Formulation-by-Design of Efinaconazole Spanlastic Nanovesicles for Transungual Delivery Using Statistical Risk Management and Multivariate Analytical Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spanlastic Nanovesicles Loaded with Efinaconazole
2.3. Characterization of Efinaconazole Spanlastics
2.3.1. Particle Size and Zeta Potential Measurement
2.3.2. Relative Deformability and Transmittance
2.3.3. Estimation of Efinaconazole Content
2.3.4. Dissolution Efficiency of Efinaconazole-Loaded Spanlastics
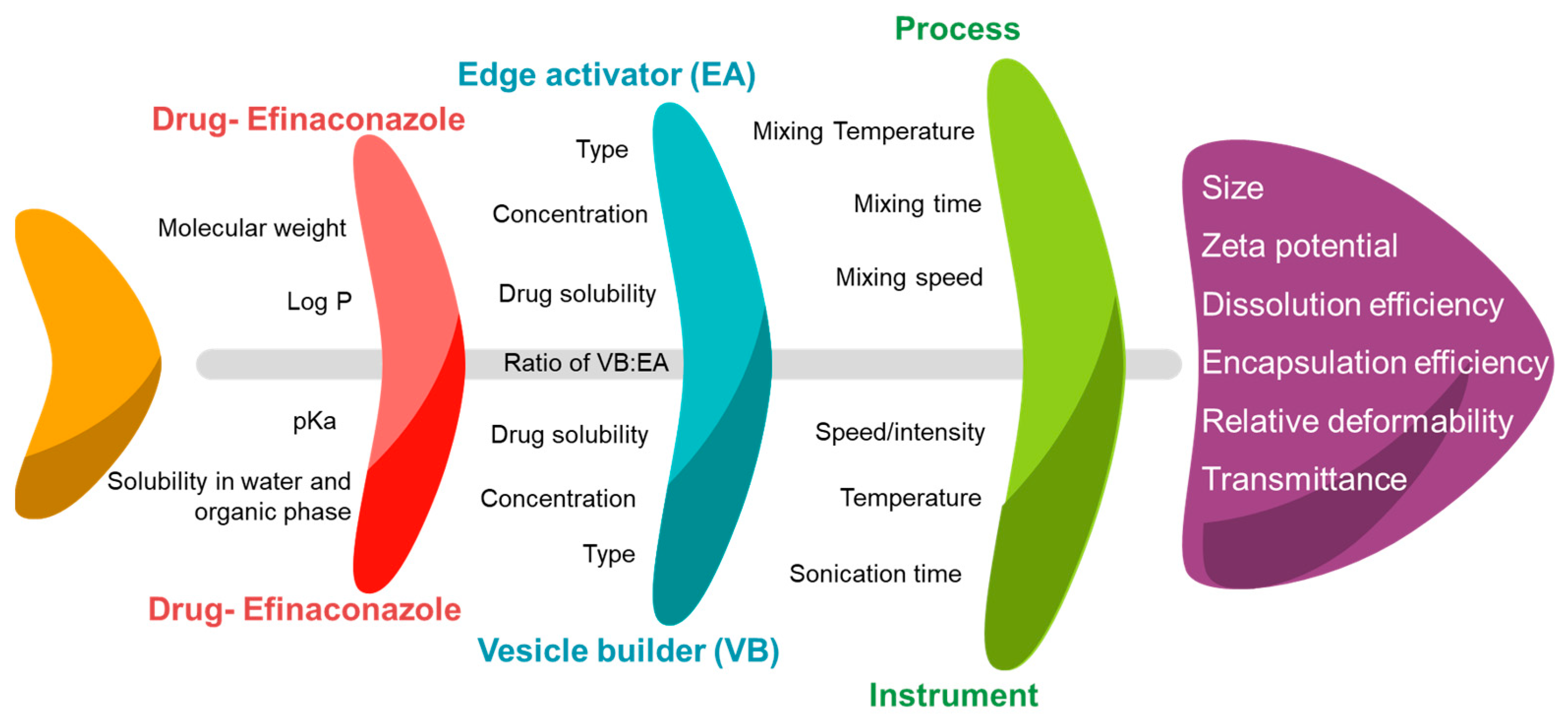
2.4. Identification of Cause–Effect Relationship Using Ishikawa Fishbone Diagram
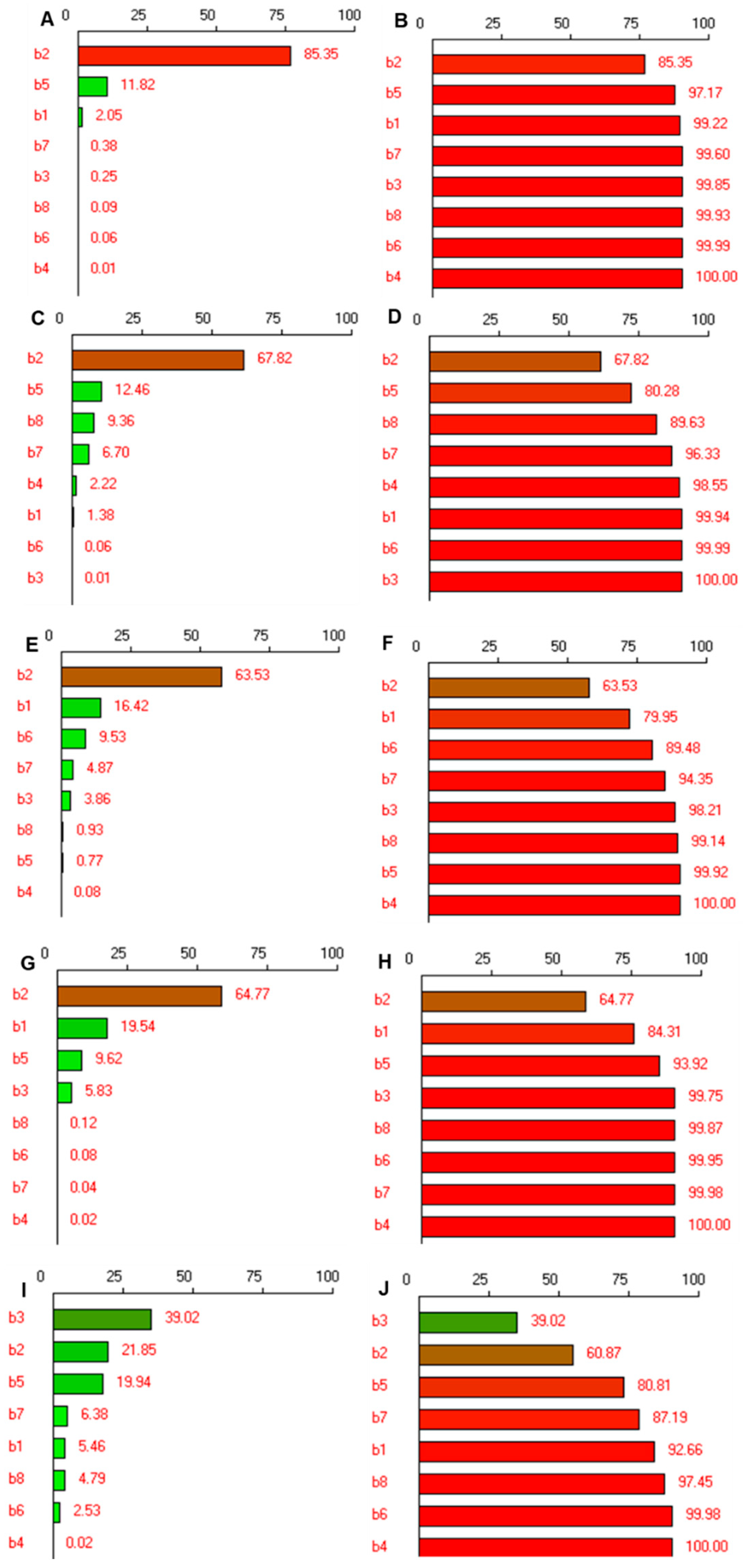
2.5. Screening of Risk Assessment of Several Variables
2.6. Optimization of Efinaconazole Spanlastics Using Full Factorial Design
2.7. Validation of Experimental Design and Optimization of Formulation
2.8. Efinaconazole Spanlastic Optimized Formulation and Converting into Gel
2.8.1. Topography Using Electron Microscopy
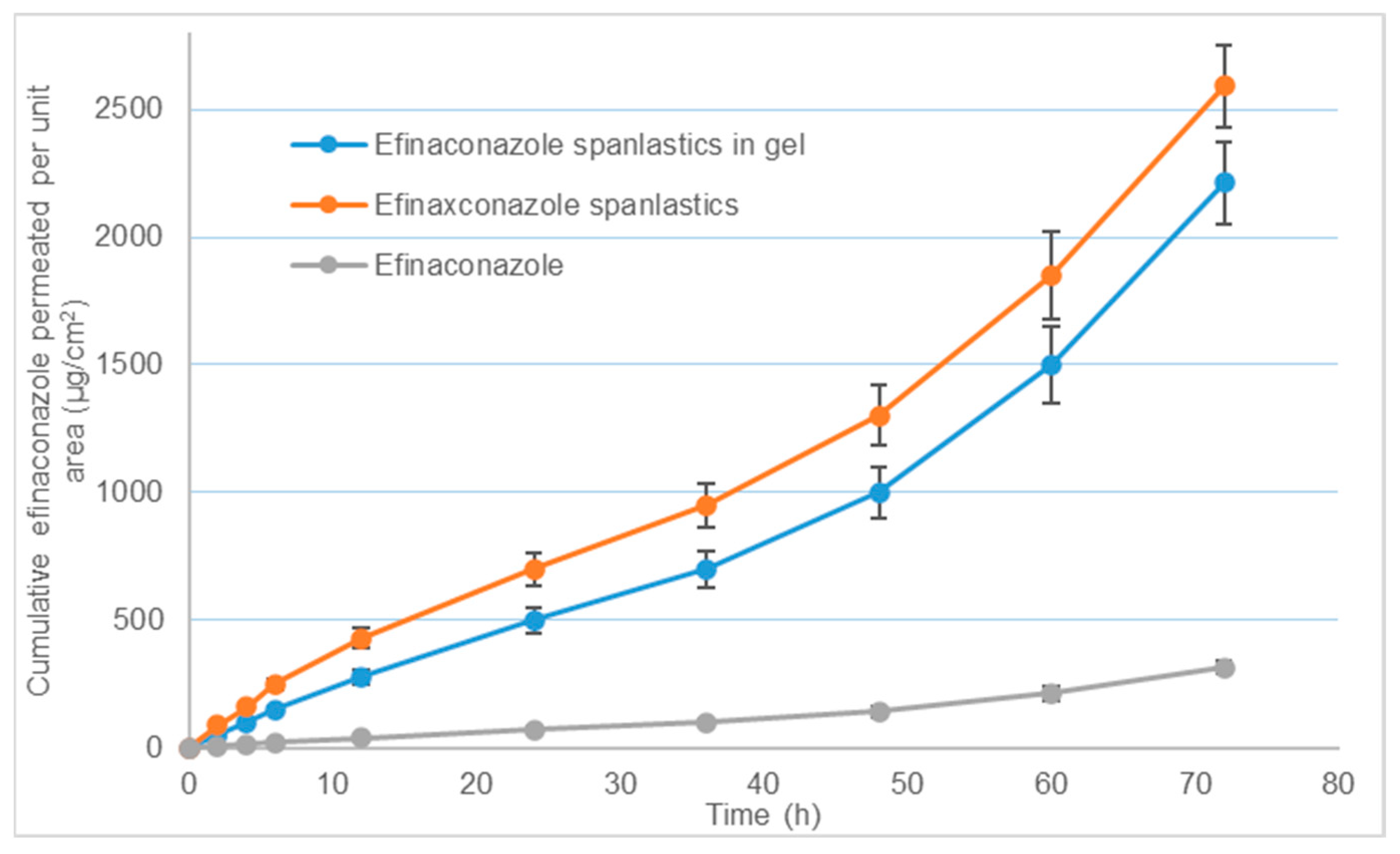
2.8.2. Ex Vivo Permeation through Bovine Hoof Membranes
3. Results and Discussion
3.1. Cause–Effect Relationship for Efinaconazole-Loaded Spanlastics Using Ishikawa Fishbone Diagram
3.2. Risk Assessment Screening Using Pareto Charts
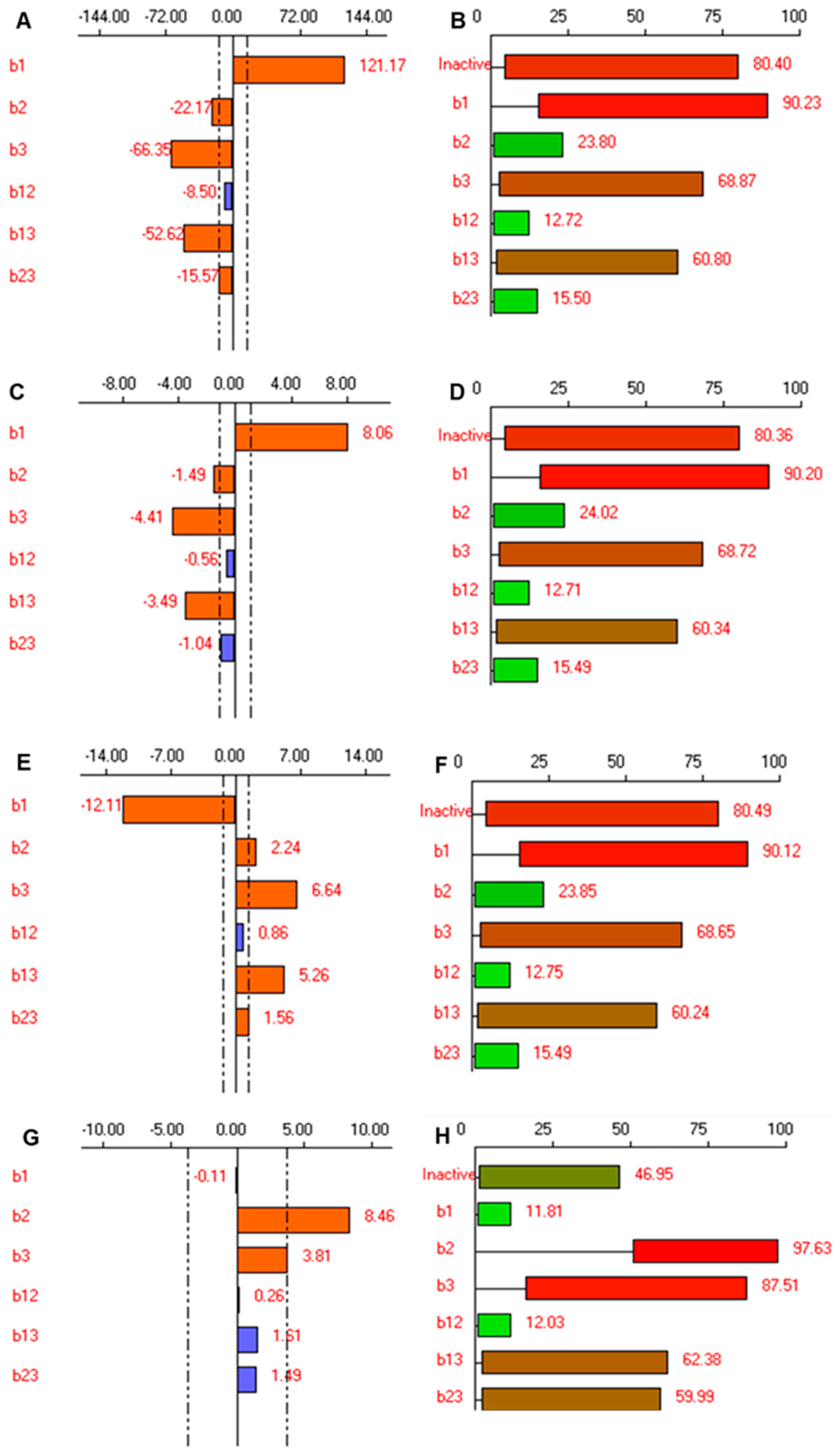
3.3. Full Factorial Experimental Design (23, 3 Factors at Two Levels)
3.3.1. Effect on the Particle Size of Spanlastics
3.3.2. Effect on Relative Deformability of Spanlastics
3.3.3. Effect on Transmittance
3.3.4. Effect on Dissolution Efficiency
3.4. Optimization and Validation of the Developed Mathematical Model
3.5. Evaluation of Efinaconazole Spanlastic Gel
3.5.1. Topographic Imaging Using SEM
3.5.2. Ex Vivo Permeation through Bovine Hoof Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Gorain, B.; Jacob, S.; Attimarad, M.; Sreeharsha, N.; Venugopala, K.N.; Morsy, M.A. Constant Voltage Iontophoresis Technique to Deliver Terbinafine via Transungual Delivery System: Formulation Optimization Using Box–Behnken Design and In Vitro Evaluation. Pharmaceutics 2021, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.; Khanna, D. Onychomycosis: Diagnosis and management. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Vaka, S.R.; Sammeta, S.M.; Kim, H.D.; Friden, P.M.; Chakraborty, B.; Murthy, S.N. Trans-ungual iontophoretic delivery of terbinafine. J. Pharm. Sci. 2009, 98, 1788–1796. [Google Scholar] [CrossRef]
- Nair, A.B.; Kim, H.D.; Chakraborty, B.; Singh, J.; Zaman, M.; Gupta, A.; Friden, P.M.; Murthy, S.N. Ungual and trans-ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis. J. Pharm. Sci. 2009, 98, 4130–4140. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Kim, H.D.; Davis, S.P.; Etheredge, R.; Barsness, M.; Friden, P.M.; Murthy, S.N. An ex vivo toe model used to assess applicators for the iontophoretic ungual delivery of terbinafine. Pharm. Res. 2009, 26, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, H.N.; Juluri, A.; Desai, B.G.; Murthy, S.N. Ungual and Transungual drug delivery. Drug Dev. Ind. Pharm. 2012, 38, 901–911. [Google Scholar] [CrossRef]
