Engineering of a Long-Acting Bone Morphogenetic Protein-7 by Fusion with Albumin for the Treatment of Renal Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
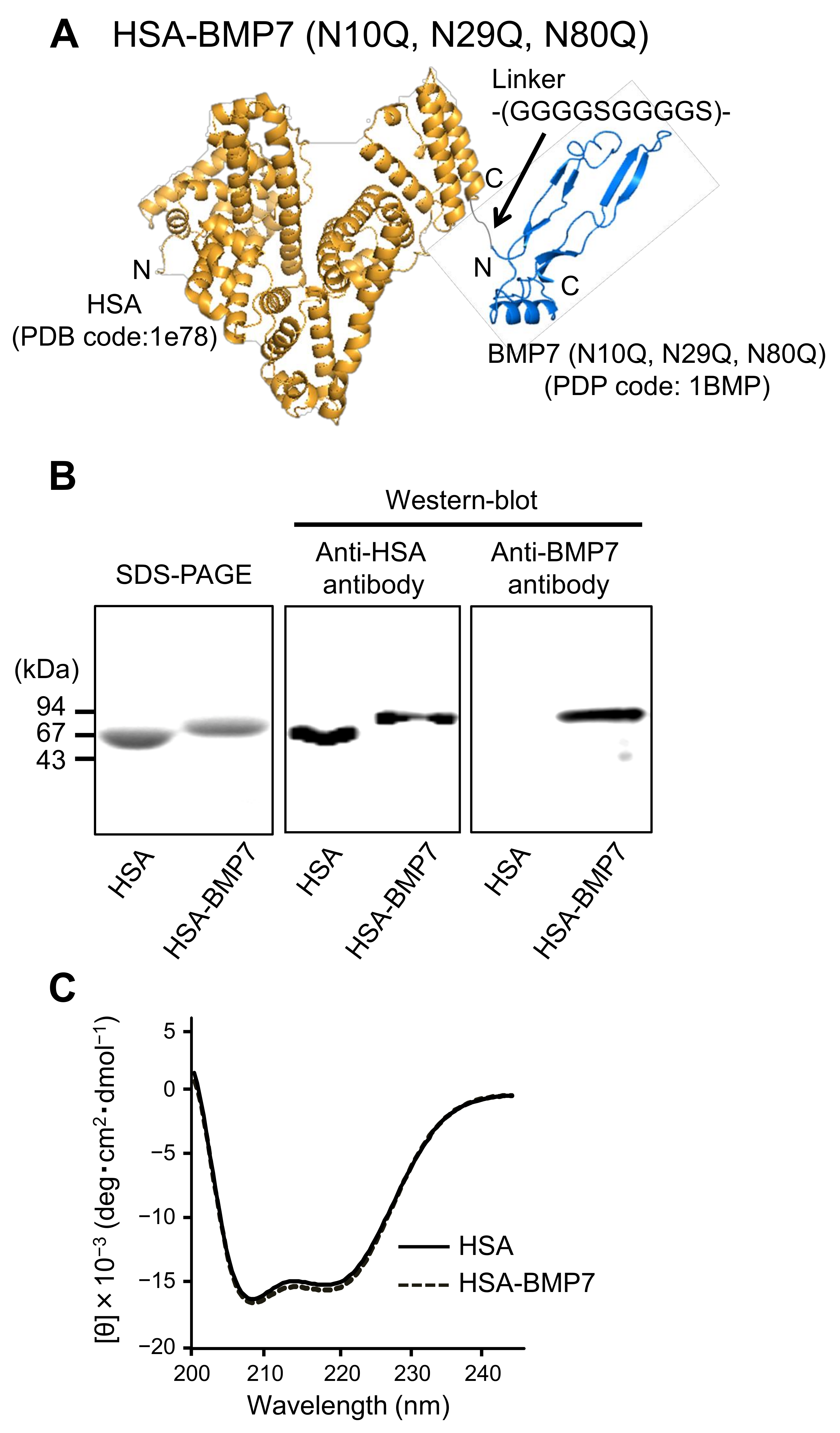
2.2. Production of HSA-BMP7 Fusion Protein
2.3. Circular Dichroism Spectral Measurement
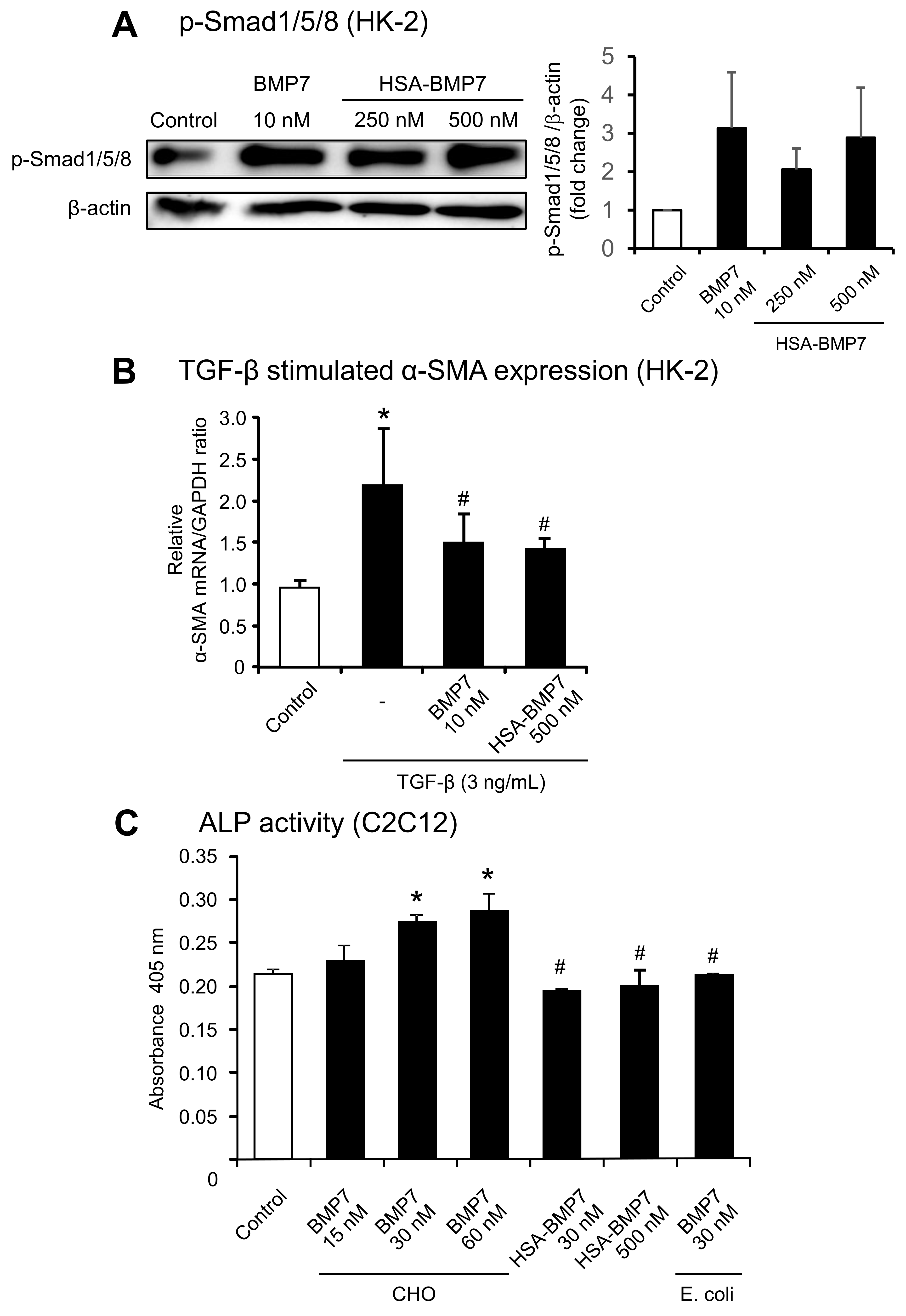
2.4. In Vitro Activity of HSA-BMP7
2.5. Pharmacokinetic Analyses of HSA-BMP7
2.6. Mouse Model of Unilateral Ureteral Obstruction (UUO) Induced Renal Fibrosis
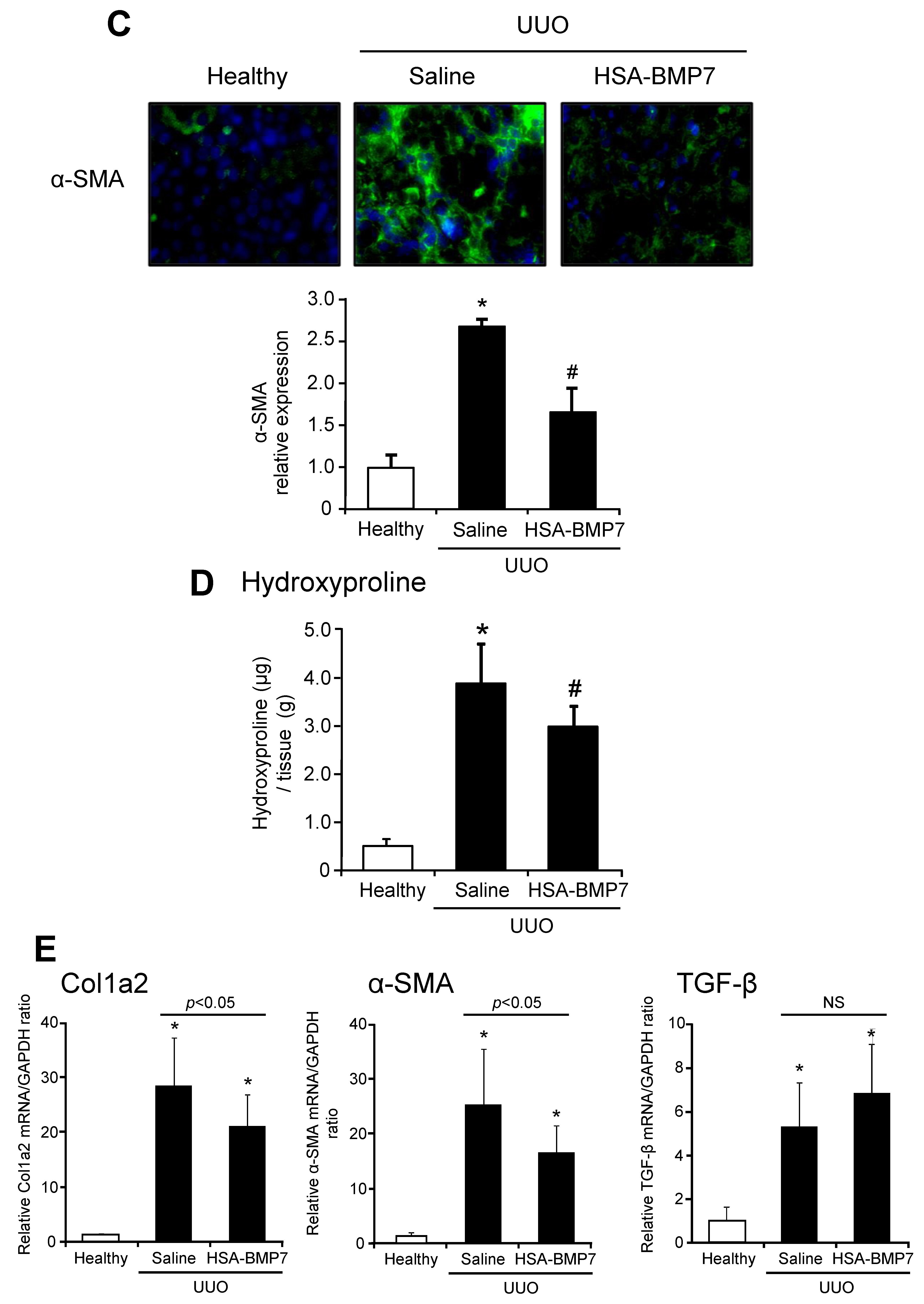
2.7. Histopathological Analysis
2.8. Determination of Hydroxyproline Levels
2.9. Quantitative RT-PCR Analysis
2.10. Cisplatin-Induced Acute Kidney Injury Model
2.11. Biochemical Evaluation of Blood and Urine Samples
2.12. Histologic Examination of Renal Tissues
2.13. Statistical Analyses
3. Results
3.1. Structural Properties of HSA-BMP7 Fusion Protein
3.2. In Vitro Activity of HSA-BMP7 Relative to Native Glycosylated BMP7
3.3. Pharmacokinetic Profile of HSA-BMP7 in Mice
3.4. Effect of HSA-BMP7 on the Renal Fibrosis in Unilateral Ureteral Obstruction Mice
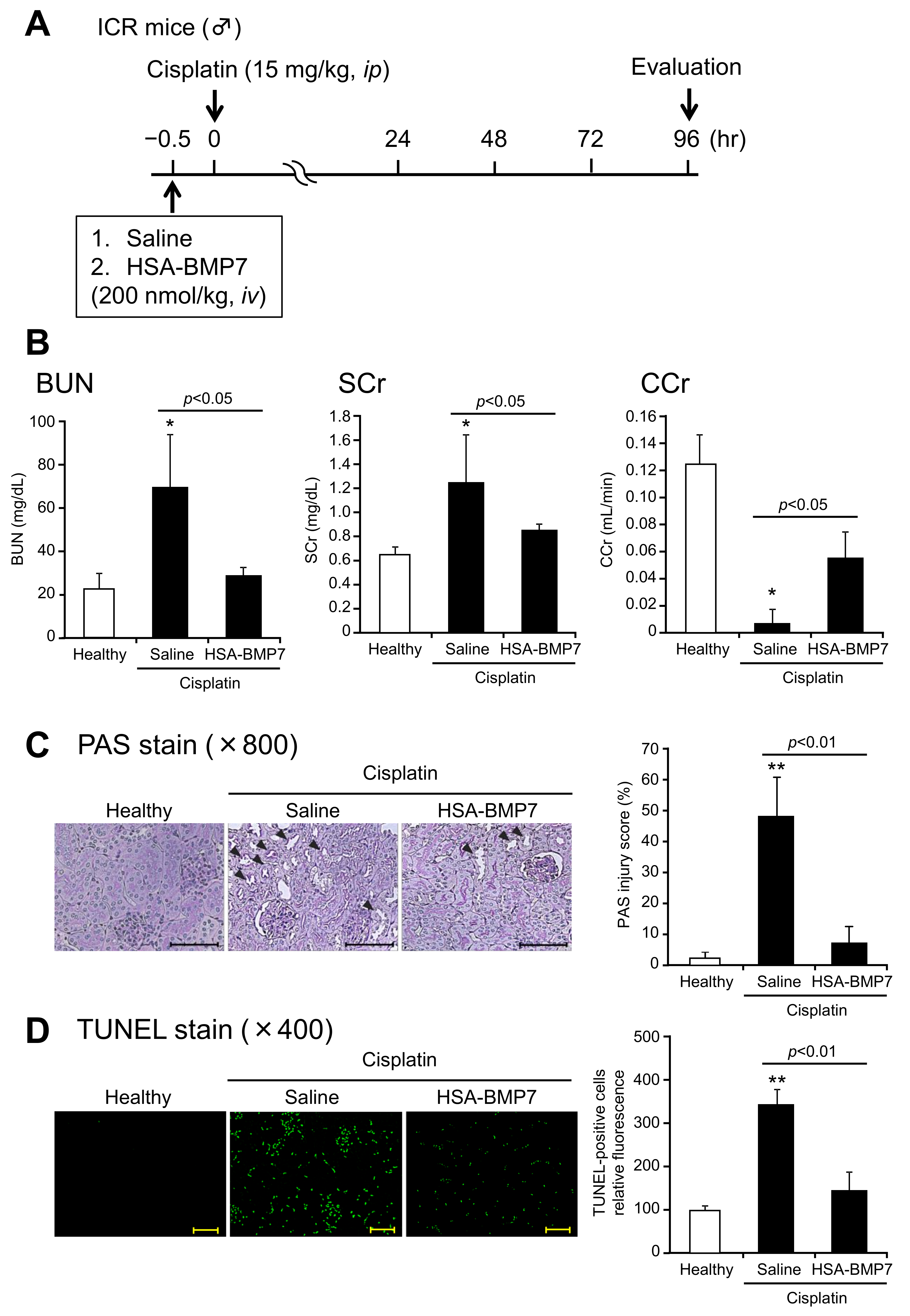
3.5. Effect of HSA-BMP7 on Cisplatin-Induced Nephropathy in Mice
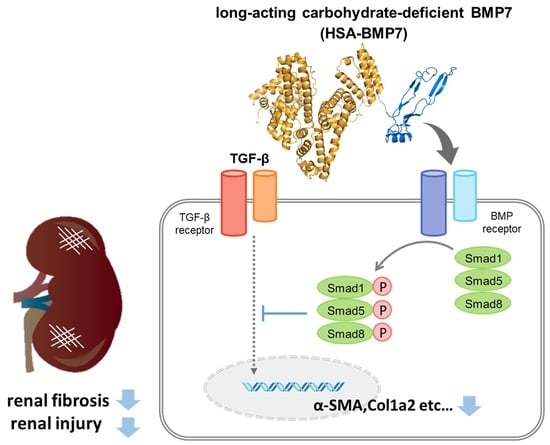
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Declèves, A.E.; Sharma, K. New pharmacological treatments for improving renal outcomes in diabetes. Nat. Rev. Nephrol. 2010, 6, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Böttinger, E.P. TGF-beta in renal injury and disease. Semin. Nephrol. 