Intravitreal Application: Physicochemical Properties of Drugs Dissolved in Silicone Oils of Different Density in Comparison to the Porcine Vitreous Body
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
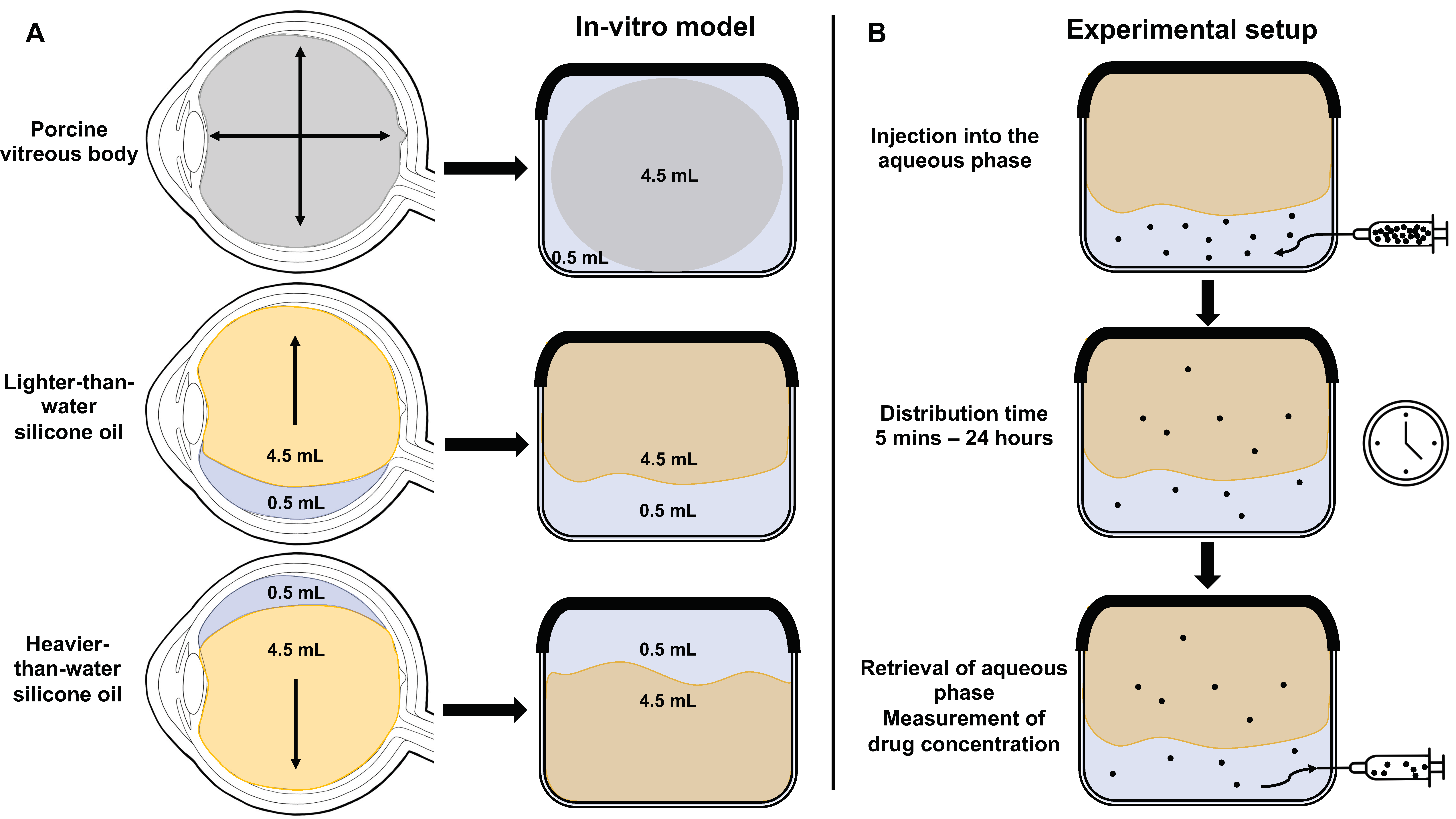
2.1.1. Preparation of the In Vitro Model of the Vitreous Cavity
2.1.2. Preparation of the Hydrophilic Phase with Solution of Voriconazole, Vancomycin and Ceftazidime
2.2. Methods
2.2.1. Experimental Setup
2.2.2. Quantification of Drugs by High-Performance Liquid Chromatography (HPLC)
2.2.3. Statistical Analysis
3. Results
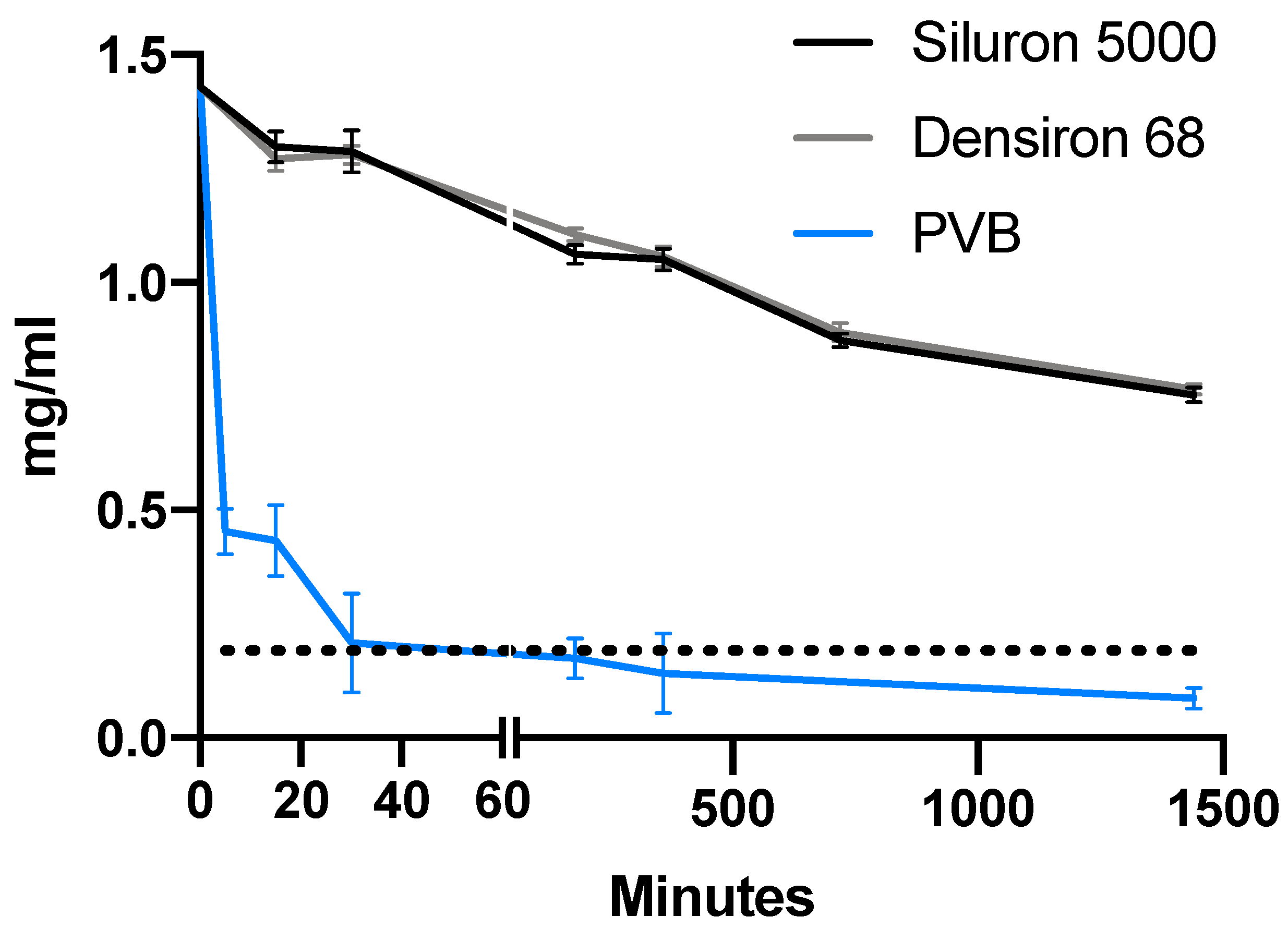
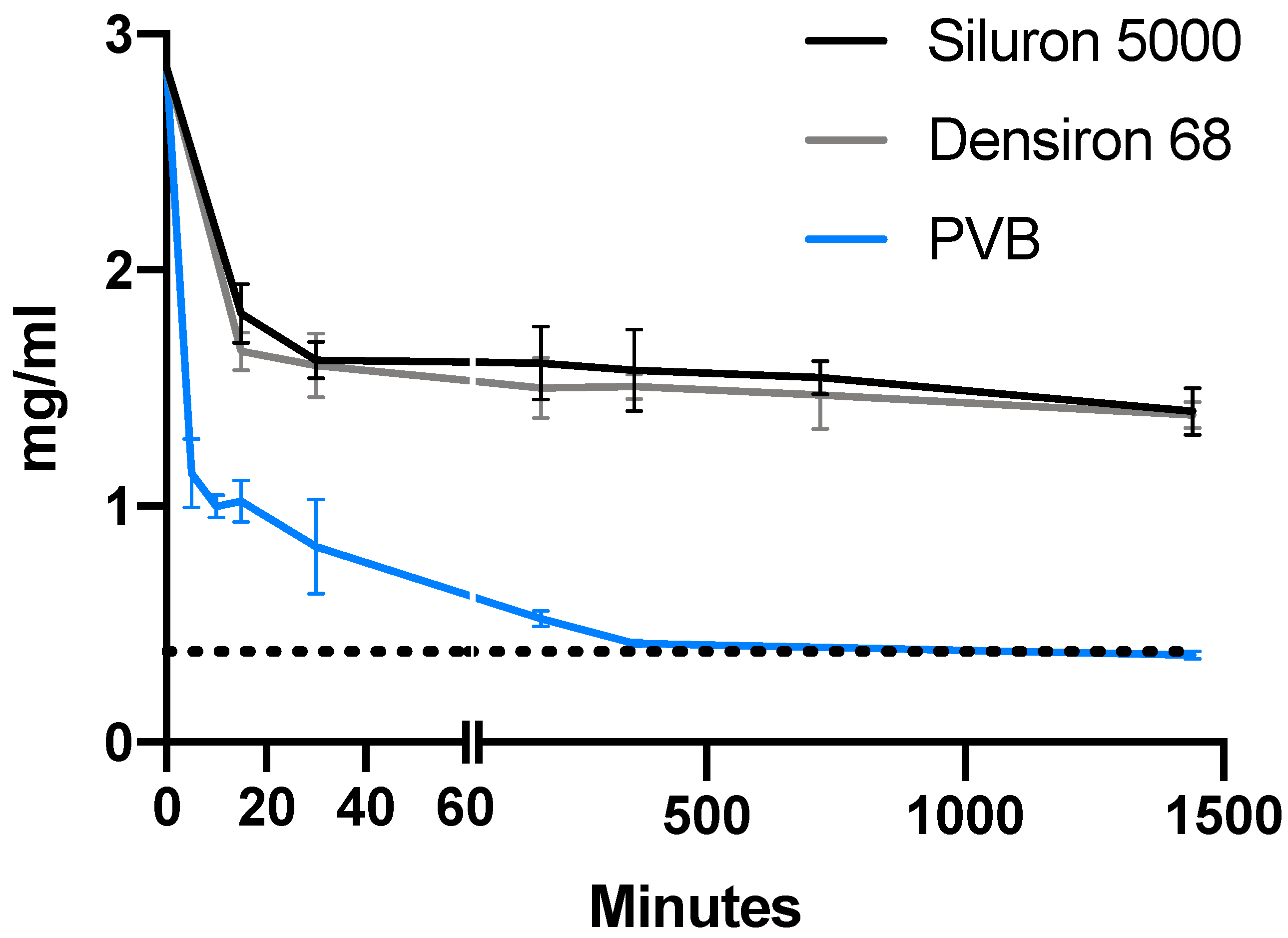
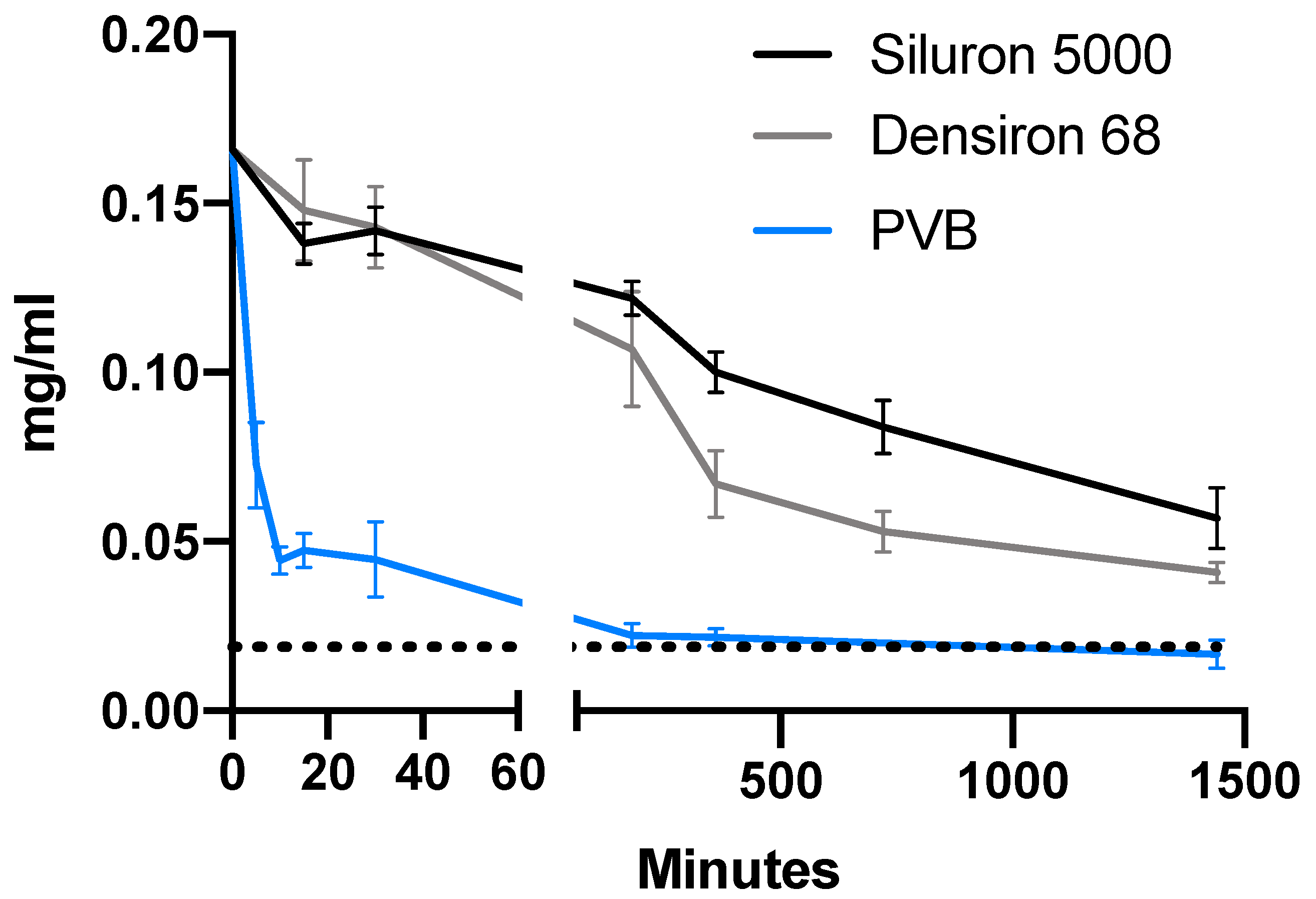
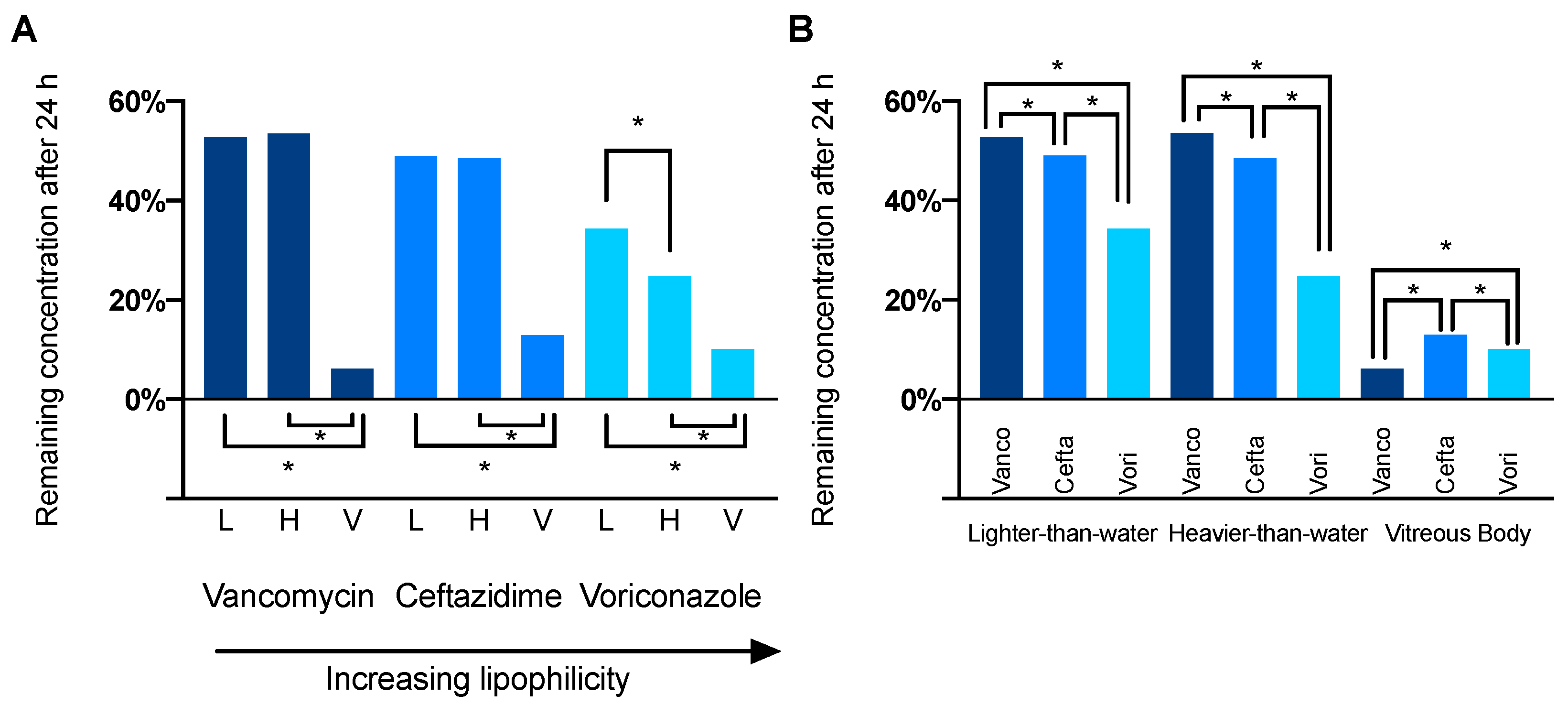
3.1. Comparison of Drug Distribution between Porcine Vitreous Bodies and Silicone Oils
3.2. Differences in Drug Distribution between Light and Heavy Silicone Oil
3.3. Differences in Solubility
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armaly, M.F. Ocular Tolerance to Silicones. I. Replacement of Aqueous and Vitreous by Silicone Fluids. Arch. Ophthalmol. 1962, 68, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Cibis, P.A.; Becker, B.; Okun, E.; Canaan, S. The Use of Liquid Silicone in Retinal Detachment Surgery. Arch. Ophthalmol. 1962, 68, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, A. Vitrectomy with Silicone Oil Infusion in Severe Diabetic Retinopathy. Br. J. Ophthalmol. 2003, 87, 318–321. [Google Scholar] [CrossRef]
