Short-Interval, Low-Dose Peptide Receptor Radionuclide Therapy in Combination with PD-1 Checkpoint Immunotherapy Induces Remission in Immunocompromised Patients with Metastatic Merkel Cell Carcinoma
Abstract
:1. Introduction
2. Material and Methods
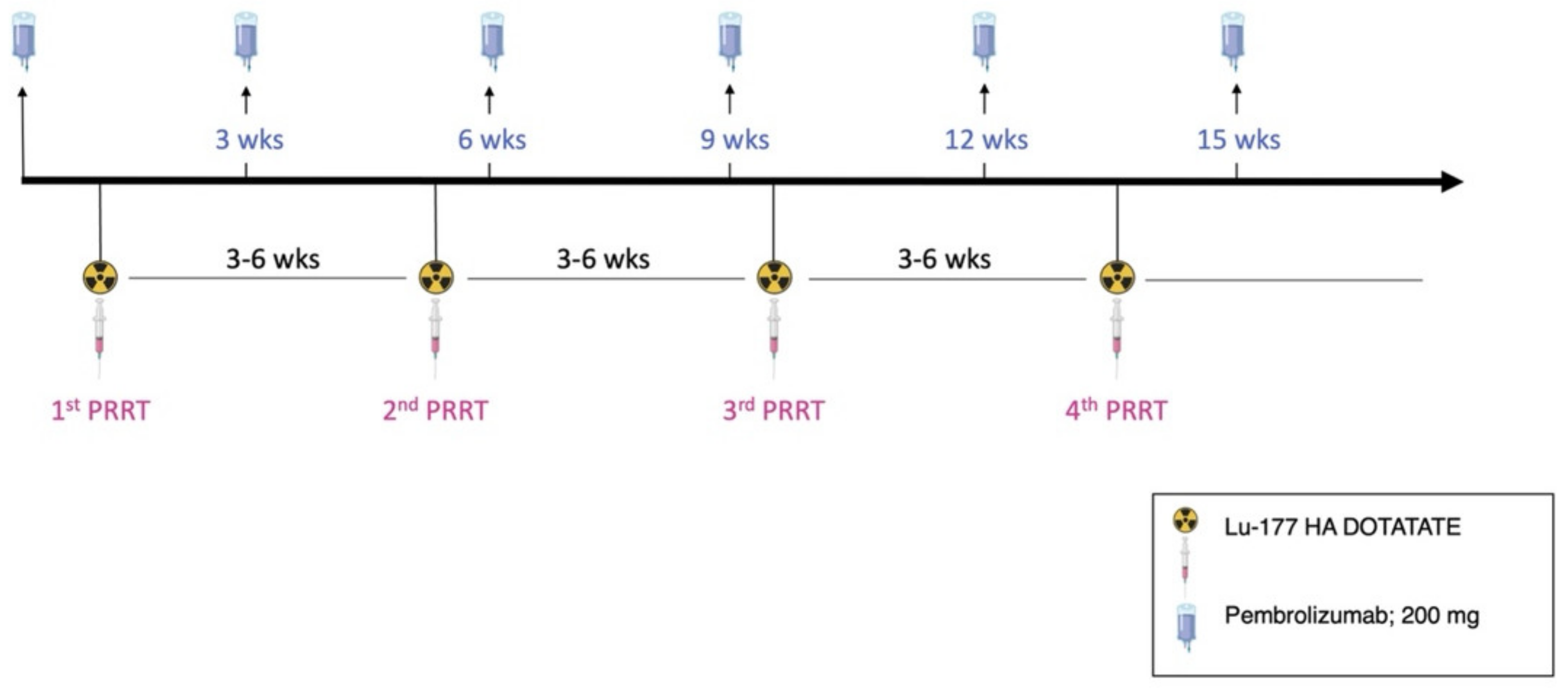
2.1. Radiopharmaceutical Preparation, PRRT and Imaging
2.2. Ethical Approval
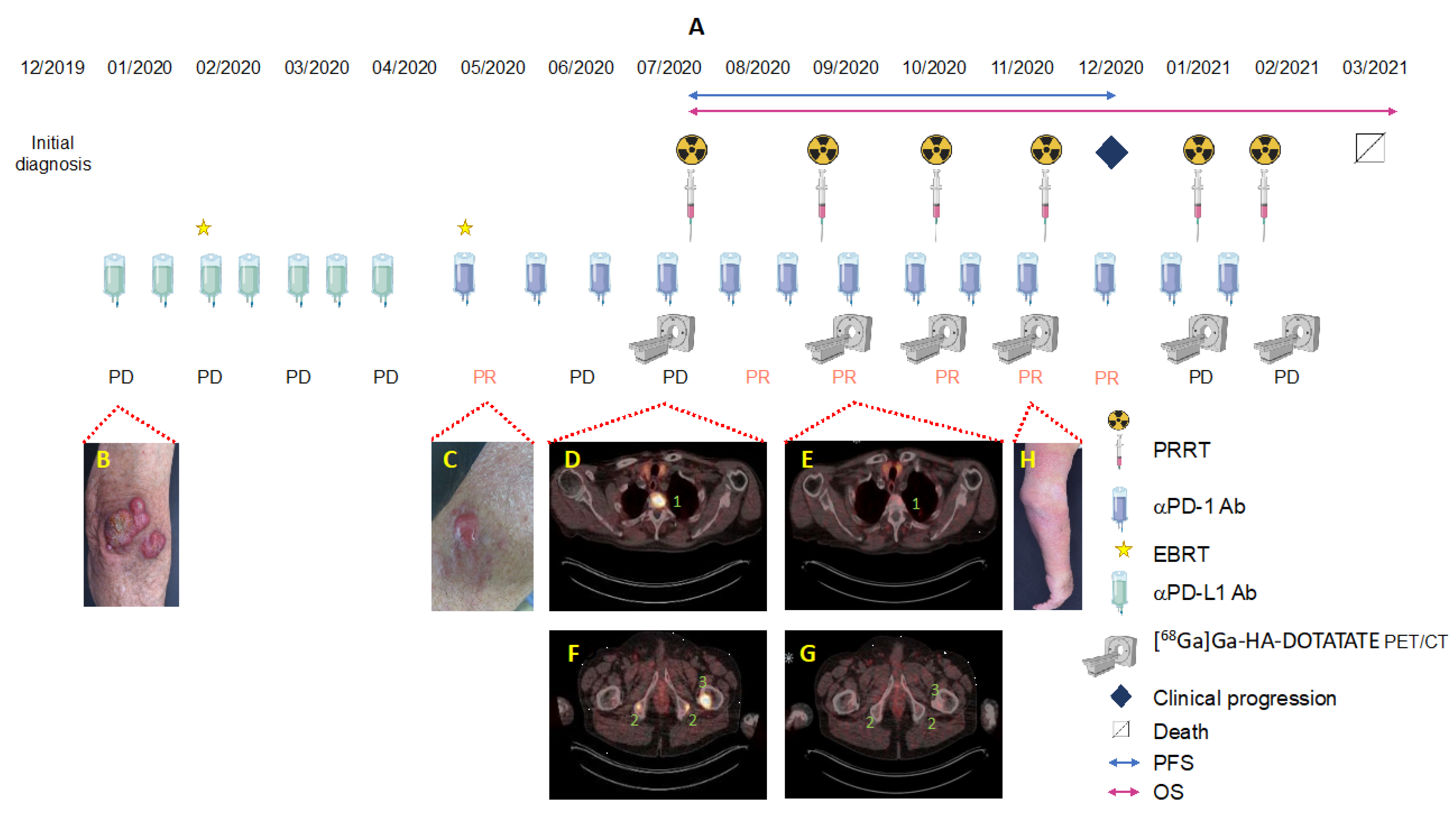
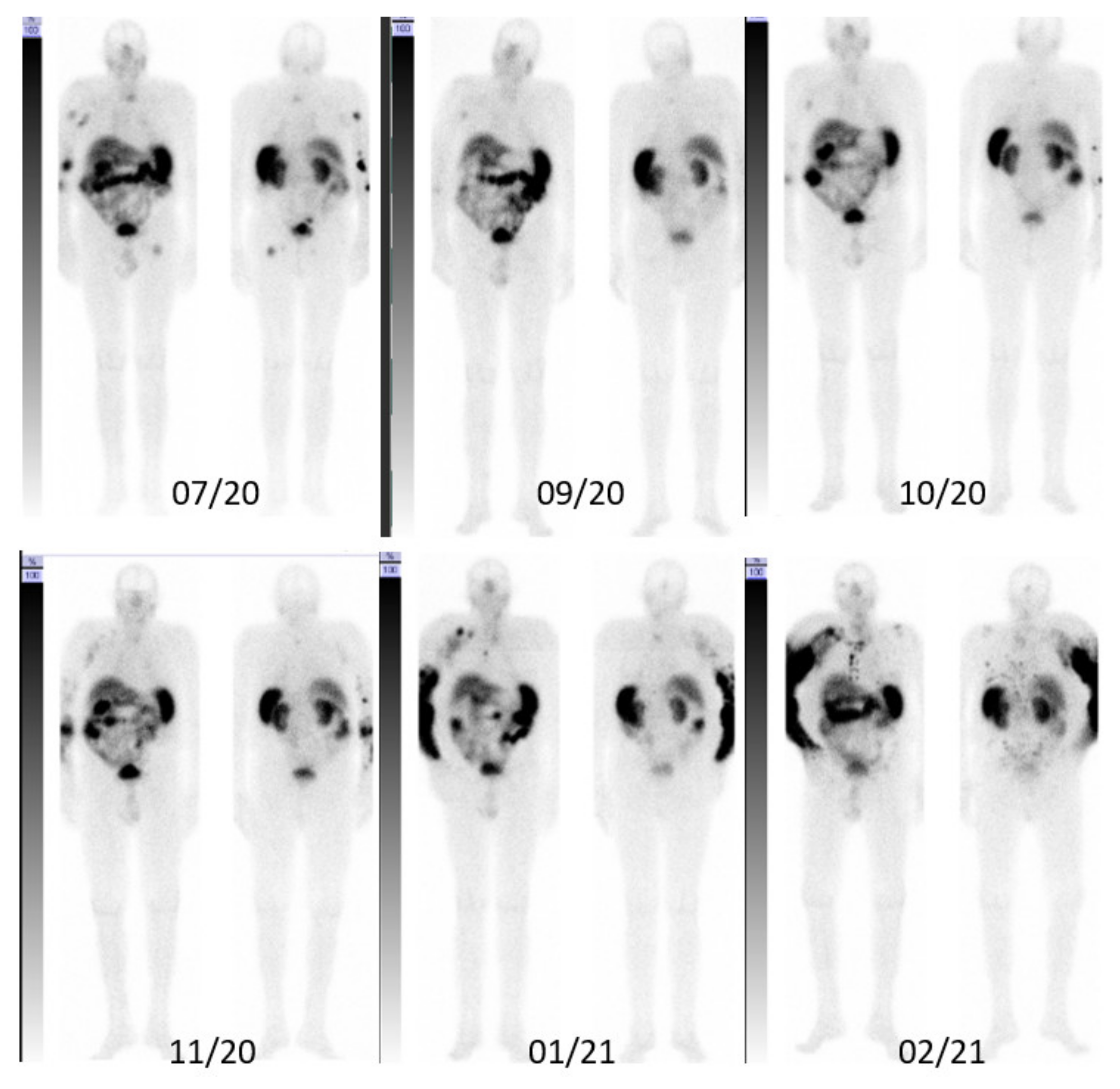
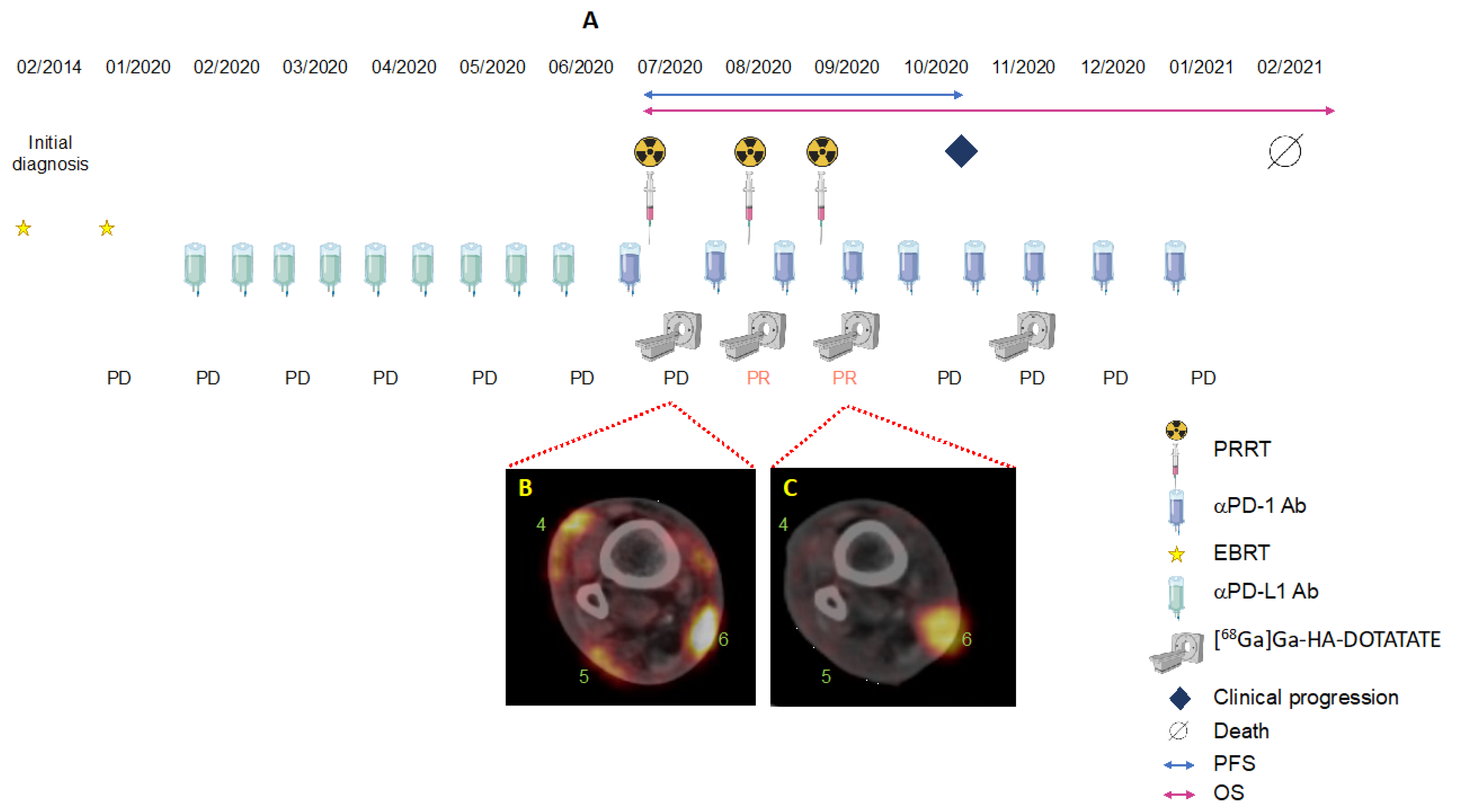
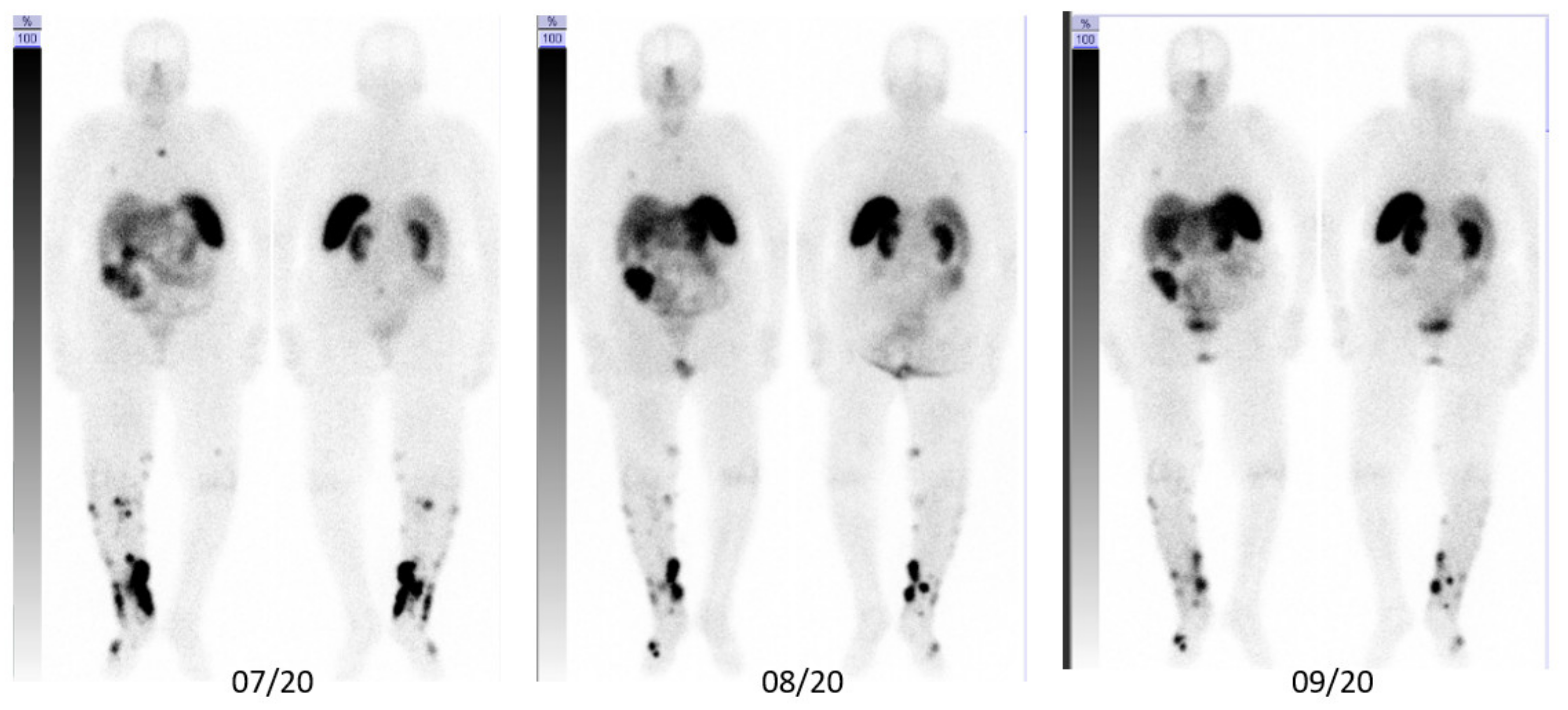
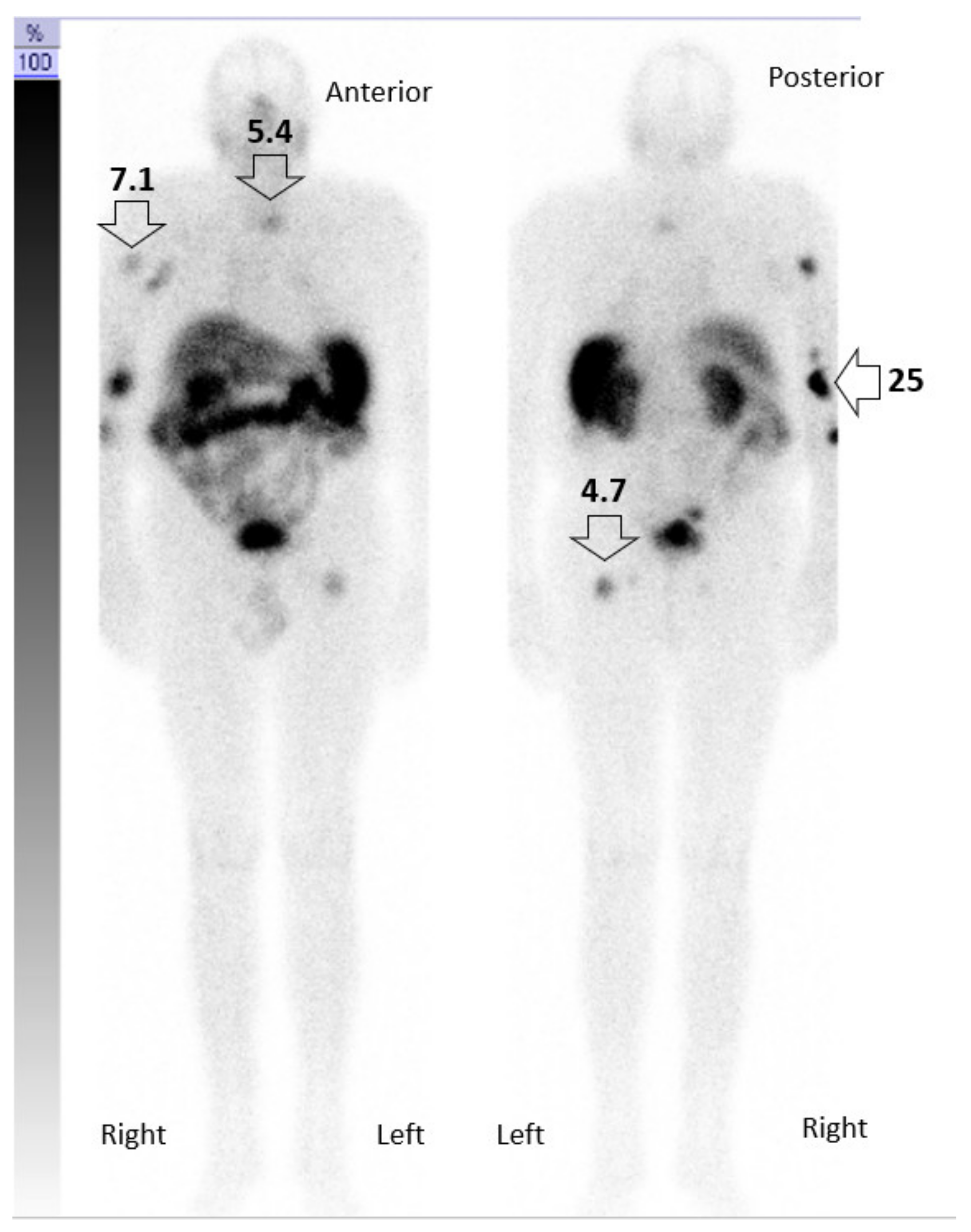
3. Case Description
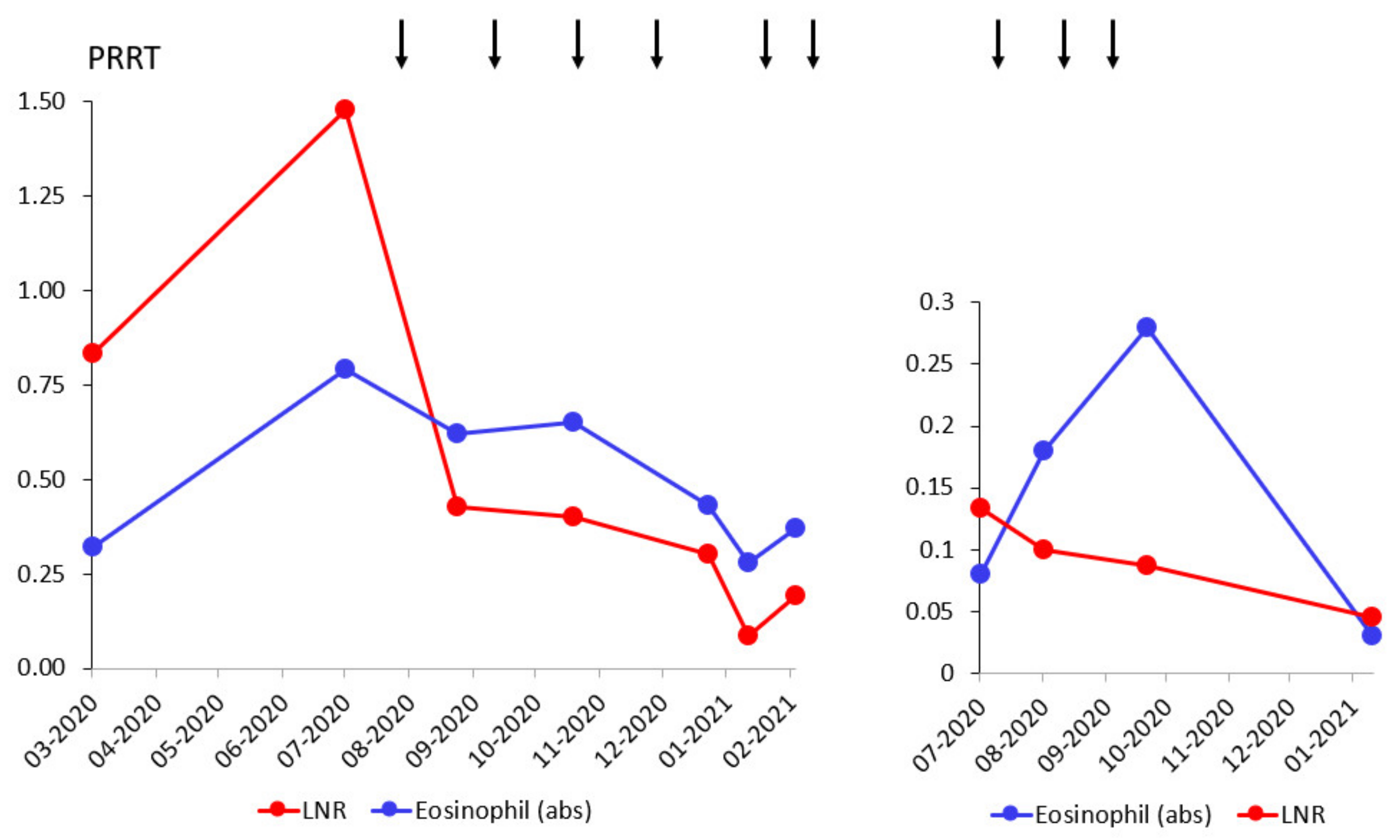
3.1. Integral Dose Assessment
3.2. Toxicity Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schadendorf, D.; Lebbe, C.; Zur Hausen, A.; Avril, M.F.; Hariharan, S.; Bharmal, M.; Becker, J.C. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur. J. Cancer 2017, 71, 53–69. [Google Scholar] [CrossRef] [Green Version]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Penas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463.e452. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Harms, P.W.; Vats, P.; Verhaegen, M.E.; Robinson, D.R.; Wu, Y.M.; Dhanasekaran, S.M.; Palanisamy, N.; Siddiqui, J.; Cao, X.; Su, F.; et al. The Distinctive Mutational Spectra of Polyomavirus-Negative Merkel Cell Carcinoma. Cancer Res. 2015, 75, 