Removal of Circulating Tumor Cells from Blood Samples of Cancer Patients Using Highly Magnetic Nanoparticles: A Translational Research Project
Abstract
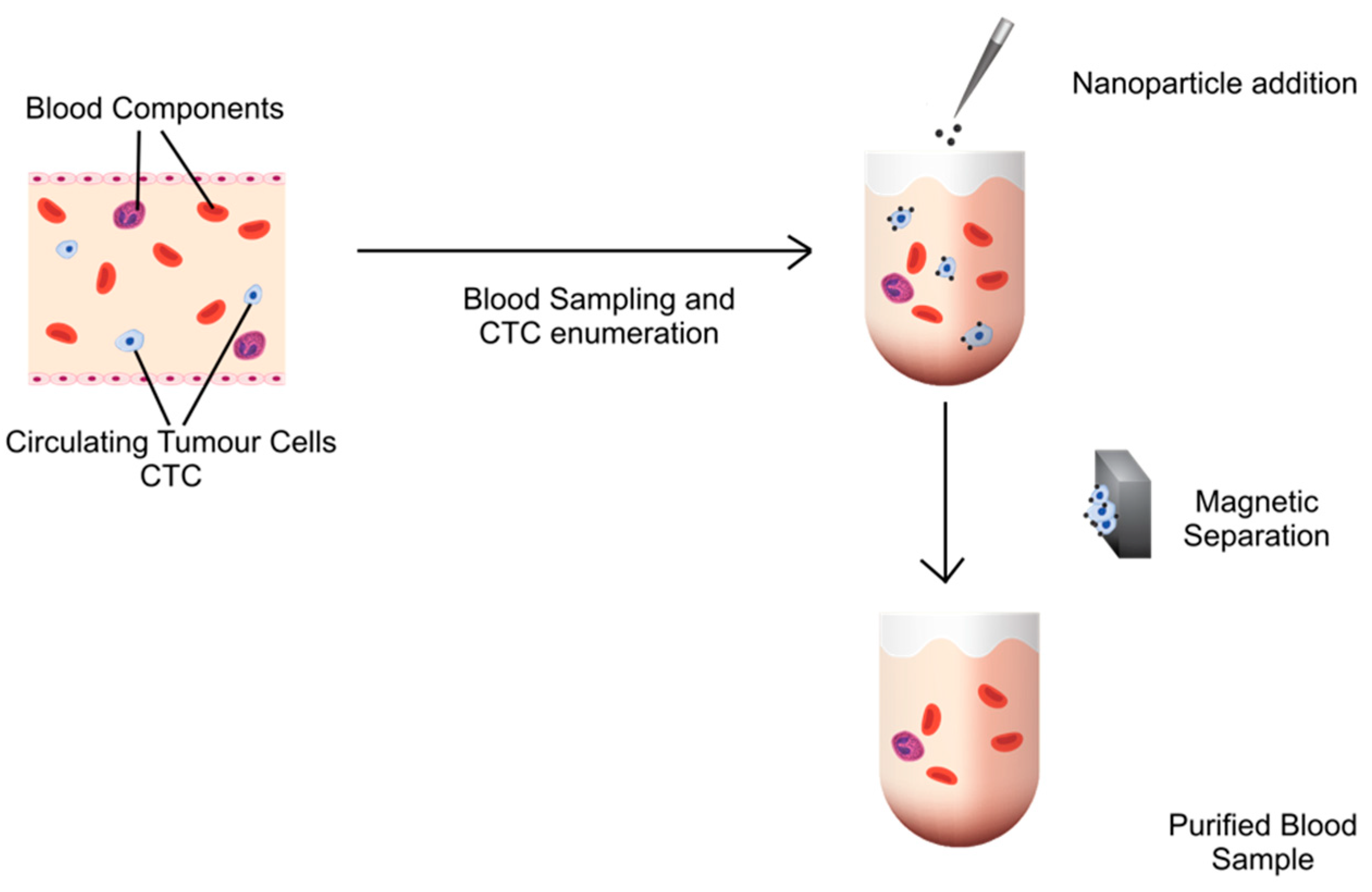
:1. Introduction
2. Methods
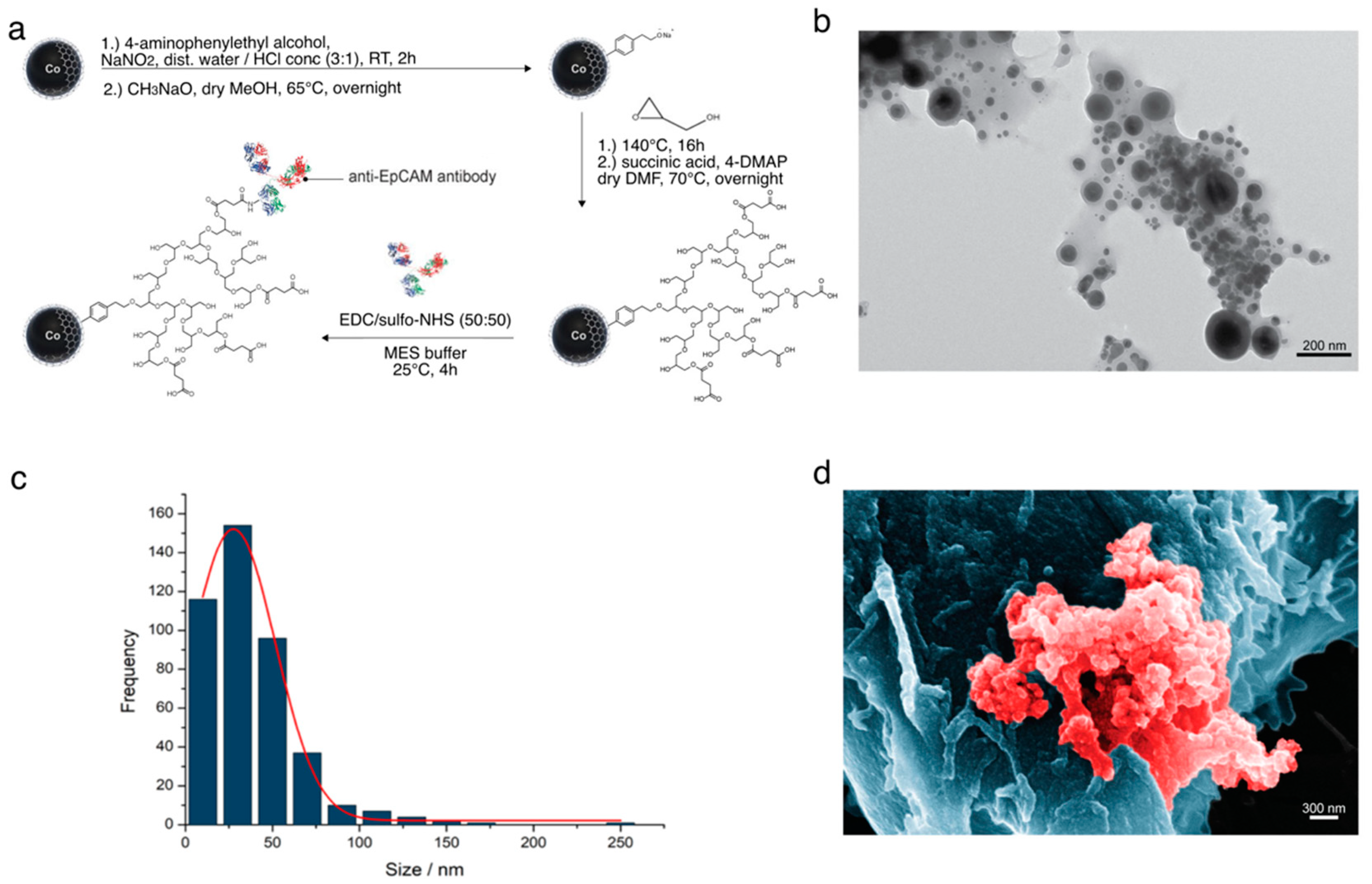
2.1. Synthesis of the Nanoparticles
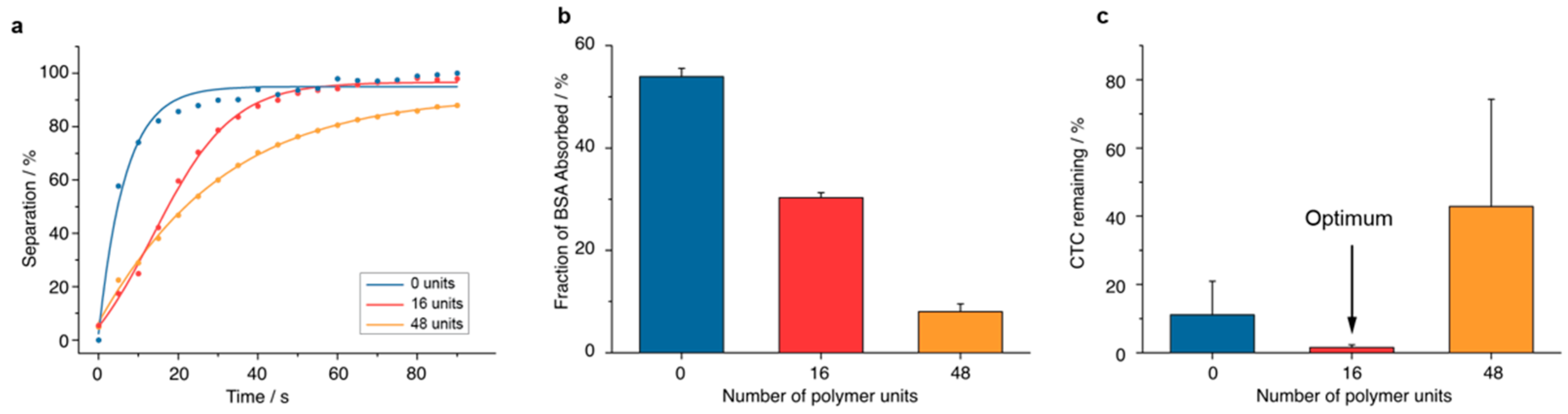
2.2. Characterisation of Separation Property
2.3. Characterisation of Antifouling Property
2.4. Cell Line Experiments
2.5. Ethics Ex Vivo Part, Healthy Volunteers
2.6. Removal of CTCs from the Blood from Healthy Subjects Spiked with Tumor Cells
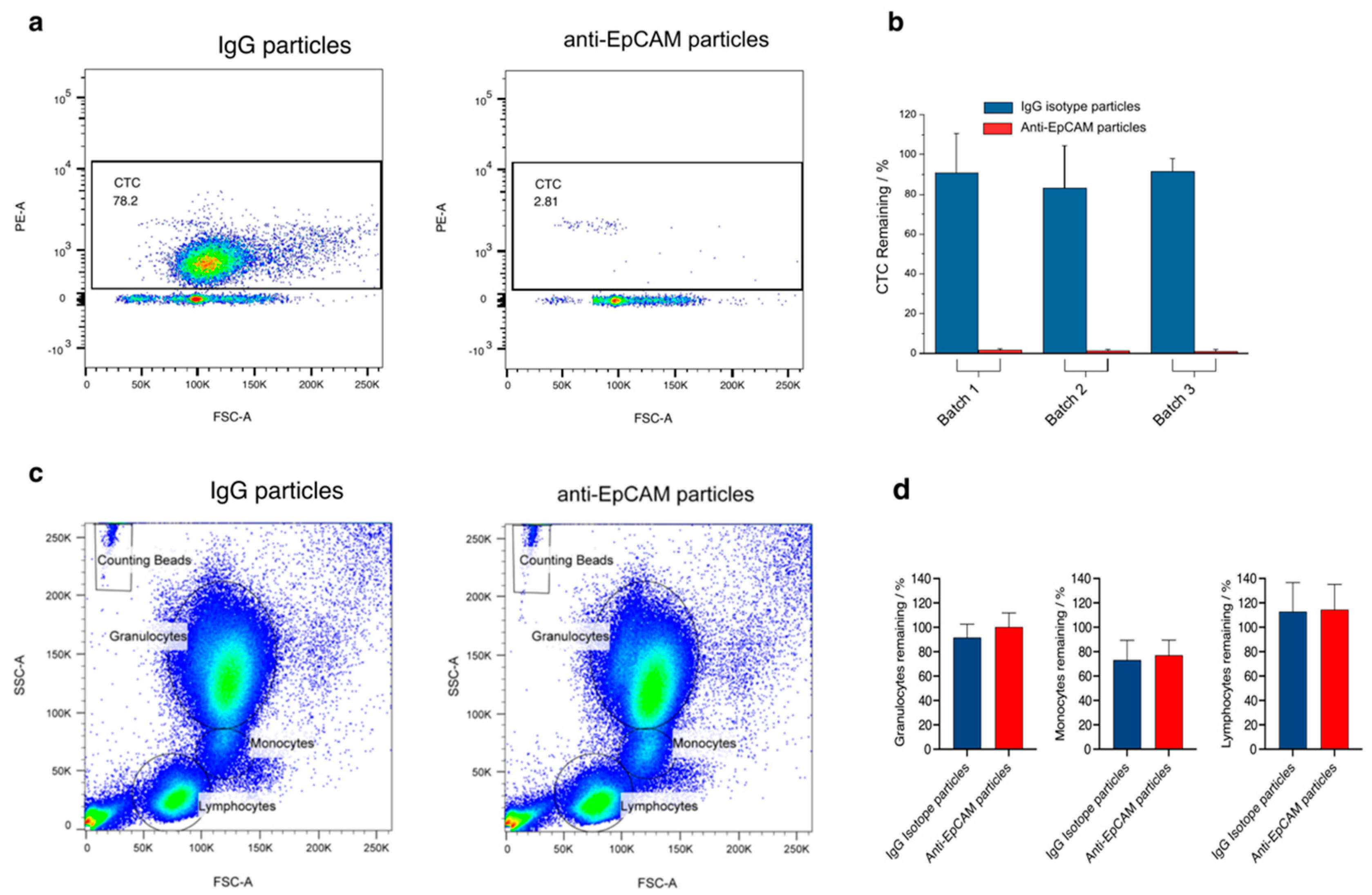
2.7. Analysis of Samples Using Fluorescence-Activated Cell Sorting (FACS)
2.8. Evaluation of a Possible Effect of Nanoparticles on Blood Cells
2.9. Evaluation of a Possible Effect of Nanoparticles on the Coagulation System
2.10. Ethics Ex Vivo Part Cancer Patients
2.11. Removal of CTCs from Blood Samples of Cancer Patients
2.12. Data presentation and Statistical Analyses
3. Results
3.1. Optimal Nanoparticle Characteristics
3.1.1. Synthesis and Separation Capability
3.1.2. Antifouling Properties
3.1.3. CTC Removal Efficiency
3.1.4. Specificity of CTC Removal
3.1.5. Testing of Possible Adverse Effects
3.2. From the CTC In Vitro Model to Blood from Cancer Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, H.; Kim, J.; Song, H.; Sohn, K.Y.; Jeon, M.; Han, K.H. Microfluidic technologies for circulating tumor cell isolation. Analyst 2018, 143, 2936–2970. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Koutsilieris, M. Diagnostic value of circulating tumor cell detection in bladder and urothelial cancer: Systematic review and meta-analysis. BMC Cancer 2011, 11, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Fan, W.; Deng, Q.; Tang, S.; Wang, P.; Xu, P.; Wang, J.; Yu, M. The prognostic and diagnostic value of circulating tumor cells in bladder cancer and upper tract urothelial carcinoma: A meta-analysis of 30 published studies. Oncotarget 2017, 8, 59527–59538. [Google Scholar] [CrossRef] [Green Version]