- Williams, R.L.; Day, M.J.; Garvey, M.J.; Morphis, G.; Irigoyen, C.; Wong, D.; Stappler, T. Injectability of Silicone Oil-Based Tamponade Agents. Br. J. Ophthalmol. 2011, 95, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Schickhardt, S.K.; Munro, D.; Khoramnia, R.; Scheuerle, A.; Mayer, C.S.; Auffarth, G.U. The Novel Use of High-Flow Polyimide Cannulas to Improve Silicone Oil Injectability in Vitreoretinal Surgery. Retina 2022, 42, 1170–1175. [Google Scholar] [CrossRef]
- Hammer, M.; Schickhardt, S.; Munro, D.J.; Scheuerle, A.; Mayer, C.S.; Auffarth, G.U. Physicochemical Properties of Explanted Silicone Oil after Use as an Intraocular Tamponade. Trans. Vis. Sci. Technol. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L.; Day, M.; Garvey, M.J.; English, R.; Wong, D. Increasing the Extensional Viscosity of Silicone Oil Reduces the Tendency for Emulsification. Retina 2010, 30, 300–304. [Google Scholar] [CrossRef]
- Caramoy, A.; Schröder, S.; Fauser, S.; Kirchhof, B. In Vitro Emulsification Assessment of New Silicone Oils. Br. J. Ophthalmol. 2010, 94, 509–512. [Google Scholar] [CrossRef]
- Imamura, T.; Kakinoki, M.; Hira, D.; Kitagawa, T.; Ueshima, S.; Kakumoto, M.; Terada, T.; Kawamoto, I.; Murase, M.; Ohji, M. Pharmacokinetics of Intravitreal Vancomycin and Ceftazidime in Silicone Oil-Filled Macaque Eyes. Trans. Vis. Sci. Technol. 2021, 10, 1. [Google Scholar] [CrossRef]
