Antioxidative NAC-Loaded Silk Nanoparticles with Opening Mucosal Tight Junctions for Nasal Drug Delivery: An In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Experimental Methods
2.1. Preparing SF Solutions
2.2. Fabrications of SF/NAC NPs
2.3. Characteristics of SF and SF/NAC NPs
2.4. ATR-FTIR Spectra to Analyze the Functional Groups of SF and SF/NAC NPs
2.5. H2O2 Scavenging Abilities of SF and SF/NAC NPs Using a MitoSOX Immunostaining
2.6. Cytotoxicity of SF and SF/NAC NPs
2.7. Encapsulation and Loading Efficiencies of SF/NAC NPs
2.8. Determining NAC Releases for SF/NAC NPs
2.9. TEER Measurements for Human Nasal RPMI2650 Cells
2.10. ZO-1 Staining for Examining the Opening Tight Junctions in RPMI 2650 Cells
2.11. Rats Studies for Nasal Delivery of FITC-NAC to Brain by Administrations of SF/FITC-NAC NPs into Their Nasal Cavities
2.12. Statistical Analysis
3. Results and Discussion
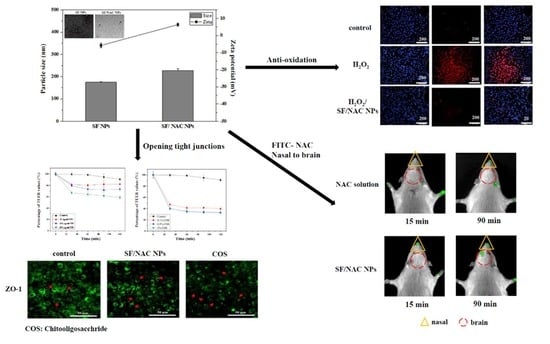
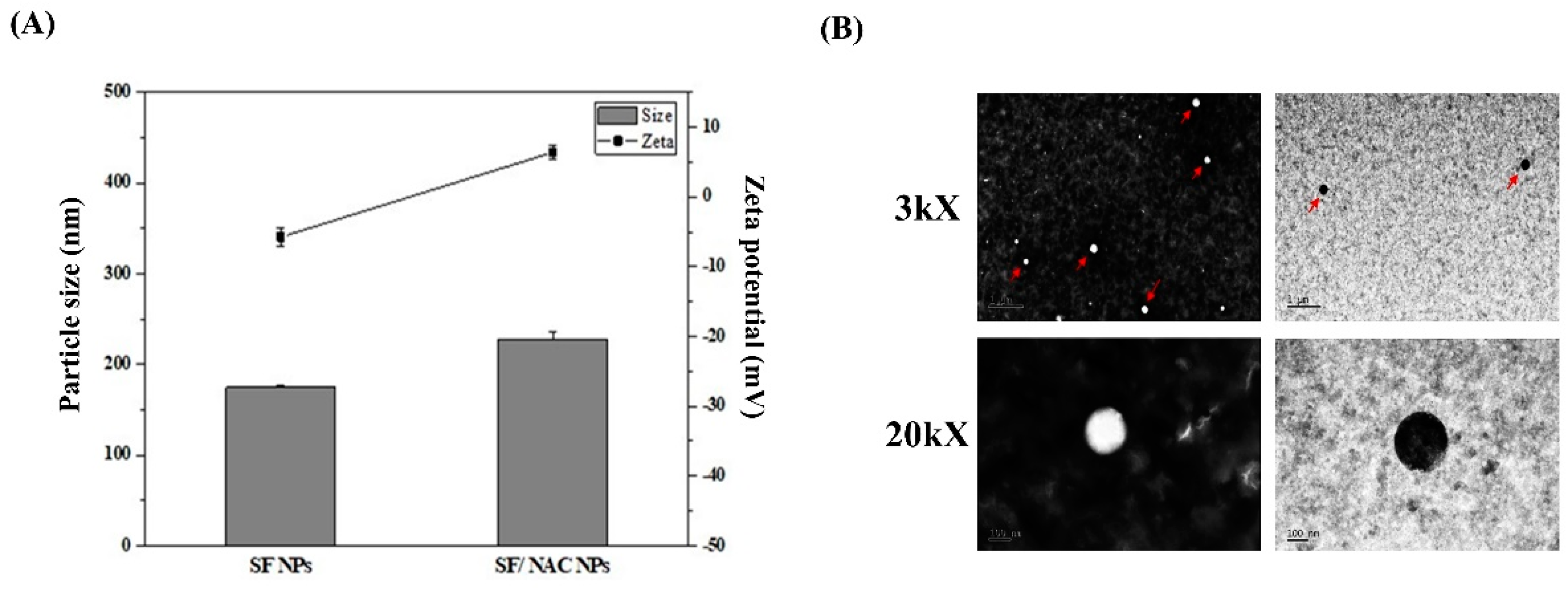
3.1. Producing SF/NAC NPs and the Characteristics of the NPs
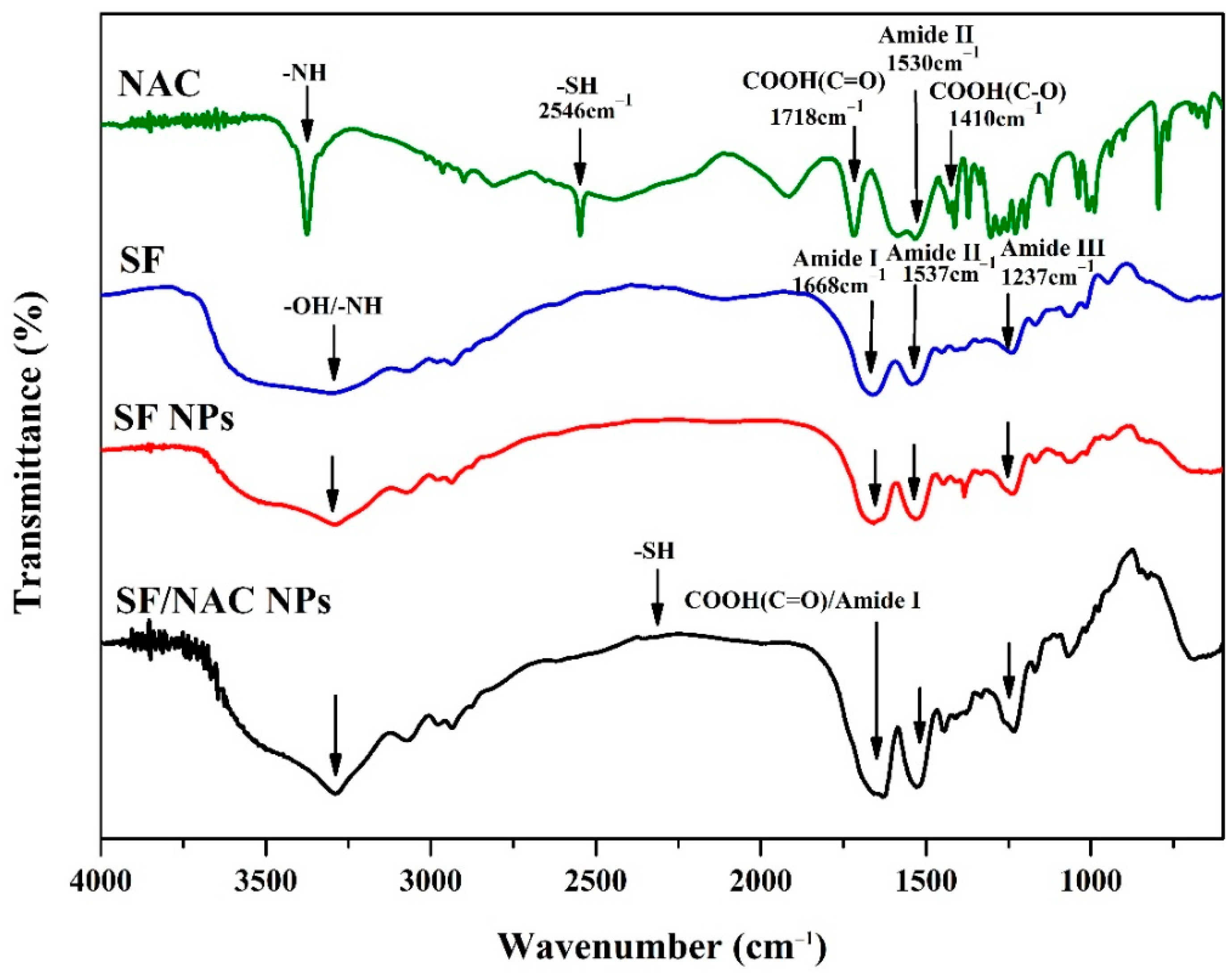
3.2. A Analysis of ATR-FTIR Spectra for Examining Functions Groups SF/NAC NPs
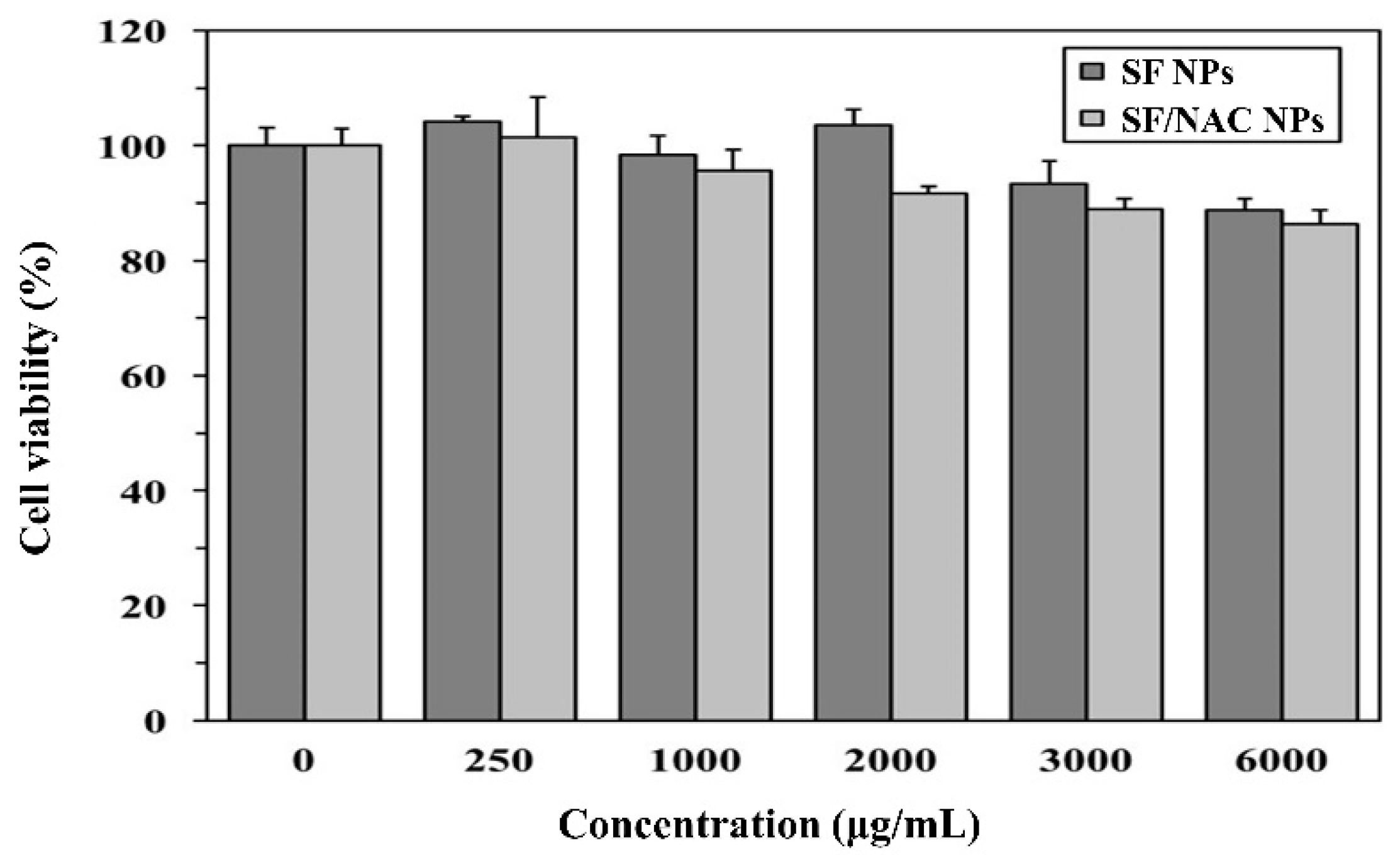
3.3. Cytotoxicity Examinations for SF and SF/NAC NPs
3.4. Anti-Oxidative Abilities for SF/NAC NPs by Determining the hBMSC Viability
3.5. Paracellular Transport: Opening Tight Junctions in RPMI 2650 Cells Using SF/NAC NPs by TEER Examinations
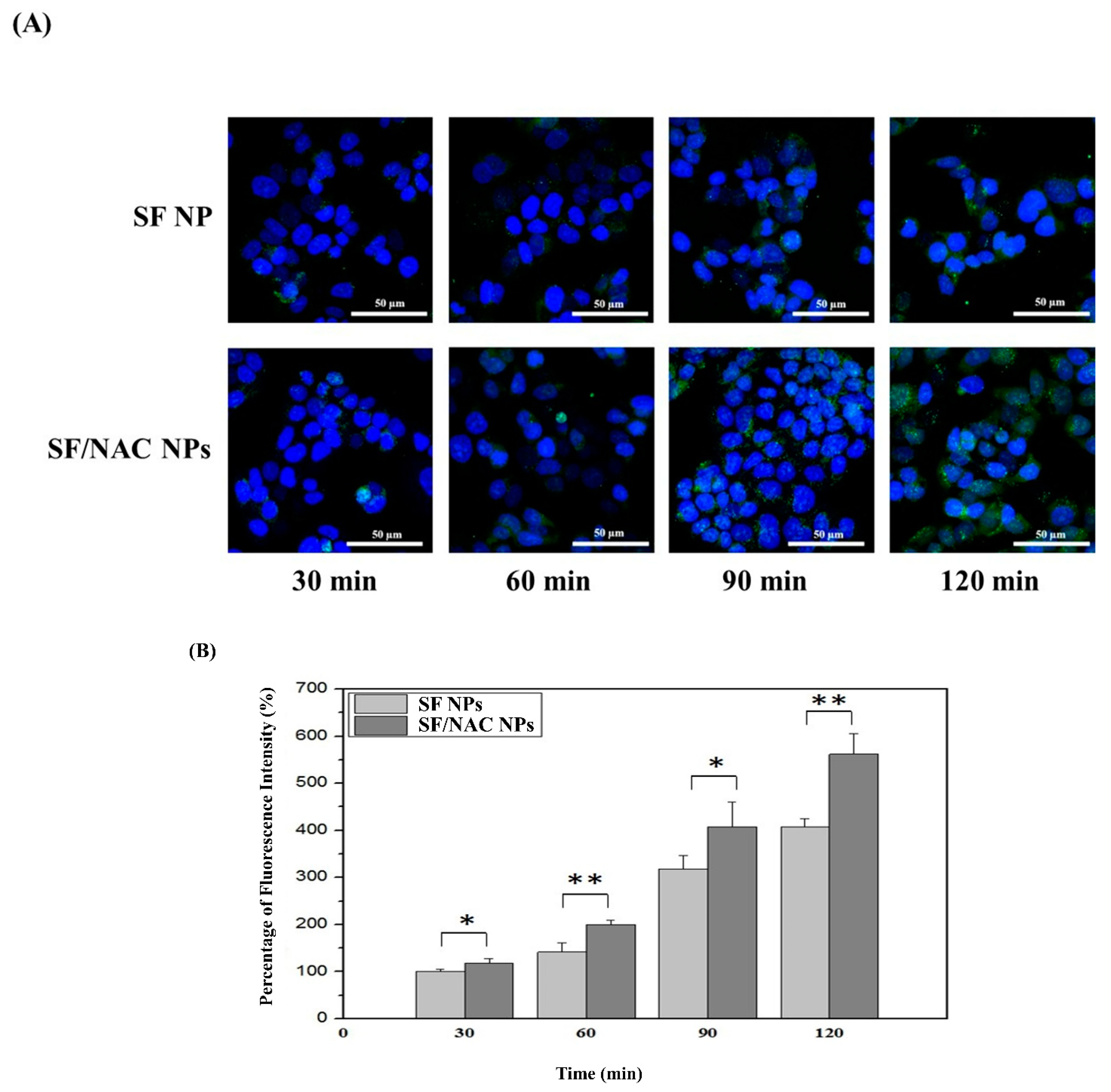
3.6. Transcellular Transport of SF/NAC NPs: Uptake of NPs by RPMI 2650 Cells
3.7. Cumulative Releases of NAC for NAC/SF NPs