- Murthy, S.N.; Vaka, S.R.; Sammeta, S.M.; Nair, A.B. TranScreen-N: Method for rapid screening of trans-ungual drug delivery enhancers. J. Pharm. Sci. 2009, 98, 4264–4271. [Google Scholar] [CrossRef]
- Nair, A.B.; Vaka, S.R.; Murthy, S.N. Transungual delivery of terbinafine by iontophoresis in onychomycotic nails. Drug Dev. Ind. Pharm. 2011, 37, 1253–1258. [Google Scholar] [CrossRef]
- Nair, A.B.; Sammeta, S.M.; Kim, H.D.; Chakraborty, B.; Friden, P.M.; Murthy, S.N. Alteration of the diffusional barrier property of the nail leads to greater terbinafine drug loading and permeation. Int. J. Pharm. 2009, 375, 22–27. [Google Scholar] [CrossRef]
- Lipner, S.R.; Scher, R.K. Efinaconazole in the treatment of onychomycosis. Infect. Drug Resist. 2015, 8, 163–172. [Google Scholar] [CrossRef]
- Nair, A.B.; Sammeta, S.M.; Vaka, S.R.; Narasimha Murthy, S. A study on the effect of inorganic salts in transungual drug delivery of terbinafine. J. Pharm. Pharmacol. 2009, 61, 431–437. [Google Scholar] [CrossRef] [PubMed]
- El-sherif, N.I.; Shamma, R.N.; Abdelbary, G. In-situ gels and nail lacquers as potential delivery systems for treatment of onychomycosis. A comparative study. J. Drug Deliv. Sci. Technol. 2018, 43, 253–261. [Google Scholar] [CrossRef]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine Hydrochloride Trans-ungual Delivery via Nanovesicular Systems: In Vitro Characterization and Ex Vivo Evaluation. AAPS PharmSciTech 2017, 18, 551–562. [Google Scholar] [CrossRef]
- Nair, A.B.; Chakraborty, B.; Murthy, S.N. Effect of polyethylene glycols on the trans-ungual delivery of terbinafine. Curr. Drug Deliv. 2010, 7, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Pund, S.; Shete, Y.; Jagadale, S. Multivariate analysis of physicochemical characteristics of lipid based nanoemulsifying cilostazol—Quality by design. Colloids Surf. B Biointerfaces 2014, 115, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mazyed, E.A.; Helal, D.A.; Elkhoudary, M.M.; Abd Elhameed, A.G.; Yasser, M. Formulation and Optimization of Nanospanlastics for Improving the Bioavailability of Green Tea Epigallocatechin Gallate. Pharmaceuticals 2021, 14, 68. [Google Scholar] [CrossRef]
- Dhat, S.; Pund, S.; Kokare, C.; Sharma, P.; Shrivastava, B. Risk management and statistical multivariate analysis approach for design and optimization of satranidazole nanoparticles. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 96, 273–283. [Google Scholar] [CrossRef]
