Bispecific Antibodies in Cancer Immunotherapy: A Novel Response to an Old Question
Abstract
1. Introduction
2. Pharmacology of Bispecific Antibodies
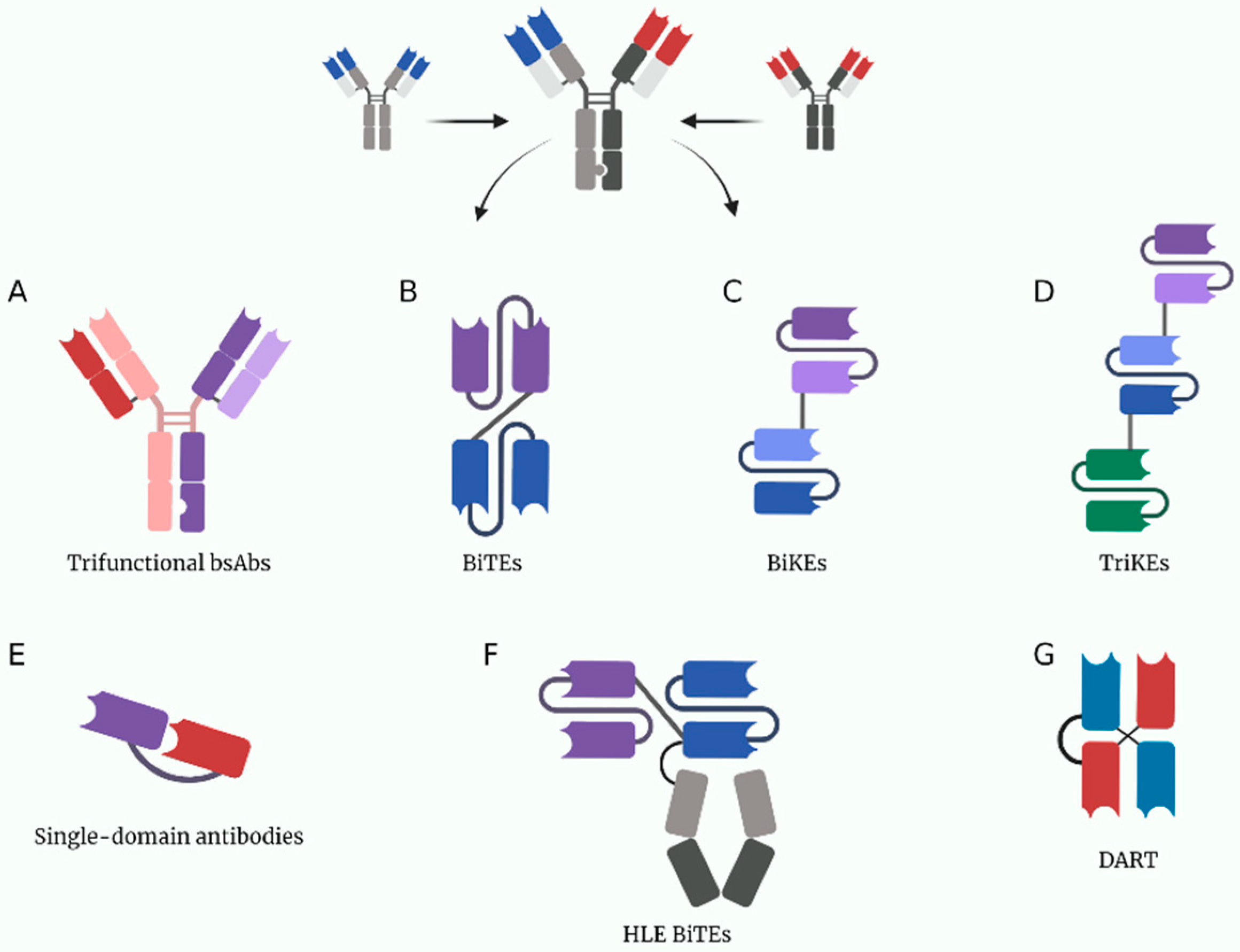
3. T-Cell Engaging Bispecific Antibodies
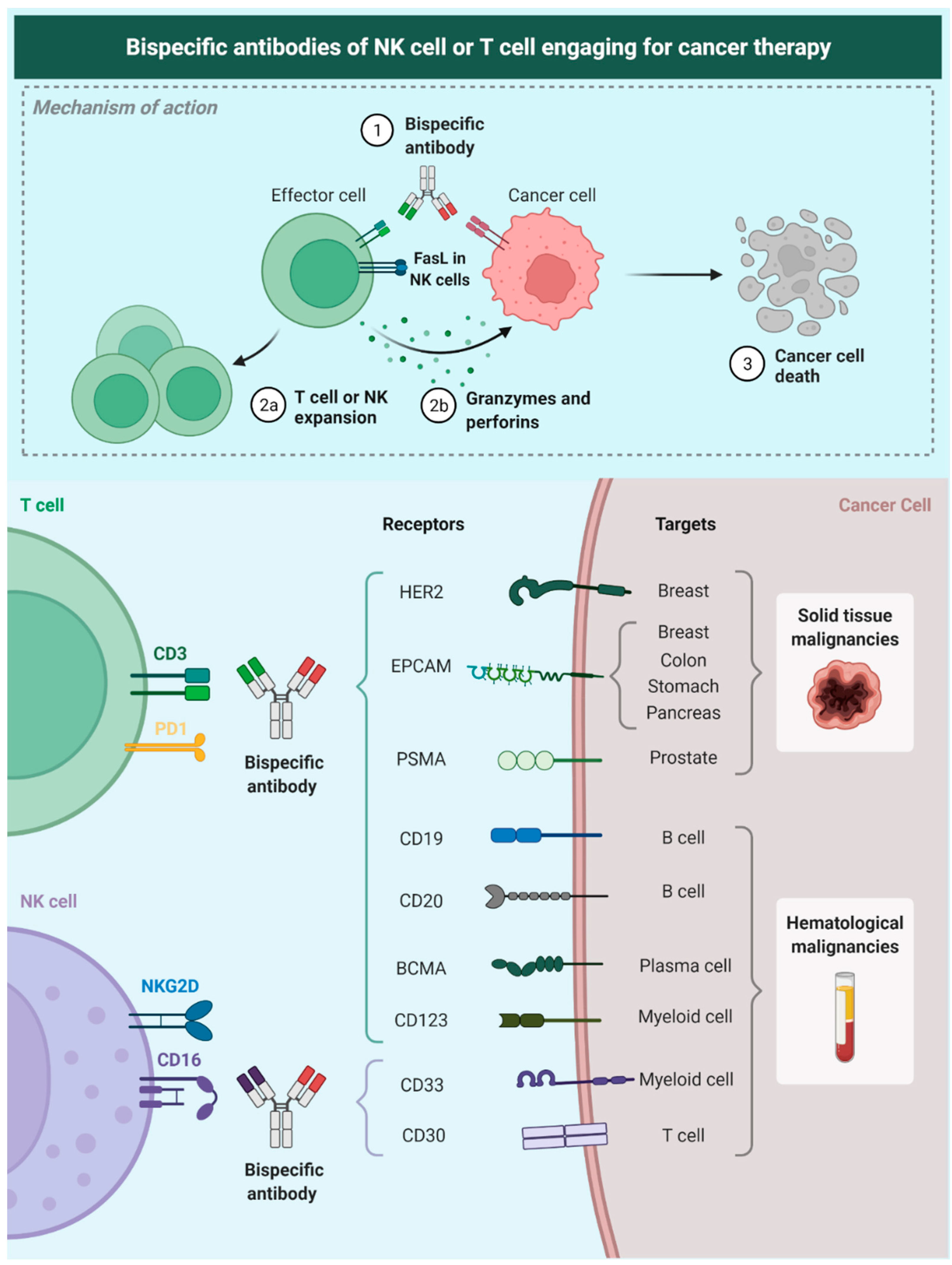
4. T-biAbs Action on Immune Effector Cells
5. T-biAbs for Redirecting Cells of the Innate Immune System
6. T-biAbs for the Restoration and Enhancement of Antitumor Immunity
7. Delivery Strategies for T-biAbs
7.1. Delivery of T-biAbs as Recombinant Proteins
7.2. Delivery of T-biAbs In Vivo Production
8. T-biAbs in Hematologic Malignancies
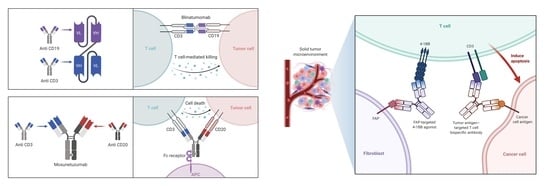
9. T-biAbs in Solid Malignancies
10. CAR-T Switches
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nuñez-Prado, N.; Compte, M.; Harwood, S.; Álvarez-Méndez, A.; Lykkemark, S.; Sanz, L.; Álvarez-Vallina, L. The Coming of Age of Engineered Multivalent Antibodies. Drug Discov. Today 2015, 20, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Labrijn, A.; Janmaat, M.; Reichert, J.; Parren, P. Bispecific Antibodies: A Mechanistic Review of the Pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Viardot, A.; Bargou, R. Bispecific Antibodies in Haematological Malignancies. Cancer Treat. Rev. 2018, 65, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Trabolsi, A.; Arumov, A.; Schatz, J.H.; Alerts, E. T Cell—Activating Bispecific Antibodies in Cancer Therapy. J. Immunol. 2022, 203, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Blanco, B.; Compte, M.; Lykkemark, S.; Sanz, L.; Alvarez-Vallina, L. T Cell-Redirecting Strategies to ‘sTAb’ Tumors: Beyond CARs and Bispecific Antibodies. Trends Immunol. 2019, 40, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Schirrmacher, V. Bispecific Antibodies and Trispecific Immunocytokines for Targeting the Immune System Against Cancer. BioDrugs 2013, 27, 35–53. [Google Scholar] [CrossRef]
- Duell, J.; Lammers, P.E.; Djuretic, I.; Chunyk, A.G.; Alekar, S.; Jacobs, I.; Gill, S. Bispecific Antibodies in the Treatment of Hematologic Malignancies. Clin. Pharmacol. Ther. 2019, 106, 781–791. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, M.; Zhu, S.; Wang, H.; Wu, K. Recent Advances and Challenges of Bispecific Antibodies in Solid Tumors. Exp. Hematol. Oncol. 2021, 10, 56. [Google Scholar] [CrossRef]
- Goulet, D.R.; Atkins, W.M. Considerations for the Design of Antibody-Based Therapeutics. J. Pharm. Sci. 2021, 109, 74–103. [Google Scholar] [CrossRef]
- Chiu, M.; Goulet, D.; Teplyakov, A.; Gilliland, G. A Review of Bispecific Antibodies and Antibody Constructs in Oncology and Clinical Challenges. Pharmacol. Ther. 2019, 201, 103–119. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Park, J.; Liu, X.; Hu, Y.; Wang, T.; Mcfarland, K.; Betenbaugh, M.J. Design and Production of Bispecific Antibodies.Pdf. Antibodies 2019, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, K.; Lei, Q.; Ma, P.; Yuan, A.Q.; Zhao, Y.; Jiang, Y.; Fang, H.; Xing, S.; Fang, Y.; et al. The State of the Art of Bispecific Antibodies for Treating Human Malignancies. EMBO Mol. Med. 2021, 13, e14291. [Google Scholar] [CrossRef]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616. [Google Scholar] [CrossRef] [PubMed]
- Lobo, E.D.; Hansen, R.J.; Balthasar, J.P. Antibody Pharmacokinetics and Pharmacodynamics. J. Pharm. Sci. 2004, 93, 2645–2668. [Google Scholar] [CrossRef]
- Chen, Y. Pharmacokinetics of Bispecific Antibody. Curr. Pharmacol. Rep. 2017, 3, 126–137. [Google Scholar] [CrossRef]
- Wu, Z.; Cheung, N. T Cell Engaging Bispecific Antibody (T-BsAb): From Technology to Therapeutics. Pharmacol. Ther. 2018, 182, 161–175. [Google Scholar] [CrossRef]
- Linke, R.; Klein, A.; Seimetz, D. Clinical Development and Future Directions Catumaxomab. Mabs 2010, 2, 129–136. [Google Scholar] [CrossRef]
- Chow, V. Clinical Use of Blinatumomab for B-Cell Acute Lymphoblastic Leukemia in Adults. Ther. Clin. Risk Manag. 2016, 12, 1301–1310. [Google Scholar]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of Immunoglobulin-T-Cell Receptor Chimeric Molecules as Functional Receptors with Antibody-Type Specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef]
- Kamakura, D.; Asano, R. T Cell Bispecific Antibodies: An Antibody-Based Delivery System for Inducing Antitumor Immunity. Pharmaceuticals 2021, 14, 1172. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.J.; Crean, R.M.; Pentier, J.M.; De Wet, B.; Lloyd, A.; Srikannathasan, V.; Lissin, N.; Lloyd, K.A.; Blicher, T.H.; Conroy, P.J.; et al. Specificity of Bispecific T Cell Receptors and Antibodies Targeting Peptide-HLA. J. Clin. Investig. 2020, 130, 2673–2688. [Google Scholar] [CrossRef] [PubMed]
- Huehls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T Cell Engagers for Cancer Immunotherapy. Immunol. Cell Biol. 2015, 93, 290–296. [Google Scholar] [CrossRef]
- Meermeier, E.W.; Welsh, S.J.; Sharik, M.E.; Du, M.T.; Garbitt, V.M.; Riggs, D.L.; Shi, C.; Stein, C.K.; Bergsagel, M.; Chau, B.; et al. Tumor Burden Limits Bispecific Antibody Efficacy through T-Cell Exhaustion Averted by Concurrent Cytotoxic Therapy. Blood Cancer Discov. 2021, 2, 354–396. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Han, C.-J.; Driver, I.; Olow, A.; Sewell, A.K.; Zhang, Z.; Ouyang, W.; Egen, J.G.; Yu, X. LILRB1 Blockade Enhances Bispecific T Cell Engager Antibody–Induced Tumor Cell Killing by Effector CD8+ T Cells. J. Immunol. 2019, 203, 1076–1087. [Google Scholar] [CrossRef]