- Hira, D.; Kitagawa, T.; Imamura, T.; Kakinoki, M.; Ueshima, S.; Okano, T.; Ohji, M.; Kakumoto, M.; Terada, T. Impact of Silicone Oil Tamponade on Intravitreally Injected Vancomycin Pharmacokinetics in Cynomolgus Monkey Eyes. Int. J. Pharm. 2021, 609, 121185. [Google Scholar] [CrossRef]
- Dutescu, R.M.; Panfil, C.; Merkel, O.M.; Schrage, N. Semifluorinated Alkanes as a Liquid Drug Carrier System for Topical Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2014, 88, 123–128. [Google Scholar] [CrossRef]
- del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Rimpelä, A.-K.; Reunanen, S.; Hagström, M.; Kidron, H.; Urtti, A. Binding of Small Molecule Drugs to Porcine Vitreous Humor. Mol. Pharm. 2018, 15, 2174–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petternel, V.; Krepler, K.; Schauersberger, J.; Wedrich, A. Fosfomycin in Human Vitreous: -In-Vitro Investigation of the Protein Binding of Fosfomycin in Human Vitreous-Fosfomycin Levels in the Vitreous Cavity after Intravenous Administration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4930. [Google Scholar]
- Schauersberger, J.; Jager, W. In-Vitro Investigation of the Protein Binding of Different Antibiotics in the Human Vitreous. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1853. [Google Scholar]
- Schulz, A.; Wahl, S.; Rickmann, A.; Ludwig, J.; Stanzel, B.V.; von Briesen, H.; Szurman, P. Age-Related Loss of Human Vitreal Viscoelasticity. Trans. Vis. Sci. Technol. 2019, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhl, P.; Pantze, S.; Storck, P.; Parmentier, J.; Witzigmann, D.; Hofhaus, G.; Huwyler, J.; Mier, W.; Fricker, G. Oral Delivery of Vancomycin by Tetraether Lipid Liposomes. Eur. J. Pharm. Sci. 2017, 108, 111–118. [Google Scholar] [CrossRef]
