New Drug Development and Clinical Trial Design by Applying Genomic Information Management
Abstract
:1. Background: Effective Clinical Trials Using Genomic Information
2. Integrated Interpretation: Tissue Specificity and Environment of Cancer
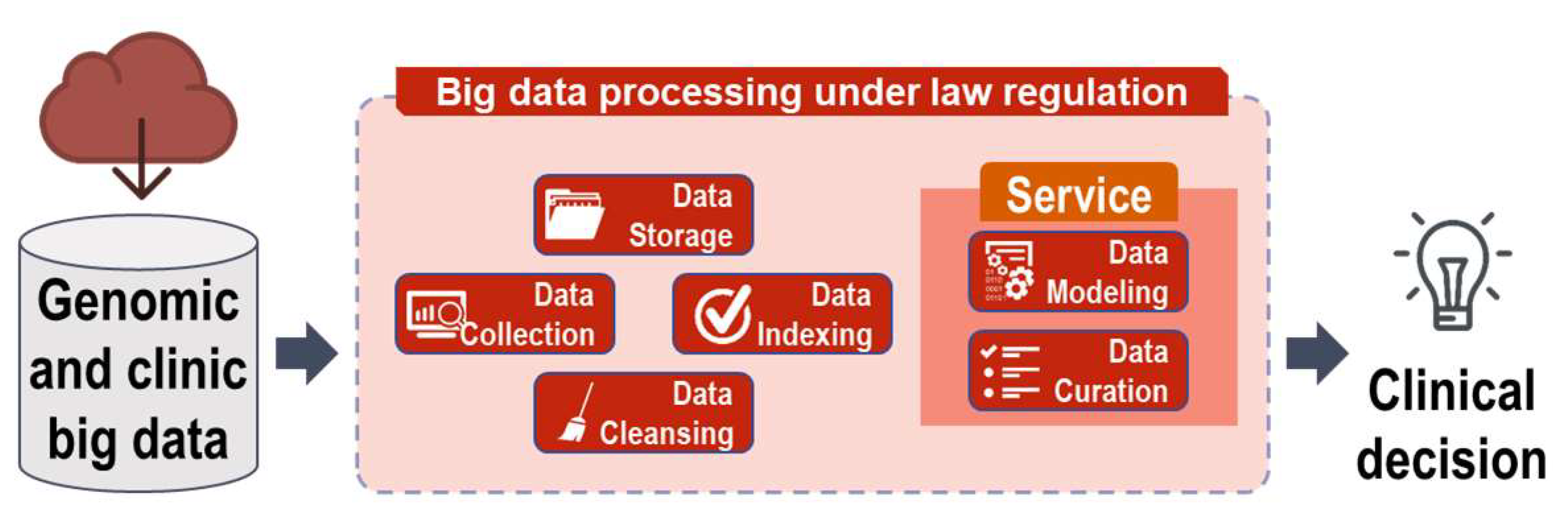
3. Deposition, Application, and Indexing of Genomic Variation Information
| Nation | Project Name | Data Size | Link | Ref. |
|---|---|---|---|---|
| Multi-national consortium | 1000 Genomes Project | 4974 people | https://www.internationalgenome.org (accessed on 21 July 2022) | [55] |
| USA | Precision Medicine Initiative cohort program (All-of-Us) | 1M people (To be completed in 2022) | https://allofus.nih.gov (accessed on 21 July 2022) | [76] |
| England | The 100,000 Genomes Project | 5M people (To be completed in 2023) | https://www.england.nhs.uk/genomics/genomic-research/100000-genomes-project (accessed on 21 July 2022) | [77] |
| Iceland | deCODE Genetics | 100K people (Completed) | https://www.decode.com (accessed on 21 July 2022) | [78] |
| Finland | FinnGen Research Project | 50K (To be completed) | https://www.finngen.fi/en/for_researchers (accessed on 21 July 2022) | [79] |
| Korea | Korea Bio-resource Information System | 500 people | https://www.kobic.re.kr/kobis (accessed on 21 July 2022) | [80] |
| Korea | Clinical & Omics Data Archive | 780 people | https://coda.nih.go.kr (accessed on 21 July 2022) | [81] |
| Netherlands | The Genome of the Netherlands project (GoNL) | 150K people (Completed) | https://www.nlgenome.nl (accessed on 21 July 2022) | [82] |
| Singapore | Genome Institute of Singapore (GIS) | 1M people (To be completed in 2028) | https://www.a-star.edu.sg/gis (accessed on 21 July 2022) | [83] |
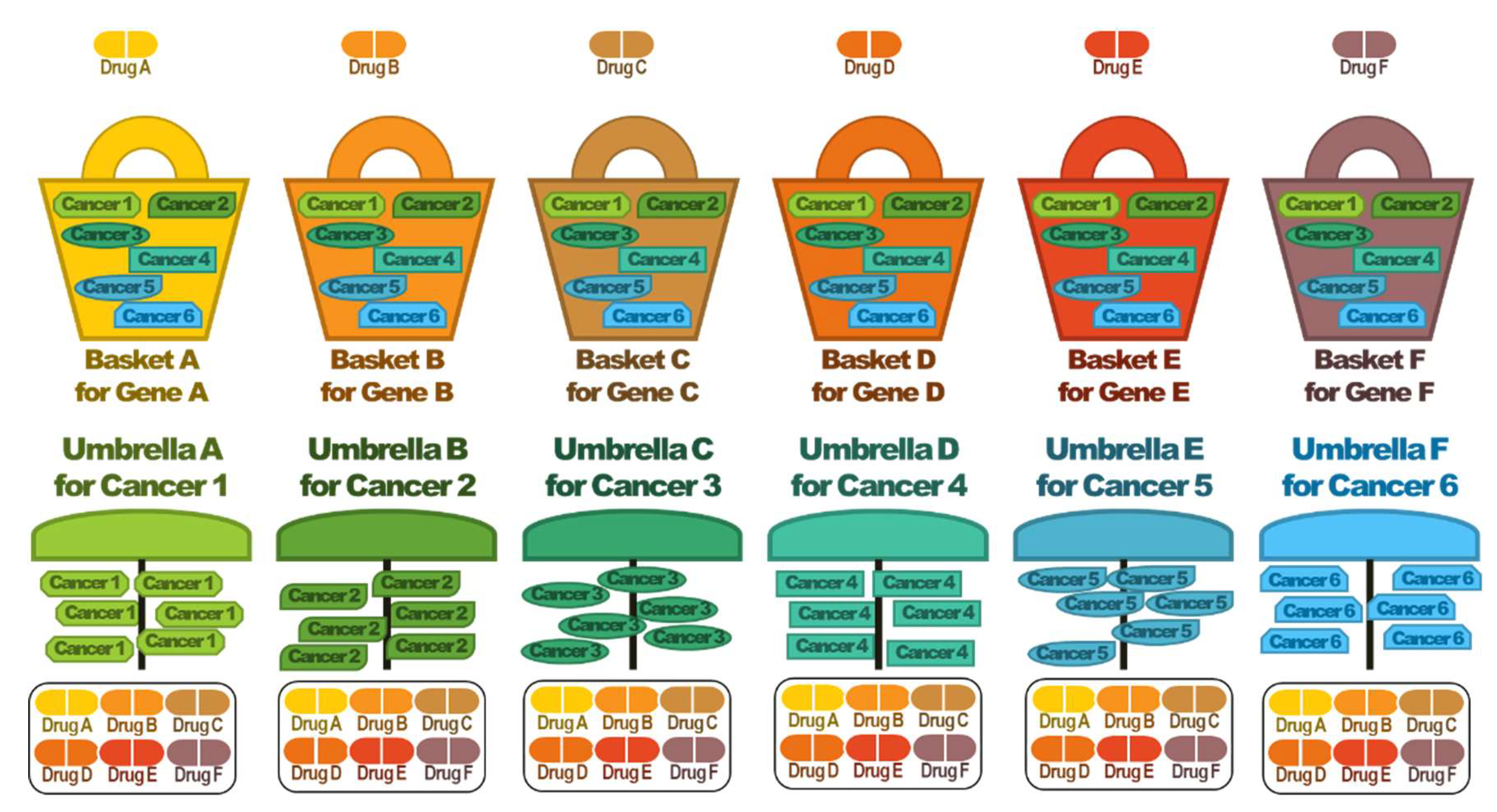
4. Basket and Umbrella Trials
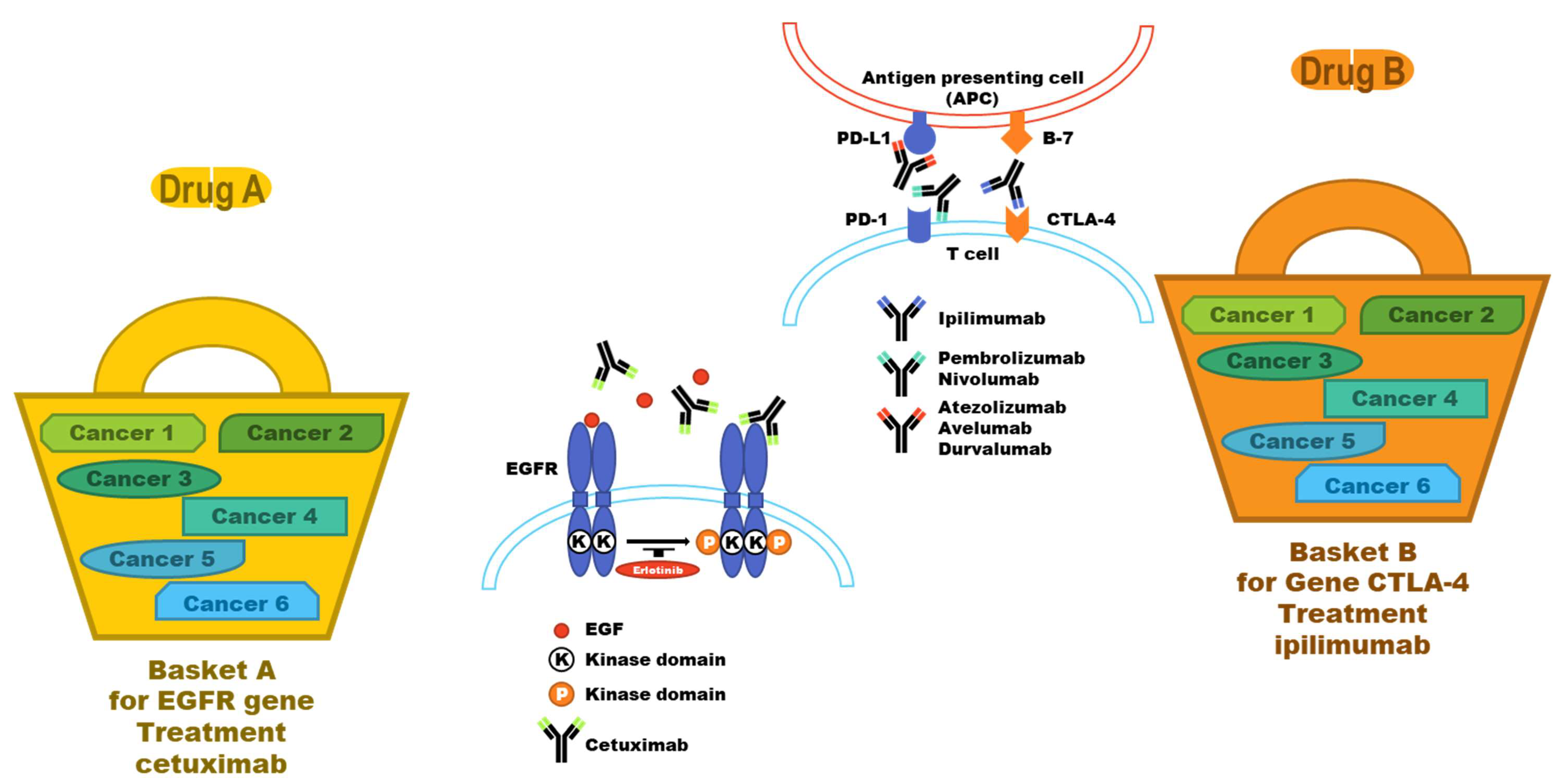
5. Companion Diagnosis: From the Genomics Point of View
| Gene/Protein | Anticancer Agent | Indications | Biomarker | Routine Testing | Ref. Papers | Ref. CT |
|---|---|---|---|---|---|---|
| ALK | Crizotinib, ceritinib, alectinib, lolatinib, brigatinib | NSCLC | ALK translocation | FISH, IHC | [90,91] | NCT00932451 |
| AR | Abiraterone, enzalutamide, dalurotamide, apalutamide | Prostate cancer | AR expression | IHC | [92] | NCT02485691 |
| BCL-2 | Venetoclax | CML | BCL-2 protein expression, BCL-2 amplification/translocation | IHC (FISH, DNA/RNA sequencing), PCR | [93] | NCT03552692 |
| BCR/ABL | Imatinib, dasatinib, nilotinib, bosutinib, ponatinib | CML | BCR/ABL1 fusion | IHC, PCR, DNA sequencing | [5] | NCT00070499 |
| BRAF | Dabrafenib+trametinib, vemurafenib+cobimetinib, encorafenib+binimetinib | Melanoma, NSCLC, ATC, HCL | BRAF V600E/K mutations | IHC, PCR, DNA sequencing | [6,7,8] | NCT01597908 |
| C-KIT, PDGFR | Imatinib | GIST | c-KIT Exon 9 and 11 mutations, PDGFR mutations | IHC, DNA sequencing | [94] | NCT00117299 |
| PDGFRB | Imatinib | Myelodysplastic/myeloproliferative syndromes | PDGFRB rearrangement | FISH | [95] | NCT00038675 |
| BRCA | Olaparib, talazoparib, rucaparib | Breast cancer, ovarian cancer, prostate cancer | Germline/somatic BRCA 1/2 mutations | DNA sequencing | [96] | NCT03286842 |
| CTLA-4 | Ipilimumab | Melanoma | DNA sequencing, PCR | [97] | NCT01216696 | |
| ER/PR | Tamoxifen, raloxifene, fulvestrant, toremifine | Breast cancer | ER/PR expression | IHC | [98,99] | NCT00066690 |
| erBB2/HER-2 | Trastuzumab, pertuzumab, ado-trastuzumab, emtansine, neratinib | Breast cancer, gastric cancer | HER-2 protein expression, HER-2 amplification | IHC, FISH | [100] | NCT01702558 |
| EGFR | Gefitinib, erlotinib, afatinib, dacomitinib | NSCLC | EGFR exon 19 deletion, exon 21 L858R mutation | DNA sequencing, PCR | [4] | NCT01955421 |
| Osimertinib | EGFR T790M mutation | [3] | NCT02474355 | |||
| FGFR2/3 | Erdafitinib | Bladder cancer | FGFR3 mutations, FGFR2/3 fusions | DNA sequencing, FISH | [101] | NCT05052372 |
| FLT3 | Midostaurin, gilteritinib | AML | FLT3 mutations | DNA sequencing, PCR | [102] | NCT04027309 |
| IDH1/2 | Ivosidenib, enasidenib | AML | IDH1/2 mutations | IHC, DNA sequencing | [103] | NCT02632708 |
| MET | Crizotinib | NSCLC | MET amplification, MET exon 14 alterations | FISH, DNA/RNA sequencing | [104] | NCT00585195 |
| MSI-H or dMMR | Pembrolizumab | MSI-H or dMMR solid tumors | MLH1, MSH2, MSH6, PMS2 protein expression, MSI high | IHC, DNA sequencing, PCR | [105] | NCT04082572 |
| Nivolumab and ipilimumab | Colorectal cancer | [106] | NCT04008030 | |||