- Benonisson, H.; Labrijn, A.F.; Schuurhuis, D.H.; Houtkamp, M.A.; Verbeek, J.S.; Schuurman, J.; Hall, T. Van CD3-Bispeci Fi c Antibody Therapy Turns Solid Tumors into Inflammatory Sites but Does Not Install Protective Memory. Mol. Cancer Ther. 2019, 18, 16–18. [Google Scholar] [CrossRef]
- Gupta, V.R.; Root, A.; Fisher, T.; Norberg, R.; David, J.; Cohen, J.; May, C.; Giddabasappa, A. Molecular Imaging Reveals Biodistribution of P-Cadherin LP- DART Bispecific and Trafficking of Adoptively Transferred T Cells in Mouse Xenograft Model. Oncotarget 2020, 11, 1344–1357. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Ybarra, R.; Mak, J.; Herault, A.; De Almeida, P.; Arrazate, A.; Ziai, J.; Totpal, K.; Junttila, M.R.; Walsh, K.B.; et al. IFN g -Induced Chemokines Are Required for CXCR3-Mediated T-Cell Recruitment and Antitumor Ef Fi Cacy of Anti-HER2/CD3 Bispeci Fi c Antibody. Clin. Cancer Res. 2018, 24, 6447–6458. [Google Scholar] [CrossRef]
- Franciszkiewicz, K.; Boissonnas, A.; Boutet, M.; Mami-chouaib, F. Role of Chemokines and Chemokine Receptors in Shaping the Effector Phase of the Antitumor Immune Response. Cancer Res. 2012, 72, 6325–6332. [Google Scholar] [CrossRef]
- Cremasco, F.; Menietti, E.; Speziale, D.; Sam, J.; Sammicheli, S.; Richard, M.; Varol, A.; Klein, C.; Umana, P.; Bacac, M.; et al. Cross-Linking of T Cell to B Cell Lymphoma by the T Cell Bispecific Antibody CD20-TCB Induces IFN γ/CXCL10-Dependent Peripheral T Cell Recruitment in Humanized Murine Model. PLoS ONE 2021, 16, e0241091. [Google Scholar] [CrossRef]
- Gasteiger, G.; Osualdo, D.; Schubert, D.A.; Bruscia, E.M. Cellular Innate Immunity: An Old Game with New Players. J. Innate Immun. 2017, 9, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Del Bano, J.; Chames, P.; Baty, D.; Kerfelec, B. Taking up Cancer Immunotherapy Challenges: Bispecific Antibodies, the Path Forward? Antibodies 2016, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Pettini, E.; Medaglini, D.; Dunkley, M.L. CD4 + T Cell Priming as Biomarker to Study Immune Response to Preventive Vaccines. Front. Immunol. 2013, 4, 421. [Google Scholar] [CrossRef] [PubMed]
- Eissler, N.; Mysliwietz, J.; Deppisch, N.; Ruf, P.; Lindhofer, H.; Mocikat, R. Potential of the Trifunctional Bispecific Antibody Surek Depends on Dendritic Cells: Rationale for a New Approach Of Tumor Immunotherapy. Mol. Med. 2013, 19, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hoseini, S.; Xu, H.; Ponomarev, V.; Cheung, N. Silencing Fc Domains in T Cell–Engaging Bispecific Antibodies Improves T-Cell Trafficking and Antitumor Potency. Cancer Immunol. Res. 2019, 7, 2013–2024. [Google Scholar] [CrossRef]
- Demaria, O.; Gauthier, L.; Debroas, G.; Vivier, E. Natural Killer Cell Engagers in Cancer Immunotherapy: Next Generation of Immuno-Oncology Treatments. Eur. J. Immunol. 2021, 81, 1934–3942. [Google Scholar] [CrossRef]
- Gauthier, L.; Morel, A.; Anceriz, N.; Morel, Y.; Narni-mancinelli, E.; Vivier, E.; Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity Article Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713. [Google Scholar] [CrossRef]
- Ellwanger, K.; Reusch, U.; Fucek, I.; Wingert, S.; Ross, T.; Müller, T.; Schniegler-mattox, U.; Haneke, T.; Rajkovic, E.; Koch, J.; et al. Redirected Optimized Cell Killing (ROCK®): A Highly Versatile Multispecific Fit-for- Purpose Antibody Platform for Engaging Innate Immunity. mAbs 2019, 11, 899–918. [Google Scholar] [CrossRef]
- Han, D.; Xu, Z.; Zhuang, Y.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Hematological Malignancies. J. Cancer 2021, 12, 326–334. [Google Scholar] [CrossRef]
- Moreno Cortes, E.; Garcia Robledo, J.; Booth, N.; Forero, J.; Castro, J. Optimization of Third Generation Chimeric Antigen Receptor T Cells Targeting ROR1 for Hematological Malignancies. Blood 2021, 138, 4804. [Google Scholar] [CrossRef]
- Forero, J.; Moreno Cortes, E.; Garcia Robledo, J.; Booth, N.; Castro, J. Preclinical NK Cell Platform for CAR Directed Therapies: Functional and Phenotypic Comparison Using a Rechallenge Cytotoxicity Assay. Blood 2021, 138, 4805. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Galo, J.; Bruni, D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Bae, J.; Li, H.; Sun, Z.; Moore, C.; Hsu, E.; Han, C.; Qiao, J.; Fu, Y. Rejuvenation of Tumour-Specific T Cells through Bispecific Antibodies Targeting PD-L1 on Dendritic Cells. Nat. Biomed. Eng. 2021, 5, 1261–1273. [Google Scholar] [CrossRef]
- Kraman, M.; Faroudi, M.; Allen, N.L.; Kmiecik, K.; Gliddon, D.; Seal, C.; Koers, A.; Wydro, M.M.; Batey, S.; Winnewisser, J.; et al. FS118, a Bispeci Fi c Antibody Targeting LAG-3 and PD-L1, Enhances T-Cell Activation Resulting in Potent Antitumor Activity. Clin. Cancer Res. 2020, 44, 3333–3344. [Google Scholar] [CrossRef]