- Leung, E.H.; Stout, J.T. Antibiotics and Antifungals in Silicone Oil. Int. J. Retin. Vitr. 2019, 5, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelkader, E.; Siddiqui, R.M.; Ramalingam, S.; Murrary, A.; Lois, N. Heavier-than-Water Silicone Oil Mixture as a Long-Term Tamponade Agent: A Pilot Study. Ophthalmologica 2011, 226, 60–65. [Google Scholar] [CrossRef]
- Prazeres, J.; Magalhães, O.; Lucatto, L.F.A.; Navarro, R.M.; Moraes, N.S.; Farah, M.E.; Maia, A.; Maia, M. Heavy Silicone Oil as a Long-Term Endotamponade Agent for Complicated Retinal Detachments. BioMed. Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Günther, B.; Meinert, H. A New, Heavier-than-Water Silicone Oil: A Solution of Perfluorohexyloctane in Polydimethylsiloxane. Eur. J. Ophthalmol. 2005, 15, 627–637. [Google Scholar] [CrossRef]
- De Majumdar, S.; Subinya, M.; Korward, J.; Pettigrew, A.; Scherer, D.; Xu, H. A Low Concentration of Tacrolimus/Semifluorinated Alkane (SFA) Eyedrop Suppresses Intraocular Inflammation in Experimental Models of Uveitis. Curr. Mol. Med. 2017, 17, 211–220. [Google Scholar] [CrossRef]
- Gehlsen, U.; Braun, T.; Notara, M.; Krösser, S.; Steven, P. A Semifluorinated Alkane (F4H5) as Novel Carrier for Cyclosporine A: A Promising Therapeutic and Prophylactic Option for Topical Treatment of Dry Eye. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, P.; Scherer, D.; Günther, B.; Rupenthal, I.D. Semifluorinated Alkane Based Systems for Enhanced Corneal Penetration of Poorly Soluble Drugs. Int. J. Pharm. 2018, 538, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Tsagogiorgas, C.; Jung, T.; Krebs, J.; Theisinger, B.; Beck, G.; Yard, B.A.; Quintel, M. Aerosolized Semifluorinated Alkanes as Excipients Are Suitable for Inhalative Drug Delivery—A Pilot Study. Int. J. Pharm. 2012, 422, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Tsagogiorgas, C.; Theisinger, S.; Heesch, E.; Krebs, J.; Holm, R.; Beck, G.; Yard, B. Evaluation of Pharmacokinetic Properties and Anaesthetic Effects of Propofol in a New Perfluorohexyloctane (F6H8) Emulsion in Rats—A Comparative Study. Int. J. Pharm. 2015, 486, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kralinger, M.T.; Kieselbach, G.F.; Voigt, M.; Parel, J.-M. Slow Release of Acetylsalicylic Acid by Intravitreal Silicone Oil. Retina 2001, 21, 513–520. [Google Scholar] [CrossRef]
- Cauldbeck, H.; Le Hellaye, M.; McDonald, T.O.; Long, M.; Williams, R.L.; Rannard, S.P.; Kearns, V.R. Modulated Release from Implantable Ocular Silicone Oil Tamponade Drug Reservoirs. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 938–946. [Google Scholar] [CrossRef]
