Relevance of Therapeutic Drug Monitoring of Tyrosine Kinase Inhibitors in Routine Clinical Practice: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment and Blood Sampling
2.2.1. Erlotinib
2.2.2. Imatinib
2.2.3. Lapatinib
2.2.4. Sorafenib
2.3. Pharmacokinetic Analysis
2.3.1. Erlotinib
2.3.2. Imatinib
2.3.3. Lapatinib
2.3.4. Sorafenib
2.4. Statistical Methods
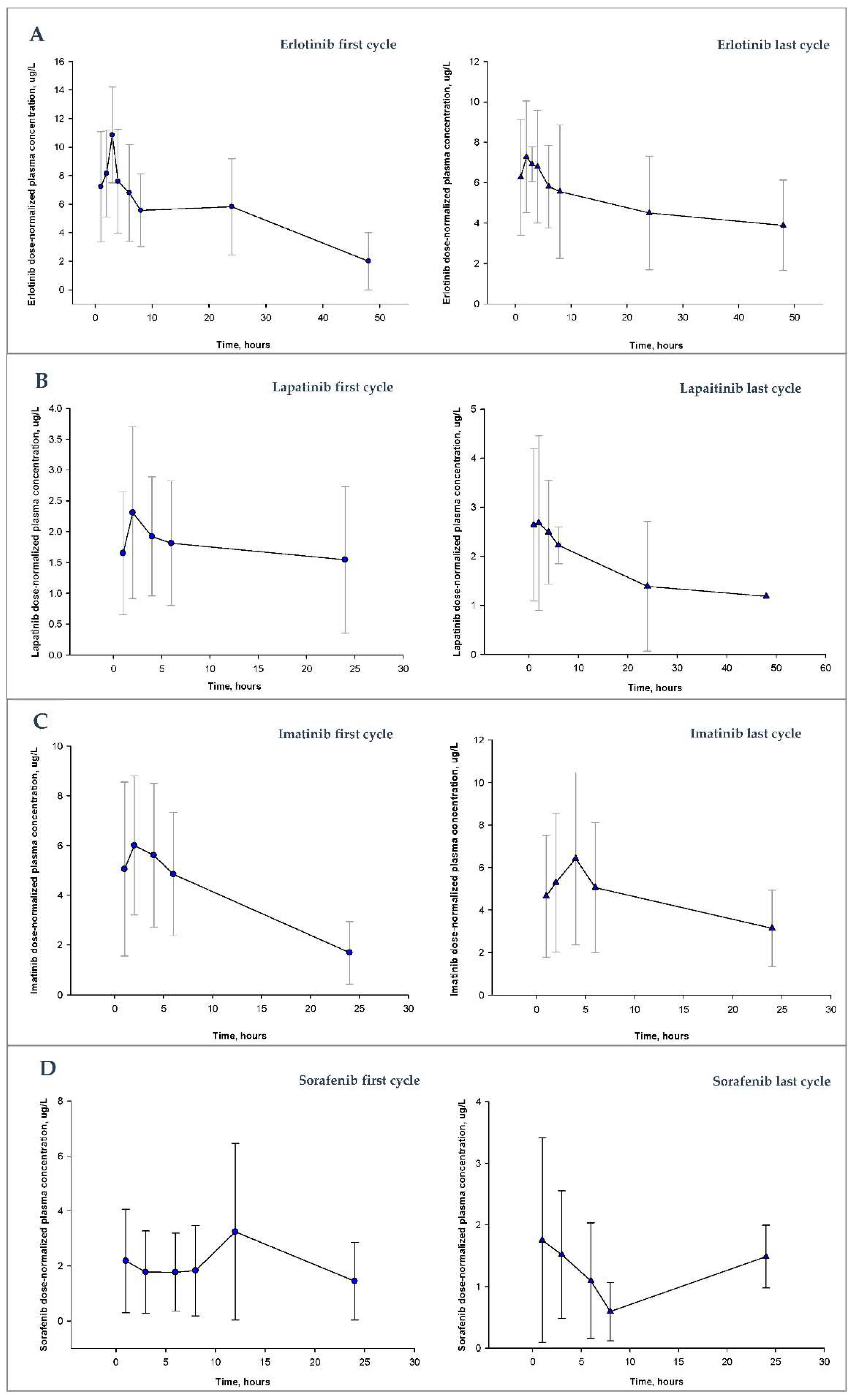
3. Results
3.1. Patients
3.1.1. Erlotinib
3.1.2. Imatinib
3.1.3. Lapatinib
3.1.4. Sorafenib
3.2. Response and Survival
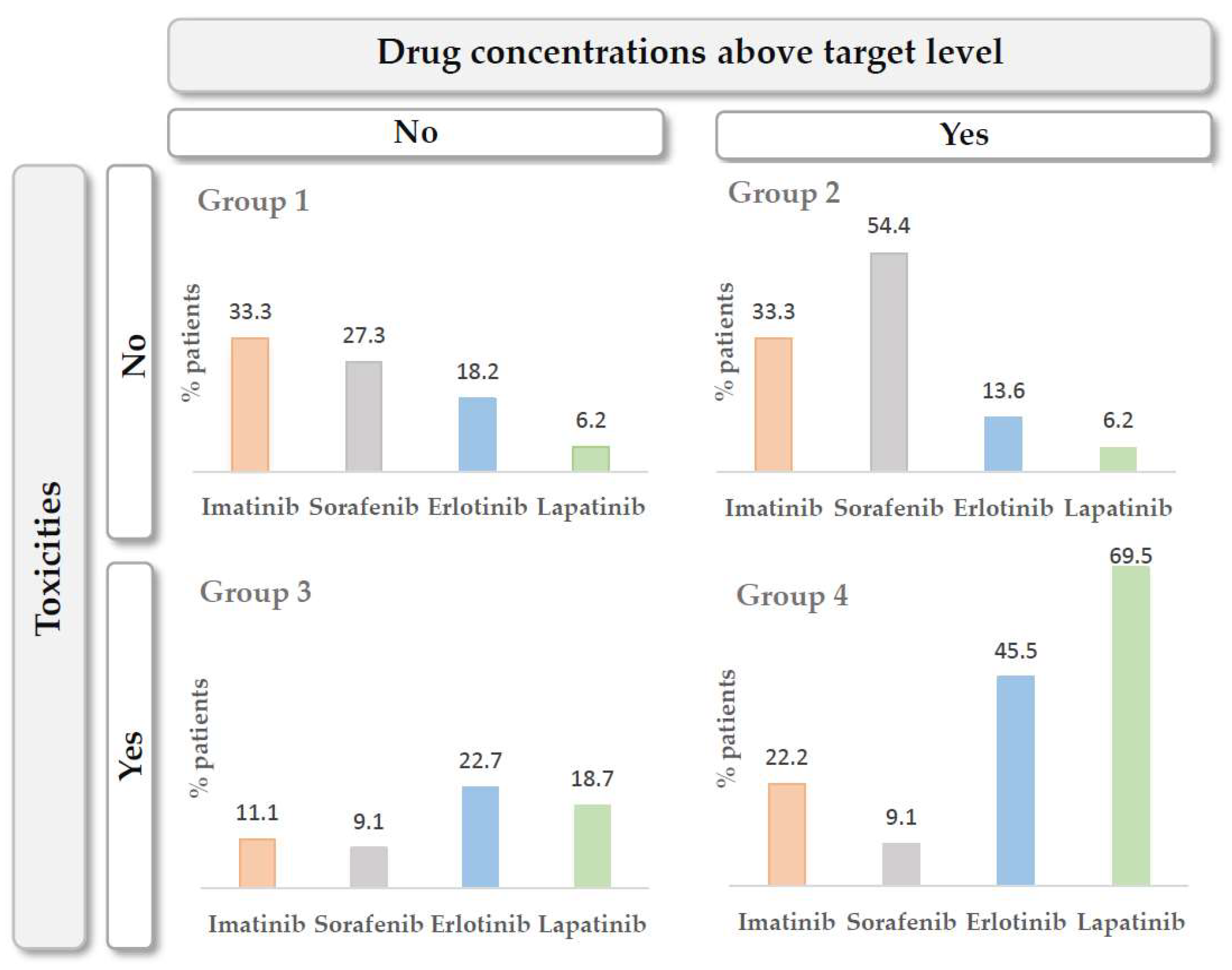
3.3. The Exposure–Toxicity Relationship
4. Discussion
4.1. Erlotinib
4.2. Imatinib
4.3. Lapatinib
4.4. Sorafenib
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holford, N.H.; Sheiner, L.B. Kinetics of pharmacologic response. Pharmacol. Ther. 1982, 16, 143–166. [Google Scholar] [CrossRef]
- Sheiner, L.B.; Beal, S.; Rosenberg, B.; Marathe, V.V. Forecasting individual pharmacokinetics. Clin. Pharmacol. Ther. 1979, 26, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Lee, M.H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Thomas, H.D.; Batey, M.A.; Cowell, I.G.; Richardson, C.J.; Griffin, R.J.; Calvert, A.H.; Newell, D.R.; Smith, G.C.; Curtin, N.J. Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor NU7441. Cancer Res. 2006, 66, 5354–5362. [Google Scholar] [CrossRef]
- Giamas, G.; Man, Y.L.; Hirner, H.; Bischof, J.; Kramer, K.; Khan, K.; Ahmed, S.S.; Stebbing, J.; Knippschild, U. Kinases as targets in the treatment of solid tumors. Cell. Signal. 2010, 22, 984–1002. [Google Scholar] [CrossRef]
- Cohen, P. Protein kinases--the major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, P.L.; Gray, N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 2009, 9, 28–39. [Google Scholar] [CrossRef]
- Gao, B.; Yeap, S.; Clements, A.; Balakrishnar, B.; Wong, M.; Gurney, H. Evidence for therapeutic drug monitoring of targeted anticancer therapies. J. Clin. Oncol. 2012, 30, 4017–4025. [Google Scholar] [CrossRef]
- von Mehren, M.; Widmer, N. Correlations between imatinib pharmacokinetics, pharmacodynamics, adherence, and clinical response in advanced metastatic gastrointestinal stromal tumor (GIST): An emerging role for drug blood level testing? Cancer Treat. Rev. 2011, 37, 291–299. [Google Scholar] [CrossRef]