2007, 27, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Chung, A.C.; Lan, H.Y. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin. Sci. 2013, 124, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.T.; Lyons, K.M.; Robertson, E.J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995, 9, 2795–2807. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; de Caestecker, M.; Kopp, J.; Mitu, G.; Lapage, J.; Hirschberg, R. Renal bone morphogenetic protein-7 protects against diabetic nephropathy. J. Am. Soc. Nephrol. 2006, 17, 2504–2512. [Google Scholar] [CrossRef]
- Vukicevic, S.; Basic, V.; Rogic, D.; Basic, N.; Shih, M.S.; Shepard, A.; Jin, D.; Dattatreyamurty, B.; Jones, W.; Dorai, H.; et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J. Clin. Investig. 1998, 102, 202–214. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Q.; Simon, T.C.; Strebeck, F.; Chaudhary, L.; Morrissey, J.; Liapis, H.; Klahr, S.; Hruska, K.A. Bone morphogenic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney Int. 2003, 63, 2037–2049. [Google Scholar] [CrossRef]
- Sugimoto, H.; Grahovac, G.; Zeisberg, M.; Kalluri, R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes 2007, 56, 1825–1833. [Google Scholar] [CrossRef]
- Zeisberg, M.; Hanai, J.; Sugimoto, H.; Mammoto, T.; Charytan, D.; Strutz, F.; Kalluri, R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003, 9, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Bottiglio, C.; Kumar, N.; Maeshima, Y.; Strutz, F.; Müller, G.A.; Kalluri, R. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am. J. Physiol. Renal. Physiol. 2003, 285, F1060–F1067. [Google Scholar] [CrossRef] [PubMed]
- Yeh, L.C.; Adamo, M.L.; Olson, M.S.; Lee, J.C. Osteogenic protein-1 and insulin-like growth factor I synergistically stimulate rat osteoblastic cell differentiation and proliferation. Endocrinology 1997, 138, 4181–4190. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, V.; Aspera-Werz, R.H.; Tendulkar, G.; Reumann, M.K.; Freude, T.; Breitkopf-Heinlein, K.; Dooley, S.; Pscherer, S.; Ochs, B.G.; Flesch, I.; et al. BMP9 a possible alternative drug for the recently withdrawn BMP7? New perspectives for (re-)implementation by personalized medicine. Arch. Toxicol. 2017, 91, 1353–1366. [Google Scholar] [CrossRef]
- Spiro, A.S.; Beil, F.T.; Baranowsky, A.; Barvencik, F.; Schilling, A.F.; Nguyen, K.; Khadem, S.; Seitz, S.; Rueger, J.M.; Schinke, T.; et al. BMP-7-induced ectopic bone formation and fracture healing is impaired by systemic NSAID application in C57BL/6-mice. J. Orthop. Res. 2010, 28, 785–791. [Google Scholar] [CrossRef]
- Saremba, S.; Nickel, J.; Seher, A.; Kotzsch, A.; Sebald, W.; Mueller, T.D. Type I receptor binding of bone morphogenetic protein 6 is dependent on N-glycosylation of the ligand. FEBS J. 2008, 275, 172–183. [Google Scholar] [CrossRef]
- Chen, R.F. Removal of fatty acids from serum albumin by charcoal treatment. J. Biol. Chem. 1967, 242, 173–181. [Google Scholar] [CrossRef]