- Sreeharsha, N.; Narayan, R.; Vijaya, J.; Nair, A. Proof-of-concept for site-specific delivery of mesalamine nanoparticles for effective therapy in colitis. Indian J. Pharm. Educ. Res. 2021, 55, S448–S456. [Google Scholar] [CrossRef]
- Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and optimization of naringenin-loaded chitosan-coated nanoemulsion for topical therapy in wound healing. Pharmaceutics 2020, 12, 893. [Google Scholar] [CrossRef]
- Van den Bergh, B.A.; Wertz, P.W.; Junginger, H.E.; Bouwstra, J.A. Elasticity of vesicles assessed by electron spin resonance, electron microscopy and extrusion measurements. Int. J. Pharm. 2001, 217, 13–24. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Vikas, A.; Rashmin, P.; Mrunali, P.; Sandip, M.; Kaushik, T. RP-HPLC method for quantitative estimation of Efinaconazole in topical microemulsion and microemulsion-based-gel formulations and in presence of its degradation products. Microchem. J. 2020, 155, 104753. [Google Scholar] [CrossRef]
- Agrawal, V.; Patel, R.; Patel, M.; Thanki, K.; Mishra, S. Design and evaluation of microemulsion-based efinaconazole formulations for targeted treatment of onychomycosis through transungual route: Ex vivo and nail clipping studies. Colloids Surf. B: Biointerfaces 2021, 201, 111652. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Binmahfouz, L.S.; Bakhaidar, R.B.; Sreeharsha, N.; Nair, A.B.; Ramnarayanan, C. Lung Targeted Lipopolymeric Microspheres of Dexamethasone for the Treatment of ARDS. Pharmaceutics 2021, 13, 1347. [Google Scholar] [CrossRef]
- Sreeharsha, N.; Rajpoot, K.; Tekade, M.; Kalyane, D.; Nair, A.B.; Venugopala, K.N.; Tekade, R.K. Development of Metronidazole Loaded Chitosan Nanoparticles Using QbD Approach—A Novel and Potential Antibacterial Formulation. Pharmaceutics 2020, 12, 920. [Google Scholar] [CrossRef]
- Deokar, G.S.; Kakulte, H.D.; Kshirsagar, S.J.; Waghchoure, D.R. QBD Approach to Predict the in-vivo Performance Based on in-vitro Results using Mucuna pruriens Seed Mucilage as a Novel Tablet Dosage Form Excipient and Dicofenac Sodium as Model drug Candidate. Indian J. Pharm. Educ. Res. 2021, 55, 715–727. [Google Scholar] [CrossRef]
- Badhwar, R.; Singh, R.; Popli, H. Implementation of Quality by Design (QbD) Approach in Development of QCT-SMEDDS with Combination of AgNPs for Diabetic Foot Ulcer Management. Indian J. Pharm. Educ. Res. 2021, 55, 1207–1223. [Google Scholar]
- Nagaraja, S.; Basavarajappa, G.M.; Attimarad, M.; Pund, S. Topical Nanoemulgel for the Treatment of Skin Cancer: Proof-of-Technology. Pharmaceutics 2021, 13, 902. [Google Scholar] [CrossRef]