- Pernot, S.; Badoual, C.; Terme, M.; Castan, F.; Cazes, A.; Bouche, O.; Bennouna, J.; Francois, E.; Ghiringhelli, F.; De La Fouchardiere, C.; et al. Dynamic evaluation of circulating tumour cells in patients with advanced gastric and oesogastric junction adenocarcinoma: Prognostic value and early assessment of therapeutic effects. Eur. J. Cancer 2017, 79, 15–22. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, X.; Bi, F.; Liu, M. Clinical significance of circulating tumor cells in gastric cancer patients. Oncotarget 2017, 8, 25713–25720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Kienle, P.; Lacroix, J.; Willeke, F.; Benner, A.; Lehnert, T.; Herfarth, C.; von Knebel Doeberitz, M. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin. Cancer Res. 1998, 4, 343–348. [Google Scholar]
- Hashimoto, M.; Tanaka, F.; Yoneda, K.; Takuwa, T.; Matsumoto, S.; Okumura, Y.; Kondo, N.; Tsubota, N.; Tsujimura, T.; Tabata, C.; et al. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact. CardioVascular Thorac. Surg. 2014, 18, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Engilbertsson, H.; Aaltonen, K.E.; Bjornsson, S.; Kristmundsson, T.; Patschan, O.; Ryden, L.; Gudjonsson, S. Transurethral bladder tumor resection can cause seeding of cancer cells into the bloodstream. J. Urol. 2015, 193, 53–57. [Google Scholar] [CrossRef]
- Daskalakis, M.; Mavroudis, D.; Sanidas, E.; Apostolaki, S.; Askoxylakis, I.; de Bree, E.; Georgoulias, V.; Melissas, J. Assessment of the effect of surgery on the kinetics of circulating tumour cells in patients with operable breast cancer based on cytokeratin-19 mRNA detection. Eur. J. Surg. Oncol. 2011, 37, 404–410. [Google Scholar] [CrossRef]