| NTRK | Larotrectinib, entrectinib | Solid tumors with NTRK fusions | NTRK protein expression, NTRK fusion | IHC, FISH, DNA/RNA sequencing | [107] | NCT02576431 |
| PI3KCA | Alpelisib | Breast cancer | PI3KCA mutation | DNA sequencing | [108] | NCT02437318 |
| PI3KCA (alpha and delta) | Copanlisib | FL | PI3K mutation | DNA sequencing | [109] | NCT01660451 |
| PI3K (delta and gamma) | Duvelisib | CLL, SLL | PI3K mutation | DNA sequencing | [110] | NCT01476657 |
| RAS | Cetuximab, panitumumab | CRC | KRAS/NRAS wildtype | DNA sequencing | [111] | NCT04117945 |
| RET | LOXO-292 | NSCLC, MTC | RET fusion, RET mutation | FISH, DNA/RNA sequencing | [112] | NCT03157128 |
| ROS1 | Crizotinib, entrectinib | NSCLC | ROS translocation | FISH, DNA/RNA sequencing | [113] | NCT04603807 |
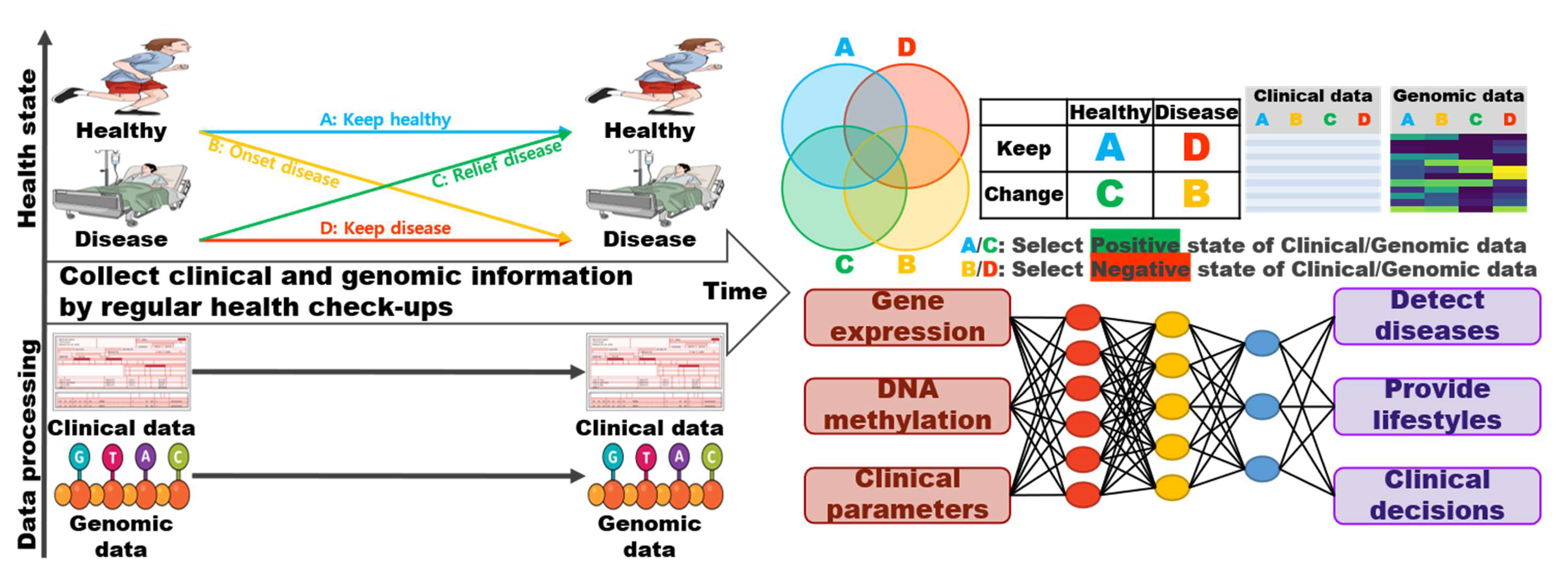
6. Genomic Information and Pharmacometrics
7. Challenge: Genomic Information Management
8. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; McLeod, H.L.; Weinshilboum, R.M. Genomics and drug response. N. Engl. J. Med. 2011, 364, 1144–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Yang, J.C.-H.; Kim, D.-W.; Planchard, D.; Ohe, Y.; Ramalingam, S.S.; Ahn, M.-J.; Kim, S.-W.; Su, W.-C.; Horn, L. AZD9291 in EGFR inhibitor–resistant non–small-cell lung cancer. N. Engl. J. Med. 2015, 372, 1689–1699. [Google Scholar] [CrossRef]
- Riely, G.J.; Pao, W.; Pham, D.; Li, A.R.; Rizvi, N.; Venkatraman, E.S.; Zakowski, M.F.; Kris, M.G.; Ladanyi, M.; Miller, V.A. Clinical course of patients with non–small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin. Cancer Res. 2006, 12, 839–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radich, J.P.; Kopecky, K.J.; Appelbaum, F.R.; Kamel-Reid, S.; Stock, W.; Malnassy, G.; Paietta, E.; Wadleigh, M.; Larson, R.A.; Emanuel, P. A randomized trial of dasatinib 100 mg versus imatinib 400 mg in newly diagnosed chronic-phase chronic myeloid leukemia. Blood J. Am. Soc. Hematol. 2012, 120, 3898–3905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grob, J.J.; Amonkar, M.M.; Karaszewska, B.; Schachter, J.; Dummer, R.; Mackiewicz, A.; Stroyakovskiy, D.; Drucis, K.; Grange, F.; Chiarion-Sileni, V. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): Results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015, 16, 1389–1398. [Google Scholar] [PubMed]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell 2018, 173, 400–416.e411. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Baran, J.; Cros, A.; Guberman, J.M.; Haider, S.; Hsu, J.; Liang, Y.; Rivkin, E.; Wang, J.; Whitty, B. International Cancer Genome Consortium Data Portal—a one-stop shop for cancer genomics data. Database 2011, 2011, bar026. [Google Scholar] [CrossRef] [Green Version]
- Bamford, S.; Dawson, E.; Forbes, S.; Clements, J.; Pettett, R.; Dogan, A.; Flanagan, A.; Teague, J.; Futreal, P.A.; Stratton, M.R. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br. J. Cancer 2004, 91, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarty, D.; Gao, J.; Phillips, S.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H. OncoKB: A precision oncology knowledge base. JCO Precis. Oncol. 2017, 1, PO.17.00011. [Google Scholar] [CrossRef] [PubMed]
- Goncearenco, A.; Rager, S.L.; Li, M.; Sang, Q.-X.; Rogozin, I.B.; Panchenko, A.R. Exploring background mutational processes to decipher cancer genetic heterogeneity. Nucleic Acids Res. 2017, 45, W514–W522. [Google Scholar] [CrossRef]
- Tamborero, D.; Rubio-Perez, C.; Deu-Pons, J.; Schroeder, M.P.; Vivancos, A.; Rovira, A.; Tusquets, I.; Albanell, J.; Rodon, J.; Tabernero, J. Cancer Genome Interpreter annotates the biological and clinical relevance of tumor alterations. Genome Med. 2018, 10, 25. [Google Scholar] [CrossRef]
- Forbes, S.A.; Beare, D.; Boutselakis, H.; Bamford, S.; Bindal, N.; Tate, J.; Cole, C.G.; Ward, S.; Dawson, E.; Ponting, L. COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017, 45, D777–D783. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhary, K.; Gupta, S.; Singh, H.; Kumar, S.; Gautam, A.; Kapoor, P.; Raghava, G.P. CancerDR: Cancer drug resistance database. Sci. Rep. 2013, 3, 1445. [Google Scholar] [CrossRef]
- Zarin, D.A.; Tse, T.; Williams, R.J.; Califf, R.M.; Ide, N.C. The ClinicalTrials. gov results database—update and key issues. N. Engl. J. Med. 2011, 364, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, J.J.; Zhao, X.; Mays, J.C.; Davoli, T. Not all cancers are created equal: Tissue specificity in cancer genes and pathways. Curr. Opin. Cell Biol. 2020, 63, 135–143. [Google Scholar] [CrossRef]