3.8. In Vivo IVIS Study to Evaluate the Enhancement of Nasal Delivery of FITC-NAC to the Brain Using SF/FITC-NAC NPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, Z.; Gao, W.; Bai, H. Silk-based bioinspired structural and functional materials. iScience 2022, 25, 103940. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Srichana, T. Monitoring of inflammatory mediators induced by silk sericin. J. Biosci. Bioeng. 2009, 107, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Lee, A.S.; Chang, S.L.; Lin, S.F.; Weng, C.H.; Lo, H.Y.; Chou, P.C.; Tsai, Y.N.; Sung, Y.L.; Chen, C.C.; et al. Biomaterial-induced conversion of quiescent cardiomyocytes into pacemaker cells in rats. Nat. Biomed. Eng. 2022, 6, 421–434. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef]
- Pritchard, E.M.; Hu, X.; Finley, V.; Kuo, C.K.; Kaplan, D.L. Effect of silk protein processing on drug delivery from silk films. Macromol. Biosci. 2013, 13, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Chung, T.W.; Tyan, Y.C.; Lin, S.W.; Yang, M.H.; Liu, Y.H.; Wang, R.P. Developing photothermal-responsive and anti-oxidative silk/dopamine nanoparticles decorated with drugs which were incorporated into silk films as a depot-based drug delivery. Int. J. Biol. Macromol. 2021, 185, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, Q.; Li, R.; Wang, J.; Zhen, X.; Yue, G.; Wang, H.; Cui, F.; Wu, F.; Yang, M.; et al. Facile preparation of paclitaxel loaded silk fibroin nanoparticles for enhanced antitumor efficacy by locoregional drug delivery. ACS Appl. Mater. Interfaces 2013, 5, 12638–12645. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Xie, M.B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Dong, P.; Huang, D.; Mei, L.; Xia, Y.; Wang, Z.; Pan, X.; Li, G.; Wu, C. Fabrication and characterization of silk fibroin-coated liposomes for ocular drug delivery. Eur. J. Pharm. Biopharm. 2015, 91, 82–90. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Mato, Y.L. Nasal route for vaccine and drug delivery: Features and current opportunities. Int. J. Pharm. 2019, 572, 118813. [Google Scholar] [CrossRef] [PubMed]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; John, J.A.S.; Ekberg, J.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens penetrating the central nervous system: Infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef] [Green Version]
- Beule, A.G. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2010, 9, Doc07. [Google Scholar] [CrossRef]
- Marasini, N.; Skwarczynski, M.; Toth, I. Intranasal delivery of nanoparticle-based vaccines. Ther. Deliv. 2017, 8, 151–167. [Google Scholar] [CrossRef]
- Chung, T.W.; Liu, D.Z.; Yang, J.S. Effects of interpenetration of thermo-sensitive gels by crosslinking of chitosan on nasal delivery of insulin: In vitro characterization and in vivo study. Carbohydr. Polym. 2010, 82, 316–322. [Google Scholar] [CrossRef]
- Wu, J.; Wei, W.; Wang, L.Y.; Su, Z.G.; Ma, G.H. A thermos-sensitive hydrogel based on quaternized chitosan and poly (ethylene glycol) for nasal drug delivery system. Biomaterials 2017, 28, 2220–2232. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Van Itallie, C.M.; Anderson, J.M. Architecture of tight junctions and principles of molecular composition. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2014; Volume 36, pp. 157–165. [Google Scholar]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: Effect of size and surface charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef]
- Holmay, M.J.; Terpstra, M.; Coles, L.D.; Mishra, U.; Ahlskog, M.; Oz, G.; Cloyd, J.C.; Tuite, P. N-acetylcysteine boosts brain and blood glutathione in gaucher and Parkinson’s diseases. Clin. Neuropharmacol. 2013, 36, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markoutsa, E.; Xu, P. Redox potential-sensitive N-acetyl cysteine-prodrug nanoparticles inhibit the activation of microglia and improve neuronal survival. Mol. Pharm. 2017, 14, 1591–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahumane, G.D.; Kumar, P.; Pillay, V.; Choonara, Y.E. Repositioning N-acetylcysteine (NAC): NAC-loaded electrospun drug delivery scaffolding for potential neural tissue engineering application. Pharmaceutics 2020, 12, 934. [Google Scholar] [CrossRef]
- Chung, T.W.; Chen, W.P.; Tai, P.W.; Lo, H.Y.; Wu, T.Y. Roles of silk fibroin on characteristics of hyaluronic acid/silk fibroin hydrogels for tissue engineering of nucleus pulposus. Materials 2020, 13, 2750. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Zan, B.S.; Chen, L.T.; Chung, T.W. Multifunctional PLGA-based nanoparticles as a controlled release drug delivery system for antioxidant and anticoagulant therapy. Int. J. Nanomed. 2019, 14, 1533. [Google Scholar] [CrossRef] [Green Version]