- Wind, J.; Tuynman, J.B.; Tibbe, A.G.; Swennenhuis, J.F.; Richel, D.J.; van Berge Henegouwen, M.I.; Bemelman, W.A. Circulating tumour cells during laparoscopic and open surgery for primary colonic cancer in portal and peripheral blood. Eur. J. Surg. Oncol. 2009, 35, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Huang, J.; Zhou, Y.; Li, L.; Bao, G.; Feng, J.; Sha, H. Hematogenous dissemination of lung cancer cells during surgery: Quantitative detection by flow cytometry and prognostic significance. Lung Cancer 2002, 37, 293–301. [Google Scholar] [CrossRef]
- Kienle, P.; Koch, M.; Autschbach, F.; Benner, A.; Treiber, M.; Wannenmacher, M.; von Knebel Doeberitz, M.; Buchler, M.; Herfarth, C.; Weitz, J. Decreased detection rate of disseminated tumor cells of rectal cancer patients after preoperative chemoradiation: A first step towards a molecular surrogate marker for neoadjuvant treatment in colorectal cancer. Ann. Surg. 2003, 238, 324–330; discussion 321–330. [Google Scholar] [CrossRef]
- Martin, O.A.; Anderson, R.L.; Narayan, K.; MacManus, M.P. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat. Rev. Clin. Oncol. 2017, 14, 32–44. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Elbers, H.; Askoxylakis, V.; Motschall, E.; Bork, U.; Buchler, M.W.; Weitz, J.; Koch, M. Neoadjuvant radiotherapy for rectal cancer: Meta-analysis of randomized controlled trials. Ann. Surg. Oncol. 2013, 20, 4169–4182. [Google Scholar] [CrossRef]
