Multiparticulate Systems of Meloxicam for Colonic Administration in Cancer or Autoimmune Diseases
Abstract
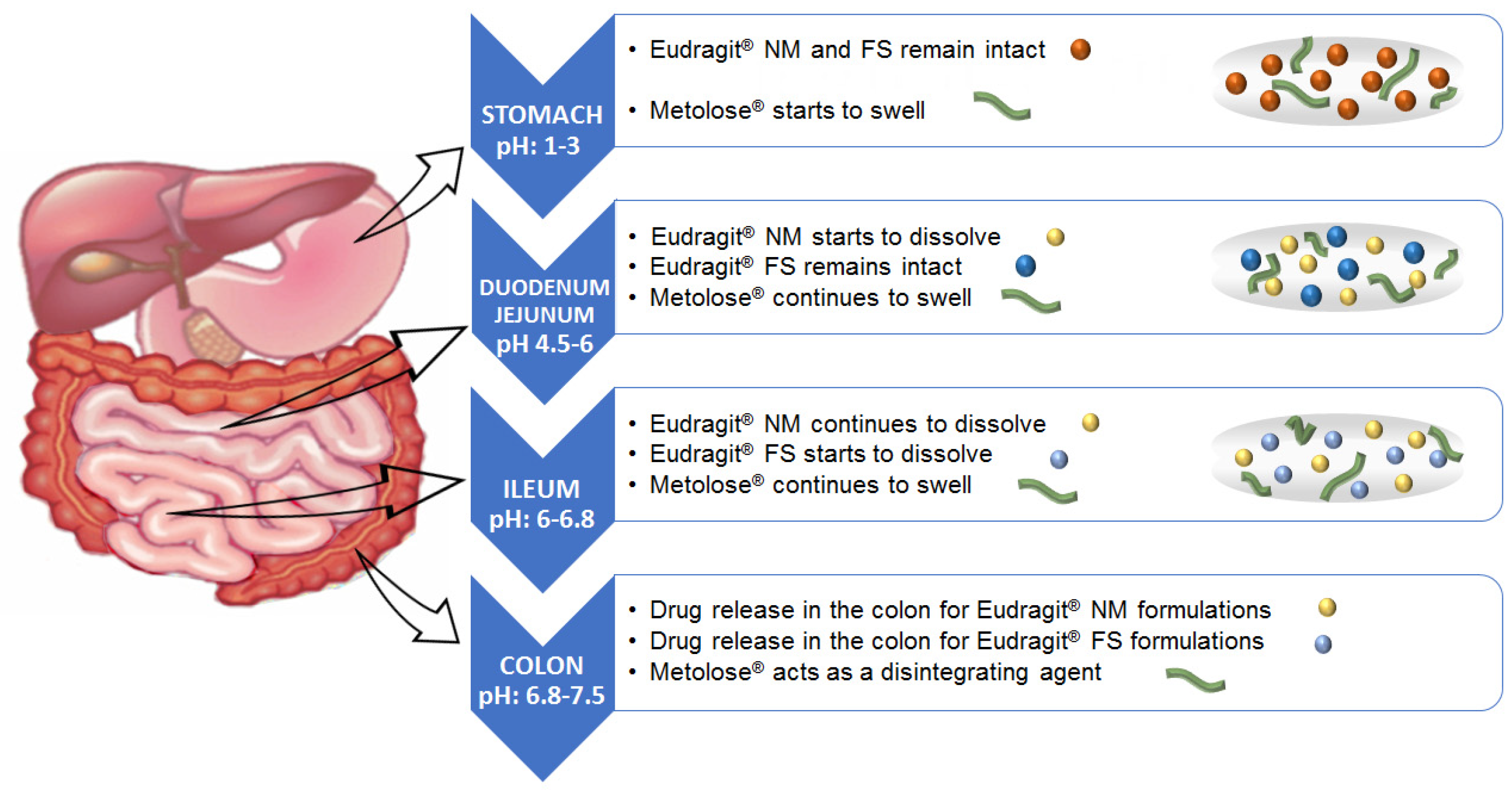
1. Introduction
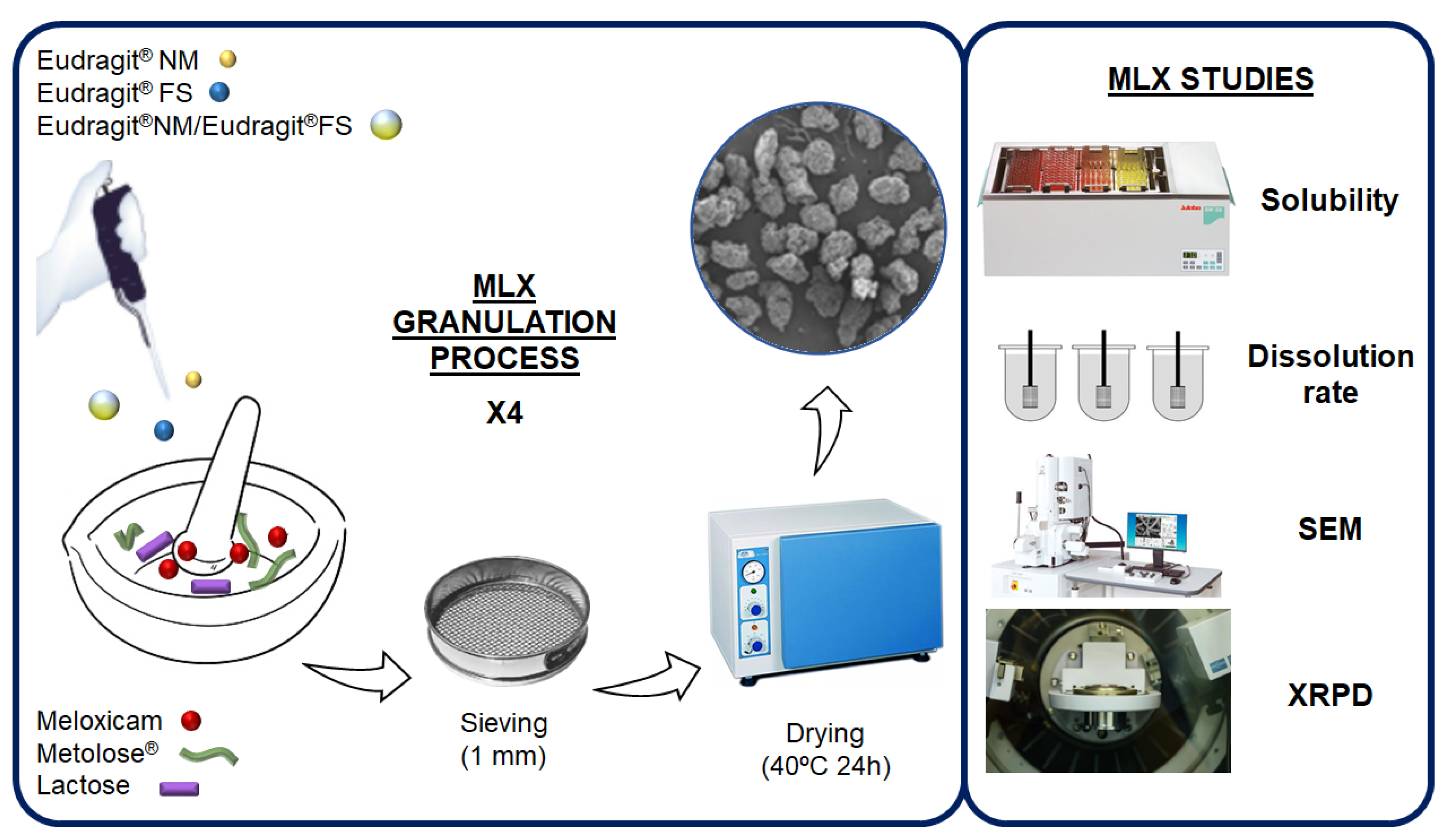
2. Materials and Methods
2.1. Materials
2.2. Preparation of Formulations
2.3. Solubility Study
2.4. In Vitro Release Profile Study
2.5. Scanning Electron Microscopy (SEM)
2.6. X-ray Powder Diffractometry (XRPD) and Grazing Incidence X-ray Diffraction (GID)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Solubility Studies at pH 6.8 with MLX Raw Material and 0.50–1.41 mm Granules of Formulations: FS, NM, NM + FS, CFS, CNM and CNM + CFS
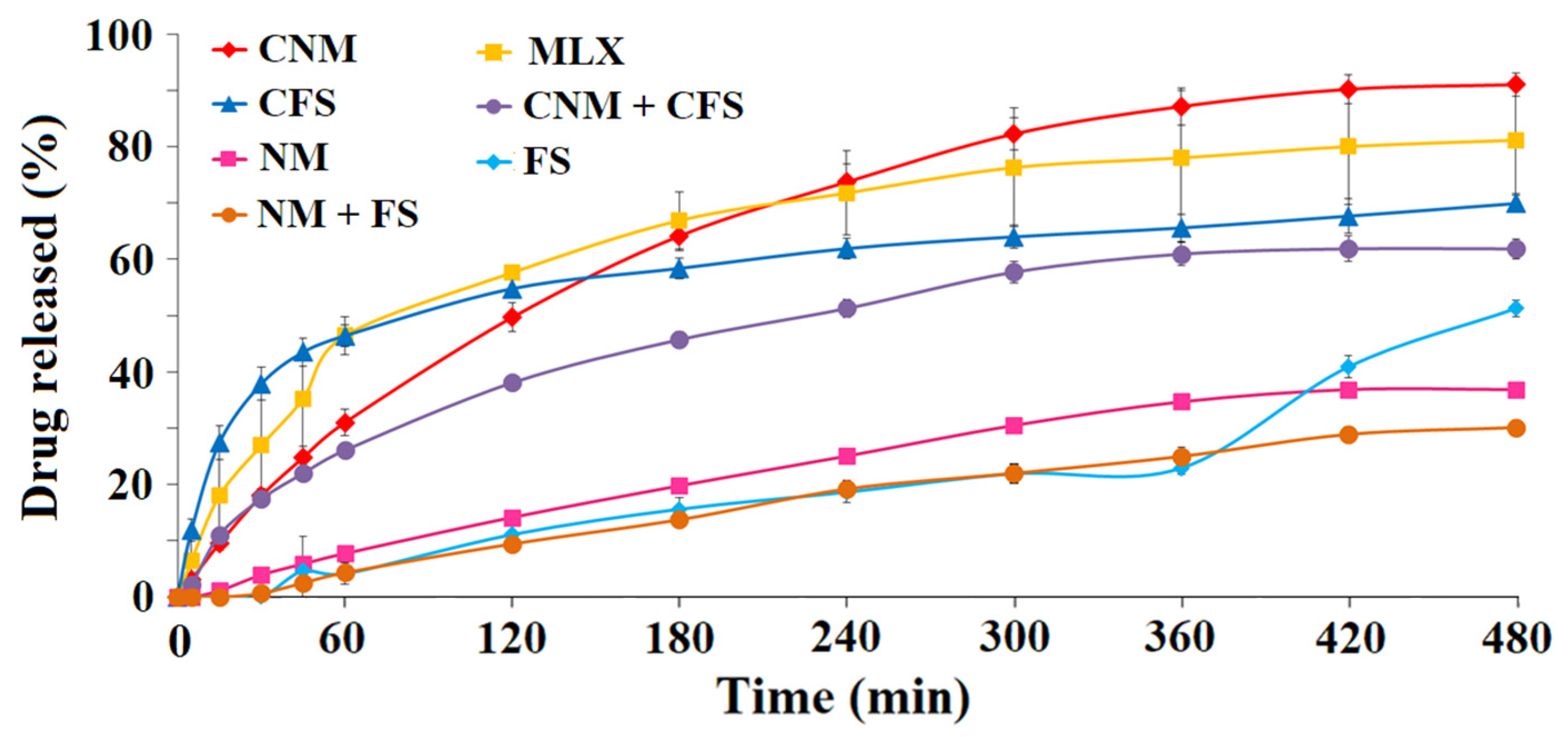
3.2. In Vitro Release Profile Study
3.2.1. First Dissolution Tests at pH 6.8 Were Carried out with 0.5–1.41 mm Granules for FS, NM, FS + NM, CFS, CNM and CNM + CFS Samples
3.2.2. Dissolution Rate of Granules of Size 0.35–1.50 mm at Different pHs (6.8 and 7.4)
3.2.3. Dissolution Rate of MLX at pH 1.2, 6.8 and 7.4, of 0.85–1.00 mm Diameter Granules of CFS, CNM and CNM + CFS with Buffer Changes (Simulating the Gastrointestinal Environment)
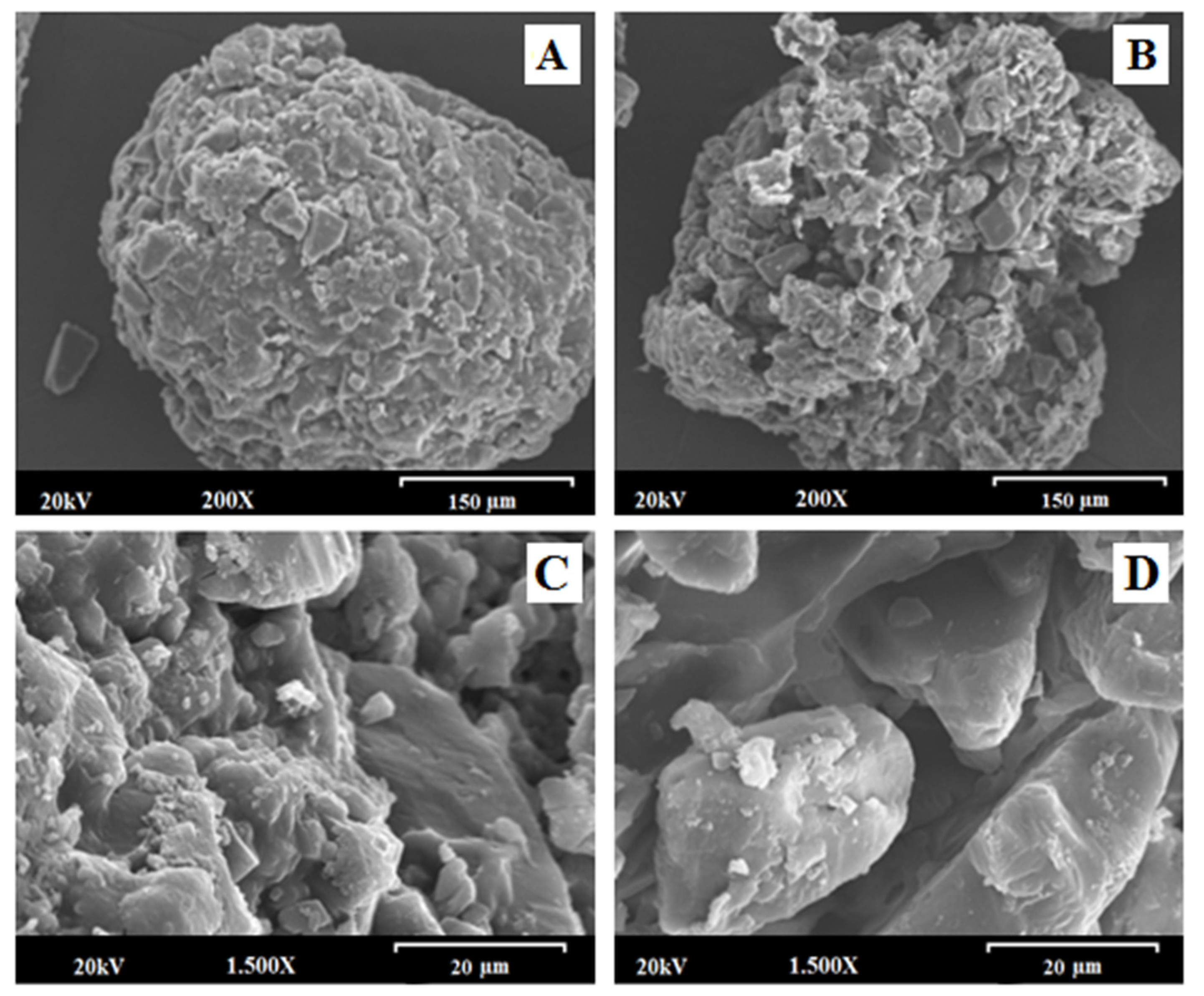
3.3. Scanning Electron Microscopy (SEM)
3.4. X-ray Powder Diffractometry (XRPD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, H.; Huang, S.; Huang, G. Design and application of oral colon administration system. J. Enzyme Inhib. Med. Chem. 2019, 34, 1590–1596. [Google Scholar] [CrossRef]
- Newton, A.; Prabakaran, L.; Jayaveera, K. Pectin-HPMC E15LV vs pH sensitive polymer coating films for delayed drug delivery to colon: A comparison of two dissolution models to asses colonic targeting performance In-Vitro. Int. J. Appl. Res. Nat. Prod. 2012, 5, 1–16. [Google Scholar]
- Philip, A.K.; Philip, B. Colon targeted drug delivery systems: A review on primary and novel approaches. Oman Med. J. 2010, 25, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.; Jain, S. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Pharm. Sci. 2003, 6, 33–66. [Google Scholar] [PubMed]
- Basit, A.; Bloor, J. Perspectives on colonic drug delivery business briefing. Pharm. Technol. 2003, 185–190. [Google Scholar]
- Asghar, L.; Chandran, S. Multiparticulate formulation approach to colon specific drug delivery: Current prospective. J. Pharm. Pharm. Sci. 2006, 9, 327–338. [Google Scholar]