- Schaefer, M.H.; Serrano, L. Cell type-specific properties and environment shape tissue specificity of cancer genes. Sci. Rep. 2016, 6, 20707. [Google Scholar] [CrossRef] [Green Version]
- Haigis, K.M.; Cichowski, K.; Elledge, S.J. Tissue-specificity in cancer: The rule, not the exception. Science 2019, 363, 1150–1151. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.J.; Mankaney, G.; Sarvapelli, S.; Abushamma, S.; Lopez, R.; Cruise, M.; LaGuardia, L.; O’Malley, M.; Church, J.M.; Kalady, M.F. Endoscopic and histologic features associated with gastric cancer in familial adenomatous polyposis. Gastrointest. Endosc. 2019, 89, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Blair, V.R.; McLeod, M.; Carneiro, F.; Coit, D.G.; D’Addario, J.L.; van Dieren, J.M.; Harris, K.L.; Hoogerbrugge, N.; Oliveira, C.; van der Post, R.S. Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet Oncol. 2020, 21, e386–e397. [Google Scholar] [CrossRef]
- Tabano, S.; Azzollini, J.; Pesenti, C.; Lovati, S.; Costanza, J.; Fontana, L.; Peissel, B.; Miozzo, M.; Manoukian, S. Analysis of BRCA1 and RAD51C promoter methylation in italian families at high-risk of breast and ovarian cancer. Cancers 2020, 12, 910. [Google Scholar] [CrossRef] [Green Version]
- Tiacci, E.; Trifonov, V.; Schiavoni, G.; Holmes, A.; Kern, W.; Martelli, M.P.; Pucciarini, A.; Bigerna, B.; Pacini, R.; Wells, V.A. BRAF mutations in hairy-cell leukemia. N. Engl. J. Med. 2011, 364, 2305–2315. [Google Scholar] [CrossRef] [Green Version]
- Ottaviano, M.; Giunta, E.F.; Tortora, M.; Curvietto, M.; Attademo, L.; Bosso, D.; Cardalesi, C.; Rosanova, M.; De Placido, P.; Pietroluongo, E. BRAF gene and melanoma: Back to the future. Int. J. Mol. Sci. 2021, 22, 3474. [Google Scholar] [CrossRef]
- Li, X.; Kwon, H. The Impact of BRAF mutation on the recurrence of papillary thyroid carcinoma: A meta-analysis. Cancers 2020, 12, 2056. [Google Scholar] [CrossRef]
- Li, Z.-N.; Zhao, L.; Yu, L.-F.; Wei, M.-J. BRAF and KRAS mutations in metastatic colorectal cancer: Future perspectives for personalized therapy. Gastroenterol. Rep. 2020, 8, 192–205. [Google Scholar] [CrossRef]
- Levine, A.J. P53 and the immune response: 40 years of exploration—A plan for the future. Int. J. Mol. Sci. 2020, 21, 541. [Google Scholar] [CrossRef] [Green Version]
- Malekzadeh, P.; Pasetto, A.; Robbins, P.F.; Parkhurst, M.R.; Paria, B.C.; Jia, L.; Gartner, J.J.; Hill, V.; Yu, Z.; Restifo, N.P. Neoantigen screening identifies broad TP53 mutant immunogenicity in patients with epithelial cancers. J. Clin. Investig. 2019, 129, 1109–1114. [Google Scholar] [CrossRef]
- Saxton, S.N.; Clark, B.J.; Withers, S.B.; Eringa, E.C.; Heagerty, A.M. Mechanistic links between obesity, diabetes, and blood pressure: Role of perivascular adipose tissue. Physiol. Rev. 2019, 99, 1701–1763. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Pilot, C.; Marunaka, Y.; Fais, S. Targeting acidity in cancer and diabetes. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2019, 1871, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.; Liao, Z. Diabetes and cancer: Epidemiological and biological links. World J. Diabetes 2020, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Abdalkareem, E.A.; Yin, K.B. A Current Perspective of in Association with Colorectal Carcinogenesis. Open Infect. Dis. J. 2019, 11, 7–12. [Google Scholar] [CrossRef]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and cancer: Epidemiology and biological mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef]
- Zhou, Q.; Xi, S. A review on arsenic carcinogenesis: Epidemiology, metabolism, genotoxicity and epigenetic changes. Regul. Toxicol. Pharmacol. 2018, 99, 78–88. [Google Scholar] [CrossRef]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Xu, M.; Jung, X.; Hines, O.J.; Eibl, G.; Chen, Y. Obesity and pancreatic cancer: Overview of epidemiology and potential prevention by weight loss. Pancreas 2018, 47, 158. [Google Scholar] [CrossRef]
- Ye, P.; Xi, Y.; Huang, Z.; Xu, P. Linking obesity with colorectal cancer: Epidemiology and mechanistic insights. Cancers 2020, 12, 1408. [Google Scholar] [CrossRef]
- Lim, A.R.; Rathmell, W.K.; Rathmell, J.C. The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. Elife 2020, 9, e55185. [Google Scholar] [CrossRef]
