Differential Pharmacokinetics of Liver Tropism for Iron Sucrose, Ferric Carboxymaltose, and Iron Isomaltoside: A Clue to Their Safety for Dialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Anemia Treatment and Iron Therapy
2.3. Longitudinal Analysis of Liver Iron and Spleen Iron Concentrations
2.4. Biological Markers
2.5. Quantification of LIC and SIC by MRI
2.6. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
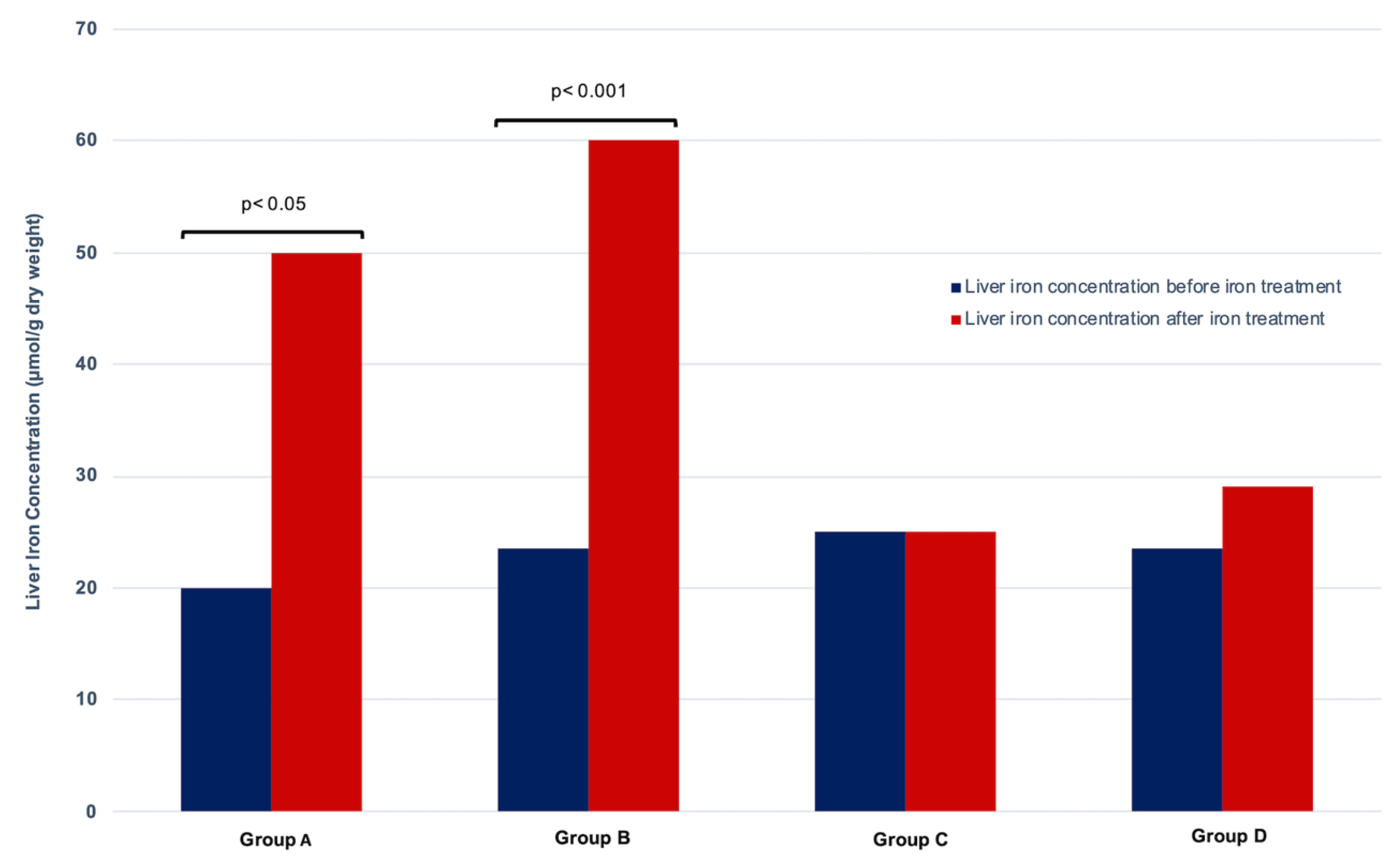
3.2. Evolution of Liver Iron Load by MRI
3.3. Evolution of Splenic Iron Load by MRI
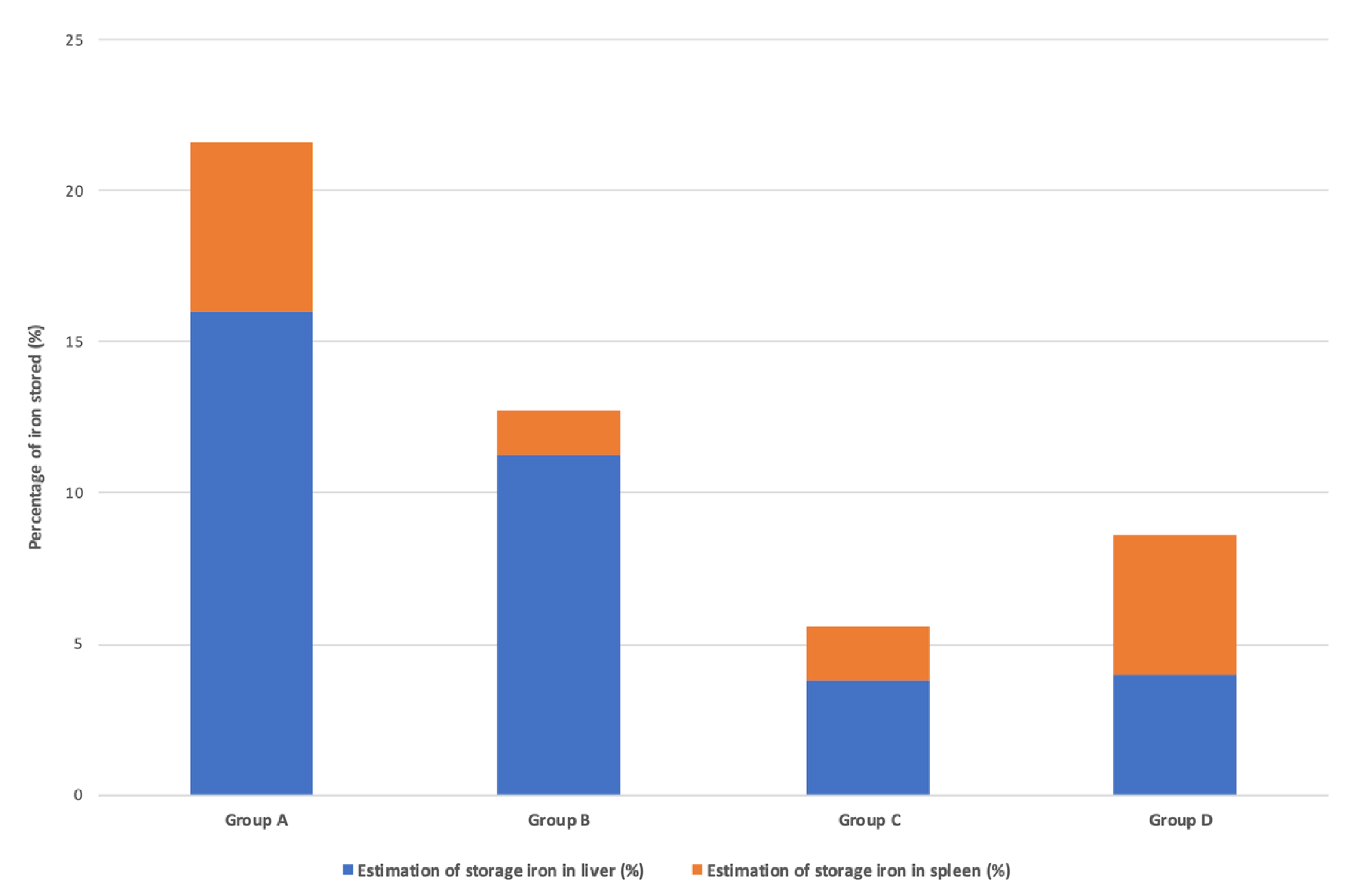
3.4. Quantification of Iron Sequestered in the Liver and Spleen after IV Iron Infusions
3.5. Evolution of Iron Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martà nez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozzelino, R.; Arosio, P. Iron homeostasis in health and disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, Redox Biology and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daher, R.; Manceau, H.; Karim, Z. Iron metabolism and the role of iron-regulating hormone hepcidin in health and disease. Presse Med. 2017, 46, e272–e278. [Google Scholar] [CrossRef] [PubMed]
- Hörl, W.H. Clinical aspects of iron use in the anemia of kidney disease. J. Am. Soc. Nephrol. 2007, 18, 382–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eschbach, J.W.; Egrie, J.C.; Downing, M.R.; Browne, J.K.; Adamson, J.W. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N. Engl. J. Med. 1987, 316, 73–78. [Google Scholar] [CrossRef]
- Rottembourg, J.; Rostoker, G. Use of intravenous iron supplementation in chronic kidney disease: Interests, limits, and recommendations for a better practice. Nephrol. Ther. 2015, 11, 531–542. [Google Scholar] [CrossRef]
- Locatelli, F.; Bárány, P.; Covic, A.; De Francisco, A.; Del Vecchio, L.; Goldsmith, D.; Hörl, W.; London, G.; Vanholder, R.; Van Biesen, W.; et al. Kidney disease: Improving global outcomes guidelines on anaemia management in chronic kidney disease: A European Renal Best Practice position statement. Nephrol. Dial Transpl. 2013, 28, 1346–1359. [Google Scholar] [CrossRef]
- Auerbach, M.; Gafter-Gvili, A.; Macdougall, I.C. Intravenous iron: A framework for changing the management of iron deficiency. Lancet Haematol. 2020, 7, e342–e350. [Google Scholar] [CrossRef]