- Ikuta, S.; Chuang, V.T.; Ishima, Y.; Nakajou, K.; Furukawa, M.; Watanabe, H.; Maruyama, T.; Otagiri, M. Albumin fusion of thioredoxin--the production and evaluation of its biological activity for potential therapeutic applications. J. Control. Release 2010, 147, 17–23. [Google Scholar] [CrossRef]
- Nishida, K.; Watanabe, H.; Ogaki, S.; Kodama, A.; Tanaka, R.; Imafuku, T.; Ishima, Y.; Chuang, V.T.; Toyoda, M.; Kondoh, M.; et al. Renoprotective effect of long acting thioredoxin by modulating oxidative stress and macrophage migration inhibitory factor against rhabdomyolysis-associated acute kidney injury. Sci. Rep. 2015, 5, 14471. [Google Scholar] [CrossRef]
- Tanaka, R.; Watanabe, H.; Kodama, A.; Chuang, V.T.; Ishima, Y.; Hamasaki, K.; Tanaka, K.; Mizushima, T.; Otagiri, M.; Maruyama, T. Long-acting human serum albumin-thioredoxin fusion protein suppresses bleomycin-induced pulmonary fibrosis progression. J. Pharmacol. Exp. Ther. 2013, 345, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, B.L.; Imamura, T.; Okadome, T.; Cox, G.N.; Yamashita, H.; ten Dijke, P.; Heldin, C.H.; Miyazono, K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 7632–7636. [Google Scholar] [CrossRef]
- ten Dijke, P.; Yamashita, H.; Sampath, T.K.; Reddi, A.H.; Estevez, M.; Riddle, D.L.; Ichijo, H.; Heldin, C.H.; Miyazono, K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 1994, 269, 16985–16988. [Google Scholar] [CrossRef]