- Cui, X.; Jia, H.; Xin, H.; Zhang, L.; Chen, S.; Xia, S.; Li, X. A Novel Bispecific Antibody Targeting PD-L1 and VEGF With Combined Anti-Tumor Activities. Front. Immunol. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Chen, S.-H.; Dominik, P.K.; Stanfield, J.; Ding, S.; Yang, W.; Kurd, N.; Llewellyn, R.; Heyen, J.; Wang, C.; Melton, Z.; et al. Dual Checkpoint Blockade of CD47 and L1 Using an Affinity-Tuned Bispecific Antibody Maximizes Antitumor Immunity. J. Immunother. Cancer 2021, 9, e003464. [Google Scholar] [CrossRef]
- Ramaswamy, M.; Taeil, K.; Jones, D.C.; Ghadially, H.; Mahmoud, T.; Garcia, A.; Browne, G.; Zenonos, Z.; Puplampu-Dove, Y.; Riggs, J.M.; et al. Immunomodulation of T- and NK-Cell Responses by a Bispecific Antibody Targeting CD28 Homolog and PD-L1. Cancer Immunol. Res. 2022, 10, 200–214. [Google Scholar] [CrossRef]
- Peper-gabriel, J.K.; Pavlidou, M.; Pattarini, L.; Morales-kastresana, A.; Jaquin, J.; Gallou, C.; Hansbauer, E.; Richter, M.; Lelievre, H.; Bossenmaier, B.; et al. The PD-L1/4-1BB Bispecific Antibody-Anticalin Fusion Protein PRS-344/S095012 Elicits Strong T_cell Stimulation in a Tumor-Localized Manne. Clin. Cancer Resear 2022, 1078–1082. [Google Scholar]
- De Miguel, M.; Umana, P.; Luiza, A.; De Morais, G.; Moreno, V.; Calvo, E. T-Cell—Engaging Therapy for Solid Tumors. Clin. Cancer Res. 2021, 27, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Rader, C. Bispecific Antibodies in Cancer Immunotherapy. Curr. Opin. Biotechnol. 2020, 65, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, N.G.; Hollander, E.E.; Powell, D.J., Jr. The Emergence of Universal Immune Receptor T Cell Therapy for Cancer. Front. Oncol. 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Arndt, C.; Koristka, S.; Berndt, N.; Bergmann, R.; Bachmann, M.P. Conventional CARs versus Modular CARs. Cancer Immunol. Immunother. 2019, 68, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, H.; Wirth, T.; Schenkwein, D.; Räty, J.K.; Ylä-herttuala, S. Challenges in Monoclonal Antibody-Based Therapies. Ann. Med. 2009, 41, 3890. [Google Scholar] [CrossRef]
- Sanz, L.; Luis, A.; Sa, D. Engineering Human Cells for in Vivo Secretion of Antibody and Non-Antibody Therapeutic Proteins. Curr. Opin. Biotechnol. 2011, 22, 924–930. [Google Scholar] [CrossRef]
- Blanco, B.; Dominguez-Alonso, C.; Alvarez-Vallina, L. Bispecific Immunomodulatory Antibodies for Cancer Immunotherapy. Clin. Cancer Res. 2021, 27, 5457–5464. [Google Scholar] [CrossRef]
- Shaw, A.R.; Suzuki, M. Oncolytic Viruses Partner With T-Cell Therapy for Solid Tumor Treatment. Front. Immunol. 2018, 9, 2103 . [Google Scholar] [CrossRef]
- Fajardo, C.A.; Guedan, S.; Rojas, L.A.; Moreno, R.; Arias-badia, M.; De Sostoa, J.; June, C.H.; Alemany, R. Oncolytic Adenoviral Delivery of an EGFR-Targeting T-Cell Engager Improves Antitumor Ef Fi Cacy. Cancer Res. 2017, 77, 2052–2063. [Google Scholar] [CrossRef]
- Stadler, C.R.; Bähr-mahmud, H.; Celik, L.; Hebich, B.; Roth, A.S.; Roth, R.P.; Karikó, K.; Türeci, Ö.; Sahin, U. Elimination of Large Tumors in Mice by MRNA-Encoded Bispecific Antibodies. Nat. Med. 2017, 23, 815–817. [Google Scholar] [CrossRef]
- De Sostoa, J.; Fajardo, C.A.; Moreno, R.; Ramos, M.D.; Farrera-sal, M.; Alemany, R. Targeting the Tumor Stroma with an Oncolytic Adenovirus Secreting a Fibroblast Activation Protein-Targeted Bispecific T-Cell Engager. J. Immunother. Cancer 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wen, W.; Qin, W. Bispecific Antibodies as a Development Platform for New Concepts and Treatment Strategies. Int. J. Mol. Sci. 2016, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.P.; Bonifant, C.L.; Gottschalk, S.; Gottschalk, S.; Place, D.T. Redirecting T Cells to Hematological Malignancies with Bispecific Antibodies. Blood 2017, 131, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lam, C.K.; Long, V.; Widjaja, L.; Yang, Y.; Li, H.; Jin, L.; Burke, S.; Gorlatov, S.; Brown, J.; et al. MGD011, A CD19 x CD3 Dual-Af Fi Nity Retargeting Bi-Speci Fi c Molecule Incorporating Extended Circulating Half-Life for the Treatment of B-Cell Malignancies. Clin. Cancer Res. 2017, 23, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Fielding, A.K.; Ph, D.; Schuh, A.C.; Dombret, H.; Foà, R.; Bassan, R.; Arslan, Ö.; Sanz, M.A.; Ph, D.; Bergeron, J.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Martinelli, G.; Boissel, N.; Chevallier, P.; Ottmann, O.; Go, N.; Rambaldi, A.; Ritchie, E.K.; Papayannidis, C.; Tuglus, C.A.; Morris, J.D.; et al. Long-Term Follow-up of Blinatumomab in Patients with Relapsed/Refractory Philadelphia Chromosome e Positive B-Cell Precursor Acute Lymphoblastic Leukaemia: Final Analysis of ALCANTARA Study. Eur. J. Cancer 2021, 146, 107–114. [Google Scholar] [CrossRef]
- DRUGS Blincyo FDA Approval History. Available online: https://www.drugs.com/history/blincyto.html (accessed on 17 March 2022).
- Frey, N.V.; Porter, D.L. Cytokine Release Syndrome with Novel Therapeutics for Acute Lymphoblastic Leukemia. Hematology 2016, 2016, 567–572. [Google Scholar] [CrossRef]
- Conde-royo, D.; Juárez-salcedo, L.M.; Dalia, S. Management of Adverse Effects of New Monoclonal Antibody Treatments in Acute Lymphoblastic Leukemia Blinatumomab. Drugs Context 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Uy, G.; Stewart, S.; Baughman, J.; Rettig, M.; Chichili, G.; Bonvini, E.; Wigginton, J.; Lechleider, R.; Dipersio, J. A Phase I Trial of MGD006 in Patients with Relapsed Acute Myeloid Leukemia (AML). J. Immunother. Cancer 2014, 2, P87. [Google Scholar] [CrossRef]