- Lo, H.Y.; Huang, A.L.; Lee, P.C.; Chung, T.W.; Wang, S.S. Morphological transformation of h BMSC from 2 D monolayer to 3 D microtissue on low-crystallinity SF-PCL patch with promotion of cardiomyogenesis. J. Tissue Eng. Regen. Med. 2018, 12, 1852–1864. [Google Scholar] [CrossRef]
- Jin, X.; Asghar, S.; Zhang, M.; Chen, Z.; Huang, L.; Ping, Q.; Xiao, Y. N-acetylcysteine modified hyaluronic acid-paclitaxel conjugate for efficient oral chemotherapy through mucosal bioadhesion ability. Colloids Surf. B Biointerfaces 2018, 172, 655–664. [Google Scholar] [CrossRef]
- Reichl, S.; Becker, K. Cultivation of RPMI 2650 cells as an in-vitro model for human transmucosal nasal drug absorption studies: Optimization of selected culture conditions. J. Pharm. Pharmacol. 2012, 64, 1621–1630. [Google Scholar] [CrossRef]
- Dolberg, A.M.; Reichl, S. Expression of P-glycoprotein in excised human nasal mucosa and optimized models of RPMI 2650 cells. Int. J. Pharm. 2016, 508, 22–33. [Google Scholar] [CrossRef]
- Gonçalves, V.S.; Matias, A.A.; Poejo, J.; Serra, A.T.; Duarte, C.M. Application of RPMI 2650 as a cell model to evaluate solid formulations for intranasal delivery of drugs. Int. J. Pharm. 2016, 515, 1–10. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kahar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sung, D.K.; Kim, H.; Kong, W.H.; Kim, Y.E.; Hahn, S.K. Nose-to-brain delivery of hyaluronate–FG loop peptide conjugate for non-invasive hypoxic-ischemic encephalopathy therapy. J. Control. Release 2019, 307, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Yellepeddi, V.K.; Mohammadpour, R.; Kambhampati, S.P.; Sayre, C.; Mishra, M.K.; Kannan, R.M.; Ghandehari, H. Pediatric oral formulation of dendrimer-N-acetyl-l-cysteine conjugates for the treatment of neuroinflamma-tion. Int. J. Pharm. 