- Liu, F.; Ventura, F.; Doody, J.; Massagué, J. Human type II receptor for bone morphogenic proteins (BMPs): Extension of the two-kinase receptor model to the BMPs. Mol. Cell. Biol. 1995, 15, 3479–3486. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; LeBleu, V.S.; Bosukonda, D.; Keck, P.; Taduri, G.; Bechtel, W.; Okada, H.; Carlson, W.; Bey, P.; Rusckowski, M.; et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat. Med. 2012, 18, 396–404. [Google Scholar] [CrossRef]
- Youngster, S.; Wang, Y.S.; Grace, M.; Bausch, J.; Bordens, R.; Wyss, D.F. Structure, biology, and therapeutic implications of pegylated interferon alpha-2b. Curr. Pharm. Des. 2002, 8, 2139–2157. [Google Scholar] [CrossRef] [PubMed]
- Metzner, H.J.; Pipe, S.W.; Weimer, T.; Schulte, S. Extending the pharmacokinetic half-life of coagulation factors by fusion to recombinant albumin. Thromb. Haemost. 2013, 110, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Wang, X.; Wang, Y.; Niu, A.; Wang, S.; Zou, C.; Harris, R.C. IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int. 2017, 91, 375–386. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Wang, S.L.; Yang, C.Q.; Qi, X.L.; Yuan, M.; Chang, Y.Z.; Yang, L.; Gao, H.J. Inhibitory effect of bone morphogenetic protein-7 on hepatic fibrosis in rats. Int. J. Clin. Exp. Pathol. 2013, 6, 897–903. [Google Scholar] [PubMed]