3720–3727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Angelo, S.P.; Bhatia, S.; Brohl, A.S.; Hamid, O.; Mehnert, J.M.; Terheyden, P.; Shih, K.C.; Brownell, I.; Lebbe, C.; Lewis, K.D.; et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: Long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J. Immunother. Cancer 2020, 8, e000674. [Google Scholar] [CrossRef] [PubMed]
- Vandeven, N.A.; Nghiem, P. Merkel Cell Carcinoma: An Unusually Immunogenic Cancer Proves Ripe for Immune Therapy. J. Oncol. Pract. 2016, 12, 649–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, S.; Storer, B.E.; Iyer, J.G.; Moshiri, A.; Parvathaneni, U.; Byrd, D.; Sober, A.J.; Sondak, V.K.; Gershenwald, J.E.; Nghiem, P. Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases from the National Cancer Data Base. J. Natl. Cancer Inst. 2016, 108, djw042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastrelli, M.; Del Fiore, P.; Russo, I.; Tartaglia, J.; Dal Monico, A.; Cappellesso, R.; Nicole, L.; Piccin, L.; Fabozzi, A.; Biffoli, B.; et al. Merkel Cell Carcinoma: Evaluation of the Clinico-Pathological Characteristics, Treatment Strategies and Prognostic Factors in a Monocentric Retrospective Series (n = 143). Front. Oncol. 2021, 11, 737842. [Google Scholar] [CrossRef]
- Xu, M.J.; Wu, S.; Daud, A.I.; Yu, S.S.; Yom, S.S. In-field and abscopal response after short-course radiation therapy in patients with metastatic Merkel cell carcinoma progressing on PD-1 checkpoint blockade: A case series. J. Immunother. Cancer 2018, 6, 43. [Google Scholar] [CrossRef]