- SreeHarsha, N.; Hiremath, J.G.; Rawre, B.K.; Puttaswamy, N.; Al-Dhubiab, B.E.; Venugopala, K.N.; Akrawi, S.H.; Meravanige, G.; Attimarad, M.; Nair, A.B. Formulation and Evaluation of Tamoxifen Citrate Loaded Transdermal Reservoir Gel Drug Delivery Systems. Indian J. Pharma Educ. Res. 2019, 53, S596–S606. [Google Scholar] [CrossRef]
- Pund, S.; Rasve, G.; Borade, G. Ex vivo permeation characteristics of venlafaxine through sheep nasal mucosa. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 48, 195–201. [Google Scholar] [CrossRef]
- Nair, A.B.; Singh, K.; Shinu, P.; Harsha, S.; Al-Dhubiab, B.E. A comprehensive study to evaluate the effect of constant low voltage iontophoresis on transungual delivery. Drug Dev. Ind. Pharm. 2013, 39, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, A.; Anwar, M.; Mallick, N.; Naz, Z.; Jain, G.K.; Ahmad, F.J. Transungual Delivery of Ketoconazole Nanoemulgel for the Effective Management of Onychomycosis. AAPS PharmSciTech 2016, 17, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.K.; Jain, V. Screening of process variables using Plackett–Burman design in the fabrication of gedunin-loaded liposomes. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1011–1022. [Google Scholar] [CrossRef]
- Badria, F.; Fayed, H.A.; Ibraheem, A.K.; Mazyed, E.A. Formulation of sodium valproate nanospanlastics as a promising approach for drug repurposing in the treatment of androgenic alopecia. Pharmaceutics 2020, 12, 866. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Chapter 10—Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 369–400. [Google Scholar]
- Al-Mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Enhanced non invasive trans-tympanic delivery of ciprofloxacin through encapsulation into nano-spanlastic vesicles: Fabrication, in-vitro characterization, and comparative ex-vivo permeation studies. Int. J. Pharm. 2017, 522, 157–164. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 2013, 20, 338–344. [Google Scholar] [CrossRef]
- Aggarwal, R.; Targhotra, M.; Sahoo, P.K.; Chauhan, M.K. Onychomycosis: Novel strategies for treatment. J. Drug Deliv. Sci. Technol. 2020, 57, 101774. [Google Scholar] [CrossRef]
- Sakai, T.; Kurosawa, H.; Okada, T.; Mishima, S. Vesicle formation in mixture of a PEO-PPO-PEO block copolymer (Pluronic P123) and a nonionic surfactant (Span 65) in water. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 82–89. [Google Scholar] [CrossRef]
- Kakkar, S.; Pal Kaur, I. A novel nanovesicular carrier system to deliver drug topically. Pharm. Dev. Technol. 2013, 18, 673–685. [Google Scholar] [CrossRef]
- Abdelbary, G.A.; Amin, M.M.; Zakaria, M.Y. Ocular ketoconazole-loaded proniosomal gels: Formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017, 24, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.A. Flexibility and phase transitions of polymers. J. Appl. Polym. Sci. 2003, 88, 1590–1599. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Pereira, S.V.; Colombo, F.B.; de Freitas, L.A.P. Ultrasound influence on the solubility of solid dispersions prepared for a poorly soluble drug. Ultrason. Sonochem. 2016, 29, 461–469. [Google Scholar] [CrossRef]