- Ravandi, F.; Bashey, A.; Stock, W.; Egan, D. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of Vibecotamab (XmAb14045), a CD123 x CD3 T Cell-Engaging Bispecific Antibody; Initial Results of a Phase 1 Study. Blood 2020, 136, 4–5. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-marchant, K.; et al. Gemtuzumab Ozogamicin for de Novo Acute Myeloid Leukemia: Final Efficacy and Safety Updates from the Open-Label, Phase III ALFA-0701 Trial. Hematologica 2019, 104, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, M.; Zhang, Y.; Wang, X. Bispecific T Cell Engagers: An Emerging Therapy for Management of Hematologic Malignancies. J. Hematol. Oncol. 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.S.; Duell, J.; Zugmaier, G.; Attal, M.; Moreau, P.; Langer, C. Anti—B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma Abstract. J. Clin. Oncol. 2022, 38, 775–783. [Google Scholar] [CrossRef] [PubMed]
- De Zafra, C.L.Z.; Fajardo, F.; Zhong, W.; Bernett, M.J.; Muchhal, U.S.; Moore, G.L.; Stevens, J.; Case, R.; Pearson, J.T.; Liu, S.; et al. Targeting Multiple Myeloma with AMG 424, a Novel Anti-CD38/CD3 Bispeci Fi c T-Cell—Recruiting Antibody Optimized for Cytotoxicity and Cytokine Release. Clin. Cancer Res. 2019, 25, 3921–3933. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Kochi, Y.; Nakai, W.; Mizuno, H.; Baba, T.; Habu, K.; Sawada, N.; Tsunoda, H.; Shima, T.; Miyawaki, K.; et al. Anti-GPRC5D/CD3 Bispeci Fi c T-Cell -- Redirecting Antibody for the Treatment of Multiple Myeloma. Mol. Cancer Ther. 2019, 18, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Huang, M.; Lum, L.G. Bispecific Antibody Based Therapeutics: Strengths and Challenges. Blood Rev. 2018, 32, 339–347. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.Y.O.S.; Cui, Z.Y.U.N.; Lee, H.; Ahn, J.I.N.S.; Park, C.K.; Park, K.; Ahn, M. Clinicopathological Implications of EpCAM Expression in Adenocarcinoma of the Lung. Anticancer Res. 2009, 1822, 1817–1822. [Google Scholar]
- Dudnichenko, A.S.; Aleknaviciene, B.; Razbadauskas, A.; Gore, M.; Ganea-motan, E. The Trifunctional Antibody Catumaxomab for the Treatment of Malignant Ascites Due to Epithelial Cancer: Results of a Prospective Randomized Phase II/III Trial. Int. J. Cancer 2010, 2221, 2209–2221. [Google Scholar] [CrossRef]
- Brinkmann, U.; Kontermann, R.E. Bispecific Antibodies. Science 2021, 372, 916–917. [Google Scholar] [CrossRef]
- Kebenko, M.; Goebeler, M.; Wolf, M.; Hasenburg, A.; Seggewiss-bernhardt, R. A Multicenter Phase 1 Study of Solitomab (MT110, AMG 110), a Bispecific EpCAM/CD3 T-Cell Engager (BiTE Ò) Antibody Construct, in Patients with Refractory Solid Tumors. Oncoimmunology 2018, 7, e1450710. [Google Scholar] [CrossRef]
- Haruyama, Y.; Kataoka, H. Glypican-3 Is a Prognostic Factor and an Immunotherapeutic Target in Hepatocellular Carcinoma. World J. Gastroenterol. 2016, 22, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, X.; Huang, N.; Lang, Q.; He, Q.; Jian-hua, W.; Liang-peng, G. A Novel Targeted GPC3/CD3 Bispecific Antibody for the Treatment Hepatocellular Carcinoma. Cancer Biol. Ther. 2020, 21, 597–603. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.; Fong, L.; Beer, T.; Gao, X.; Geynisman, D.; Burris, H. Results of an Ongoing Phase 1/2a Dose Escalation Study of HPN424, a Tri-Specific Half-Life Extended PSMA-Targeting T-Cell Engager, in Patients with Metastatic Castration-Resistant Prostate Cancer (MCRPC). J. Clin. Oncol. 2021, 39, 5013. [Google Scholar] [CrossRef]
- Petra, D.; Thomas, O.; Nolan-Stevaux, O.; Li, S.; Wahl, J.; Bogner, P.; Aeffner, F.; Friedrich, M.; Liao, M.; Matthes, K.; et al. The PSMA-Targeting Half-Life Extended BiTE Therapy AMG 160 Has Potent Antitumor Activity in Preclinical Models of Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2021, 27, 2928–2937. [Google Scholar]
- Geyer, M. First CAR to Pass the Road Test: Tisagenlecleucel’s Drive to FDA Approval. Clin. Cancer Res. 2020, 25, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, D.T.; Mazagova, M.; Hampton, E.N.; Cao, Y.; Ramadoss, N.S.; Hardy, I.R.; Schulman, A.; Du, J.; Wang, F.; Singer, O.; et al. Switch-Mediated Activation and Retargeting of CAR-T Cells for B-Cell Malignancies. Proc. Natl. Acad. Sci. USA 2016, 113, E459–E468. [Google Scholar] [CrossRef]
- Moghanloo, E.; Mollanoori, H.; Talebi, M.; Pashangzadeh, S. Translational Oncology Remote Controlling of CAR-T Cells and Toxicity Management: Molecular Switches and next Generation CARs. Transl. Oncol. 2021, 14, 101070. [Google Scholar] [CrossRef]
| ClinicalTrials.gov Identifier | Title | Conditions | Interventions | Phase |
|---|---|---|---|---|
| Recruiting | ||||
| NCT03146637 | Study of Activated CIK Armed With Bispecific Antibody for Advanced Liver Cancer | Advanced Liver Cancer | Biological: Activated CIK|Biological: CIK | Phase 2 |
| NCT05125016 | REGN4336 (a PSMAXCD3 Bispecific Antibody) Administered Alone or in Combination With Cemiplimab in Adult Male Patients With Metastatic Castration-Resistant Prostate Cancer | Metastatic Castration-resistant Prostate Cancer | Drug: REGN4336|Drug: Cemiplimab|Other: 18F-DCFPyL | Phase 2 |