- Macdougall, I.C.; White, C.; Anker, S.D.; Bhandari, S.; Farrington, K.; Kalra, P.A.; McMurray, J.J.V.; Murray, H.; Tomson, C.R.V.; Wheeler, D.C.; et al. Intravenous iron in patients undergoing maintenance hemodialysis. N. Engl. J. Med. 2019, 380, 447–458. [Google Scholar] [CrossRef]
- Ribeiro, S.; Belo, L.; Reis, F.; Santos-Silva, A. Iron therapy in chronic kidney disease: Recent changes, benefits and risks. Blood Rev. 2015, 30, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, G.; Vaziri, N.D.; Fishbane, S. Iatrogenic iron overload in dialysis patients at the beginning of the 21st century. Drugs 2016, 76, 741–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoti, H.; Rachmilewitz, E.A.; Simon-Lopez, R.; Gaber, R.; Katzir, Z.; Konen, E.; Kushnir, T.; Girelli, D.; Campostrini, N.; Fibach, E.; et al. Evidence for tissue iron overload in long-term hemodialysis patients and the impact of withdrawing parenteral iron. Eur. J. Haematol. 2012, 8, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, G.; Griuncelli, M.; Loridon, C.; Couprie, R.; Benmaadi, A.; Bounhiol, C.; Roy, M.; Machado, G.; Janklewicz, P.; Drahi, G.; et al. Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents: An MRI study. Am. J. Med. 2012, 125, 991–999. [Google Scholar] [CrossRef]
- Rostoker, G.; Vaziri, N.D. Risk of iron overload with chronic indiscriminate use of intravenous iron products in ESRD and IBD populations. Heliyon 2019, 5, e02045. [Google Scholar] [CrossRef] [Green Version]
- Carrilho, P.; Lima, A.; Manso, R.; Nobrega, L.; Lima Santos, A.; Matos Soares, E.; Bastos, L.; Fidalgo, P. Post-mortem hepatic and bone marrow iron content in hemodialysis patients: A prospective cohort study. In Proceedings of the 58th EDTA Congress, Berlin, Germany, 5–8 June 2021. [Google Scholar] [CrossRef]
- Van der Weerd, N.C.; Grooteman, M.P.C.; Bots, M.L.; Van den Dorpel, M.A.; Den Hoedt, C.H.; Mazairac, A.H.A.; Nubé, M.J.; Penne, E.L.; Wetzels, J.F.M.; Wiegerinck, E.T.; et al. Hepcidin-25 is related to cardiovascular events in chronic haemodialysis patients. Nephrol. Dial Transpl. 2013, 28, 3062–3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Zou, L.X.; Lu, Y.; Deng, N.; Wang, H.X. Effect of serum hepcidin on predicting mortality in hemodialysis patients: A prospective cohort study. Iran. Red Crescent Med. J. 2019, 21, e87091. [Google Scholar] [CrossRef]