- Wood, L. A review on adherence management in patients on oral cancer therapies. Eur. J. Oncol. Nurs. 2012, 16, 432–438. [Google Scholar] [CrossRef]
- Tsang, J.; Rudychev, I.; Pescatore, S.L. Prescription compliance and persistency in chronic myelogenous leukemia (CML) and gastrointestinal stromal tumor (GIST) patients (pts) on imatinib (IM). J. Clin. Oncol. 2006, 24, 6119. [Google Scholar] [CrossRef]
- Feng, W.; Henk, H.; Thomas, S.; Baladi, J.; Hatfield, A.; Goldberg, G.A. Compliance and persistency with imatinib. J. Clin. Oncol. 2006, 24, 6038. [Google Scholar] [CrossRef]
- Levine, A.M.; Richardson, J.L.; Marks, G.; Chan, K.; Graham, J.; Selser, J.N.; Kishbaugh, C.; Shelton, D.R.; Johnson, C.A. Compliance with oral drug therapy in patients with hematologic malignancy. J. Clin. Oncol. 1987, 5, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Gater, A.; Heron, L.; Abetz-Webb, L.; Coombs, J.; Simmons, J.; Guilhot, F.; Rea, D. Adherence to oral tyrosine kinase inhibitor therapies in chronic myeloid leukemia. Leuk. Res. 2012, 36, 817–825. [Google Scholar] [CrossRef]
- Jabbour, E.J.; Kantarjian, H.; Eliasson, L.; Cornelison, A.M.; Marin, D. Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Am. J. Hematol. 2012, 87, 687–691. [Google Scholar] [CrossRef]
- de Almeida, M.H.; Pagnano, K.B.; Vigorito, A.C.; Lorand-Metze, I.; de Souza, C.A. Adherence to tyrosine kinase inhibitor therapy for chronic myeloid leukemia: A Brazilian single-center cohort. Acta Haematol. 2013, 130, 16–22. [Google Scholar] [CrossRef]
- Bui, B.N.; Italiano, A.; Miranova, A.; Bouchet, S.; Molimard, M. Trough imatinib plasma levels in patients treated for advanced gastrointestinal stromal tumors evidence of large interpatient variations under treatment with standard doses. J. Clin. Oncol. 2008, 26, 10564. [Google Scholar] [CrossRef]
- Marin, D.; Bazeos, A.; Mahon, F.X.; Eliasson, L.; Milojkovic, D.; Bua, M.; Apperley, J.F.; Szydlo, R.; Desai, R.; Kozlowski, K.; et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J. Clin. Oncol. 2010, 28, 2381–2388. [Google Scholar] [CrossRef]
- Tanaka, C.; Yin, O.Q.; Sethuraman, V.; Smith, T.; Wang, X.; Grouss, K.; Kantarjian, H.; Giles, F.; Ottmann, O.G.; Galitz, L.; et al. Clinical pharmacokinetics of the BCR-ABL tyrosine kinase inhibitor nilotinib. Clin. Pharmacol. Ther. 2010, 87, 197–203. [Google Scholar] [CrossRef]