- Bloom, B.C.; Augustyn, A.; Pezzi, T.A.; Menon, H.; Mayo, L.L.; Shah, S.J.; Schwartz, D.L.; Chmura, S.J.; Johnson, F.M.; Welsh, J.W.; et al. Rescue of Immunotherapy-Refractory Metastatic Merkel Cell Carcinoma With Conventionally Fractionated Radiotherapy and Concurrent Pembrolizumab. Front. Oncol. 2019, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Keam, S.; Gill, S.; Ebert, M.A.; Nowak, A.K.; Cook, A.M. Enhancing the efficacy of immunotherapy using radiotherapy. Clin. Transl. Immunol. 2020, 9, e1169. [Google Scholar] [CrossRef]
- Deroose, C.M.; Hindie, E.; Kebebew, E.; Goichot, B.; Pacak, K.; Taieb, D.; Imperiale, A. Molecular Imaging of Gastroenteropancreatic Neuroendocrine Tumors: Current Status and Future Directions. J. Nucl. Med. 2016, 57, 1949–1956. [Google Scholar] [CrossRef] [Green Version]
- Akaike, T.; Qazi, J.; Anderson, A.; Behnia, F.S.; Shinohara, M.M.; Akaike, G.; Hippe, D.S.; Thomas, H.; Takagishi, S.R.; Lachance, K.; et al. High somatostatin receptor expression and efficacy of somatostatin analogues in patients with metastatic Merkel cell carcinoma. Br. J. Dermatol. 2021, 184, 319–327. [Google Scholar] [CrossRef]