- Friend, R. New oral delivery systems for treatment of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2004, 57, 247–265. [Google Scholar] [CrossRef]
- Arriagada, F.; Ugarte, C.; Günther, G.; Larraín, M.A.; Guarnizo-Herrero, V.; Nonell, S.; Morales, J. Carminic acid linked to silica nanoparticles as pigment/antioxidant bifunctional excipient for pharmaceutical emulsions. Pharmaceutics 2020, 12, 376. [Google Scholar] [CrossRef]
- Marks, S.; Schneider, J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Bak, A.; Ashford, M.; Brayden, D. Local delivery of macromolecules to treat diseases associated with the colon. Adv. Drug Deliv. Rev. 2018, 136–137, 2–27. [Google Scholar] [CrossRef]
- Naeem, M.; Choi, M.; Cao, J.; Lee, Y.; Ikram, M.; Yoon, S.; Lee, J.; Moon, H.; Kim, M.; Jung, Y.; et al. Colon-targeted delivery of budesonide using dual pH-and timedependent polymeric nanoparticles for colitis therapy. Drug Des. Dev. Ther. 2015, 9, 3789–3799. [Google Scholar] [CrossRef]
- Maroni, A.; Moutaharrik, S.; Zema, L.; Gazzaniga, A. Enteric coating for colonic drug delivery: State of the art. Expert Opin. Drug Deliv. 2017, 14, 1027–1029. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.M.; Kaur, V.; Hallan, S.; Sharma, S.; Mishra, N. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis: A brief review. Saudi Pharm. J. 2016, 24, 458–472. [Google Scholar] [CrossRef]
- Naik, J.B.; Waghulde, M.R. Development of vildagliptin loaded Eudragit® microspheres by screening design: In Vitro evaluation. J. Pharm. Investig. 2018, 48, 627–637. [Google Scholar] [CrossRef]
- Hua, S. Orally administered liposomal formulations for colon targeted drug delivery. Front. Pharmacol. 2014, 5, 138. [Google Scholar] [CrossRef]
- Patel, M. Cutting-edge technologies in colon-targeted drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 1247–1258. [Google Scholar] [CrossRef]
- Raish, M.; Kalam, M.A.; Ahmad, A.; Shahid, M.; Ansari, M.A.; Ahad, A.; Ali, R.; Bin Jardan, Y.A.; Alshamsan, A.; Alkholief, M.; et al. Eudragit-coated sporopollenin exine microcapsules (SEMC) of phoenix dactylifera l. of 5-fluorouracil for colon-specific drug delivery. Pharmaceutics 2021, 13, 1921. [Google Scholar] [CrossRef]