2018, 545, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Liao, L.; Yang, L.; Qin, A.; Liang, A. Aqueous synthesis of func-tionalized copper sulfide quantum dots as near-infrared luminescent probes for detection of Hg2+, Ag+ and Au3+. Sci. Rep. 2017, 7, 11451. [Google Scholar] [CrossRef]
- Rajawat, G.S.; Shinde, U.A.; Nair, H.A. Chitosan-N-acetyl cysteine microspheres for ocular delivery of acyclovir: Synthesis and in vitro/in vivo evaluation. J. Drug Deliv. Sci. Technol. 2016, 35, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Pérez, A.A.; Rodriguez-Nogales, A.; Ortiz-Cullera, V.; Algieri, F.; Garrido-Mesa, J.; Zorrilla, P.; Rodriguez-Cabezas, M.E.; Garrido-Mesa, N.; Utrilla, M.P.; De Matteis, L.; et al. Silk fibroin nanoparticles constitute a vector for controlled release of resveratrol in an experimental model of inflammatory bowel disease in rats. Int. J. Nanomed. 2014, 9, 4507. [Google Scholar]
- Zhang, Y.; Du, X.; Zhang, Y.; Li, G.; Cai, C.; Xu, J.; Tang, X. Thiolated eudragit-based nanoparticles for oral insulin delivery: Preparation, characterization, and evaluation using intestinal epithelial cells in vitro. Macromol. Biosci. 2014, 14, 842–852. [Google Scholar] [CrossRef]



| Time | 0.5 h | 1 h | 2 h | 4 h | 8 h |
|---|---|---|---|---|---|
| NAC (%) | 46.0 ± 0.1 | 69.6 ± 0.3 | 82.3 ± 0.7 | 84.7 ± 0.9 | 86.1 ± 1.4 |
| SF/NAC NP(%) | 32.6 ± 2.8 | 45.6 ± 3.7 | 55.3 ± 3.6 | 58.8 ± 1.7 | 60.3 ± 1.5 |
| FITC-NAC (ph/s/cm2/sr, ×106) | 15 min | 30 min | 60 min | 90 min | 120 min | Carries (n = 4) |