- Kasi, P.M.; Sharma, A.; Jain, M.K. Expanding the Indication for Novel Theranostic 177Lu-Dotatate Peptide Receptor Radionuclide Therapy: Proof-of-Concept of PRRT in Merkel Cell Cancer. Case Rep. Oncol. 2019, 12, 98–103. [Google Scholar] [CrossRef]
- Hicks, R.J.; Kwekkeboom, D.J.; Krenning, E.; Bodei, L.; Grozinsky-Glasberg, S.; Arnold, R.; Borbath, I.; Cwikla, J.; Toumpanakis, C.; Kaltsas, G.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology 2017, 105, 295–309. [Google Scholar] [CrossRef]
- Schreiter, V.; Steffen, I.; Huebner, H.; Bredow, J.; Heimann, U.; Kroencke, T.J.; Poellinger, A.; Doellinger, F.; Buchert, R.; Hamm, B.; et al. Ventilation/perfusion SPECT/CT in patients with pulmonary emphysema. Evaluation of software-based analysing. Nuklearmedizin 2015, 54, 31–35. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Horsch, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Vonken, E.P.A.; Bruijnen, R.C.G.; Snijders, T.J.; Seute, T.; Lam, M.; Keizer, B.; Braat, A. Intraarterial Administration Boosts (177)Lu-HA-DOTATATE Accumulation in Salvage Meningioma Patients. J. Nucl. Med. 2022, 63, 406–409. [Google Scholar] [CrossRef]
- Moreira, A.; Leisgang, W.; Schuler, G.; Heinzerling, L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy 2017, 9, 115–121. [Google Scholar] [CrossRef]
- Kitagawa, S.; Hakozaki, T.; Kitadai, R.; Hosomi, Y. Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: Case series and literature review. Thorac. Cancer 2020, 11, 1927–1933. [Google Scholar] [CrossRef]