- Cauldbeck, H.; Le Hellaye, M.; Long, M.; Kennedy, S.M.; Williams, R.L.; Kearns, V.R.; Rannard, S.P. Controlling Drug Release from Non-Aqueous Environments: Moderating Delivery from Ocular Silicone Oil Drug Reservoirs to Combat Proliferative Vitreoretinopathy. J. Control. Release 2016, 244, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Chan, Y.K.; Lau, L.H.; Wong, D.; Wong, J.K.W.; Shih, K.C.; Lai, S.M.; Shum, H.C. Amphiphilic Additives in Silicone Oil Tamponade and Emulsification: An Eye-on-a-chip Study. Acta Ophthalmol. 2020, 98, e232–e237. [Google Scholar] [CrossRef]
- Barot, M.; Bagui, M.; Gokulgandhi, M.R.; Mitra, A.K. Prodrug Strategies in Ocular Drug Delivery. Med. Chem. 2012, 8, 753–768. [Google Scholar] [CrossRef] [Green Version]
- Macha, S.; Duvvuri, S.; Mitra, A.K. Ocular Disposition of Novel Lipophilic Diester Prodrugs of Ganciclovir Following Intravitreal Administration Using Microdialysis. Curr. Eye Res. 2004, 28, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tirucherai, G.S.; Dias, C.; Mitra, A.K. Corneal Permeation of Ganciclovir: Mechanism of Ganciclovir Permeation Enhancement by Acyl Ester Prodrug Design. J. Ocul. Pharmacol. Ther. 2002, 18, 535–548. [Google Scholar] [CrossRef] [PubMed]
- For the International Retina Group (IRG); Iglicki, M.; Zur, D.; Fung, A.; Gabrielle, P.-H.; Lupidi, M.; Santos, R.; Busch, C.; Rehak, M.; Cebeci, Z.; et al. TRActional DIabetic ReTInal Detachment Surgery with Co-Adjuvant Intravitreal DexamethasONe Implant: The Tradition Study. Acta Diabetol. 2019, 56, 1141–1147. [Google Scholar] [CrossRef]