- Rostoker, G.; Loridon, C.; Griuncelli, M.; Rabaté, C.; Lepeytre, F.; Ureña-Torres, P.; Issad, B.; Ghali, N.; Cohen, Y. Liver iron load influences hepatic fat fraction in end-stage renal disease patients on dialysis: A proof of concept study. EBioMedicine 2019, 39, 461–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Span, K.; Pieters, E.H.E.; Hennink, W.E.; Van der Toorn, A.; Brinks, V.; Dijkhuizen, R.M.; Van Tilborg, G.A.F. The Use of Magnetic Resonance Imaging for Non-Invasive Assessment of Venofer® Biodistribution in Rats. Pharm. Res. 2018, 35, 88. [Google Scholar] [CrossRef] [Green Version]
- FDA Briefing Document. Cardiovascular and Renal Drugs Advisory Committee. Meeting 15 July 2021 Roxadustat. Available online: https://www.fda.gov/media/150728/download (accessed on 9 May 2022).
- Roxadustat EMA Label. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/evrenzo (accessed on 9 May 2022).
- A Cohort Study of Haemodialysis Patients Based on Hepatic Magnetic Resonance Imaging. Available online: https://www.isrctn.com/ISRCTN80100088 (accessed on 9 May 2022).
- Iron Sucrose French Label—RCP ANSM. Available online: http://agence-prd.ansm.sante.fr/php/ecodex/rcp/R0309204.htm (accessed on 9 May 2022).
- Ferinject French Label. Available online: https://base-donnees-publique.medicaments.gouv.fr/extrait.php?specid=60960624 (accessed on 9 May 2022).
- Monover Label. Available online: https://docetp.mpa.se/LMF/Monofer%20solution%20for%20injection%20or%20infusion%20ENG%20PAR_09001bee807a625c.pdf (accessed on 9 May 2022).
- Gandon, Y.; Olivié, D.; Guyader, D.; Aubé, C.; Oberti, F.; Sebille, V.; Deugnier, Y. Non-invasive assessment of hepatic iron stores by MRI. Lancet 2004, 363, 357–362. [Google Scholar] [CrossRef]
- Rostoker, G.; Laroudie, M.; Blanc, R.; Griuncelli, M.; Loridon, C.; Lepeytre, F.; Rabaté, C.; Cohen, Y. Histological scores validate the accuracy of hepatic iron load measured by Signal Intensity Ratio and R2* relaxometry MRI in dialysis patients. J. Clin. Med. 2019, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T.D. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef] [Green Version]