- Wang, X. Stem cells in tissues, organoids, and cancers. Cell. Mol. Life Sci. 2019, 76, 4043–4070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, H.; Loh, K.M.; Nusse, R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Dewi, D.L.; Ishii, H.; Kano, Y.; Nishikawa, S.; Haraguchi, N.; Sakai, D.; Satoh, T.; Doki, Y.; Mori, M. Cancer stem cell theory in gastrointestinal malignancies: Recent progress and upcoming challenges. J. Gastroenterol. 2011, 46, 1145. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; von der Ohe, J.; Ungefroren, H. Impact of the tumor microenvironment on tumor heterogeneity and consequences for cancer cell plasticity and stemness. Cancers 2020, 12, 3716. [Google Scholar] [CrossRef]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013, 32, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 501, 338–345. [Google Scholar] [CrossRef]
- Chung, W.; Eum, H.H.; Lee, H.-O.; Lee, K.-M.; Lee, H.-B.; Kim, K.-T.; Ryu, H.S.; Kim, S.; Lee, J.E.; Park, Y.H. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017, 8, 15081. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.W.-H.; Tsui, Y.-M.; Chan, L.-K.; Sze, K.M.-F.; Zhang, X.; Cheu, J.W.-S.; Chiu, Y.-T.; Lee, J.M.-F.; Chan, A.C.-Y.; Cheung, E.T.-Y. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat. Commun. 2021, 12, 3684. [Google Scholar] [CrossRef]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 2021, 12, 2540. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [PubMed] [Green Version]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp. Oncol. 2015, 19, A68. [Google Scholar] [CrossRef] [PubMed]
- Neuhausen, S.L. Ethnic differences in cancer risk resulting from genetic variation. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1999, 86, 2575–2582. [Google Scholar]
- Weise, N.; Shaya, J.; Javier-Desloges, J.; Cheng, H.H.; Madlensky, L.; McKay, R.R. Disparities in germline testing among racial minorities with prostate cancer. Prostate Cancer Prostatic Dis. 2021, 1–8. [Google Scholar] [CrossRef]
- Siva, N. 1000 Genomes project. Nat. Biotechnol. 2008, 26, 256–257. [Google Scholar] [CrossRef]
- Kanchi, K.L.; Johnson, K.J.; Lu, C.; McLellan, M.D.; Leiserson, M.D.; Wendl, M.C.; Zhang, Q.; Koboldt, D.C.; Xie, M.; Kandoth, C. Integrated analysis of germline and somatic variants in ovarian cancer. Nat. Commun. 2014, 5, 3156. [Google Scholar] [CrossRef]
- Vosoughi, A.; Zhang, T.; Shohdy, K.S.; Vlachostergios, P.J.; Wilkes, D.C.; Bhinder, B.; Tagawa, S.T.; Nanus, D.M.; Molina, A.M.; Beltran, H. Common germline-somatic variant interactions in advanced urothelial cancer. Nat. Commun. 2020, 11, 6195. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhang, J.; Zhu, M.; Zhang, X.; Li, Z.; Dai, J.; Ma, H.; Hu, Z.; Jin, G. Interaction analysis between germline susceptibility loci and somatic alterations in lung cancer. Int. J. Cancer 2018, 143, 878–885. [Google Scholar] [CrossRef] [Green Version]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E. COSMIC: The catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [Green Version]
- Knudson, A.G. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [Green Version]
- Sanders, S.J.; Murtha, M.T.; Gupta, A.R.; Murdoch, J.D.; Raubeson, M.J.; Willsey, A.J.; Ercan-Sencicek, A.G.; DiLullo, N.M.; Parikshak, N.N.; Stein, J.L. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 2012, 485, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.; Frigge, M.L.; Masson, G.; Besenbacher, S.; Sulem, P.; Magnusson, G.; Gudjonsson, S.A.; Sigurdsson, A.; Jonasdottir, A.; Jonasdottir, A. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012, 488, 471–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, A.; Katoh, H.; Komura, D.; Kakiuchi, M.; Tagashira, A.; Yamamoto, S.; Tatsuno, K.; Ueda, H.; Nagae, G.; Fukuda, S. Defined lifestyle and germline factors predispose Asian populations to gastric cancer. Sci. Adv. 2020, 6, eaav9778. [Google Scholar] [CrossRef]
- Katoh, H.; Ishikawa, S. Lifestyles, genetics, and future perspectives on gastric cancer in east Asian populations. J. Hum. Genet. 2021, 66, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Ngeow, J.; Eng, C. Precision medicine in heritable cancer: When somatic tumour testing and germline mutations meet. NPJ Genom. Med. 2016, 1, 15006. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, S.; Kim, S.I.; Kim, J.-H.; Eoh, K.J.; Lee, C.; Kim, Y.T.; Suh, D.-S.; Park, T.; Song, Y.S. Development of Machine Learning Models to Predict Platinum Sensitivity of High-Grade Serous Ovarian Carcinoma. Cancers 2021, 13, 1875. [Google Scholar] [CrossRef]