- Jain, V.; Singh, R. Development and characterization of Eudragit RS100 loaded microsponges and its colonic delivery using natural polysaccharides. Acta Pol. Pharm. Drug Res. 2010, 67, 407–415. [Google Scholar]
- Chourasia, M.K.; Jain, S.K. Polysaccharides for colon targeted drug delivery. Drug Deliv. 2004, 11, 129–148. [Google Scholar] [CrossRef]
- Maurer, A.H. Gastrointestinal motility, part 2: Small-bowel and colon transit. J. Nucl. Med. Technol. 2016, 44, 12–18. [Google Scholar] [CrossRef]
- Guo, Y.; Zong, S.; Pu, Y.; Xu, B.; Zhang, T.; Wang, B. Advances in pharmaceutical strategies enhancing the efficiencies of oral colon-targeted delivery systems in inflammatory bowel disease. Molecules 2018, 23, 1622. [Google Scholar] [CrossRef] [PubMed]
- Vemula, S.K.; Veerareddy, P.R. Development, evaluation and pharmacokinetics of time-dependent ketorolac tromethamine tablets. Exper. Opin. Drug Deliv. 2013, 10, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jung, H.; Ho, M.; Lee, D.R.; Cho, H.R.; Choi, Y.S.; Jun, J.; Son, M.; Kang, M.J. Colon-targeted delivery of solubilized bisacodyl by doubly enteric-coated multiple-unit tablet. Eur. J. Pharm. Sci. 2017, 102, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Myla Lôbo, M.; de Albuquerque, V.; Pereira, L.; dos Santos, W.; de Moura, L.; Siqueira, G.; dos Santos, L.; Araújo, L.; Rolim Neto, P. A systematic review of functionalized polymeric nanoparticles to improve intestinal permeability of drugs and biological products. Curr. Pharm. Des. 2022, 28, 410–426. [Google Scholar] [CrossRef]
- Patel, M.; Amin, A. Formulation and development of release modulated colon targeted system of meloxicam for potential application in the prophylaxis of colorectal cancer. Drug Deliv. 2011, 18, 281–293. [Google Scholar] [CrossRef]
- Ochi, M.; Inoue, R.; Yamauchi, Y.; Yamada, S.; Onoue, S. Development of meloxicam salts with improved dissolution and pharmacokinetic behaviors in rats with impaired gastric motility. Pharm. Res. 2013, 30, 377–386. [Google Scholar] [CrossRef]
- Tsujii, M. COX-2 inhibitor and colon cancer. Gan Kagaku Ryoho 2001, 28, 1799–1805. [Google Scholar]
- Tsubouchi, Y.; Mukai, S.; Kawahito, Y.; Yamada, R.; Kohno, M.; Inoue, K.; Sano, H. Meloxicam inhibits the growth of non-small cell lung cancer. Anticancer Res. 2000, 20, 2867–2872. [Google Scholar]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.W.; Park, B.J.; Han, H.K. Strategic approaches for colon targeted drug delivery: An overview of recent advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef]