- Garbowski, M.W.; Carpenter, J.P.; Smith, G.; Roughton, M.; Alam, M.H.; He, T.; Pennell, D.J.; Porter, J.B. Biopsy-based calibration of T2* magnetic resonance for estimation of liver iron concentration and comparison with R2 Ferriscan. J. Cardiovasc. Magn. Reson. 2014, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Sheskin, D.J. Handbook of Parametric and Nonparametric Statistical Procedures, 4th ed.; Chapman and Hall, Taylor and Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Brissot, P.; Bourel, M.; Herry, D.; Verger, J.P.; Messner, M.; Beaumont, C.; Regnouard, F.; Ferrand, B.; Simon, M. Assessment of liver iron content in 271 patients: A reevaluation of direct and indirect methods. Gastroenterology 1981, 80, 557–565. [Google Scholar] [CrossRef]
- Paisant, A.; d’Assignies, G.; Bannier, E.; Bardou-Jacquet, E.; Gandon, Y. MRI for the measurement of liver iron content and for the diagnosis and follow-up of iron overload disorders. Presse Med. 2017, 46, e279–e287. [Google Scholar] [CrossRef] [PubMed]
- França, M.; Carvalho, J.G. MR imaging assessment and quantification of liver iron. Abdom. Radiol. (NY) 2020, 45, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Kulkarni, H.; Dheda, S.; Betti, B.; Harrison, C.; St Pierre, T.G.; Olynyk, J.K. Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beshara, S.; Lundqvist, H.; Sundin, J.; Lubberink, M.; Tolmachev, V.; Valind, S.; Antoni, G.; Långström, B.; Danielson, B.G. Pharmacokinetics and red cell utilization of iron (III) hydroxide-sucrose complex in anaemic patients: A study using positron emission tomography. Br. J. Haematol. 1999, 104, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Beshara, S.; Sörensen, J.; Lubberink, M.; Tolmachev, V.; Långström, B.; Antoni, G.; Danielson, B.G.; Lundqvist, H. Pharmacokinetics and red cell utilization of 52 Fe/59Fe-labelled iron polymaltose in anaemic patients using positron emission tomography. Br. J. Haematol. 2003, 120, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Pharmacovigilance Risk Assessment Committee (PRAC). Minutes for the Meeting on 2017. Available online: https://www.ema.europa.eu/en/documents/minutes/minutes-prac-meeting-29-august-1-september2017_en.pdf (accessed on 9 May 2022).