- Patil, S.; Pund, S.; Joshi, A.; Shishoo, C.J.; Shahiwala, A. Chronomodulated press-coated pulsatile therapeutic system for aceclofenac: Optimization of factors influencing drug release and lag time. ChronoPhysiology Ther. 2011, 1, 1–10. [Google Scholar] [CrossRef][Green Version]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Elmowafy, E.; El-Gogary, R.I.; Ragai, M.H.; Nasr, M. Novel antipsoriatic fluidized spanlastic nanovesicles: In vitro physicochemical characterization, ex vivo cutaneous retention and exploratory clinical therapeutic efficacy. Int. J. Pharm. 2019, 568, 118556. [Google Scholar] [CrossRef]
- Dora, C.P.; Singh, S.K.; Kumar, S.; Datusalia, A.K.; Deep, A. Development and characterization of nanoparticles of glibenclamide by solvent displacement method. Acta Pol. Pharm. 2010, 67, 283–290. [Google Scholar]
- Salama, H.A.; Mahmoud, A.A.; Kamel, A.O.; Abdel Hady, M.; Awad, G.A. Brain delivery of olanzapine by intranasal administration of transfersomal vesicles. J. Liposome Res. 2012, 22, 336–345. [Google Scholar] [CrossRef]
- Li, P.H.; Chiang, B.H. Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason. Sonochem. 2012, 19, 192–197. [Google Scholar] [CrossRef]
- El-Say, K.M.; Abd-Allah, F.I.; Lila, A.E.; Hassan Ael, S.; Kassem, A.E. Diacerein niosomal gel for topical delivery: Development, in vitro and in vivo assessment. J. Liposome Res. 2016, 26, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Foulon, M.; Bonnemain, B.; Vandamme, T.F. Niosomes as carriers of radiopaque contrast agents for X-ray imaging. J. Microencapsul. 2000, 17, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Dwivedi, P.; Gupta, P.K.; Mishra, P.R. Optimization of novel tocopheryl acetate nanoemulsions for parenteral delivery of curcumin for therapeutic intervention of sepsis. Expert Opin. Drug Deliv. 2014, 11, 1697–1712. [Google Scholar] [CrossRef] [PubMed]
- Ngan, C.L.; Basri, M.; Lye, F.F.; Fard Masoumi, H.R.; Tripathy, M.; Karjiban, R.A.; Abdul-Malek, E. Comparison of process parameter optimization using different designs in nanoemulsion-based formulation for transdermal delivery of fullerene. Int. J. Nanomed. 2014, 9, 4375–4386. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.M.; El-Setouhy, D.A.; Habib, B.A.; Tayel, S.A. Enhancement of Transdermal Delivery of Haloperidol via Spanlastic Dispersions: Entrapment Efficiency vs. Particle Size. AAPS PharmSciTech 2019, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- El-Nabarawy, N.A.; Teaima, M.H.; Helal, D.A. Assessment of Spanlastic Vesicles of Zolmitriptan for Treating Migraine In Rats. Drug Des. Dev. Ther. 2019, 13, 3929–3937. [Google Scholar] [CrossRef]