| NCT04868877 | A Phase 1/2 Study Evaluating MCLA-129, a Human Anti-EGFR and Anti-c-MET Bispecific Antibody, in Patients With Advanced NSCLC and Other Solid Tumors | Non-Small Cell Lung Cancer Metastatic|Gastric Cancer|Head and Neck Cancer | Drug: MCLA-129 | Phase 2 |
| NCT05090566 | MagnetisMM-4: Umbrella Study of Elranatamab (PF-06863135) in Combination With Anti-Cancer Treatments in Multiple Myeloma | Multiple Myeloma | Drug: Elranatamab + Nirogacestat|Drug: Elranatamab + lenalidomide + dexamethasone | Phase 2 |
| NCT03860207 | Study of the Safety and Efficacy of Humanized 3F8 Bispecific Antibody (Hu3F8-BsAb) in Patients With Relapsed/Refractory Neuroblastoma, Osteosarcoma and Other Solid Tumor Cancers | Neuroblastoma|Osteosarcoma|Other Solid Tumor Cancers | Biological: Humanized 3F8 Bispecific Antibody|Other: Blood draw | Phase 2 |
| NCT04380805 | A Study of AK104, a PD-1/CTLA-4 Bispecific Antibody in Subjects With Recurrent/Metastatic Cervical Cancer | Recurrent Cervical Cancer|Metastatic Cervical Cancer | Biological: AK104 | Phase 2 |
| NCT04886271 | Recombinant Humanized Anti-CD47/PD-1 Bifunctional Antibody HX009 Injection in the Treatment of Advanced Solid Tumors | Advanced Solid Tumor | Drug: HX009 | Phase 2 |
| NCT04276493 | Anti-HER2 Bispecific Antibody ZW25 Activity in Combination With Chemotherapy With/Without Tislelizumab | Breast Cancer|Gastric Cancer|Gastroesophageal Junction Cancer | Biological: ZW25|Drug: Docetaxel|Biological: Tislelizumab|Drug: Capecitabine|Drug: Oxaliplatin | Phase 2 |
| NCT04999605 | A Study of AK112 Combined With PARP Inhibitor in the Treatment of Recurrent Ovarian Cancer | Ovarian Neoplasms|Recurrent Ovarian Carcinoma|Relapsed Ovarian Cancer|Ovarian Cancer | Drug: AK112 low dose|Drug: AK112 medium dose|Drug: AK112 high dose | Phase 2 |
| NCT04618393 | A Study of EMB-02 in Participants With Advanced Solid Tumors | Advanced Solid Tumors | Biological: EMB-02 | Phase 2 |
| NCT05214482 | A Study of AK112 in Advanced Malignant Tumors | Advanced Malignant Tumors | Drug: AK112|Drug: AK117|Drug: Chemotherapy | Phase 2 |
| NCT03406858 | Pembrolizumab and HER2Bi-Armed Activated T Cells in Treating Patients With Metastatic Castration Resistant Prostate Cancer | Castration Levels of Testosterone|Castration-Resistant Prostate Carcinoma|Prostate Carcinoma Metastatic in the Bone|PSA Progression|Stage IV Prostate Adenocarcinoma AJCC v7 | Biological: HER2Bi-Armed Activated T Cells|Other: Laboratory Biomarker Analysis|Biological: Pembrolizumab | Phase 2 |
| NCT02912949 | A Study of Zenocutuzumab (MCLA-128) in Patients With Solid Tumors Harboring an NRG1 Fusion | Solid Tumours Harboring NRG1 Fusion|NSCLC Harboring NRG1 Fusion|Pancreatic Cancer Harboring NRG1 Fusion|NRG1 Fusion | Drug: zenocutuzumab (MCLA-128) | Phase 2 |
| NCT04995523 | A Study to Assess the Safety and Efficacy of AZD2936 in Participants With Advanced or Metastatic Non-small Cell Lung Cancer (NSCLC) | Non-Small-Cell Lung Carcinoma | Drug: AZD2936 | Phase 2 |
| NCT05102214 | HLX301 (TIGIT × PDL1 Bispecific) in Patients With Locally Advanced or Metastatic Solid Tumors | Locally Advanced or Metastatic Solid Tumors|Non-small Cell Lung Cancer | Drug: HLX301 | Phase 2 |
| NCT03269526 | BATs Treatment for Pancreatic Cancer, Phase Ib/II | Locally Advanced Pancreatic Adenocarcinoma|Metastatic Pancreatic Adenocarcinoma | Drug: EGFR BATs after standard of care chemo | Phase 2 |
| NCT04547101 | A Study of AK104 in Subjects With Locally Advanced Unresectable or Metastatic MSI-H/dMMR Solid Tumors | MSI-H/dMMR Solid Tumor | Drug: AK104 | Phase 2 |
| NCT03564340 | Study of REGN4018 Administered Alone or in Combination With Cemiplimab in Adult Patients With Recurrent Ovarian Cancer | Recurrent Ovarian Cancer|Recurrent Fallopian Tube Cancer|Recurrent Primary Peritoneal Cancer | Drug: REGN4018|Drug: cemiplimab | Phase 2 |
| NCT05159388 | A Study of PRS-344/S095012 (PD-L1 × 4-1BB Bispecific Antibody-Anticalin Fusion) in Patients With Solid Tumors | Solid Tumor | Drug: PRS-344/S095012 | Phase 2 |
| NCT04626635 | REGN7075 in Combination With Cemiplimab in Adult Participants With Advanced Solid Tumors | Advanced Solid Tumors | Drug: REGN7075|Drug: cemiplimab | Phase 2 |
| NCT04930432 | Study of MCLA-129, a Human Bispecific EGFR and cMet Antibody, in Patients With Advanced NSCLC and Other Solid Tumors | Solid Tumor|Non-Small Cell Lung Cancer|Head and Neck Cancer|Colorectal Cancer | Drug: MCLA-129 | Phase 2 |
| NCT04750239 | Safety and Clinical Activity of Nivatrotamab in Relapsed/Recurrent Metastatic Small-cell Lung Cancer | SCLC | Drug: Nivatrotamab | Phase 2 |
| NCT04931654 | A Study to Assess the Safety and Efficacy of AZD7789 in Participants With Advanced or Metastatic Solid Cancer | Carcinoma, Non-Small-Cell Lung | Drug: AZD7789 | Phase 2 |
| NCT03440437 | FS118 First in Human Study in Patients With Advanced Malignancies | Advanced Cancer|Metastatic Cancer|Squamous Cell Carcinoma of Head and Neck | Drug: FS118 | Phase 2 |
| NCT04900363 | A Trial of AK112 (PD-1/VEGF Bispecific Antibody) in Patients With NSCLC | Non-small Cell Lung Cancer | Drug: AK112 | Phase 2 |
