Placenta-Derived Mesenchymal-like Adherent Stromal Cells as an Effective Cell Therapy for Cocaine Addiction in a Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Animals
2.3. Catheter and Guide Cannula Implantation
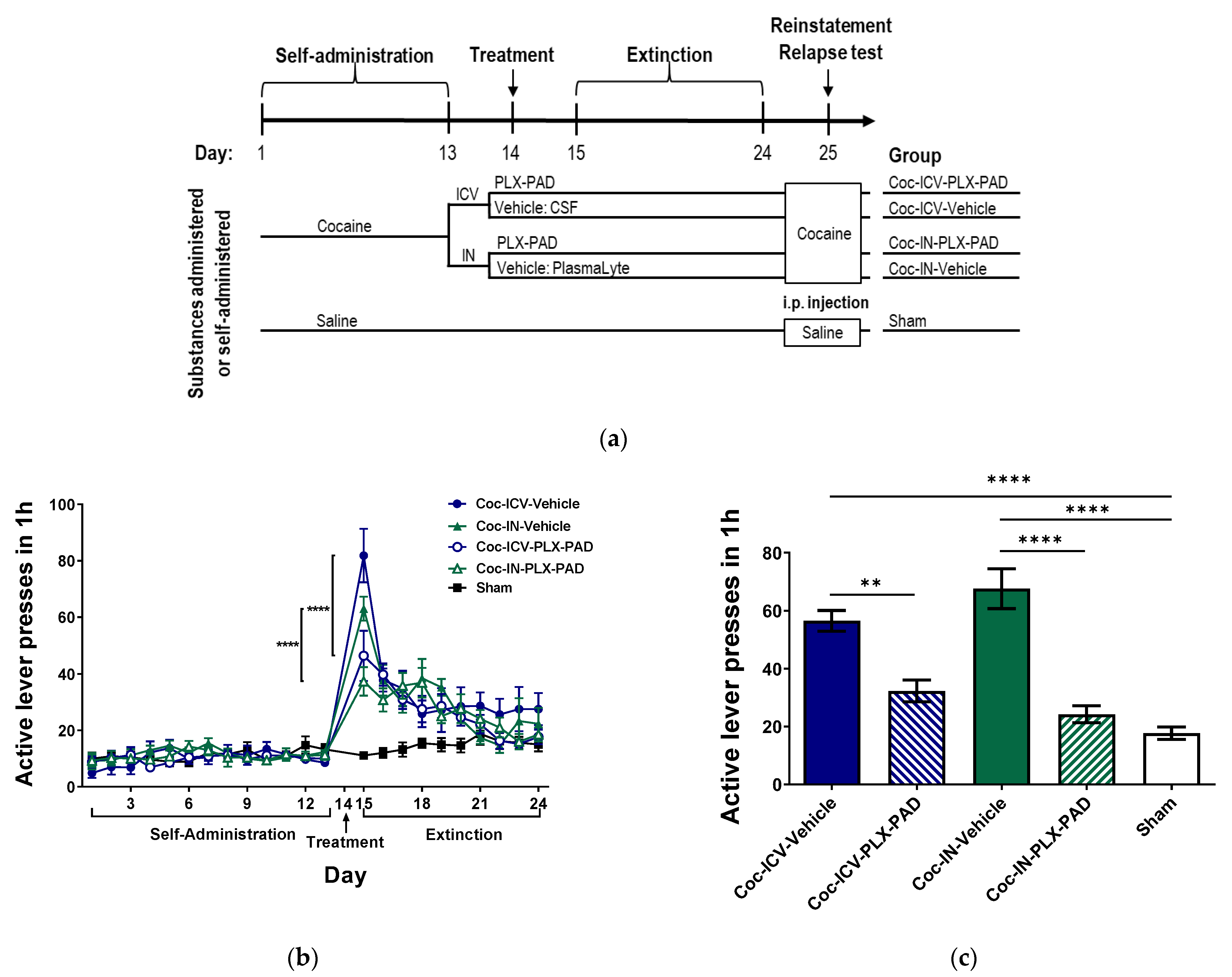
2.4. Cocaine Self-Administration
2.5. Extinction Phase and Reinstatement Test (Relapse)
2.6. Intracerebroventricular Injection
2.7. Intranasal Administration
2.8. Tracking PLX-PAD Cell Navigation Using Gold Nanoparticle Labeling
2.9. Gold Nanoparticle Labeling of PLX-PAD Cells
2.10. Cell Uploading with Gold Nanoparticle
2.11. In Vivo Micro Computed Tomography Scans
2.12. Inductively Coupled Plasma Optical Emission Spectrometry
2.13. Identifying PLX-PAD Cell Navigation with Immunohistochemistry
2.14. Quantification of Neurogenesis
3. Results
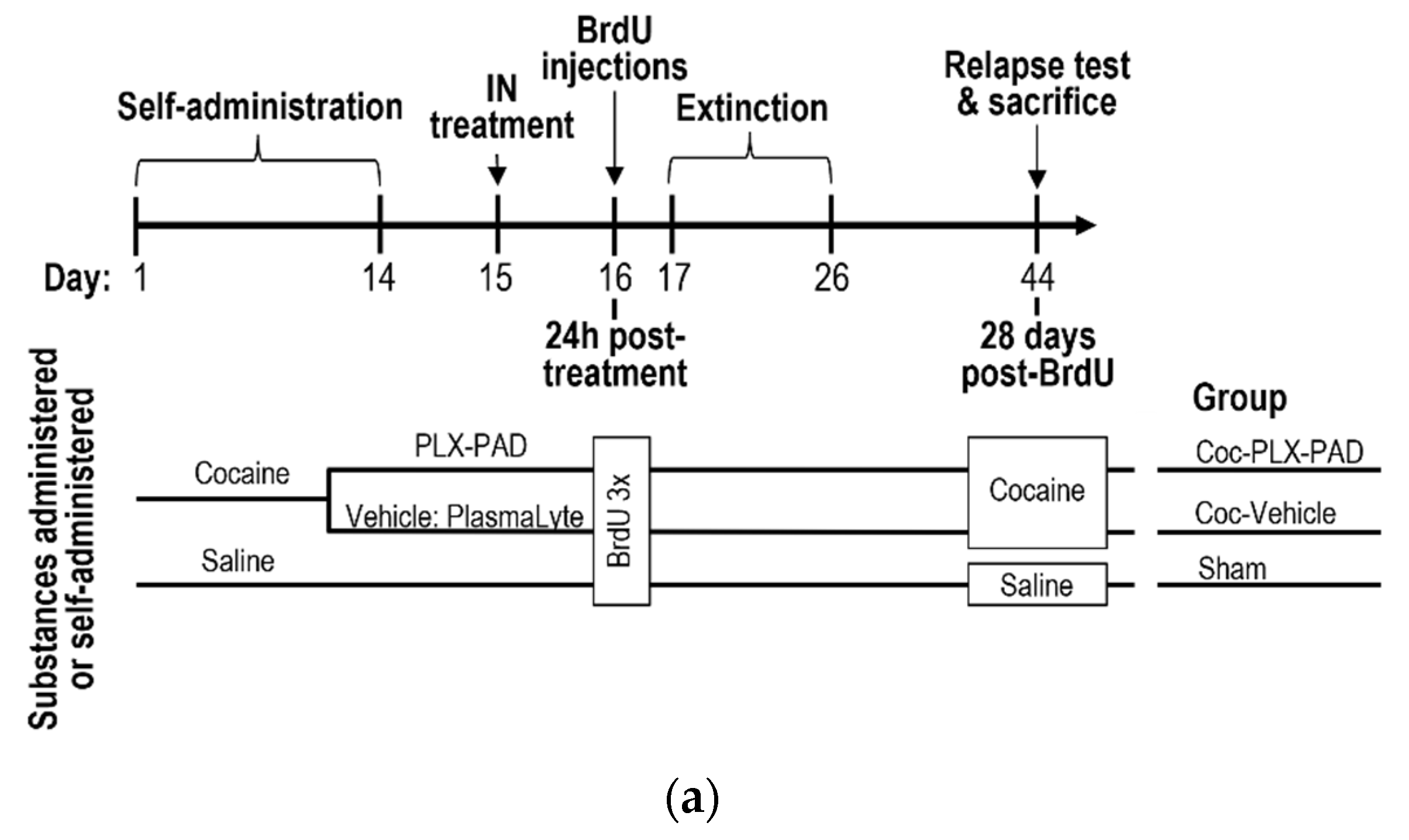
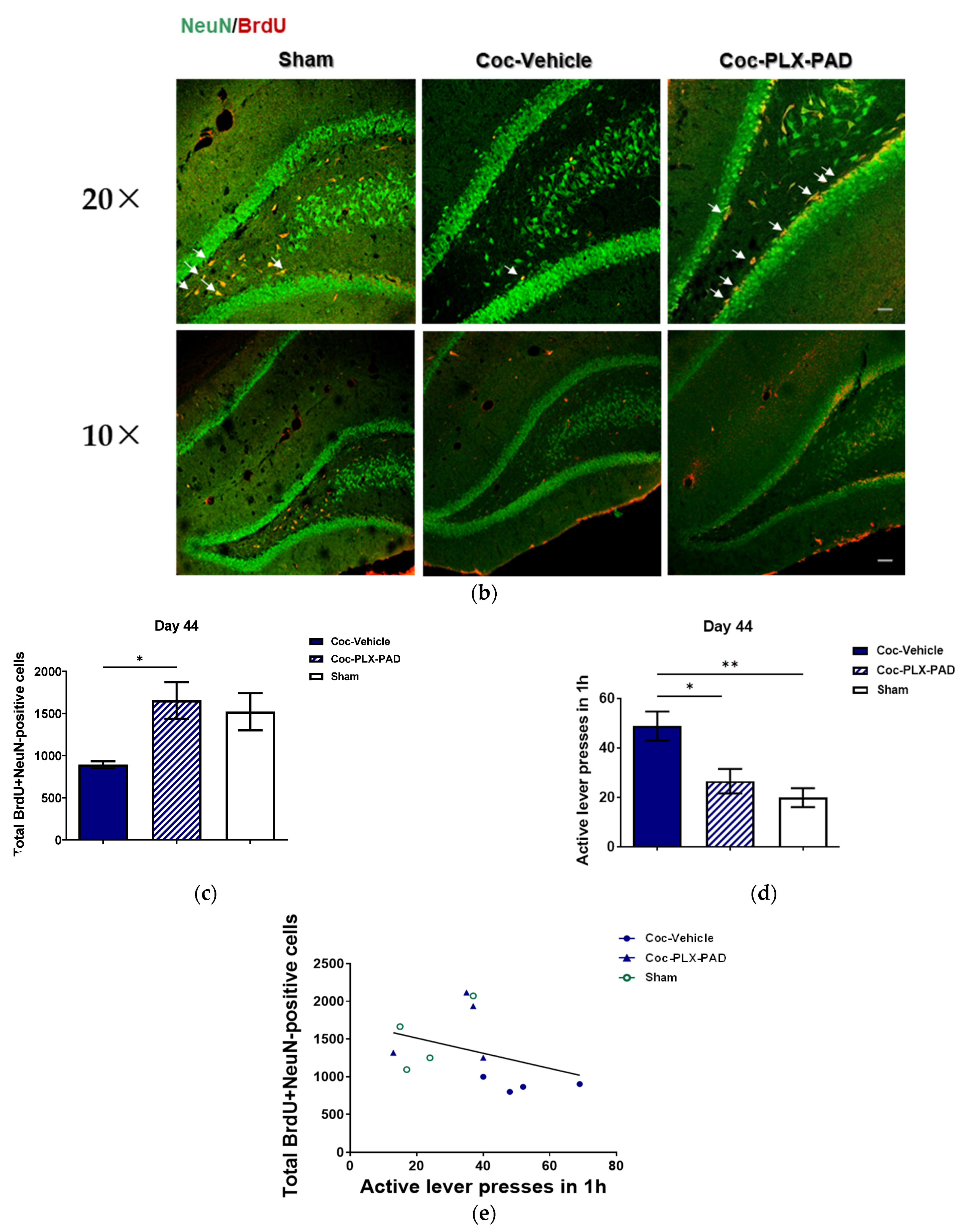
3.1. Intracerebroventricular (ICV) and Intranasal (IN) Administration of PLX-PAD Cells Alleviates Cocaine Extinction and Relapse of Usage Behavior
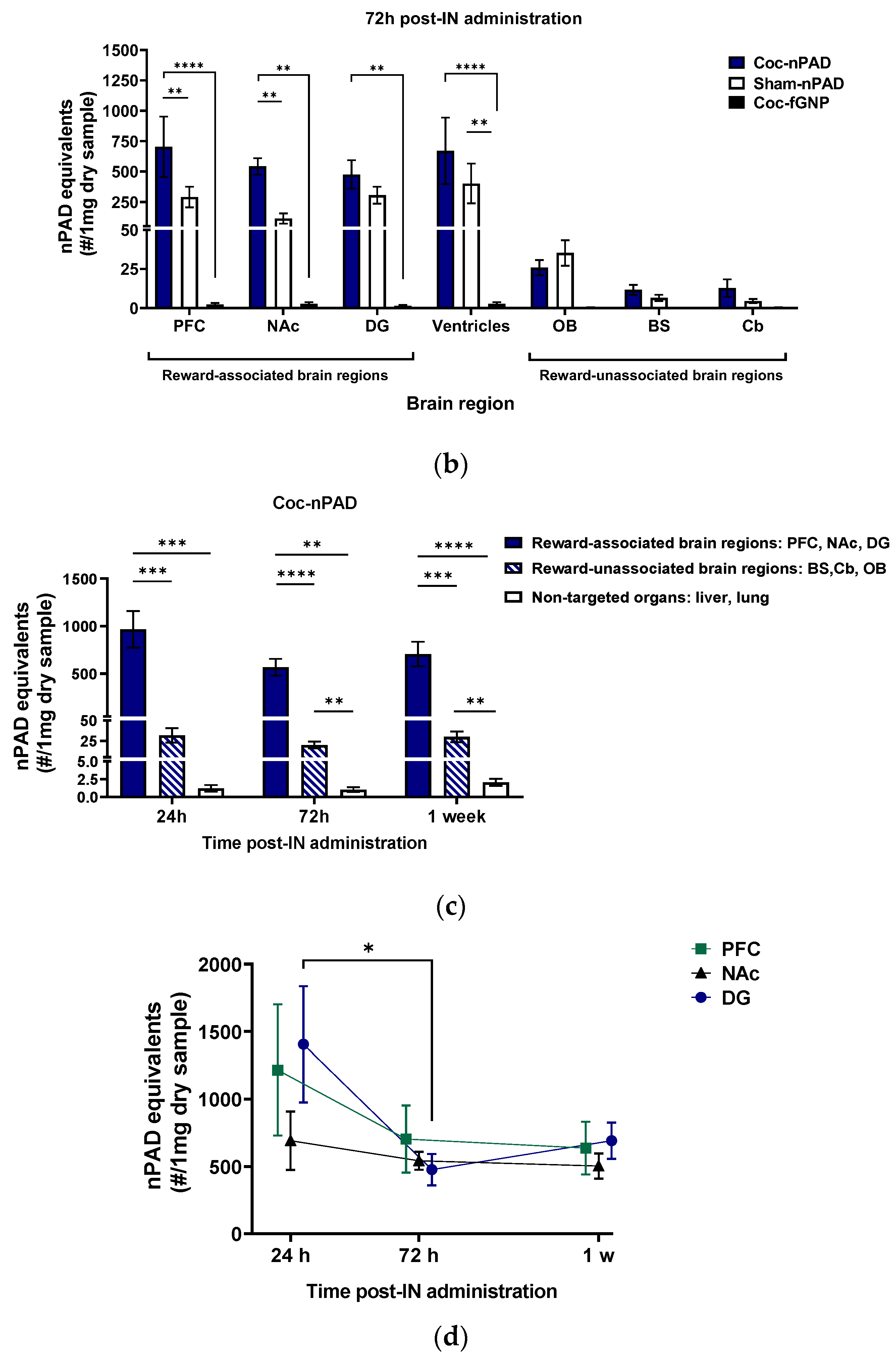
3.2. PLX-PAD Cells Can Navigate to Specific Pathological Addiction-Associated Brain Regions
3.2.1. IN-Administrated PLX-PAD Cells Reach the Brain Mainly through the Olfactory Route
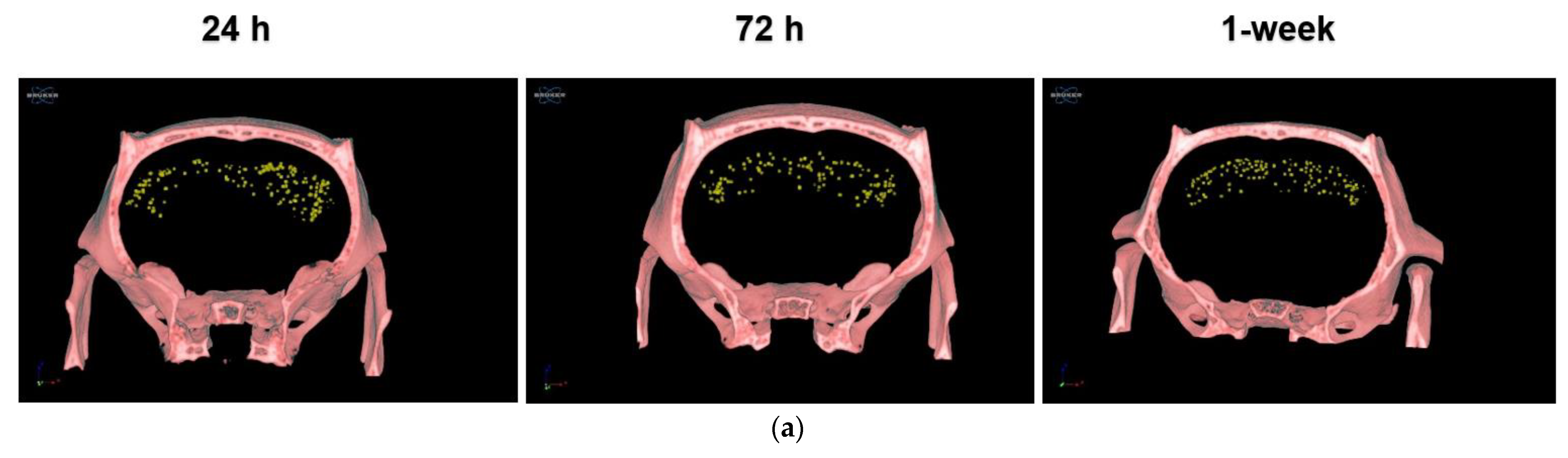
3.2.2. PLX-PAD Cells Reach the Brain in the First 24 h Post-IN Administration
3.2.3. PLX-PAD Cells’ Particular Kinetic Pattern in the Dentate Gyrus
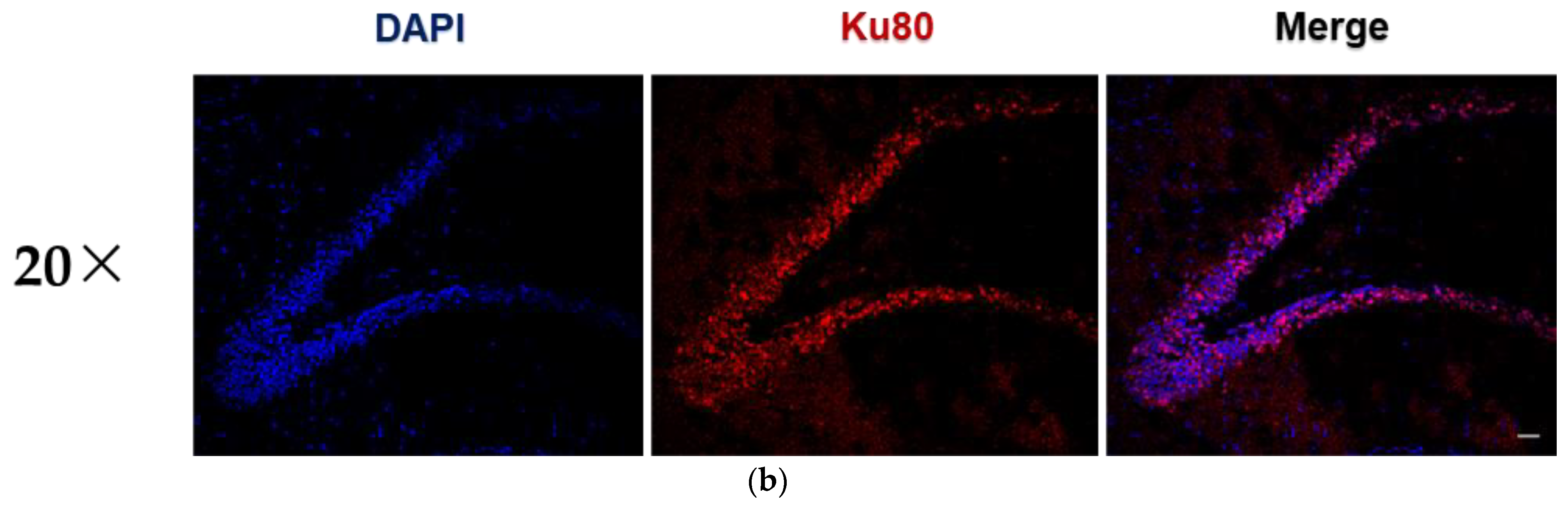
3.3. PLX-PAD Cells Induce Renewal of Hippocampal Neuronal Cells (Neurogenesis)
4. Discussion
Forward-Looking Potential Improvements to the Therapeutic Method Proposed
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Everitt, B.J.; Dickinson, A.; Robbins, T.W. The Neuropsychological Basis of Addictive Behaviour. Brain Res. Rev. 2001, 36, 129–138. [Google Scholar] [CrossRef]
- Rounsaville, B.J.; Anton, S.F.; Carroll, K.; Budde, D.; Prusoff, B.A.; Gawin, F. Psychiatric Diagnoses of Treatment-Seeking Cocaine Abusers. Arch. Gen. Psychiatry 1991, 48, 43–51. [Google Scholar] [CrossRef]