- Baptiste, M.; Moinuddeen, S.S.; Soliz, C.L.; Ehsan, H.; Kaneko, G. Making Sense of Genetic Information: The Promising Evolution of Clinical Stratification and Precision Oncology Using Machine Learning. Genes 2021, 12, 722. [Google Scholar] [CrossRef]
- Rehm, H.L.; Berg, J.S.; Brooks, L.D.; Bustamante, C.D.; Evans, J.P.; Landrum, M.J.; Ledbetter, D.H.; Maglott, D.R.; Martin, C.L.; Nussbaum, R.L. ClinGen—The clinical genome resource. N. Engl. J. Med. 2015, 372, 2235–2242. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.X.; He, Y.; Sanford, E.; Montesion, M.; Frampton, G.M.; Vignot, S.; Soria, J.-C.; Ross, J.S.; Miller, V.A.; Stephens, P.J. A computational approach to distinguish somatic vs. germline origin of genomic alterations from deep sequencing of cancer specimens without a matched normal. PLoS Comput. Biol. 2018, 14, e1005965. [Google Scholar] [CrossRef] [Green Version]
- Qing, T.; Mohsen, H.; Marczyk, M.; Ye, Y.; O’Meara, T.; Zhao, H.; Townsend, J.P.; Gerstein, M.; Hatzis, C.; Kluger, Y. Germline variant burden in cancer genes correlates with age at diagnosis and somatic mutation burden. Nat. Commun. 2020, 11, 2438. [Google Scholar] [CrossRef]
- Tennessen, J.A.; Bigham, A.W.; O’Connor, T.D.; Fu, W.; Kenny, E.E.; Gravel, S.; McGee, S.; Do, R.; Liu, X.; Jun, G. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science 2012, 337, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorn, C.F.; Klein, T.E.; Altman, R.B. PharmGKB: The pharmacogenomics knowledge base. In Pharmacogenomics; Springer: Berlin, Germany, 2013; pp. 311–320. [Google Scholar]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Madhukar, N.S.; Elemento, O. Bioinformatics approaches to predict drug responses from genomic sequencing. Cancer Syst. Biol. 2018, 1711, 277–296. [Google Scholar]
- Mak, A.C.; White, M.J.; Eckalbar, W.L.; Szpiech, Z.A.; Oh, S.S.; Pino-Yanes, M.; Hu, D.; Goddard, P.; Huntsman, S.; Galanter, J. Whole-genome sequencing of pharmacogenetic drug response in racially diverse children with asthma. Am. J. Respir. Crit. Care Med. 2018, 197, 1552–1564. [Google Scholar] [CrossRef]
- Sankar, P.L.; Parker, L.S. The Precision Medicine Initiative’s All of Us Research Program: An agenda for research on its ethical, legal, and social issues. Genet. Med. 2017, 19, 743–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco-Ramiro, F.; Peiró-Pastor, R.; Aguado, B. Human genomics projects and precision medicine. Gene Ther. 2017, 24, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Helgason, H.; Gudjonsson, S.A.; Zink, F.; Oddson, A.; Gylfason, A.; Besenbacher, S.; Magnusson, G.; Halldorsson, B.V.; Hjartarson, E. Large-scale whole-genome sequencing of the Icelandic population. Nat. Genet. 2015, 47, 435–444. [Google Scholar] [CrossRef]
- Ritari, J.; Hyvärinen, K.; Clancy, J.; FinnGen; Partanen, J.; Koskela, S. Increasing accuracy of HLA imputation by a population-specific reference panel in a FinnGen biobank cohort. NAR Genom. Bioinform. 2020, 2, lqaa030. [Google Scholar] [CrossRef]
- Seong-Jin, P.; Cho, K.H.; Daeyeon, K.; Jeon, Y.J.; Jin, T.E.; Choe, Y.K. Korean Bio-resource Information System (KOBIS): The Nationwide Infrastructure for Collecting and Integrating Biological Resource Information in Korea. Biodivers. Inf. Sci. Stand. 2018, 2, e26286. [Google Scholar]
- Yun, Y.; Kim, H.-N.; Kim, S.E.; Heo, S.G.; Chang, Y.; Ryu, S.; Shin, H.; Kim, H.-L. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 2017, 17, 151. [Google Scholar] [CrossRef] [Green Version]
- Boomsma, D.I.; Wijmenga, C.; Slagboom, E.P.; Swertz, M.A.; Karssen, L.C.; Abdellaoui, A.; Ye, K.; Guryev, V.; Vermaat, M.; Van Dijk, F. The Genome of the Netherlands: Design, and project goals. Eur. J. Hum. Genet. 2014, 22, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, Y.-Y.; Sim, X.; Ong, R.T.; Tan, A.K.; Chen, J.; Tantoso, E.; Small, K.S.; Ku, C.-S.; Lee, E.J.; Seielstad, M. Singapore Genome Variation Project: A haplotype map of three Southeast Asian populations. Genome Res. 2009, 19, 2154–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, R.S.; Gandara, D.R.; Hirsch, F.R.; Redman, M.W.; LeBlanc, M.; Mack, P.C.; Schwartz, L.H.; Vokes, E.; Ramalingam, S.S.; Bradley, J.D. Lung Master Protocol (Lung-MAP)—A biomarker-driven protocol for accelerating development of therapies for squamous cell lung cancer: SWOG S1400. Clin. Cancer Res. 2015, 21, 1514–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Garralda, E.; Dienstmann, R.; Piris-Giménez, A.; Braña, I.; Rodon, J.; Tabernero, J. New clinical trial designs in the era of precision medicine. Mol. Oncol. 2019, 13, 549–557. [Google Scholar] [CrossRef]