| NCT04870177 | Study of AK112 in the Treatment of Advanced Gynecological Tumors | Gynecologic Cancer|Cancer Metastatic|Ovarian Neoplasms|Cervical Neoplasm|Endometrial Neoplasms | Drug: AK112 | Phase 2 |
| NCT04557098 | A Study of Teclistamab, in Participants With Relapsed or Refractory Multiple Myeloma | Hematological Malignancies | Drug: Teclistamab | Phase 2 |
| NCT04634552 | A Study of Talquetamab in Participants With Relapsed or Refractory Multiple Myeloma | Hematological Malignancies | Drug: Talquetamab | Phase 2 |
| NCT05180474 | Research Trial to Study Safety of GEN1047 (DuoBody®-CD3xB7H4) in Participants With Malignant Solid Tumors | Breast Cancer|Uterine Cancer|Ovarian Cancer|Non Small Cell Lung Cancer (NSCLC)|Cervical Cancer|Head and Neck Squamous Cell Carcinoma (HNSCC), Except for Nasopharyngeal Carcinoma|Urothelial Cancer|Cholangiocarcinoma (CCA) | Biological: GEN1047 is a bispecific antibody that induces T-cell-mediated cytotoxicity of B7H4-positive cells. | Phase 2 |
| NCT03888105 | Assess the Anti-Tumor Activity and Safety of Odronextamab in Patients With Relapsed or Refractory B-cell Non-Hodgkin Lymphoma | B-cell Non-Hodgkin Lymphoma (NHL) | Drug: Odronextamab | Phase 2 |
| NCT04696809 | A Study of Teclistamab in Japanese Participants With Relapsed or Refractory Multiple Myeloma | Hematologic Malignancies | Drug: Teclistamab | Phase 2 |
| NCT04496674 | Bispecific PSMAxCD3 Antibody CC-1 in Patients With Squamous Cell Carcinoma of the Lung | Lung Cancer Squamous Cell | Drug: CC-1 and Toczilizumab | Phase 2 |
| NCT05228470 | MagnetisMM-8: Study Of Elranatamab (PF-06863135) Monotherapy in Chinese Participants With Refractory Multiple Myeloma | Elranatamab|Myeloma|Multiple Myeloma|Relapsed Multiple Myeloma|Refractory Multiple Myeloma|PF-06863135|BCMA|Bispecific|Bispecific Antibody|BCMA-CD3 Bispecific|MagnetisMM-8 | Drug: Elranatamab | Phase 2 |
| NCT04590781 | Safety and Efficacy of XmAb18087 ± Pembrolizumab in Advanced Merkel Cell Carcinoma or Extensive-stage Small Cell Lung Cancer | Merkel Cell Carcinoma|Small Cell Lung Cancer | Biological: XmAb18087|Drug: XmAb18087 ± Pembrolizumab | Phase 2 |
| NCT03272334 | Her2-BATS and Pembrolizumab in Metastatic Breast Cancer | Metastatic Breast Cancer | Drug: HER2 BATs with Pembrolizumab | Phase 2 |
| NCT04492033 | A Study of ABL001 in Combination With Irinotecan or Paclitaxel in Patients With Advanced or Metastatic Solid Tumors | P1b: Advanced Solid Tumors|P2: Biliary Tract Cancer | Drug: ABL001|Drug: Paclitaxel|Drug: Irinotecan | Phase 2 |
| NCT04466891 | A Study of ZW25 (Zanidatamab) in Subjects With Advanced or Metastatic HER2-Amplified Biliary Tract Cancers | HER2-amplified Biliary Tract Cancers | Drug: ZW25 (Zanidatamab)|Diagnostic Test: In situ hybridization (ISH)-based companion diagnostic assay|Diagnostic Test: Immunohistochemistry (IHC)-based companion diagnostic assay | Phase 2 |
| NCT03929666 | A Safety and Efficacy Study of ZW25 (Zanidatamab) Plus Combination Chemotherapy in HER2-expressing Gastrointestinal Cancers, Including Gastroesophageal Adenocarcinoma, Biliary Tract Cancer, and Colorectal Cancer | HER2-expressing Gastrointestinal Cancers, Including Gastroesophageal Adenocarcinoma, Biliary Tract Cancer, and Colorectal Cancer | Drug: ZW25 (Zanidatamab)|Drug: Capecitabine|Drug: Cisplatin|Drug: Fluorouracil|Drug: Leucovorin|Drug: Oxaliplatin|Drug: Bevacizumab|Drug: Gemcitabine | Phase 2 |
| NCT03761108 | First in Human (FIH) Study of REGN5458 in Patients With Relapsed or Refractory Multiple Myeloma | Multiple Myeloma | Drug: REGN5458 | Phase 2 |
| NCT04224272 | A Study of ZW25 (Zanidatamab) With Palbociclib Plus Fulvestrant in Patients With HER2+/HR+ Advanced Breast Cancer | HER2+/HR+ Breast Cancer | Drug: ZW25 (Zanidatamab)|Drug: Palbociclib|Drug: Fulvestrant | Phase 2 |
| NCT05176665 | EMB-01 in Patients With Advanced/Metastatic Gastrointestinal Cancers | Neoplasms|Neoplasm Metastasis|Metastatic Gastrointestinal Carcinoid Tumor | Drug: EMB-01 | Phase 2 |
| NCT03797391 | A Dose Escalation With Expansion Study of EMB-01 in Participants With Advanced/Metastatic Solid Tumors | Neoplasms|Neoplasm Metastasis|Non-Small-Cell Lung Cancer | Drug: EMB-01 | Phase 2 |
| NCT04785820 | A Study of RO7121661 and RO7247669 Compared With Nivolumab in Participants With Advanced or Metastatic Squamous Cell Carcinoma of the Esophagus | Advanced or Metastatic Esophageal Squamous Cell Carcinoma | Drug: RO7121661|Drug: RO7247669|Drug: Nivolumab | Phase 2 |
| NCT04735575 | A Ph1/2 Study of EMB-06 in Participants With Recurrent or Refractory Myeloma | Relapsed or Refractory Multiple Myeloma | Biological: EMB-06 | Phase 2 |
| NCT05014412 | A Study to Learn About the Study Medicine (Elranatamab) in Participants With Multiple Myeloma That Has Come Back After Responding to Treatment or Has Not Responded to Treatment | Multiple Myeloma | Drug: Elranatamab | Phase 2 |
| NCT05189093 | Recombinant Humanized Anti-CD47/PD-1 Bifunctional Antibody HX009 in Patients With Relapsed/Refractory Lymphoma | Relapsed/Refractory Lymphoma | Drug: Recombinant humanized anti-CD47/PD-1 bifunctional antibody HX009 injection | Phase 2 |