- Cevc, G. Polymorphism of the bilayer membranes in the ordered phase and the molecular origin of the lipid pretransition and rippled lamellae. Biochim. Et Biophys. Acta 1991, 1062, 59–69. [Google Scholar] [CrossRef]
- Shamma, R.N.; Elsayed, I. Transfersomal lyophilized gel of buspirone HCl: Formulation, evaluation and statistical optimization. J. Liposome Res. 2013, 23, 244–254. [Google Scholar] [CrossRef]
- Nair, A.B.; Singh, B.; Shah, J.; Jacob, S.; Aldhubiab, B.; Sreeharsha, N.; Morsy, M.A.; Venugopala, K.N.; Attimarad, M.; Shinu, P. Formulation and Evaluation of Self-Nanoemulsifying Drug Delivery System Derived Tablet Containing Sertraline. Pharmaceutics 2022, 14, 336. [Google Scholar] [CrossRef]
- Joshi, A.; Pund, S.; Nivsarkar, M.; Vasu, K.; Shishoo, C. Dissolution test for site-specific release isoniazid pellets in USP apparatus 3 (reciprocating cylinder): Optimization using response surface methodology. Eur. J. Pharm. Biopharm. 2008, 69, 769–775. [Google Scholar] [CrossRef]

| Parameter | CFAs and CPPs | Low Level (−1) | High Level (+1) |
|---|---|---|---|
| X1 | Type of vesicle builder | Span 65 | Span 60 |
| X2 | Type of edge activator | Sodium deoxycholate | Tween 80 |
| X3 | Mixing time (min) | 10 | 20 |
| X4 | Amount of organic phase (mL) | 5 | 10 |
| X5 | Sonication time (min) | 0 | 5 |
| X6 | Amount of efinaconazole | 10 | 15 |
| X7 | Mixing speed (rpm) | 50 | 100 |
| X8 | Volume of aqueous phase (mL) | 50 | 100 |
| CQA | Unit | ||
| Y1 | Particle size | nm | |
| Y2 | Zeta potential | mV | |
| Y3 | Relative deformability | min | |
| Y4 | Transmittance | % | |
| Y5 | Encapsulation efficiency | % | |
| Batch | CFAs and CPPs Selected for Efinaconazole Spanlastics | CQAs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 (min) | X4 (mL) | X5 (min) | X6 (mg) | X7 (rpm) | X8 (mL) | Y1 (nm) | Y2 (mV) | Y3 (min) | Y4 (%) | Y5 (%) | |
| P1 | 1 | 1 | −1 | 1 | 1 | 1 | 1 | −1 | 252.1 ± 4.1 | −16.5 ± 0.5 | 16.8 ± 1.2 | 84.8 ± 2.1 | 63.1 ± 1.8 |
| P2 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 309.2 ± 3.7 | −17.8 ± 0.7 | 30.6 ± 1.8 | 49.1 ± 1.2 | 65.2 ± 2.2 |
| P3 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 778.3 ± 6.5 | −14.3 ± 0.5 | 41.9 ± 2.3 | 32.2 ± 1.1 | 45.5 ± 1.8 |
| P4 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 313.2 ± 3.2 | −16.7 ± 0.4 | 49.9 ± 3.6 | 68.7 ± 2.2 | 65.3 ± 3.1 |
| P5 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 734.1 ± 6.0 | −15.9 ± 0.4 | 48.9 ± 3.1 | 36.6 ± 1.2 | 55.9 ± 1.6 |
| P6 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 851.3 ± 4.9 | −18.8 ± 0.6 | 56.7 ± 4.2 | 24.9 ± 0.9 | 81.9 ± 0.8 |
| P7 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 613.3 ± 3.8 | −16.2 ± 0.3 | 55.9 ± 2.2 | 48.7 ± 1.1 | 79.8 ± 1.2 |