- Ciccarone, D. Stimulant Abuse: Pharmacology, Cocaine, Methamphetamine, Treatment, Attempts at Pharmacotherapy. Prim. Care 2011, 38, 41–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penberthy, J.K.; Ait-Daoud, N.; Vaughan, M.; Fanning, T. Review of Treatment for Cocaine Dependence. Curr. Drug Abuse Rev. 2010, 3, 49–62. [Google Scholar] [CrossRef]
- Girczys-Połedniok, K.; Pudlo, R.; Jarząb, M.; Szymlak, A. Cocaine—Characteristics and Addiction. Med. Pr. 2016, 67, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Riley, A.L.; Manke, H.N.; Huang, S. Impact of the Aversive Effects of Drugs on Their Use and Abuse. Behav. Neurol. 2022, 2022, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Sampedro-Piquero, P.; Santín, L.J.; Castilla-Ortega, E. Aberrant Brain Neuroplasticity and Function in Drug Addiction: A Focus on Learning-Related Brain Regions. In Behavioral Neuroscience; IntechOpen: London, UK, 2019; pp. 1–24. [Google Scholar]
- Kutlu, M.G.; Gould, T.J. Effects of Drugs of Abuse on Hippocampal Plasticity and Hippocampus-Dependent Learning and Memory: Contributions to Development and Maintenance of Addiction. Learn. Mem. 2016, 23, 515–533. [Google Scholar] [CrossRef] [Green Version]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The Role of Adult Hippocampal Neurogenesis in Brain Health and Disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Tzeng, W.Y.; Chuang, J.Y.; Lin, L.C.; Cherng, C.G.; Lin, K.Y.; Chen, L.H.; Su, C.C.; Yu, L. Companions Reverse Stressor-Induced Decreases in Neurogenesis and Cocaine Conditioning Possibly by Restoring BDNF and NGF Levels in Dentate Gyrus. Psychoneuroendocrinology 2013, 38, 425–437. [Google Scholar] [CrossRef]
- Sudai, E.; Croitoru, O.; Shaldubina, A.; Abraham, L.; Gispan, I.; Flaumenhaft, Y.; Roth-Deri, I.; Kinor, N.; Aharoni, S.; Ben-Tzion, M.; et al. High Cocaine Dosage Decreases Neurogenesis in the Hippocampus and Impairs Working Memory. Addict. Biol. 2011, 16, 251–260. [Google Scholar] [CrossRef]