- Kraus, V.B. Biomarkers as drug development tools: Discovery, validation, qualification and use. Nat. Rev. Rheumatol. 2018, 14, 354–362. [Google Scholar] [CrossRef]
- Pant, S.; Weiner, R.; Marton, M.J. Navigating the rapids: The development of regulated next-generation sequencing-based clinical trial assays and companion diagnostics. Front. Oncol. 2014, 4, 78. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.E.; Chester, J.D. Personalised cancer medicine. Int. J. Cancer 2015, 137, 262–266. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, E.E.; Usari, T.; Polli, A.; Lewis, I.; Wilner, K.D. Renal Effects of Crizotinib in Patients With ALK-Positive Advanced NSCLC. J. Thorac. Oncol. 2019, 14, 1077–1085. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wang, Y.-F.; Yang, J.C.-H.; Yu, C.-J.; Wu, S.-G.; Shih, J.-Y.; Yang, P.-C. Development of renal cysts after crizotinib treatment in advanced ALK-positive non–small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 1720–1725. [Google Scholar] [CrossRef] [Green Version]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.-C.; Bamias, A.; Carles, J. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef] [PubMed]
- Ballotta, L.; Zinzani, P.L.; Pileri, S.; Bruna, R.; Tani, M.; Casadei, B.; Tabanelli, V.; Volpetti, S.; Luminari, S.; Corradini, P. Venetoclax Shows Low Therapeutic Activity in BCL2-Positive Relapsed/Refractory Peripheral T-Cell Lymphoma: A Phase 2 Study of the Fondazione Italiana Linfomi. Front. Oncol. 2021, 11, 789891. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, D.; Cooke, L.; Riley, C.; Swart, R.; Simons, B.; Della Croce, K.; Wisner, L.; Iorio, M.; Shakalya, K.; Garewal, H. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene 2007, 26, 3909–3919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, E.J.; Kulkarni, S.; Vizmanos, J.L.; Jaju, R.; Martinelli, G.; Testoni, N.; Hughes, G.; Salamanchuk, Z.; Calasanz, M.J.; Lahortiga, I. Novel translocations that disrupt the platelet-derived growth factor receptor β (PDGFRB) gene in BCR–ABL-negative chronic myeloproliferative disorders. Br. J. Haematol. 2003, 120, 251–256. [Google Scholar] [CrossRef]
- Gelmon, K.A.; Fasching, P.A.; Couch, F.J.; Balmaña, J.; Delaloge, S.; Labidi-Galy, I.; Bennett, J.; McCutcheon, S.; Walker, G.; O’Shaughnessy, J. Clinical effectiveness of olaparib monotherapy in germline BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: Phase IIIb LUCY interim analysis. Eur. J. Cancer 2021, 152, 68–77. [Google Scholar] [CrossRef]
- Haag, G.; Zoernig, I.; Hassel, J.; Halama, N.; Dick, J.; Lang, N.; Podola, L.; Funk, J.; Ziegelmeier, C.; Juenger, S. Phase II trial of ipilimumab in melanoma patients with preexisting humoural immune response to NY-ESO-1. Eur. J. Cancer 2018, 90, 122–129. [Google Scholar] [CrossRef]
- Francis, P.A.; Regan, M.M.; Fleming, G.F.; Láng, I.; Ciruelos, E.; Bellet, M.; Bonnefoi, H.R.; Climent, M.A.; Da Prada, G.A.; Burstein, H.J. Adjuvant ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 2015, 372, 436–446. [Google Scholar] [CrossRef] [Green Version]
- Elledge, R.M.; Green, S.; Pugh, R.; Allred, D.C.; Clark, G.M.; Hill, J.; Ravdin, P.; Martino, S.; Osborne, C.K. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: A Southwest Oncology Group study. Int. J. Cancer 2000, 89, 111–117. [Google Scholar] [CrossRef]
- Cortés, J.; Diéras, V.; Lorenzen, S.; Montemurro, F.; Riera-Knorrenschild, J.; Thuss-Patience, P.; Allegrini, G.; De Laurentiis, M.; Lohrisch, C.; Oravcová, E. Efficacy and safety of Trastuzumab Emtansine plus Capecitabine vs Trastuzumab Emtansine alone in patients with previously treated ERBB2 (HER2)-positive metastatic breast Cancer: A phase 1 and randomized phase 2 trial. JAMA Oncol. 2020, 6, 1203–1209. [Google Scholar] [CrossRef]