- Spassova, I.; Ugurel, S.; Kubat, L.; Zimmer, L.; Terheyden, P.; Mohr, A.; Bjorn Andtback, H.; Villabona, L.; Leiter, U.; Eigentler, T.; et al. Clinical and molecular characteristics associated with response to therapeutic PD-1/PD-L1 inhibition in advanced Merkel cell carcinoma. J. Immunother. Cancer 2022, 10, e003198. [Google Scholar] [CrossRef]
- Glutsch, V.; Kneitz, H.; Gesierich, A.; Goebeler, M.; Haferkamp, S.; Becker, J.C.; Ugurel, S.; Schilling, B. Activity of ipilimumab plus nivolumab in avelumab-refractory Merkel cell carcinoma. Cancer Immunol. Immunother. 2021, 70, 2087–2093. [Google Scholar] [CrossRef]
- LoPiccolo, J.; Schollenberger, M.D.; Dakhil, S.; Rosner, S.; Ali, O.; Sharfman, W.H.; Silk, A.W.; Bhatia, S.; Lipson, E.J. Rescue therapy for patients with anti-PD-1-refractory Merkel cell carcinoma: A multicenter, retrospective case series. J. Immunother. Cancer 2019, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Ferdinandus, J.; Fendler, W.P.; Lueckerath, K.; Berliner, C.; Kurzidem, S.; Hadaschik, E.; Klode, J.; Zimmer, L.; Livingstone, E.; Schadendorf, D.; et al. Response to combined peptide receptor radionuclide therapy and checkpoint immunotherapy with ipilimumab plus nivolumab in metastatic Merkel cell carcinoma. J. Nucl. Med. 2022, 63, 396–398. [Google Scholar] [CrossRef]