- Recinto, P.; Samant, A.R.H.; Chavez, G.; Kim, A.; Yuan, C.J.; Soleiman, M.; Grant, Y.; Edwards, S.; Wee, S.; Koob, G.F.; et al. Levels of Neural Progenitors in the Hippocampus Predict Memory Impairment and Relapse to Drug Seeking as a Function of Excessive Methamphetamine Self-Administration. Neuropsychopharmacology 2012, 37, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, J.A.; Alén, F.; Mouret, A.; Viveros, M.P.; Llorente, R.; Lepousez, G.; Lledo, P.M. Converging Action of Alcohol Consumption and Cannabinoid Receptor Activation on Adult Hippocampal Neurogenesis. Int. J. Neuropsychopharmacol. 2010, 13, 191–205. [Google Scholar] [CrossRef] [Green Version]
- Eisch, A.J.; Barrot, M.; Schad, C.A.; Self, D.W.; Nestler, E.J. Opiates Inhibit Neurogenesis in the Adult Rat Hippocampus. Proc. Natl. Acad. Sci. USA 2000, 97, 7579–7584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noonan, M.A.; Bulin, S.E.; Fuller, D.C.; Eisch, A.J. Reduction of Adult Hippocampal Neurogenesis Confers Vulnerability in an Animal Model of Cocaine Addiction. J. Neurosci. 2010, 30, 304–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, B.; Kondo, K.; Freeman, M.; Ayers, C.; Montgomery, J.; Kansagara, D. Pharmacotherapy for Cocaine Use Disorder—A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2019, 34, 2858. [Google Scholar] [CrossRef]
- Chan, H.J.; Yanshree; Roy, J.; Tipoe, G.L.; Fung, M.L.; Lim, L.W. Therapeutic Potential of Human Stem Cell Implantation in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 10151. [Google Scholar] [CrossRef]
- Boika, A.; Aleinikava, N.; Chyzhyk, V.; Zafranskaya, M.; Nizheharodava, D.; Ponomarev, V. Mesenchymal Stem Cells in Parkinson’s Disease: Motor and Nonmotor Symptoms in the Early Posttransplant Period. Surg. Neurol. Int. 2020, 11. [Google Scholar] [CrossRef]
- Shwartz, A.; Betzer, O.; Kronfeld, N.; Kazimirsky, G.; Cazacu, S.; Finniss, S.; Lee, H.K.; Motiei, M.; Dagan, S.Y.; Popovtzer, R.; et al. Therapeutic Effect of Astroglia-like Mesenchymal Stem Cells Expressing Glutamate Transporter in a Genetic Rat Model of Depression. Theranostics 2017, 7, 2690. [Google Scholar] [CrossRef] [Green Version]
- Tfilin, M.; Sudai, E.; Merenlender, A.; Gispan, I.; Yadid, G.; Turgeman, G. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Counteract Depressive-like Behavior. Mol. Psychiatry 2010, 15, 1164–1175. [Google Scholar] [CrossRef]