- Palugan, L.; Cerea, M.; Zema, L.; Gazzaniga, A.; Maroni, A. Coated pellets for oral colon delivery. J. Drug Deliv. Sci. Technol. 2015, 25, 1–15. [Google Scholar] [CrossRef]
- Woraphatphadung, T.; Sajomsang, W.; Gonil, P.; Treetong, A.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Praneet, O. pH-responsive polymeric micelles based on amphiphilic chitosan derivates: Effect of hydrophobic cores on oral meloxicam delivery. Int. J. Pharm. 2016, 497, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Álvarez, C.; García, J.; Torrado, S.; Torrado, S.; Torre, P. New multi-particle systems for colon-targeted meloxicam. J. Appl. Pharm. 2016, 8, 221. [Google Scholar] [CrossRef][Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, C.; Wang, S.; Siegel, R.A.; Sun, C. Efficient development of sorafenib tablets with improved oral bioavailability enabled by coprecipitated amorphous solid dispersion. Int. J. Pharm. 2021, 610, 121216. [Google Scholar] [CrossRef]
- Maruyama, S.; Ando, S.; Yonemochi, E. Application of void forming index (VFI): Detection of the effect of physical properties of dry powder inhaler formulations on powder cohesion. Int. J. Pharm. 2020, 588, 119766. [Google Scholar] [CrossRef]
- Li, J.; Lee, I.W.; Shin, G.H.; Chen, X.; Park, H.J. Curcumin-Eudragit® E PO solid dispersion: A simple and potent method to solve the problems of curcumin. Eur. J. Pharm. Biopharm. 2015, 94, 322–332. [Google Scholar] [CrossRef]
- Mašková, E.; Naiserová, M.; Kubová, K.; Mašek, J.; Pavloková, S.; Urbanová, M.; Brus, J.; Vysloužil, J.; Vetchý, D. Highly soluble drugs directly granulated by water dispersions of insoluble Eudragit® polymers as a part of hypromellose k100m matrix systems. BioMed Res. Int. 2019, 5, 8043415. [Google Scholar] [CrossRef]
- Vaz, G.R.; Carrasco, M.C.F.; Batista, M.M.; Barros, P.A.B.; Oliveira, M.d.C.; Muccillo, A.; Yurgel, V.; Buttini, F.; Soare, F.; Cordeiro, L.M.; et al. Curcumin and quercetin-loaded lipid nanocarriers: Development of omega-3 mucoadhesive nanoemulsions for intranasal administration. Nanomaterials 2022, 12, 1073. [Google Scholar] [CrossRef]