- Koch, K.M.; Reddy, N.J.; Cohen, R.B.; Lewis, N.L.; Whitehead, B.; Mackay, K.; Stead, A.; Beelen, A.P.; Lewis, L.D. Effects of food on the relative bioavailability of lapatinib in cancer patients. J. Clin. Oncol. 2009, 27, 1191–1196. [Google Scholar] [CrossRef]
- Heath, E.I.; Chiorean, E.G.; Sweeney, C.J.; Hodge, J.P.; Lager, J.J.; Forman, K.; Malburg, L.; Arumugham, T.; Dar, M.M.; Suttle, A.B.; et al. A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high-fat or low-fat meal in patients with advanced solid tumors. Clin. Pharmacol. Ther. 2010, 88, 818–823. [Google Scholar] [CrossRef]
- Kantarjian, H.; Giles, F.; Wunderle, L.; Bhalla, K.; O’Brien, S.; Wassmann, B.; Tanaka, C.; Manley, P.; Rae, P.; Mietlowski, W.; et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N. Engl. J. Med. 2006, 354, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Hornecker, M.; Blanchet, B.; Billemont, B.; Sassi, H.; Ropert, S.; Taieb, F.; Mir, O.; Abbas, H.; Harcouet, L.; Coriat, R.; et al. Saturable absorption of sorafenib in patients with solid tumors: A population model. Investig. New Drugs. 2012, 30, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.I.; Dowlati, A.; Saini, S.; Savage, S.; Suttle, A.B.; Gibson, D.M.; Hodge, J.P.; Merkle, E.M.; Pandite, L. Phase I trial of pazopanib in patients with advanced cancer. Clin. Cancer Res. 2009, 15, 4220–4227. [Google Scholar] [CrossRef] [PubMed]
- Pavlovsky, C.; Egorin, M.J.; Shah, D.D.; Beumer, J.H.; Rogel, S.; Pavlovsky, S. Imatinib mesylate pharmacokinetics before and after sleeve gastrectomy in a morbidly obese patient with chronic myeloid leukemia. Pharmacotherapy 2009, 29, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Ryu, M.H.; Kang, B.W.; Yoon, S.K.; Ryoo, B.Y.; Chang, H.M.; Lee, J.L.; Beck, M.Y.; Kim, T.W.; Kang, Y.K. Cross-sectional study of imatinib plasma trough levels in patients with advanced gastrointestinal stromal tumors: Impact of gastrointestinal resection on exposure to imatinib. J. Clin. Oncol. 2010, 28, 1554–1559. [Google Scholar] [CrossRef]
- van Erp, N.P.; Gelderblom, H.; Guchelaar, H.J. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat. Rev. 2009, 35, 692–706. [Google Scholar] [CrossRef]