- Gobshtis, N.; Tfilin, M.; Fraifeld, V.E.; Turgeman, G. Transplantation of Mesenchymal Stem Cells Causes Long-Term Alleviation of Schizophrenia-like Behaviour Coupled with Increased Neurogenesis. Mol. Psychiatry 2021, 26, 4448–4463. [Google Scholar] [CrossRef]
- Tobin, M.K.; Stephen, T.K.L.; Lopez, K.L.; Pergande, M.R.; Bartholomew, A.M.; Cologna, S.M.; Lazarov, O. Activated Mesenchymal Stem Cells Induce Recovery Following Stroke Via Regulation of Inflammation and Oligodendrogenesis. J. Am. Heart Assoc. 2020, 9, e013583. [Google Scholar] [CrossRef]
- Do, A.D.; Kurniawati, I.; Hsieh, C.L.; Wong, T.T.; Lin, Y.L.; Sung, S.Y. Application of Mesenchymal Stem Cells in Targeted Delivery to the Brain: Potential and Challenges of the Extracellular Vesicle-Based Approach for Brain Tumor Treatment. Int. J. Mol. Sci. 2021, 22, 11187. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, E.; Klopp, A.; Dembinski, J.; Andreeff, M.; Marini, F. Inflammation and Tumor Microenvironments: Defining the Migratory Itinerary of Mesenchymal Stem Cells. Gene Ther. 2008, 15, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Wu, X.M.; Liu, Y.; He, D. Transplantation of Bone Marrow Mesenchymal Stem Cells Promotes Learning and Memory Functional Recovery and Reduces Hippocampal Damage in Rats with Alcohol-Associated Dementia. Transplantation 2015, 99, 492–499. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Neves-Carvalho, A.; Panchalingam, K.M.; Behie, L.A.; Pinto, L.; Sousa, N.; Salgado, A.J. Secretome of Mesenchymal Progenitors from the Umbilical Cord Acts as Modulator of Neural/Glial Proliferation and Differentiation. Stem Cell Rev. Rep. 2015, 11, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, F.; Morales, P.; Quintanilla, M.E.; Santapau, D.; Lespay-Rebolledo, C.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y. Intravenous Administration of Anti-Inflammatory Mesenchymal Stem Cell Spheroids Reduces Chronic Alcohol Intake and Abolishes Binge-Drinking. Sci. Rep. 2018, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Doshmanziari, M.; Shirian, S.; Kouchakian, M.-R.; Moniri, S.F.; Jangnoo, S.; Mohammadi, N.; Zafari, F. Mesenchymal Stem Cells Act as Stimulators of Neurogenesis and Synaptic Function in a Rat Model of Alzheimer’s Disease. Heliyon 2021, 7, e07996. [Google Scholar] [CrossRef]