- Gómez-Burgaz, M.; Torrado, G.; Torrado, S. Characterization and superficial transformations on mini-matrices made of interpolymer complexes of chitosan and carboxymethylcellulose during In Vitro clarithromycin release. Eur. J. Pharm. Biopharm. 2009, 73, 130–139. [Google Scholar] [CrossRef]
- Lu, J.; Obara, S.; Liu, F.; Fu, W.; Zhang, W.; Kikuchi, S. Melt extrusion for a high melting point compound with improved solubility and sustained release. AAPS PharmSciTech 2018, 19, 358–370. [Google Scholar] [CrossRef]
- Kadota, K.; Terada, H.; Fujimoto, A.; Nogami, S.; Uchiyama, H.; Tozuka, Y. Formulation and evaluation of bitter taste-masked orally disintegrating tablets of high memantine hydrochloride loaded granules coated with polymer via layering technique. Int. J. Pharm. 2021, 604, 120725. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, N.; Begg, N.; Alany, R.G.; Hassanin, H.; Elshaer, A. Oral modified release multiple-unit particulate systems: Compressed pellets, microparticles and nanoparticles. Pharmaceutics 2018, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Weyna, D.; Cheney, M.; Shan, N.; Hanna, M.; Zaworotko, M.J.; Sava, D.; Song, S.; Sanchez-Ramos, J.R. Improving solubility and pharmacokinetics of meloxicam via multiple-component crystal formation. Mol. Pharm. 2012, 7, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Bae, J.; Oshi, M.A.; Kim, M.S.; Moon, H.R.; Lee, B.L.; Jung, Y.; Yoo, J.W. Colon-targeted delivery of cyclosporine A using dual-functional Eudragit® FS30D/PLGA nanoparticles ameliorates murine experimental colitis. Int. J. Nanomed. 2018, 13, 1225–1240. [Google Scholar] [CrossRef]
- Turanli, Y.; Acartürk, F. Fabrication and characterization of budesonide loaded colon-specific nanofiber drug delivery systems using anionic and cationic polymethacrylate polymers. J. Drug Deliv. Sci. Technol. 2021, 63, 102511. [Google Scholar] [CrossRef]
- Consumi, M.; Leone, G.; Pepi, S.; Tamasi, G.; Lamponi, S.; Donati, A.; Magnani, A. Xanthan gum–chitosan: Delayed, prolonged, and burst-release tablets using same components in different ratio. Adv. Polym. Technol. 2018, 37, 2936–2945. [Google Scholar] [CrossRef]
- Zhang, F. Melt-Extruded Eudragit® FS-Based Granules for Colonic Drug Delivery. AAPS PharmSciTech 2016, 17, 56–67. [Google Scholar] [CrossRef]