| NCT04728321 | A Study of Anti-PD-1/CTLA-4 Bispecific AK104 Alone or in Combination With Lenvatinib in Advanced Hepatocellular Carcinoma | Hepatocellular Carcinoma | Biological: AK104 lenvatinib|Biological: AK104 | Phase 2 |
| NCT04889716 | CAR-T Followed by Bispecific Antibodies | Large B-cell Lymphoma | Drug: mosunetuzumab|Drug: glofitamab|Drug: obinutuzumab | Phase 2 |
| NCT04444167 | A Study of Anti-PD-1/CTLA-4 Bispecific AK104 Plus Lenvatinib in First-line Advanced Hepatocellular Carcinoma | Hepatocellular Carcinoma | Biological: AK104|Drug: Lenvatinib | Phase 2 |
| NCT04444141 | A Study of PD-1/CTLA-4 Bispecific AK104 in Relapsed or Refractory Peripheral T-cell Lymphoma | Peripheral T-cell Lymphoma | Biological: AK104 | Phase 2 |
| NCT04602065 | Evaluation of Safety and Efficacy of IBI318 Monotherapy for Relapsed/Refractory Extranodal NK/T Cell Lymphoma (Nasal Type) Trial | Extranodal NK/T Cell Lymphoma, Nasal Type | Drug: IBI318(Recombinant human anti-PD1/PD-L1 bispecific antibody) | Phase 2 |
| NCT05044897 | A Clinical Study to Evaluate the Efficacy and Safety of SI-B001 in the Treatment of Recurrent and Metastatic HNSCC | Head and Neck Squamous Cell Carcinoma | Drug: SI-B001 | Phase 2 |
| NCT04763083 | First in Human Study of NVG-111 in Chronic Lymphocytic Leukaemia and Mantle Cell Lymphoma | Chronic Lymphocytic Leukaemia|Small Lymphocytic Lymphoma|Mantle Cell Lymphoma | Drug: NVG-111|Drug: NVG-111 (RP2D) | Phase 2 |
| NCT04703686 | Treatment by a Bispecific CD3xCD20 Antibody for Relapse/Refractory Lymphomas After CAR T-cells Therapy | Diffuse Large B-Cell Lymphoma Refractory|Refractory Indolent Adult Non-Hodgkin Lymphoma|Refractory Transformed B-cell Non-Hodgkin Lymphoma|Refractory Primary Mediastinal Large B-Cell Cell Lymphoma|Refractory Mantle Cell Lymphoma | Drug: Obinutuzumab|Drug: RO7082859 | Phase 2 |
| NCT04469725 | KN046 (a Humanized PD-L1/CTLA4 Bispecific Single Domain Fc Fusion Protein Antibody) in Subjects With Thymic Carcinoma | Thymic Carcinoma | Drug: KN046 | Phase 2 |
| NCT05020236 | MagnetisMM-5: Study of Elranatamab (PF-06863135) Monotherapy and Elranatamab + Daratumumab Versus Daratumumab + Pomalidomide + Dexamethasone in Participants With Relapsed/Refractory Multiple Myeloma | Multiple Myeloma | Drug: Elranatamab|Drug: Daratumumab|Drug: Pomalidomide|Drug: Dexamethasone | Phase 3 |
| NCT05152147 | A Study of Zanidatamab in Combination With Chemotherapy Plus or Minus Tislelizumab in Patients With HER2-positive Advanced or Metastatic Gastric and Esophageal Cancers | Gastric Neoplasms|Gastroesophageal Adenocarcinoma|Esophageal Adenocarcinoma | Drug: Zanidatamab|Drug: Tislelizumab|Drug: Trastuzumab|Drug: Capecitabine|Drug: Oxaliplatin|Drug: Cisplatin|Drug: 5-Fluorouracil|Diagnostic Test: In situ hybridization (ISH)-based companion diagnostic assay|Diagnostic Test: Immunohistochemistry (IHC)-based companion diagnostic assay | Phase 3 |
| NCT03643276 | Treatment Protocol for Children and Adolescents With Acute Lymphoblastic Leukemia—AIEOP-BFM ALL 2017 | Acute Lymphoblastic Leukemia, Pediatric | Drug: Blinatumomab|Drug: Bortezomib|Drug: Cyclophosphamide|Drug: Cytarabine|Drug: Daunorubicin|Drug: Myocet|Drug: Dexamethasone|Drug: Doxorubicin|Drug: Etoposide|Drug: Fludarabine Phosphate|Drug: Ifosfamide|Drug: 6-Mercaptopurine|Drug: Methotrexate|Drug: Pegaspargase|Drug: Prednisolone|Drug: Tioguanin|Drug: Vincristine|Drug: Vindesine|Drug: Erwinase | Phase 3 |
| NCT04722848 | Sequential Treatment With Ponatinib and Blinatumomab vs. Chemotherapy and Imatinib in Newly Diagnosed Adult Ph+ ALL | Acute Lymphoblastic Leukemia (Philadelphia Chromosome Positive)|ALL, Adult|Philadelphia-Positive ALL | Drug: Ponatinib + Blinatumomab|Drug: Chemotherapy + Imatinib | Phase 3 |
| Active, not recruiting | ||||
| NCT02620865 | Bispecific Antibody Armed Activated T-cells With Aldesleukin and Sargramostim in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer | Metastatic Pancreatic Adenocarcinoma|Recurrent Pancreatic Carcinoma|Stage III Pancreatic Cancer|Stage IV Pancreatic Cancer | Biological: Aldesleukin|Biological: Antibody Therapy|Drug: Fluorouracil|Drug: Gemcitabine Hydrochloride|Drug: Irinotecan Hydrochloride|Other: Laboratory Biomarker Analysis|Drug: Leucovorin Calcium|Drug: Oxaliplatin|Drug: Paclitaxel Albumin-Stabilized Nanoparticle Formulation|Biological: Sargramostim | Phase 2 |
| NCT03321981 | MCLA-128 With Trastuzumab/Chemotherapy in HER2+ and With Endocrine Therapy in ER+ and Low HER2 Breast Cancer | Breast Cancer Metastatic | Drug: MCLA-128|Drug: Trastuzumab|Drug: Vinorelbine|Drug: Endocrine therapy | Phase 2 |
| NCT04649359 | MagnetisMM-3: Study Of Elranatamab (PF-06863135) Monotherapy in Participants With Multiple Myeloma Who Are Refractory to at Least One PI, One IMiD and One Anti-CD38 mAb | Multiple Myeloma | Drug: Elranatamab (PF-06863135) | Phase 2 |
| NCT04083534 | First In Human (FIH) Study of REGN5459 in Adult Patients With Relapsed or Refractory Multiple Myeloma (MM) | Relapsed Multiple Myeloma|Refractory Multiple Myeloma | Drug: REGN5459 | Phase 2 |
| Not yet recruiting | ||||
| NCT04868708 | A Study of AK104 (an Anti-PD-1 and Anti-CTLA-4 Bispecific Antibody) in Recurrent or Metastatic Cervical Cancer | Recurrent or Metastatic Cervical Cancer | Biological: AK104|Biological: Bevacizumab|Drug: Paclitaxel|Drug: Cisplatin or Carboplatin | Phase 2 |