- Petain, A.; Kattygnarath, D.; Azard, J.; Chatelut, E.; Delbaldo, C.; Geoerger, B.; Barrois, M.; Séronie-Vivien, S.; LeCesne, A.; Vassal, G. Innovative Therapies with Children with Cancer European consortium. Population pharmacokinetics and pharmacogenetics of imatinib in children and adults. Clin. Cancer Res. 2008, 14, 7102–7109. [Google Scholar] [CrossRef]
- Tang, S.C.; Lagas, J.S.; Lankheet, N.A.; Poller, B.; Hillebrand, M.J.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int. J. Cancer 2012, 130, 223–233. [Google Scholar] [CrossRef]
- Bazeos, A.; Marin, D.; Reid, A.G.; Gerrard, G.; Milojkovic, D.; May, P.C.; de Lavallade, H.; Garland, P.; Rezvani, K.; Apperley, J.F.; et al. hOCT1 transcript levels and single nucleotide polymorphisms as predictive factors for response to imatinib in chronic myeloid leukemia. Leukemia 2010, 24, 1243–1245. [Google Scholar] [CrossRef]
- White, D.L.; Saunders, V.A.; Dang, P.; Engler, J.; Venables, A.; Zrim, S.; Zannettino, A.; Lynch, K.; Manley, P.W.; Hughes, T. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: Higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood 2007, 110, 4064–4072. [Google Scholar] [CrossRef]
- Widmer, N.; Decosterd, L.A.; Leyvraz, S.; Duchosal, M.A.; Rosselet, A.; Debiec-Rychter, M.; Csajka, C.; Biollaz, J.; Buclin, T. Relationship of imatinib-free plasma levels and target genotype with efficacy and tolerability. Br. J. Cancer 2008, 98, 1633–1640. [Google Scholar] [CrossRef]
- Widmer, N.; Decosterd, L.A.; Csajka, C.; Leyvraz, S.; Duchosal, M.A.; Rosselet, A.; Rochat, B.; Eap, C.B.; Henry, H.; Biollaz, J.; et al. Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein. Br. J. Clin. Pharmacol. 2006, 62, 97–112. [Google Scholar] [CrossRef]
- Mir, O.; Coriat, R.; Blanchet, B.; Durand, J.P.; Boudou-Rouquette, P.; Michels, J.; Ropert, S.; Vidal, M.; Pol, S.; Chaussade, S.; et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE 2012, 7, e37563. [Google Scholar] [CrossRef]
- Antoun, S.; Baracos, V.E.; Birdsell, L.; Escudier, B.; Sawyer, M.B. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann. Oncol. 2010, 21, 1594–1598. [Google Scholar] [CrossRef]
- Thomas-Schoemann, A.; Blanchet, B.; Bardin, C.; Noé, G.; Boudou-Rouquette, P.; Vidal, M.; Goldwasser, F. Drug interactions with solid tumour-targeted therapies. Crit. Rev. Oncol. Hematol. 2014, 89, 179–196. [Google Scholar] [CrossRef]