| P8 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | −1 | 411.2 ± 2.9 | −18.3 ± 0.4 | 21.4 ± 1.8 | 68.9 ± 1.3 | 44.6 ± 2.1 |
| P9 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 423.6 ± 3.9 | −19.3 ± 0.4 | 25.2 ± 0.8 | 67.7 ± 1.7 | 27.9 ± 2.3 |
| P10 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 479.2 ± 5.1 | −18.1 ± 0.2 | 31.9 ± 1.1 | 42.1 ± 0.8 | 27.1 ± 1.6 |
| P11 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 706.5 ± 6.3 | −16.1 ± 0.4 | 47.1 ± 1.9 | 39.4 ± 1.5 | 55.8 ± 1.6 |
| P12 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 862.2 ± 5.8 | −14.9 ± 0.2 | 57.5 ± 2.0 | 23.8 ± 0.9 | 68.3 ± 1.4 |
| CFAs and CPP Were Used in 23 Factorial Design | Levels of CFAs and CPP | |
|---|---|---|
| −1 | +1 | |
| X1: Amount of vesicle builder, Span 60 (mg/mL) | 100 | 120 |
| X2: Amount of edge activator, Tween 80 (mg/mL) | 80 | 100 |
| X3: Sonication time (min) | 0 | 5 |
| Unchanged variables levels | ||
| Type of edge activator | Tween 80 | |
| Type of Vesicle builder | Span 60 | |
| Mixing time | 10 min | |
| Mixing speed | 50 rpm | |
| Amount of organic phase | 10 mL | |
| Amount of aqueous phase | 100 mL | |
| Amount of efinaconazole | 15 mg | |
| CFAs and CPP | CQAs | ||||||
|---|---|---|---|---|---|---|---|
| Formulation Run | X1 | X2 | X3 (min) | Y1 (nm) | Y2 (min) | Y3 (%) | Y4 (%) |
| FD1 | −1 | −1 | −1 | 235.3 | 15.7 | 86.5 | 60.5 |
| FD2 | 1 | −1 | −1 | 602.2 | 40.1 | 49.8 | 57.1 |
| FD3 | −1 | 1 | −1 | 241.4 | 16.1 | 85.9 | 74.5 |
| FD4 | 1 | 1 | −1 | 569.7 | 37.9 | 53.1 | 71.0 |
| FD5 | −1 | −1 | 1 | 241.3 | 16.1 | 85.9 | 62.5 |
| FD6 | 1 | −1 | 1 | 393.1 | 26.2 | 70.7 | 64.4 |
| FD7 | −1 | 1 | 1 | 180.5 | 12.0 | 92.0 | 81.3 |
| FD8 | 1 | 1 | 1 | 302.9 | 20.2 | 79.8 | 85.4 |
| Independent Variable | Coefficient | Particle Size | ZETA Potential | Relative Deformability | Transmittance | Encapsulation Efficiency | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | ||
| Constant | b0 | 560.92 | <0.0001 | −14.91 | <0.001 | 40.32 | <0.0001 | 48.91 | 0.0003 | 56.7 | <0.001 |
| Type of vesicle builder | b1 | −30.42 | 0.023 | 0.125 | 0.84 | −0.56 | 0.009 | 8.042 | 0.051 | −3.92 | 0.10 |
| Type of edge activator | b2 | −196.42 | 0.001 | −0.88 | 0.23 | −11.02 | 0.001 | 14.64 | 0.011 | −7.83 | 0.018 |
| Mixing time | b3 | 10.58 | 0.233 | −0.00 | 0.99 | −2.72 | 0.065 | −4.392 | 0.184 | −10.47 | 0.0081 |
| Amount of organic phase | b4 | 2.25 | 0.77 | 0.16 | 0.81 | 0.40 | 0.703 | −0.225 | 0.935 | −0.25 | 0.89 |
| Sonication time | b5 | −73.08 | 0.002 | 0.38 | 0.58 | 1.22 | 0.292 | 5.642 | 0.114 | 7.483 | 0.021 |
| Amount of efinaconazole | b6 | −5.08 | −0.52 | −0.03 | 0.97 | −4.27 | 0.021 | 0.508 | 0.855 | 2.667 | 0.208 |