- Han, H.; Choi, H. Improved absorption of meloxicam via salt formation with ethanolamines. Eur. J. Pharm. Biopharm. 2007, 1, 99–103. [Google Scholar] [CrossRef]
- Muhammad, H.; Ghulam, A.; Shahid, S.; Muhammad, Z.; Akhtar, R.; Abdul, M.; Mehmood, K.S.; Masood, A.M. Raft-forming system for pantoprazole and domperidone delivery: In Vitro and In Vivo study. Bioinspir. Biomim. Nanobiomater. 2020, 9, 137–146. [Google Scholar] [CrossRef]
- Solomon, S.; Iqbal, J.; Albadarin, A.B. Insights into the ameliorating ability of mesoporous silica in modulating drug release in ternary amorphous solid dispersion prepared by hot melt extrusion. Eur. J. Pharm. Biopharm. 2021, 165, 244–258. [Google Scholar] [CrossRef]
- Iurckevicz, G.; Dahmer, D.; Santos, V.; Vetvicka, V.; Barbosa-Dekker, A.; Dekker, R.; Maneck, C.R.; da Cunha, M.A. Encapsulated microparticles of (1→6)-β-d-glucan containing extract of baccharis dracunculifolia: Production and characterization. Molecules 2019, 24, 2099. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Awan, U.A.; Subhan, F.; Cao, J.; Hlaing, S.; Lee, J.; Im, E.; Jung, Y.; Yoo, J. Advances in colon-targeted nano-drug delivery systems: Challenges and solutions. Arch. Pharm. Res. 2020, 43, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Papp, J.; Marton, S.; Süvegh, K.; Zelkó, R. The influence of Metolose structure on the free volume and the consequent metoprolol tartrate release of patches. Int. J. Biol. Macromol. 2009, 44, 6–8. [Google Scholar] [CrossRef]
- Guarnizo, V.; Torrado, C.; Torres, N.S.; Torrado, G.; Morales, J.; Torrado-Santiago, S. Study of different chitosan/sodium carboxymethyl cellulose proportions in the development of polyelectrolyte complexes for the sustained release of clarithromycin from matrix tablets. Polymers 2021, 13, 2813. [Google Scholar] [CrossRef]
- Chu, K.; Lee, E.; Jeong, S.; Park, E. Effect of particle size on the dissolution behaviors of poorly water-soluble drugs. Arch. Pharm. Res. 2012, 7, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Güres, S.; Kleinebudde, P. Dissolution from solid lipid extrudates containing release modifiers. Int. J. Pharm. 2011, 412, 77–84. [Google Scholar] [CrossRef]
- Bartos, C.; Szabó-Révész, P.; Bartos, C.; Katona, G.; Jójárt-Laczkovich, O.; Ambrus, R. The effect of an optimized wet milling technology on the crystallinity, morphology and dissolution properties of micro- and nanonized meloxicam. Molecules 2016, 21, 507. [Google Scholar] [CrossRef]