- Chinnadurai, R.; Ritchie, J.; Green, D.; Kalra, P.A. Non-alcoholic fatty liver disease and clinical outcomes in chronic kidney disease. Nephrol. Dial. Transpl. 2018, 34, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, G.; Vaziri, N.D. Impact of iatrogenic iron overload on the course of hepatitis C in the dialysis population: A plea for caution. Hemodial. Int. 2017, 21, S68–S77. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Iron Sucrose 1.2 g | Iron Sucrose 2.4 g | Ferric Carboxymaltose 1 g | Iron Isomaltoside 1 g | ||
|---|---|---|---|---|---|
| (n = 11) | (n = 14) | (n = 15) | (n = 14) | p-Value | |
| Group A | Group B | Group C | Group D | ||
| Age (years) | 68 (51–89) | 52.5 (30–82) | 70 (40–89) | 56.5 (33–87) | 0.08 * |
| Sex, female | 6 (54.5%) | 8 (57.1%) | 10 (66.7%) | 3 (21.4%) | 0.09 ** |
| Dialysis duration before | 6.6 (1.1–48) | 13.5 (1.9–139.6) | 4.3 (0.7–116.8) | 2.25 (0.9–51.8) | 0.07 * |
| the study (months) | |||||
| Dialysis modality (HD, PD) | 11 (100% HD) | 14 (100% HD) | 14 (93.3% HD) | 8 (57.1% HD) | |
| 1 (6.7% PD) | 6 (42.9% PD) | ||||
| Diabetes mellitus | 4 (36.4%) | 5 (35.7%) | 6 (40%) | 2 (14.3%) | 0.45 ** |
| Modified Charlson | 6 (3–10) | 4.5 (2–8) | 6 (2–8) | 3.5 (2–11) | 0.14 * |
| comorbidity index | |||||
| Audit questionnaire on | 2 (1–5) | 1 (0–12) | 1 (0–4) | 1 (0–10) | 0.37 * |
| alcoholism | |||||
| Weight (kg) | 65 (43–97) | 73.25 (55–95) | 72.5 (52–112) | 72.25 (58.5–106) | 0.70 * |
| BMI (kg/m2) | 24 (17–34) | 25.5 (19–35) | 26 (18–40) | 26 (21–35) | 0.73 * |
| IV iron dose received | 1.2 (1–1.6) | 2.4 (2.3–2.7) | 1 (1–1.1) | 1 (1–1.2) | <0.0001 * |
| between the two MRI (g) | |||||
| ESA therapy (closest to MRI 1) | 10 (90.9%) | 13 (92.9%) | 13 (86.7%) | 10 (71.4%) | 0.38 ** |
| ESA therapy (closest to MRI 2) | 10 (90.9%) | 12 (85.7%) | 12 (80%) | 10 (71.4%) | 0.62 ** |
| Darbopoetin dose | 40 (0–100) | 40 (0–100) | 40 (0–130) | 20 (0–130) | 0.06 * |
| (μg/week closest to MRI 1) | |||||
| Darbopoetin dose | 40 (0–100) | 40 (0–100) | 50 (0–130) | 25 (0–130) | 0.57 * |
| (μg/week closest to MRI 2) |
| Group A | Group B | |||||||
| (Changes in 11 patients treated with 1.2 g iron sucrose) | (Changes in 14 patients treated with 2.4 g iron sucrose) | |||||||
| Initial | Final | Difference | p-Value | Initial | Final | Difference | p-Value | |