- Eslami, S.Z.; Cortes-Hernandez, L.E.; Alix-Panabieres, C. Epithelial Cell Adhesion Molecule: An Anchor to Isolate Clinically Relevant Circulating Tumor Cells. Cells 2020, 9, 1836. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jonkheijm, P.; Terstappen, L.; Stevens, M. Magnetic Particles for CTC Enrichment. Cancers 2020, 12, 3525. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Ribeiro-Samy, S.; Oliveira, M.I.; Pereira-Veiga, T.; Muinelo-Romay, L.; Carvalho, S.; Gaspar, J.; Freitas, P.P.; López-López, R.; Costa, C.; Diéguez, L. Fast and efficient microfluidic cell filter for isolation of circulating tumor cells from unprocessed whole blood of colorectal cancer patients. Sci. Rep. 2019, 9, 8032. [Google Scholar] [CrossRef]
- Andree, K.C.; van Dalum, G.; Terstappen, L.W.M.M. Challenges in circulating tumor cell detection by the CellSearch system. Mol. Oncol. 2016, 10, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Grass, R.N.; Athanassiou, E.K.; Stark, W.J. Covalently functionalized cobalt nanoparticles as a platform for magnetic separations in organic synthesis. Angew. Chem.-Int. Ed. 2007, 46, 4909–4912. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.; Ogrinc, G.; Davies, L.; Baker, G.R.; Barnsteiner, J.; Foster, T.C.; Gali, K.; Hilden, J.; Horwitz, L.; Kaplan, H.C.; et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: Examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual. Saf. 2016, 25, e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeltner, M.; Grass, R.N.; Schaetz, A.; Bubenhofer, S.B.; Luechinger, N.A.; Stark, W.J. Stable dispersions of ferromagnetic carbon-coated metal nanoparticles: Preparation via surface initiated atom transfer radical polymerization. J. Mater. Chem. 2012, 22, 12064. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Yang, S.; Ding, B. Growing hyperbranched polyglycerols on magnetic nanoparticles to resist nonspecific adsorption of proteins. Colloids Surf. B Biointerfaces 2008, 67, 122–126. [Google Scholar] [CrossRef]