| NCT04556253 | AK104 in Locally Advanced MSI-H/dMMR Gastric Carcinoma and Colorectal Cancer | MSI-H/dMMR Gastric Carcinoma and Colorectal Cancer | Drug: AK104 | Phase 2 |
| NCT04172454 | Safety and Efficacy of AK104, a PD-1/CTLA-4 Bispecific Antibody, in Selected Advanced Solid Tumors | Advanced Solid Tumors|Melanoma | Biological: AK104 | Phase 2 |
| NCT04597541 | A Study of AK112, a PD-1/VEGF Bispecific Antibody, for Advanced Solid Tumors | Solid Tumor, Adult | Drug: AK112 | Phase 2 |
| NCT05229497 | A Phase Ib/II Study of AK112 in Combination With AK117 in Advanced Malignant Tumors | Advanced Malignant Tumors | Drug: AK112|Drug: AK117|Drug: Carboplatin|Drug: Cisplatin|Drug: 5-Fluorouracil | Phase 2 |
| NCT05235542 | A Phase Ib/II Study of AK104 and AK117 in Combination With or Without Chemotherapy in Advanced Malignant Tumors | Advanced Malignant Tumors | Drug: AK104|Drug: AK117|Drug: Capecitabine tablets|Drug: Oxaliplatin|Drug: Cisplatin|Drug: Paclitaxel|Drug: Irinotecan|Drug: Docetaxel|Drug: 5-FU | Phase 2 |
| NCT05247684 | AK112 Neoadjuvant/Adjuvant Treatment for Resectable NSCLC | Resectable Non-small Cell Lung Cancer | Drug: AK112|Drug: Carboplatin|Drug: Cisplatin|Drug: Paclitaxel | Phase 2 |
| NCT04841538 | A Study of ES101 (PD-L1x4-1BB Bispecific Antibody) in Patients With Advanced Malignant Thoracic Tumors | Thoracic Tumors|Non-small Cell Lung Cancer|Small Cell Lung Cancer | Drug: ES101 | Phase 2 |
| NCT05227651 | AK104 in Neoadjuvant Treatment of Cervical Cancer | Cervical Cancer | Drug: AK104 | Phase 2 |
| NCT05215067 | A Phase II Trial of AK104 in Advanced Non-Small Cell Lung Cancer | Advanced Non-small Cell Lung Cancer | Drug: AK104|Drug: Docetaxel | Phase 2 |
| NCT05032040 | A Study of XmAb20717 in Patients With Selected Advanced Gynecologic and Genitourinary Malignancies | Ovarian Cancer|Clear Cell Carcinoma|Endometrial Cancer|Cervical Carcinoma|Metastatic Castration-Resistant Prostate Cancer (mCRPC) | Biological: XmAb20717 | Phase 2 |
| NCT05216835 | Safety and Preliminary Efficacy Assessment of AZD7789 in Patients With Relapsed or Refractory Classical Hodgkin Lymphoma | Relapsed or Refractory Classical Hodgkin Lymphoma | Drug: AZD7789 | Phase 2 |
| NCT04542837 | The Study of KN046 in Combination With Lenvatinib in Advanced Hepatocellular Carcinoma | HCC | Biological: KN046|Drug: Lenvatinib | Phase 2 |
| NCT05256472 | A Study of AK104 Plus Axitinib in Advanced/Metastatic Clear Cell Renal Cell Carcinoma | Clear Cell Renal Cell Carcinoma|First-line Treatment | Drug: AK104|Drug: axitinib | Phase 2 |
| Unknown status | ||||
| NCT03852251 | A Study of AK104, a PD-1/CTLA-4 Bispecific Antibody, for Advanced Solid Tumors or With mXELOX as First-line Therapy for Advanced Gastric or GEJ Adenocarcinoma | Gastric Adenocarcinoma|Advanced Solid Tumors|Gastroesophageal Junction Adenocarcinoma | Biological: AK104|Drug: Oxaliplatin|Drug: Capecitabine | Phase 2 |
| NCT02173093 | Activated T Cells Armed With GD2 Bispecific Antibody in Children and Young Adults With Neuroblastoma and Osteosarcoma | Disseminated Neuroblastoma|Recurrent Neuroblastoma | Biological: IL-2|Biological: GD2Bi-aATC|Biological: GM-CSF|Other: laboratory evaluations of immune responses | Phase 2 |
| NCT04220307 | A Study of a PD-1/CTLA-4 Bispecific Antibody AK104 in Patients With Metastatic Nasopharyngeal Carcinoma | Nasopharyngeal Carcinoma | Biological: AK-104 | Phase 2 |
| NCT02744768 | D-ALBA Frontline Sequential Dasatinib and Blinatumomab in Adult Philadelphia Positive Acute Lymphoblastic Leukemia | Acute Lymphoblastic Leukemia | Drug: Dasatinib|Drug: Blinatumomab | Phase 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordóñez-Reyes, C.; Garcia-Robledo, J.E.; Chamorro, D.F.; Mosquera, A.; Sussmann, L.; Ruiz-Patiño, A.; Arrieta, O.; Zatarain-Barrón, L.; Rojas, L.; Russo, A.; et al. Bispecific Antibodies in Cancer Immunotherapy: A Novel Response to an Old Question. Pharmaceutics 2022, 14, 1243. https://doi.org/10.3390/pharmaceutics14061243
Ordóñez-Reyes C, Garcia-Robledo JE, Chamorro DF, Mosquera A, Sussmann L, Ruiz-Patiño A, Arrieta O, Zatarain-Barrón L, Rojas L, Russo A, et al. Bispecific Antibodies in Cancer Immunotherapy: A Novel Response to an Old Question. Pharmaceutics. 2022; 14(6):1243. https://doi.org/10.3390/pharmaceutics14061243
Chicago/Turabian StyleOrdóñez-Reyes, Camila, Juan Esteban Garcia-Robledo, Diego F. Chamorro, Andrés Mosquera, Liliana Sussmann, Alejandro Ruiz-Patiño, Oscar Arrieta, Lucia Zatarain-Barrón, Leonardo Rojas, Alessandro Russo, and et al. 2022. "Bispecific Antibodies in Cancer Immunotherapy: A Novel Response to an Old Question" Pharmaceutics 14, no. 6: 1243. https://doi.org/10.3390/pharmaceutics14061243
APA StyleOrdóñez-Reyes, C., Garcia-Robledo, J. E., Chamorro, D. F., Mosquera, A., Sussmann, L., Ruiz-Patiño, A., Arrieta, O., Zatarain-Barrón, L., Rojas, L., Russo, A., de Miguel-Perez, D., Rolfo, C., & Cardona, A. F. (2022). Bispecific Antibodies in Cancer Immunotherapy: A Novel Response to an Old Question. Pharmaceutics, 14(6), 1243. https://doi.org/10.3390/pharmaceutics14061243