- Minczeles, N.S.; de Herder, W.W.; Feelders, R.A.; Verburg, F.A.; Hofland, J.; Brabander, T. Long-term outcomes of submaximal activities of peptide receptor radionuclide therapy with (177)Lu-DOTATATE in neuroendocrine tumour patients. J. Nucl. Med. 2022, 63. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.X.; Auh, S.L. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011, 71, 2488–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Rohl, I.; Hopfner, K.P.; Ludwig, J.; Hornung, V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.B.; Hernandez, R.; Carlson, P.; Grudzinski, J.; Bates, A.M.; Jagodinsky, J.C.; Erbe, A.; Marsh, I.R.; Arthur, I.; Aluicio-Sarduy, E.; et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci. Transl. Med. 2021, 13, eabb3631. [Google Scholar] [CrossRef]
- Guida, M.; D’Alo, A.; Mangia, A.; Di Pinto, F.; Sonnessa, M.; Albano, A.; Sciacovelli, A.; Asabella, A.N.; Fucci, L. Somatostatin Receptors in Merkel-Cell Carcinoma: A Therapeutic Opportunity Using Somatostatin Analog Alone or in Association With Checkpoint Inhibitors Immunotherapy. A Case Report. Front. Oncol. 2020, 10, 1073. [Google Scholar] [CrossRef]
- Krug, S.; Mordhorst, J.P.; Moser, F.; Theuerkorn, K.; Ruffert, C.; Egidi, M.; Rinke, A.; Gress, T.M.; Michl, P. Interaction between somatostatin analogues and targeted therapies in neuroendocrine tumor cells. PLoS ONE 2019, 14, e0218953. [Google Scholar] [CrossRef]