- Castilla-Ortega, E.; Santín, L.J. Adult Hippocampal Neurogenesis as a Target for Cocaine Addiction: A Review of Recent Developments. Curr. Opin. Pharmacol. 2019, 50, 109–116. [Google Scholar] [CrossRef]
- Israel, Y.; Ezquer, F.; Quintanilla, M.E.; Morales, P.; Ezquer, M.; Herrera-Marschitz, M. Intracerebral Stem Cell Administration Inhibits Relapse-like Alcohol Drinking in Rats. Alcohol Alcohol 2017, 52, 1–4. [Google Scholar] [CrossRef]
- Quintanilla, M.E.; Ezquer, F.; Morales, P.; Santapau, D.; Berríos-Cárcamo, P.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y. Intranasal Mesenchymal Stem Cell Secretome Administration Markedly Inhibits Alcohol and Nicotine Self-Administration and Blocks Relapse-Intake: Mechanism and Translational Options. Stem Cell Res. Ther. 2019, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Papait, A.; Vertua, E.; Magatti, M.; Ceccariglia, S.; De Munari, S.; Silini, A.R.; Sheleg, M.; Ofir, R.; Parolini, O. Mesenchymal Stromal Cells from Fetal and Maternal Placenta Possess Key Similarities and Differences: Potential Implications for Their Applications in Regenerative Medicine. Cells 2020, 9, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahavi-Goldstein, E.; Blumenfeld, M.; Fuchs-Telem, D.; Pinzur, L.; Rubin, S.; Aberman, Z.; Sher, N.; Ofir, R. Placenta-Derived PLX-PAD Mesenchymal-like Stromal Cells Are Efficacious in Rescuing Blood Flow in Hind Limb Ischemia Mouse Model by a Dose- and Site-Dependent Mechanism of Action. Cytotherapy 2017, 19, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Weiss, N.; Nikol, S.; Hinchliffe, R.J.; Lantis, J.C.; Patel, M.R.; Reinecke, H.; Ofir, R.; Rosen, Y.; Peres, D.; et al. PLX-PAD Cell Treatment of Critical Limb Ischaemia: Rationale and Design of the PACE Trial. J. Vasc. Surg. 2019, 57, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Winkler, T.; Perka, C.; von Roth, P.; Agres, A.N.; Plage, H.; Preininger, B.; Pumberger, M.; Geissler, S.; Hagai, E.L.; Ofir, R.; et al. Immunomodulatory Placental-Expanded, Mesenchymal Stromal Cells Improve Muscle Function Following Hip Arthroplasty. J. Cachexia. Sarcopenia Muscle 2018, 9, 880–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Blanc, K.; Tammik, L.; Sundberg, B.; Haynesworth, S.E.; Ringdén, O. Mesenchymal Stem Cells Inhibit and Stimulate Mixed Lymphocyte Cultures and Mitogenic Responses Independently of the Major Histocompatibility Complex. Scand. J. Immunol. 