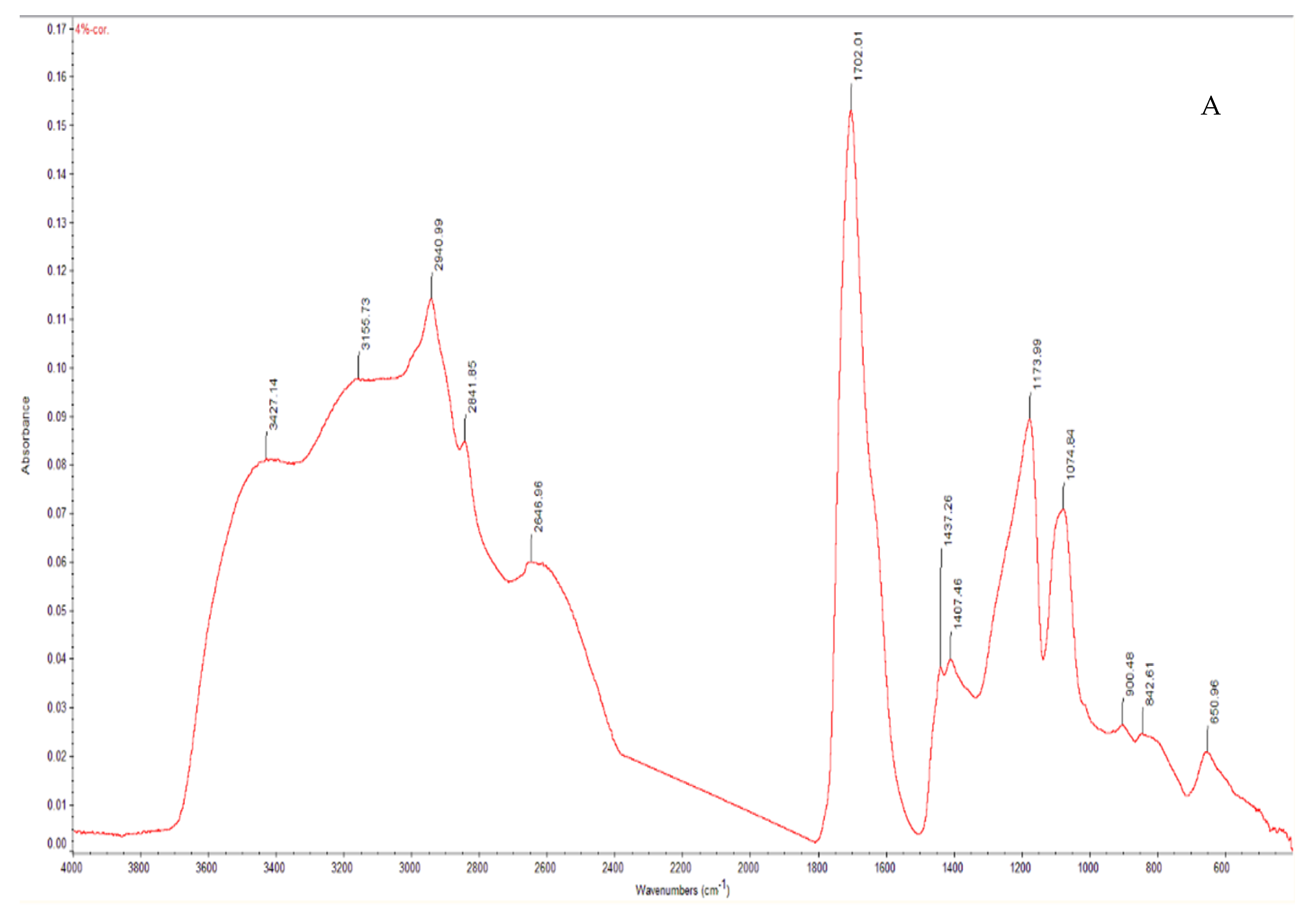


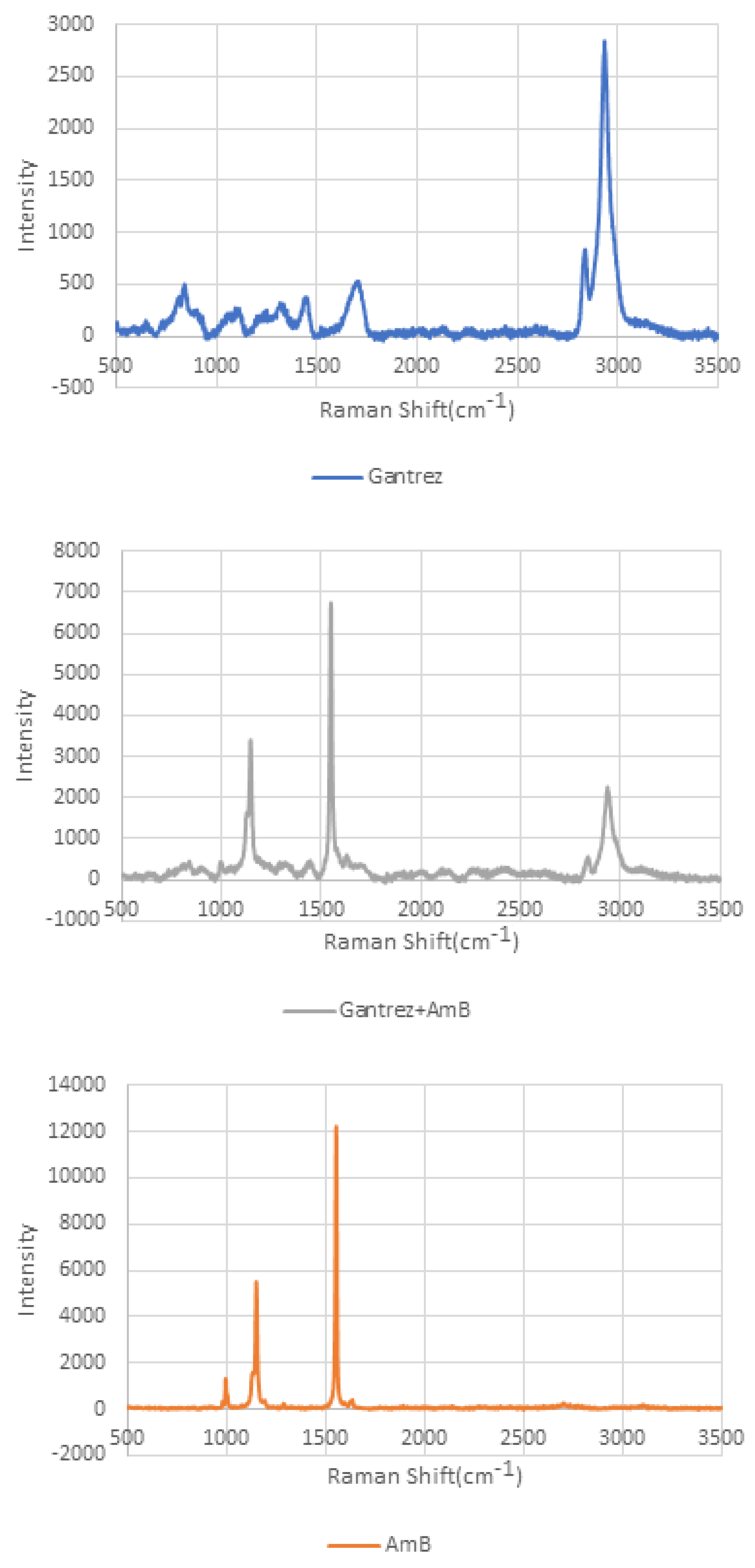


| Drug Concentration | Er (GPa) | Standard Deviation | H (GPa) | Standard Deviation | Newton/Needle | Standard Deviation |
|---|---|---|---|---|---|---|
| 4% | 8.65 | 1.1 | 0.31 | 0.85 | 0.65 | 2.02 |
| 8% | 7.05 | 1.23 | 0.43 | 0.96 | 0.54 | 2.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizi Machekposhti, S.; Nguyen, A.K.; Vanderwal, L.; Stafslien, S.; Narayan, R.J. Micromolding of Amphotericin-B-Loaded Methoxyethylene–Maleic Anhydride Copolymer Microneedles. Pharmaceutics 2022, 14, 1551. https://doi.org/10.3390/pharmaceutics14081551
Azizi Machekposhti S, Nguyen AK, Vanderwal L, Stafslien S, Narayan RJ. Micromolding of Amphotericin-B-Loaded Methoxyethylene–Maleic Anhydride Copolymer Microneedles. Pharmaceutics. 2022; 14(8):1551. https://doi.org/10.3390/pharmaceutics14081551
Chicago/Turabian StyleAzizi Machekposhti, Sina, Alexander K. Nguyen, Lyndsi Vanderwal, Shane Stafslien, and Roger J. Narayan. 2022. "Micromolding of Amphotericin-B-Loaded Methoxyethylene–Maleic Anhydride Copolymer Microneedles" Pharmaceutics 14, no. 8: 1551. https://doi.org/10.3390/pharmaceutics14081551
APA StyleAzizi Machekposhti, S., Nguyen, A. K., Vanderwal, L., Stafslien, S., & Narayan, R. J. (2022). Micromolding of Amphotericin-B-Loaded Methoxyethylene–Maleic Anhydride Copolymer Microneedles. Pharmaceutics, 14(8), 1551. https://doi.org/10.3390/pharmaceutics14081551