- Savovic, T.; Prior, J.O.; Nicod-Lalonde, M.; Bressoud, A.; Roux, S.; Schaefer, N.; Meyer, M. First experience of durable cytoreduction in chronic lymphoid leukemia with (177)Lu-DOTATATE. Med. Oncol. 2019, 36, 41. [Google Scholar] [CrossRef]
- Franzin, R.; Netti, G.S.; Spadaccino, F.; Porta, C.; Gesualdo, L.; Stallone, G.; Castellano, G.; Ranieri, E. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Front. Immunol. 2020, 11, 574271. [Google Scholar] [CrossRef]
- Salavati, A.; Prasad, V.; Schneider, C.P.; Herbst, R.; Baum, R.P. Peptide receptor radionuclide therapy of Merkel cell carcinoma using (177)lutetium-labeled somatostatin analogs in combination with radiosensitizing chemotherapy: A potential novel treatment based on molecular pathology. Ann. Nucl. Med. 2012, 26, 365–369. [Google Scholar] [CrossRef]
- Basu, S.; Ranade, R. Favorable Response of Metastatic Merkel Cell Carcinoma to Targeted 177Lu-DOTATATE Therapy: Will PRRT Evolve to Become an Important Approach in Receptor-Positive Cases? J. Nucl. Med. Technol. 2016, 44, 85–87. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aicher, A.; Sindrilaru, A.; Crisan, D.; Thaiss, W.; Steinacker, J.; Beer, M.; Wiegel, T.; Scharffetter-Kochanek, K.; Beer, A.J.; Prasad, V. Short-Interval, Low-Dose Peptide Receptor Radionuclide Therapy in Combination with PD-1 Checkpoint Immunotherapy Induces Remission in Immunocompromised Patients with Metastatic Merkel Cell Carcinoma. Pharmaceutics 2022, 14, 1466. https://doi.org/10.3390/pharmaceutics14071466