2003, 57, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Brodarac, A.; Kukucka, M.; Kurtz, A.; Becher, P.M.; Jülke, K.; Choi, Y.H.; Pinzur, L.; Chajut, A.; Tschöpe, C.; et al. Cardioprotection by Placenta-Derived Stromal Cells in a Murine Myocardial Infarction Model. J. Surg. Res. 2013, 185, 70–83. [Google Scholar] [CrossRef]
- Zhuang, W.Z.; Lin, Y.H.; Su, L.J.; Wu, M.S.; Jeng, H.Y.; Chang, H.C.; Huang, Y.H.; Ling, T.Y. Mesenchymal Stem/Stromal Cell-Based Therapy: Mechanism, Systemic Safety and Biodistribution for Precision Clinical Applications. J. Biomed. Sci. 2021, 28, 1–38. [Google Scholar] [CrossRef]
- Roth-Deri, I.; Zangen, A.; Aleli, M.; Goelman, R.G.; Pelled, G.; Nakash, R.; Gispan-Herman, I.; Green, T.; Shaham, Y.; Yadid, G. Effect of Experimenter-Delivered and Self-Administered Cocaine on Extracellular Beta-Endorphin Levels in the Nucleus Accumbens. J. Neurochem. 2003, 84, 930–938. [Google Scholar] [CrossRef] [Green Version]
- Meir, R.; Betzer, O.; Motiei, M.; Kronfeld, N.; Brodie, C.; Popovtzer, R. Design Principles for Noninvasive, Longitudinal and Quantitative Cell Tracking with Nanoparticle-Based CT Imaging. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 421–429. [Google Scholar] [CrossRef]
- Betzer, O.; Meir, R.; Dreifuss, T.; Shamalov, K.; Motiei, M.; Shwartz, A.; Baranes, K.; Cohen, C.J.; Shraga-Heled, N.; Ofir, R.; et al. In-Vitro Optimization of Nanoparticle-Cell Labeling Protocols for In-Vivo Cell Tracking Applications. Sci. Rep. 2015, 5, 15400. [Google Scholar] [CrossRef] [Green Version]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lax, E.; Friedman, A.; Croitoru, O.; Sudai, E.; Ben-Moshe, H.; Redlus, L.; Sasson, E.; Blumenfeld-Katzir, T.; Assaf, Y.; Yadid, G. Neurodegeneration of Lateral Habenula Efferent Fibers after Intermittent Cocaine Administration: Implications for Deep Brain Stimulation. Neuropharmacology 2013, 75, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ahdoot-Levi, H.; Croitoru, O.; Bareli, T.; Sudai, E.; Peér-Nissan, H.; Jacob, A.; Gispan, I.; Maayan, R.; Weizman, A.; Yadid, G. The Effect of Dehydroepiandrosterone Treatment on Neurogenesis, Astrogliosis and Long-Term Cocaine-Seeking Behavior in a Cocaine Self-Administration Model in Rats. Front. Neurosci. 