- Angi, M.; Kalirai, H.; Coupland, S.E.; Damato, B.E.; Semeraro, F.; Romano, M.R. Proteomic Analyses of the Vitreous Humour. Mediat. Inflamm. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Schnichels, S.; Schneider, N.; Hohenadl, C.; Hurst, J.; Schatz, A.; Januschowski, K.; Spitzer, M.S. Efficacy of Two Different Thiol-Modified Crosslinked Hyaluronate Formulations as Vitreous Replacement Compared to Silicone Oil in a Model of Retinal Detachment. PLoS ONE 2017, 12, e0172895. [Google Scholar] [CrossRef]
- Hurst, J.; Rickmann, A.; Heider, N.; Hohenadl, C.; Reither, C.; Schatz, A.; Schnichels, S.; Januschowski, K.; Spitzer, M.S. Long-Term Biocompatibility of a Highly Viscously Thiol-Modified Cross-Linked Hyaluronate as a Novel Vitreous Body Substitute. Front. Pharmacol. 2022, 13, 817353. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Okamoto, F.; Hoshi, S.; Katashima, T.; Zujur, D.C.; Li, X.; Shibayama, M.; Gilbert, E.P.; Chung, U.; Ohba, S.; et al. Fast-Forming Hydrogel with Ultralow Polymeric Content as an Artificial Vitreous Body. Nat. Biomed. Eng. 2017, 1, 0044. [Google Scholar] [CrossRef]
- Schulz, A.; Rickmann, A.; Wahl, S.; Germann, A.; Stanzel, B.V.; Januschowski, K.; Szurman, P. Alginate- and Hyaluronic Acid–Based Hydrogels as Vitreous Substitutes: An In Vitro Evaluation. Trans. Vis. Sci. Technol. 2020, 9, 34. [Google Scholar] [CrossRef]
| Indication | Molecular Weight (g/mol) | LogP | |
|---|---|---|---|
| vancomycin | Bacterial endophthalmitis | 1449.25 | −4.4 |
| ceftazidime | Bacterial endophthalmitis | 546.58 | −1.6 |