- Facchinetti, F.; Hollebecque, A.; Bahleda, R.; Loriot, Y.; Olaussen, K.A.; Massard, C.; Friboulet, L. Facts and new hopes on selective FGFR inhibitors in solid tumors. Clin. Cancer Res. 2020, 26, 764–774. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Zhao, S.; Qiao, X.; Knight, T.; Edwards, H.; Polin, L.; Kushner, J.; Dzinic, S.H.; White, K.; Wang, G. Inhibition of Bcl-2 synergistically enhances the antileukemic activity of midostaurin and gilteritinib in preclinical models of FLT3-mutated acute myeloid leukemia. Clin. Cancer Res. 2019, 25, 6815–6826. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; DiNardo, C.D.; Fathi, A.T.; Mims, A.S.; Pratz, K.W.; Savona, M.R.; Stein, A.S.; Stone, R.M.; Winer, E.S.; Seet, C.S. Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: A phase 1 study. Blood J. Am. Soc. Hematol. 2021, 137, 1792–1803. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.-H.I.; Bang, Y.-J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B. Crizotinib in ROS1-rearranged non–small-cell lung cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonça Gorgulho, C.; Krishnamurthy, A.; Lanzi, A.; Galon, J.; Housseau, F.; Kaneno, R.; Lotze, M.T. Gutting it Out: Developing Effective Immunotherapies for Patients With Colorectal Cancer. J. Immunother. 2021, 44, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Dreyling, M.; Morschhauser, F.; Bouabdallah, K.; Bron, D.; Cunningham, D.; Assouline, S.; Verhoef, G.; Linton, K.; Thieblemont, C.; Vitolo, U. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann. Oncol. 2017, 28, 2169–2178. [Google Scholar] [CrossRef]
- Flinn, I.W.; O’Brien, S.; Kahl, B.; Patel, M.; Oki, Y.; Foss, F.F.; Porcu, P.; Jones, J.; Burger, J.A.; Jain, N. Duvelisib, a novel oral dual inhibitor of PI3K-δ, γ, is clinically active in advanced hematologic malignancies. Blood J. Am. Soc. Hematol. 2018, 131, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Dmello, R.S.; To, S.Q.; Chand, A.L. Therapeutic Targeting of the Tumour Microenvironment in Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 2067. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.; Loong, H.H.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C. Efficacy of selpercatinib in RET fusion–positive non–small-cell lung cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Wheatley-Price, P.; Juergens, R.A.; Sacher, A.; Leighl, N.B.; Tsao, M.-S.; Cheema, P.; Snow, S.; Liu, G.; Card, P.B. The Rapidly Evolving Landscape of Novel Targeted Therapies in Advanced Non-Small Cell Lung Cancer. Lung Cancer 2021, 160, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Tsamandouras, N.; Dickinson, G.; Guo, Y.; Hall, S.; Rostami-Hodjegan, A.; Galetin, A.; Aarons, L. Identification of the effect of multiple polymorphisms on the pharmacokinetics of simvastatin and simvastatin acid using a population-modeling approach. Clin. Pharmacol. Ther. 2014, 96, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, R.; Zhao, S.; Chung, S.W.; Mager, D.E.; Gallo, J.M. Merging systems biology with pharmacodynamics. Sci. Transl. Med. 2012, 4, 126ps127. [Google Scholar] [CrossRef] [Green Version]
- Nierman, D.M. Tools that we use: If you can’t measure it, you can’t manage it. Crit. Care Med. 2007, 35, 312–313. [Google Scholar] [CrossRef]
- García, S.A.; Reyes Román, J.F.; Casamayor, J.C.; Pastor, O. Towards an effective and efficient management of genome data: An information systems engineering perspective. In Proceedings of the International Conference on Advanced Information Systems Engineering, Rome, Italy, 3–7 June 2019; pp. 99–110. [Google Scholar]
- Duyzend, M.H. Genomic medicine in a community hospital setting. J. Pediatr. 2021, 239, 1–4. [Google Scholar] [CrossRef]
- Gim, J.-A. A Genomic Information Management System for Maintaining Healthy Genomic States and Application of Genomic Big Data in Clinical Research. Int. J. Mol. Sci. 2022, 23, 5963. [Google Scholar] [CrossRef]
- Bianchi, V.; Colantoni, A.; Calderone, A.; Ausiello, G.; Ferre, F.; Helmer-Citterich, M. DBATE: Database of alternative transcripts expression. Database 2013, 2013, bat050. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.-J.; Yang, S.; Kang, T.-W.; Park, S.-M.; Kim, Y.S.; Kim, S.-Y. MENT: Methylation and expression database of normal and tumor tissues. Gene 2013, 518, 194–200. [Google Scholar] [CrossRef]
- Feng, C.; Araki, M.; Kunimoto, R.; Tamon, A.; Makiguchi, H.; Niijima, S.; Tsujimoto, G.; Okuno, Y. GEM-TREND: A web tool for gene expression data mining toward relevant network discovery. BMC Genom. 2009, 10, 411. [Google Scholar] [CrossRef] [Green Version]
- Reibe, S.; Hjorth, M.; Febbraio, M.A.; Whitham, M. GeneXX: An online tool for the exploration of transcript changes in skeletal muscle associated with exercise. Physiol. Genom. 2018, 50, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Canela-Xandri, O.; Rawlik, K.; Tenesa, A. An atlas of genetic associations in UK Biobank. Nat. Genet. 2018, 50, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sui, Y.; Xie, B.; Qu, H.; Fang, X. GliomaDB: A web server for integrating glioma omics data and interactive analysis. Genom. Proteom. Bioinform. 2019, 17, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Gabriel, B.M.; Dollet, L.; Smith, J.A.; Puig, L.S.; Botella, J.; Bishop, D.J.; Krook, A.; Zierath, J.R. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun. 2020, 11, 470. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Choi, C. Oncopression: Gene expression compendium for cancer with matched normal tissues. Bioinformatics 2017, 33, 2068–2070. [Google Scholar] [CrossRef]