2021, 15, 1552. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Meiron, M.; Toren, A.; Steiner, M.; Nyska, A. Safety and Biodistribution Profile of Placental-Derived Mesenchymal Stromal Cells (PLX-PAD) Following Intramuscular Delivery. Toxicol. Pathol. 2009, 37, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.M.L.; Ng, A.M.H.; Mohd Yunus, M.H.; Hj Idrus, R.B.; Law, J.X.; Yazid, M.D.; Chin, K.Y.; Shamsuddin, S.A.; Mohd Yusof, M.R.; Razali, R.A.; et al. Safety Study of Allogeneic Mesenchymal Stem Cell Therapy in Animal Model. Regen. Ther. 2022, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Shwartz, A.; Motiei, M.; Kazimirsky, G.; Gispan, I.; Damti, E.; Brodie, C.; Yadid, G.; Popovtzer, R. Nanoparticle-Based CT Imaging Technique for Longitudinal and Quantitative Stem Cell Tracking within the Brain: Application in Neuropsychiatric Disorders. ACS Nano 2014, 8, 9274–9285. [Google Scholar] [CrossRef]
- Yadid, G.; Redlus, L.; Barnea, R.; Doron, R. Modulation of Mood States as a Major Factor in Relapse to Substance Use. Front. Mol. Neurosci. 2012, 5, 81. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Melendez, M.; Yawno, T.; Jenkin, G.; Miller, S.L. Stem Cell Therapy to Protect and Repair the Developing Brain: A Review of Mechanisms of Action of Cord Blood and Amnion Epithelial Derived Cells. Front. Neurosci. 2013, 7, 194. [Google Scholar] [CrossRef] [Green Version]
- Lappan, S.N.; Brown, A.W.; Hendricks, P.S. Dropout Rates of In-Person Psychosocial Substance Use Disorder Treatments: A Systematic Review and Meta-Analysis. Addiction 2020, 115, 201–217. [Google Scholar] [CrossRef]
- Varga, G.; Bori, E.; Kallo, K.; Nagy, K.; Tarjan, I.; Racz, G.Z. Novel Possible Pharmaceutical Research Tools: Stem Cells, Gene Delivery and Their Combination. Curr. Pharm. Des. 2013, 19, 133–141. [Google Scholar] [CrossRef]