| [95%CI] | [95%CI] | |||||||
| LIC (μmol/g) | 20 (5–50) | 50 (30–170) | 25 [15–60] | 0.001 | 23.5 (5–45) | 60 (38–210) | 35 [30–60] | 0.0001 |
| SIC (μmol/g) | 17 (9.6–30.2) | 22.2 (11.9–91.4) | 7.8 [–5.5–44.1] | 0.049 | 17.7 (11.2–31) | 24.3 (12.3–67.7) | 4.5 [–4.3–42.2] | 0.07 |
| Spleen R2* (s−1) | 31.2 (17.9–54.9) | 40.7 (22–163.9) | 14 [–9.9–79.1] | 0.049 | 32.5 (20.7–56.5) | 44.4 (22.6–122) | 8.2 [–7.7–75.8] | 0.07 |
| Hemoglobin | 10.8 (7.2–13.3) | 10.5 (8.9–12.2) | 0.05 [–0.9–1.5] | 0.64 | 8.85 (6.3–12) | 11.8 (9–13.9) | 2.2 [1.3–4.3] | 0.001 |
| (g/dL) | ||||||||
| CHr (pg) | 28 (24.9–30.9) | 29.6 (24.6–32.5) | 1.95 [–1.3–4.9] | 0.11 | 27.7 (20.3–32.8) | 31 (27.3–35.4) | 5.5 [–5.5–8.8] | 0.25 |
| Serum ferritin | 46 (8–112) | 56 (12–220) | 28 [–6–94] | 0.05 | 21.5 (6–221) | 80 (16–443) | 47 [9–311] | 0.008 |
| (ng/mL) | 0.004 | |||||||
| Serum iron | 7.8 (2.8–19) | 8.8 (3.5–33) | 1.4 [0.7–12.6] | 0.049 | 5.3 (2.7–9.5) | 9.7 (4.8–20.9) | 2.3 [0.6–11.4] | |
| (μmol/L) | ||||||||
| Serum | 2.3 (1.7–3) | 2 (1.5–3.1) | –0.5 [–0.7–0] | 0.047 | 2.6 (1.6–3.2) | 2.1 (1.8–2.6) | –0.45 [–0.8–0] | 0.016 |
| transferrin (g/L) | ||||||||
| TSAT (%) | 11.56 (4.15–38) | 17 (4.52–67.73) | 5.44 [0.37–28] | 0.02 | 8.59 (3.38–17.27) | 17.08 (10.67–34.83) | 5.39 [2.52–27.12] | 0.008 |
| CRP (mg/L) | 3.1 (1–14.8) | 1.3 (1–8.4) | –1 [–2.7–1.5] | 0.3 | 2.2 (1–15.9) | 3.7 (1–24.2) | 0 [–2.6–11.9] | 0.31 |
| Group C | Group D | |||||||
| (Changes in 15 patients treated with 1 g ferric carboxymaltose) | (Changes in 14 patients treated with 1 g iron isomaltoside) | |||||||
| Initial | Final | Difference | p-Value | Initial | Final | Difference | p-Value | |
| [95%CI] | [95%CI] | |||||||
| LIC (μmol/g) | 25 (5–69) | 25 (5–73) | 5 [2–9] | 0.07 | 23.5 (19–41) | 29 (20–42) | 5.5 [–6–9] | 0.14 |
| SIC (μmol/g) | 21.2 (8.9–58.3) | 25 (9.9–103.4) | 2.3 [–3.7–21.9] | 0.17 | 17.5 (8.8–87.1) | 34 (12.3–79.5) | 6 [2.4–15.5] | 0.007 |
| Spleen R2* (s−1) | 38.8 (16.6–105.3) | 45.7 (18.3–185.2) | 4.1 [–6.7–38.9] | 0.17 | 32.2 (16.4–156.3) | 61.7 (22.7–142.9) | 10.8 [4.2–28] | 0.007 |
| Hemoglobin | 10.1 (7.3–11.7) | 11.3 (9–14.5) | 1.4 [–0.3–3.5] | 0.042 | 9.8 (7.1–13.5) | 11.2 (9.1–15) | 1 [0.2–2.5] | 0.002 |
| (g/dL) | ||||||||