- Ono, H.; Ogasawara, O.; Okubo, K.; Bono, H. RefEx, a reference gene expression dataset as a web tool for the functional analysis of genes. Sci. Data 2017, 4, 170105. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Ramírez, J.C.; Deng, N.; Qiu, X.; Wu, C.; Zheng, W.J.; Wu, H. Restructured GEO: Restructuring Gene Expression Omnibus metadata for genome dynamics analysis. Database 2019, 2019, bay145. [Google Scholar] [CrossRef] [Green Version]
| Case | Query | Source | Output | Accessibility | Ref. |
|---|---|---|---|---|---|
| DBATE | Gene symbols | 13 large RNA-seq from human healthy and disease tissues from NCBI GEO | Expression values that can be visualized in several ways | http://bioinformatica.uniroma2.it/DBATE (accessed on 21 July 2022) | [120] |
| MENT | Gene symbols or conditions of genomic data | NCBI GEO and TCGA | Patterns and gene list of DNA methylation, gene expression and their correlation in diverse cancers | https://mgrc.kribb.re.kr:8080/MENT/ (Unconnected) | [121] |
| GEM-TREND | Gene symbols | GEO, ArrayExpress, researchers’ websites | GEO series and platform ID, series title, similarity score, and p-value are displayed, network visualization | https://openebench.bsc.es/tool/gem-trend/ (accessed on 21 July 2022) | [122] |
| GeneXX | Gene symbols | NCBI GEO, transcriptome data | Stratified by exercise type, training status, sex, and time point postexercise | https://genexx.shinyapps.io/genexx (accessed on 21 July 2022) | [123] |
| GeneATLAS | GWAS catalog no. | UK Biobank | A large database of associations between hundreds of traits and millions of variants using the UK Biobank cohort | http://geneatlas.roslin.ed.ac.uk (accessed on 21 July 2022) | [124] |
| GliomaDB | Gene symbols | NCBI GEO, TCGA, CGGA, MSK-IMPACT, US FDA, PharmGKB of Genomic, transcriptomic, epigenomic, clinical information | Kaplan-Meier plot. The interactive heatmap visualization of the multi-omics data | http://cgga.org.cn:9091/gliomasdb (accessed on 21 July 2022) | [125] |
| Metamex | Gene symbols | Oligo package, limma package, DESeq2 package, NCBI GEO. | Skeletal muscle transcriptional responses to different modes of exercise and an online interface to readily interrogate the database | https://metamex.serve.scilifelab.se (accessed on 21 July 2022) | [126] |
| Oncopression | Gene symbols | NCBI GEO, ArrayExpress, ICGC, ExpressionAtlas, cBioPortal, ExAc Browser, oncomine (Rhodes) | Sample statistics of oncopression, Validity of dataset integration, Use of oncopression in cancer research | http://www.oncopression.com (accessed on 21 July 2022) | [127] |
| RefEx | Gene symbols, disease names | ESTs, Affymetrix GeneChip, CAGE, RNA-Seq, NCBI gene ID | Integration of publicly available gene expression data, visualize with BodyParts3D, extraction of genes with tissue-specific expression patterns, gene expression visualization of the FANTOM5 CAGE data | https://refex.dbcls.jp (accessed on 21 July 2022) | [128] |
| ReGEO | Gene symbols | GEO, NCBI, Search by keyword, GSE Accession, Pubmed ID, Experiment Type, Organism, Disease, Timepoints | Identify and categorize data for their integrative data analysis | https://regeo.org (accessed on 21 July 2022) | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, Y.K.; Gim, J.-A. New Drug Development and Clinical Trial Design by Applying Genomic Information Management. Pharmaceutics 2022, 14, 1539. https://doi.org/10.3390/pharmaceutics14081539
Ko YK, Gim J-A. New Drug Development and Clinical Trial Design by Applying Genomic Information Management. Pharmaceutics. 2022; 14(8):1539. https://doi.org/10.3390/pharmaceutics14081539
Chicago/Turabian StyleKo, Young Kyung, and Jeong-An Gim. 2022. "New Drug Development and Clinical Trial Design by Applying Genomic Information Management" Pharmaceutics 14, no. 8: 1539. https://doi.org/10.3390/pharmaceutics14081539
APA StyleKo, Y. K., & Gim, J.-A. (2022). New Drug Development and Clinical Trial Design by Applying Genomic Information Management. Pharmaceutics, 14(8), 1539. https://doi.org/10.3390/pharmaceutics14081539