- Tascon, E.; Torre, P.; Garcia, J.; Pena, M.A.; Alvarez, C. Enhancement of the dissolution rate of indomethacin by solid dispersions in low-substituted hydroxypropyl cellulose. Indian J. Pharm. Sci. 2019, 81, 824–833. [Google Scholar] [CrossRef]
- Benavent, C.; Torrado-Salmerón, C.; Torrado-Santiago, S. Development of a solid dispersion of nystatin with maltodextrin as a carrier agent: Improvements in antifungal efficacy against Candida spp. Biofilm Infections. Pharmaceuticals 2021, 14, 397. [Google Scholar] [CrossRef]
- Mustafa, W.W.; Fletcher, J.; Khoder, M.; Alany, R.G. Solid dispersions of gefitinib prepared by spray drying with improved mucoadhesive and drug dissolution properties. AAPS PharmSciTech 2022, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Chawla, A.; Sharma, P.; Pawar, P. Formulation and in vitro evaluation of Eudragit S-100 coated naproxen matrix tablets for colon-targeted drug delivery system. J. Adv. Pharm. Technol. Res. 2013, 4, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Jacon, J.; Santos, O.; Bonfilio, R.; Doriguetto, A.; de Araújo, M. Analysis of polymorphic contamination in meloxicam raw materials and its effects on the physicochemical quality of drug product. Eur. J. Pharm. Sci. 2017, 109, 347–358. [Google Scholar] [CrossRef]
- Abu Fara, D.; Rashid, I.; Alkhamis, K.; Al-Omari, M.; Chowdhry, B.Z.; Badwan, A. Modification of α-lactose monohydrate as a direct compression excipient using roller compaction. Drug Dev. Ind. Pharm. 2018, 44, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Medarević, D.; Kachrimanis, K.; Djurić, Z.; Ibrić, S. Influence of hydrophilic polymers on the complexation of carbamazepine with hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Sci. 2015, 78, 273–285. [Google Scholar] [CrossRef]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Alami-Milani, M.; Jelvehgari, M. Formulation and evaluation of Eudragit RL-100 nanoparticles loaded in-situ forming gel for intranasal delivery of rivastigmine. Adv. Pharm. Bull. 2020, 10, 20–29. [Google Scholar] [CrossRef]




| Process Parameters | Setting |
|---|---|
| 0.50–1.41 mm granule size | Dissolution rate at pH 6.8, apparatus I. |
| 0.85–1.00 mm granule size | Dissolution rate at pH 1.2, 6.8 and 7.4, apparatus I. |