- Khan, M.; Huck, W.T.S. Hyperbranched Polyglycidol on Si/SiO2 Surfaces via Surface-Initiated Polymerization. Macromolecules 2003, 36, 5088–5093. [Google Scholar] [CrossRef]
- Das, P.; Jana, N.R. Highly Colloidally Stable Hyperbranched Polyglycerol Grafted Red Fluorescent Silicon Nanoparticle as Bioimaging Probe. ACS Appl. Mater. Interfaces 2014, 6, 4301–4309. [Google Scholar] [CrossRef]
- Li, Z.; Chau, Y. Synthesis of Linear Polyether Polyol Derivatives As New Materials for Bioconjugation. Bioconjugate Chem. 2009, 20, 780–789. [Google Scholar] [CrossRef]
- Hofer, C.J.; Grass, R.N.; Zeltner, M.; Mora, C.A.; Krumeich, F.; Stark, W.J. Hollow Carbon Nanobubbles: Synthesis, Chemical Functionalization, and Container-Type Behavior in Water. Angew. Chem. Int. Ed. 2016, 55, 8761–8765. [Google Scholar] [CrossRef]
- Marquardt, F.; Mommer, S.; Lange, J.; Jeschenko, P.; Keul, H.; Möller, M. Homoserine Lactone as a Structural Key Element for the Synthesis of Multifunctional Polymers. Polymers 2017, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Kainthan, R.K.; Janzen, J.; Levin, E.; Devine, D.V.; Brooks, D.E. Biocompatibility Testing of Branched and Linear Polyglycidol. Biomacromolecules 2006, 7, 703–709. [Google Scholar] [CrossRef]
- Frey, H.; Haag, R. Dendritic polyglycerol: A new versatile biocompatible material. Rev. Mol. Biotechnol. 2002, 90, 257–267. [Google Scholar] [CrossRef]
- Shen, J.; Ly, K.; Hoang, Y. Cell Culture Medium. Hum. Stem Cell Man. 2012, 53–69. [Google Scholar] [CrossRef]
- Lang, T.; Bauters, A.; Braun, S.L.; Pötzsch, B.; von Pape, K.-W.; Kolde, H.-J.; Lakner, M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2005, 16, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.F.; Clark, G.C.F. The Corrosion of Pure Cobalt in Physiological Media. J. Mater. Sci. 1982, 17, 1675–1682. [Google Scholar] [CrossRef]