| voriconazole | Fungal endophthalmitis | 349.3 | 1.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammer, M.; Schickhardt, S.K.; Merz, P.R.; Khoramnia, R.; Scheuerle, A.F.; Mier, W.; Uhl, P.; Auffarth, G.U. Intravitreal Application: Physicochemical Properties of Drugs Dissolved in Silicone Oils of Different Density in Comparison to the Porcine Vitreous Body. Pharmaceutics 2022, 14, 1364. https://doi.org/10.3390/pharmaceutics14071364
Hammer M, Schickhardt SK, Merz PR, Khoramnia R, Scheuerle AF, Mier W, Uhl P, Auffarth GU. Intravitreal Application: Physicochemical Properties of Drugs Dissolved in Silicone Oils of Different Density in Comparison to the Porcine Vitreous Body. Pharmaceutics. 2022; 14(7):1364. https://doi.org/10.3390/pharmaceutics14071364
Chicago/Turabian StyleHammer, Maximilian, Sonja K. Schickhardt, Patrick R. Merz, Ramin Khoramnia, Alexander F. Scheuerle, Walter Mier, Philipp Uhl, and Gerd U. Auffarth. 2022. "Intravitreal Application: Physicochemical Properties of Drugs Dissolved in Silicone Oils of Different Density in Comparison to the Porcine Vitreous Body" Pharmaceutics 14, no. 7: 1364. https://doi.org/10.3390/pharmaceutics14071364
APA StyleHammer, M., Schickhardt, S. K., Merz, P. R., Khoramnia, R., Scheuerle, A. F., Mier, W., Uhl, P., & Auffarth, G. U. (2022). Intravitreal Application: Physicochemical Properties of Drugs Dissolved in Silicone Oils of Different Density in Comparison to the Porcine Vitreous Body. Pharmaceutics, 14(7), 1364. https://doi.org/10.3390/pharmaceutics14071364