- Nowakowski, A.; Walczak, P.; Janowski, M.; Lukomska, B. Genetic Engineering of Mesenchymal Stem Cells for Regenerative Medicine. Stem Cells Dev. 2015, 24, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, J.H.; Kim, S.H.; Park, H.; Lee, D.; Kim, G.J. Efficacy of Gene Modification in Placenta-Derived Mesenchymal Stem Cells Based on Nonviral Electroporation. Int. J. Stem Cells 2021, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, T.; Kawauchi, S.; Kin, K.; Morimoto, J.; Kameda, M.; Sasaki, T.; Bonsack, B.; Kingsbury, C.; Tajiri, N.; Borlongan, C.V.; et al. Cell Therapy for Central Nervous System Disorders: Current Obstacles to Progress. CNS Neurosci. Ther. 2020, 26, 595–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pe’er-Nissan, H.; Ahdoot-Levi, H.; Betzer, O.; Itzhak, P.S.; Shraga-Heled, N.; Gispan, I.; Motiei, M.; Doroshev, A.; Anker, Y.; Popovtzer, R.; et al. Placenta-Derived Mesenchymal-like Adherent Stromal Cells as an Effective Cell Therapy for Cocaine Addiction in a Rat Model. Pharmaceutics 2022, 14, 1311. https://doi.org/10.3390/pharmaceutics14071311
Pe’er-Nissan H, Ahdoot-Levi H, Betzer O, Itzhak PS, Shraga-Heled N, Gispan I, Motiei M, Doroshev A, Anker Y, Popovtzer R, et al. Placenta-Derived Mesenchymal-like Adherent Stromal Cells as an Effective Cell Therapy for Cocaine Addiction in a Rat Model. Pharmaceutics. 2022; 14(7):1311. https://doi.org/10.3390/pharmaceutics14071311
Chicago/Turabian StylePe’er-Nissan, Hilla, Hadas Ahdoot-Levi, Oshra Betzer, Pnina Shirel Itzhak, Niva Shraga-Heled, Iris Gispan, Menachem Motiei, Arthur Doroshev, Yaakov Anker, Rachela Popovtzer, and et al. 2022. "Placenta-Derived Mesenchymal-like Adherent Stromal Cells as an Effective Cell Therapy for Cocaine Addiction in a Rat Model" Pharmaceutics 14, no. 7: 1311. https://doi.org/10.3390/pharmaceutics14071311
APA StylePe’er-Nissan, H., Ahdoot-Levi, H., Betzer, O., Itzhak, P. S., Shraga-Heled, N., Gispan, I., Motiei, M., Doroshev, A., Anker, Y., Popovtzer, R., Ofir, R., & Yadid, G. (2022). Placenta-Derived Mesenchymal-like Adherent Stromal Cells as an Effective Cell Therapy for Cocaine Addiction in a Rat Model. Pharmaceutics, 14(7), 1311. https://doi.org/10.3390/pharmaceutics14071311