- Hyun, K.A.; Koo, G.B.; Han, H.; Sohn, J.; Choi, W.; Kim, S.I.; Jung, H.I.; Kim, Y.S. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 2016, 7, 24677–24687. [Google Scholar] [CrossRef] [Green Version]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Doswald, S.; Stark, W.J.; Beck-Schimmer, B. Biochemical functionality of magnetic particles as nanosensors: How far away are we to implement them into clinical practice? J. Nanobiotechnology 2019, 17, 73. [Google Scholar] [CrossRef]
- Poli, E.C.; Rimmelé, T.; Schneider, A.G. Hemoadsorption with CytoSorb(®). Intensive Care Med. 2019, 45, 236–239. [Google Scholar] [CrossRef]
- Bougas, L.; Langenegger, L.D.; Mora, C.A.; Zeltner, M.; Stark, W.J.; Wickenbrock, A.; Blanchard, J.W.; Budker, D. Nondestructive in-line sub-picomolar detection of magnetic nanoparticles in flowing complex fluids. Sci. Rep. 2018, 8, 3491. [Google Scholar] [CrossRef] [Green Version]
| Particles | B-Lymphocytes (n Cells per 106 Counting Beads) | T-Lymphocytes (n Cells per 106 Counting Beads) | ||||
|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 3 | Test 1 | Test 2 | Test 3 | |
| IgG | 3529 | 2936 | 1641 | 34888 | 27673 | 20943 |
| Anti-EpCAM | 4233 | 2927 | 1447 | 40493 | 28682 | 19231 |
| Coagulation Parameters | EXTEM | INTEM | ||||
|---|---|---|---|---|---|---|
| CT (s) | CFT (s) | MCF (mm) | CT (s) | CFT (s) | MCF (mm) | |
| Test 1 | ||||||
| IgG | 69 | 95 | 61 | 177 | 72 | 60 |
| Anti-EpCAM | 74 | 116 | 55 | 207 | 71 | 76 |
| Test 2 | ||||||
| IgG | 68 | 162 | 48 | 213 | 139 | 48 |
| Anti-EpCAM | 109 | 185 | 44 | 211 | 150 | 47 |
| Test 3 | ||||||
| IgG | 67 | 182 | 49 | 186 | 106 | 53 |
| Anti-EpCam | 71 | 178 | 47 | 175 | 105 | 51 |
| Normal range | 38–79 | 34–159 | 50–72 | 100–240 | 30-110 | 50–72 |