Aicher A, Sindrilaru A, Crisan D, Thaiss W, Steinacker J, Beer M, Wiegel T, Scharffetter-Kochanek K, Beer AJ, Prasad V. Short-Interval, Low-Dose Peptide Receptor Radionuclide Therapy in Combination with PD-1 Checkpoint Immunotherapy Induces Remission in Immunocompromised Patients with Metastatic Merkel Cell Carcinoma. Pharmaceutics. 2022; 14(7):1466. https://doi.org/10.3390/pharmaceutics14071466
Chicago/Turabian StyleAicher, Alexandra, Anca Sindrilaru, Diana Crisan, Wolfgang Thaiss, Jochen Steinacker, Meinrad Beer, Thomas Wiegel, Karin Scharffetter-Kochanek, Ambros J. Beer, and Vikas Prasad. 2022. "Short-Interval, Low-Dose Peptide Receptor Radionuclide Therapy in Combination with PD-1 Checkpoint Immunotherapy Induces Remission in Immunocompromised Patients with Metastatic Merkel Cell Carcinoma" Pharmaceutics 14, no. 7: 1466. https://doi.org/10.3390/pharmaceutics14071466
APA StyleAicher, A., Sindrilaru, A., Crisan, D., Thaiss, W., Steinacker, J., Beer, M., Wiegel, T., Scharffetter-Kochanek, K., Beer, A. J., & Prasad, V. (2022). Short-Interval, Low-Dose Peptide Receptor Radionuclide Therapy in Combination with PD-1 Checkpoint Immunotherapy Induces Remission in Immunocompromised Patients with Metastatic Merkel Cell Carcinoma. Pharmaceutics, 14(7), 1466. https://doi.org/10.3390/pharmaceutics14071466