|---|---|---|---|---|---|---|
| Brain region | 0.85 ± 0.15 | 1.18 ± 0.42 | 1.12 ± 0.36 | 1.10 ± 0.37 | 1.05 ± 0.40 | NAC solution |
| Brain region | 2.17 ± 0.73 | 2.14 ± 0.29 | 1.48 ± 0.13 | 1.72 ± 0.27 | 1.45 ± 0.12 | SF/NAC NPs |
| Enhancement Brain (100%) | 2.60 ± 1.05 | 1.96 ± 0.61 | 1.40 ± 0.39 | 1.68 ± 0.60 | 1.61 ± 0.91 | SF/NAC NPs |
| Nasal region | 0.81 ± 0.84 | 1.26 ± 0.72 | 1.05 ± 0.38 | 1.24 ± 0.61 | 1.04 ± 0.28 | SF/NAC NPs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, T.-W.; Wu, T.-Y.; Siah, Z.-Y.; Liu, D.-Z. Antioxidative NAC-Loaded Silk Nanoparticles with Opening Mucosal Tight Junctions for Nasal Drug Delivery: An In Vitro and In Vivo Study. Pharmaceutics 2022, 14, 1288. https://doi.org/10.3390/pharmaceutics14061288
Chung T-W, Wu T-Y, Siah Z-Y, Liu D-Z. Antioxidative NAC-Loaded Silk Nanoparticles with Opening Mucosal Tight Junctions for Nasal Drug Delivery: An In Vitro and In Vivo Study. Pharmaceutics. 2022; 14(6):1288. https://doi.org/10.3390/pharmaceutics14061288
Chicago/Turabian StyleChung, Tze-Wen, Ting-Ya Wu, Zheng-Yu Siah, and Der-Zen Liu. 2022. "Antioxidative NAC-Loaded Silk Nanoparticles with Opening Mucosal Tight Junctions for Nasal Drug Delivery: An In Vitro and In Vivo Study" Pharmaceutics 14, no. 6: 1288. https://doi.org/10.3390/pharmaceutics14061288
APA StyleChung, T.-W., Wu, T.-Y., Siah, Z.-Y., & Liu, D.-Z. (2022). Antioxidative NAC-Loaded Silk Nanoparticles with Opening Mucosal Tight Junctions for Nasal Drug Delivery: An In Vitro and In Vivo Study. Pharmaceutics, 14(6), 1288. https://doi.org/10.3390/pharmaceutics14061288