| CTC | n CTC without Treatment | n CTC with Treatment | % CTC Removed |
|---|---|---|---|
| Sample 1 | 1946 | 620 | 68 |
| Sample 2 | 95 | 22 | 77 |
| Sample 3 | 161 | 1 | 99 |
| Sample 4 | 8 | 5 | 37 |
| Sample 5 | 75 | 13 | 83 |
| Sample 6 | 11 | 6 | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doswald, S.; Herzog, A.F.; Zeltner, M.; Zabel, A.; Pregernig, A.; Schläpfer, M.; Siebenhüner, A.; Stark, W.J.; Beck-Schimmer, B. Removal of Circulating Tumor Cells from Blood Samples of Cancer Patients Using Highly Magnetic Nanoparticles: A Translational Research Project. Pharmaceutics 2022, 14, 1397. https://doi.org/10.3390/pharmaceutics14071397
Doswald S, Herzog AF, Zeltner M, Zabel A, Pregernig A, Schläpfer M, Siebenhüner A, Stark WJ, Beck-Schimmer B. Removal of Circulating Tumor Cells from Blood Samples of Cancer Patients Using Highly Magnetic Nanoparticles: A Translational Research Project. Pharmaceutics. 2022; 14(7):1397. https://doi.org/10.3390/pharmaceutics14071397
Chicago/Turabian StyleDoswald, Simon, Antoine F. Herzog, Martin Zeltner, Anja Zabel, Andreas Pregernig, Martin Schläpfer, Alexander Siebenhüner, Wendelin J. Stark, and Beatrice Beck-Schimmer. 2022. "Removal of Circulating Tumor Cells from Blood Samples of Cancer Patients Using Highly Magnetic Nanoparticles: A Translational Research Project" Pharmaceutics 14, no. 7: 1397. https://doi.org/10.3390/pharmaceutics14071397
APA StyleDoswald, S., Herzog, A. F., Zeltner, M., Zabel, A., Pregernig, A., Schläpfer, M., Siebenhüner, A., Stark, W. J., & Beck-Schimmer, B. (2022). Removal of Circulating Tumor Cells from Blood Samples of Cancer Patients Using Highly Magnetic Nanoparticles: A Translational Research Project. Pharmaceutics, 14(7), 1397. https://doi.org/10.3390/pharmaceutics14071397