| CHr (pg) | 29.4 (26.4–34.4) | 30.7 (27.9–33.8) | 1 [–1.5–3.6] | 0.24 | 32.3 (22.7–35.4) | 33.8 (26.3–35.7) | 1.55 [–0.2–3.1] | 0.11 |
| Serum ferritin | 30 (15–195) | 59.5 (22–644) | 12 [–2–177] | 0.027 | 113 (19–363) | 260 (38–472) | 61 [30–227] | 0.008 |
| (ng/mL) | ||||||||
| Serum iron | 8.1 (4.5–11.9) | 8.3 (6–16.3) | 0.7 [–2.4–6.1] | 0.38 | 10.2 (5.1–18.6) | 13.8 (4.5–17.4) | 0.2 [–2.5–4.30] | 0.61 |
| (μmol/L) | ||||||||
| Serum | 2.2 (1.42–2.9) | 2.03 (1.4–2.8) | –0.29 [–0.5–0.1] | 0.02 | 2.25 (1.8–3) | 2.05 (1.6–2.8) | –0.20 [–0.32–0.10] | 0.1 |
| transferrin (g/L) | ||||||||
| TSAT (%) | 14.73 (6.92–28) | 15.88 (9.29–43.50) | 3.87 [–2.73–15.50] | 0.16 | 18.62 (7.47–39.16) | 26.66 (7.29–40) | 2.8 [–2–14.05] | 0.09 |
| CRP (mg/L) | 2.8 (1–11.6) | 2 (1–6.1) | 0 [–0.5–2.1] | 0.22 | 2.2 (1–27.6) | 3.1 (1–24.8) | 0.60 [–7.9–3.4] | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostoker, G.; Lepeytre, F.; Merzoug, M.; Griuncelli, M.; Loridon, C.; Boulahia, G.; Cohen, Y. Differential Pharmacokinetics of Liver Tropism for Iron Sucrose, Ferric Carboxymaltose, and Iron Isomaltoside: A Clue to Their Safety for Dialysis Patients. Pharmaceutics 2022, 14, 1408. https://doi.org/10.3390/pharmaceutics14071408
Rostoker G, Lepeytre F, Merzoug M, Griuncelli M, Loridon C, Boulahia G, Cohen Y. Differential Pharmacokinetics of Liver Tropism for Iron Sucrose, Ferric Carboxymaltose, and Iron Isomaltoside: A Clue to Their Safety for Dialysis Patients. Pharmaceutics. 2022; 14(7):1408. https://doi.org/10.3390/pharmaceutics14071408
Chicago/Turabian StyleRostoker, Guy, Fanny Lepeytre, Myriam Merzoug, Mireille Griuncelli, Christelle Loridon, Ghada Boulahia, and Yves Cohen. 2022. "Differential Pharmacokinetics of Liver Tropism for Iron Sucrose, Ferric Carboxymaltose, and Iron Isomaltoside: A Clue to Their Safety for Dialysis Patients" Pharmaceutics 14, no. 7: 1408. https://doi.org/10.3390/pharmaceutics14071408
APA StyleRostoker, G., Lepeytre, F., Merzoug, M., Griuncelli, M., Loridon, C., Boulahia, G., & Cohen, Y. (2022). Differential Pharmacokinetics of Liver Tropism for Iron Sucrose, Ferric Carboxymaltose, and Iron Isomaltoside: A Clue to Their Safety for Dialysis Patients. Pharmaceutics, 14(7), 1408. https://doi.org/10.3390/pharmaceutics14071408