| Mixing speed | b7 | −13.08 | 0.16 | −0.28 | 0.68 | 3.05 | 0.049 | −0.358 | 0.897 | 4.233 | 0.084 |
| Volume of aqueous phase | b8 | −6.25 | 0.44 | 0.33 | 0.63 | 1.33 | 0.26 | 0.625 | 0.823 | −3.667 | 0.115 |
| Statistical analysis of the model | |||||||||||
| Model p value | 0.0012 | 0.008 | 0.0107 | 0.007 | 0.034 | ||||||
| F value | 111.9 | 38.7 | 26.2 | 16.3 | 12.66 | ||||||
| Regression coefficient (r2) | 0.99 | 0.90 | 0.99 | 0.94 | 0.971 | ||||||
| p Values for Coefficients | |||||
|---|---|---|---|---|---|
| Factor | Coefficient | Y1 | Y2 | Y3 | Y4 |
| Intercept | b0 | 0.0021 | 0.0024 | 0.001 | 0.0026 |
| X1 | b1 | 0.0060 | 0.0069 | 0.0059 | 0.763 |
| X2 | b2 | 0.0330 | 0.0374 | 0.0320 | 0.0216 |
| X3 | b3 | 0.0110 | 0.0126 | 0.0108 | 0.0479 |
| X1X2 | b12 | 0.086 | 0.098 | 0.083 | 0.529 |
| X2X3 | b23 | 0.0139 | 0.0160 | 0.0136 | 0.112 |
| X1X3 | b13 | 0.0469 | 0.054 | 0.0458 | 0.122 |
| Response | Y1 | Y2 | Y3 | Y4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition | Experimental Value | Predicted Value | Bias (%) a | Experimental Value | Predicted Value | Bias (%) a | Experimental Value | Predicted Value | Bias (%) a | Experimental Value | Predicted Value | Bias (%) a |
| A (105, 100, 5) b | 197.00 | 196.23 | (+)0.39 | 12.50 | 13.09 | (−)4.51 | 91.00 | 90.19 | (+)0.90 | 81.23 | 82.03 | (−)0.97 |
| B (115, 85, 5) b | 330 | 335.01 | (−)1.50 | 21.00 | 22.31 | (−)5.89 | 75.00 | 76.69 | (−)2.20 | 70.25 | 69.11 | (+)1.65 |
| C (107.5, 82.5, 2.5) b | 310 | 309.64 | (+)0.12 | 20.00 | 20.65 | (−)3.15 | 80.21 | 79.02 | (+)1.51 | 65.12 | 64.46 | (+)1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuqbil, R.M.; Sreeharsha, N.; Nair, A.B. Formulation-by-Design of Efinaconazole Spanlastic Nanovesicles for Transungual Delivery Using Statistical Risk Management and Multivariate Analytical Techniques. Pharmaceutics 2022, 14, 1419. https://doi.org/10.3390/pharmaceutics14071419
Almuqbil RM, Sreeharsha N, Nair AB. Formulation-by-Design of Efinaconazole Spanlastic Nanovesicles for Transungual Delivery Using Statistical Risk Management and Multivariate Analytical Techniques. Pharmaceutics. 2022; 14(7):1419. https://doi.org/10.3390/pharmaceutics14071419
Chicago/Turabian StyleAlmuqbil, Rashed M., Nagaraja Sreeharsha, and Anroop B. Nair. 2022. "Formulation-by-Design of Efinaconazole Spanlastic Nanovesicles for Transungual Delivery Using Statistical Risk Management and Multivariate Analytical Techniques" Pharmaceutics 14, no. 7: 1419. https://doi.org/10.3390/pharmaceutics14071419
APA StyleAlmuqbil, R. M., Sreeharsha, N., & Nair, A. B. (2022). Formulation-by-Design of Efinaconazole Spanlastic Nanovesicles for Transungual Delivery Using Statistical Risk Management and Multivariate Analytical Techniques. Pharmaceutics, 14(7), 1419. https://doi.org/10.3390/pharmaceutics14071419