- Morrissey, C.; Brown, L.G.; Pitts, T.E.; Vessella, R.L.; Corey, E. Bone morphogenetic protein 7 is expressed in prostate cancer metastases and its effects on prostate tumor cells depend on cell phenotype and the tumor microenvironment. Neoplasia 2010, 12, 192–205. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takano, M.; Toda, S.; Watanabe, H.; Fujimura, R.; Nishida, K.; Bi, J.; Minayoshi, Y.; Miyahisa, M.; Maeda, H.; Maruyama, T. Engineering of a Long-Acting Bone Morphogenetic Protein-7 by Fusion with Albumin for the Treatment of Renal Injury. Pharmaceutics 2022, 14, 1334. https://doi.org/10.3390/pharmaceutics14071334
Takano M, Toda S, Watanabe H, Fujimura R, Nishida K, Bi J, Minayoshi Y, Miyahisa M, Maeda H, Maruyama T. Engineering of a Long-Acting Bone Morphogenetic Protein-7 by Fusion with Albumin for the Treatment of Renal Injury. Pharmaceutics. 2022; 14(7):1334. https://doi.org/10.3390/pharmaceutics14071334
Chicago/Turabian StyleTakano, Mei, Shota Toda, Hiroshi Watanabe, Rui Fujimura, Kento Nishida, Jing Bi, Yuki Minayoshi, Masako Miyahisa, Hitoshi Maeda, and Toru Maruyama. 2022. "Engineering of a Long-Acting Bone Morphogenetic Protein-7 by Fusion with Albumin for the Treatment of Renal Injury" Pharmaceutics 14, no. 7: 1334. https://doi.org/10.3390/pharmaceutics14071334
APA StyleTakano, M., Toda, S., Watanabe, H., Fujimura, R., Nishida, K., Bi, J., Minayoshi, Y., Miyahisa, M., Maeda, H., & Maruyama, T. (2022). Engineering of a Long-Acting Bone Morphogenetic Protein-7 by Fusion with Albumin for the Treatment of Renal Injury. Pharmaceutics, 14(7), 1334. https://doi.org/10.3390/pharmaceutics14071334