| 0.35–1.50 mm granule size | Dissolution rate at pH 6.8 and 7.4, apparatus I. |
| Formulations | Korsmeyer-Peppas | Higuchi | First-Order | |||
|---|---|---|---|---|---|---|
| n | r2 | n | r2 | n | r2 | |
| FS | 0.8287 | 0.9799 | 1.8851 | 0.9072 | −0.0009 | 0.9023 |
| NM + FS | 1.2865 | 0.944 | 1.8552 | 0.9966 | −0.0008 | 0.9943 |
| NM | 0.9818 | 0.9767 | 2.2698 | 0.9931 | −0.0012 | 0.9989 |
| CNM + CFS | 0.6754 | 0.9337 | 3.3662 | 0.9906 | −0.0023 | 0.9706 |
| CFS | 0.3425 | 0.8971 | 2.6235 | 0.8756 | −0.0021 | 0.8412 |
| MLX | 0.6073 | 0.9603 | 4.0304 | 0.946 | −0.0037 | 0.935 |
| CNM | 0.8114 | 0.9871 | 5.1589 | 0.9947 | −0.0056 | 0.9995 |
| Formulations | Korsmeyer-Peppas | Higuchi | First-Order | |||
|---|---|---|---|---|---|---|
| n | r2 | n | r2 | n | r2 | |
| CNM + CFS | 1.1911 | 0.9573 | 5.8765 | 0.9985 | −0.0082 | 0.868 |
| CFS | 0.9702 | 0.9871 | 4.2177 | 0.8693 | −0.0097 | 0.9824 |
| MLX | 0.7534 | 0.9464 | 4.1776 | 0.9135 | −0.0058 | 0.9725 |
| CNM | 0.96 | 0.9956 | 5.6754 | 0.9884 | −0.0069 | 0.9843 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Ruíz, E.; Álvarez-Álvarez, C.; Peña, M.Á.; Torrado-Salmerón, C.; Dahma, Z.; de la Torre-Iglesias, P.M. Multiparticulate Systems of Meloxicam for Colonic Administration in Cancer or Autoimmune Diseases. Pharmaceutics 2022, 14, 1504. https://doi.org/10.3390/pharmaceutics14071504
Navarro-Ruíz E, Álvarez-Álvarez C, Peña MÁ, Torrado-Salmerón C, Dahma Z, de la Torre-Iglesias PM. Multiparticulate Systems of Meloxicam for Colonic Administration in Cancer or Autoimmune Diseases. Pharmaceutics. 2022; 14(7):1504. https://doi.org/10.3390/pharmaceutics14071504
Chicago/Turabian StyleNavarro-Ruíz, Eva, Covadonga Álvarez-Álvarez, M Ángeles Peña, Carlos Torrado-Salmerón, Zaid Dahma, and Paloma Marina de la Torre-Iglesias. 2022. "Multiparticulate Systems of Meloxicam for Colonic Administration in Cancer or Autoimmune Diseases" Pharmaceutics 14, no. 7: 1504. https://doi.org/10.3390/pharmaceutics14071504
APA StyleNavarro-Ruíz, E., Álvarez-Álvarez, C., Peña, M. Á., Torrado-Salmerón, C., Dahma, Z., & de la Torre-Iglesias, P. M. (2022). Multiparticulate Systems of Meloxicam for Colonic Administration in Cancer or Autoimmune Diseases. Pharmaceutics, 14(7), 1504. https://doi.org/10.3390/pharmaceutics14071504