- Haouala, A.; Zanolari, B.; Rochat, B.; Montemurro, M.; Zaman, K.; Duchosal, M.A.; Ris, H.B.; Leyvraz, S.; Widmer, N.; Decosterd, L.A. Therapeutic Drug Monitoring of the new targeted anticancer agents imatinib, nilotinib, dasatinib, sunitinib, sorafenib and lapatinib by LC tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 1982–1996. [Google Scholar] [CrossRef]
- de Castro, D.G.; Clarke, P.A.; Al-Lazikani, B.; Workman, P. Personalized cancer medicine: Molecular diagnostics, predictive biomarkers, and drug resistance. Clin. Pharmacol. Ther. 2013, 93, 252–259. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Stephenson, J.J., Jr.; Rosen, P.; Loesch, D.M.; Borad, M.J.; Anthony, S.; Jameson, G.; Brown, S.; Cantafio, N.; Richards, D.A.; et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. 2010, 28, 4877–4883. [Google Scholar] [CrossRef]
- Rebollo, J.; Sureda, M.; Martinez, E.M.; Fernández-Morejón, F.J.; Farré, J.; Muñoz, V.; Fernández-Latorre, F.; Manzano, R.G.; Brugarolas, A. Gene Expression Profiling of Tumors From Heavily Pretreated Patients With Metastatic Cancer for the Selection of Therapy: A Pilot Study. Am. J. Clin. Oncol. 2017, 40, 140–145. [Google Scholar] [CrossRef]
- Chantry, A.S.; Quaranta, S.; Ciccolini, J.; Lacarelle, B. Applications cliniques, limites et perspectives des analyses pharmacogénétiques et pharmacocinétiques des traitements anticancéreux [Clinical application, limits and perspectives of pharmacogenetic and pharmacokinetic analysis of anticancer drugs]. Ann. Biol. Clin. 2014, 72, 527–542. [Google Scholar] [CrossRef]
- Gervasini, G.; Benítez, J.; Carrillo, J.A. Pharmacogenetic testing and therapeutic drug monitoring are complementary tools for optimal individualization of drug therapy. Eur. J. Clin. Pharmacol. 2010, 66, 755–774. [Google Scholar] [CrossRef]
- Verheijen, R.B.; Yu, H.; Schellens, J.H.M.; Beijnen, J.H.; Steeghs, N.; Huitema, A.D.R. Practical Recommendations for Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology. Clin. Pharmacol. Ther. 2017, 102, 765–776. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE); Version 5; US Department of Health and Human Services, National Institutes of Health, National Cancer Institute: Washington, DC, USA, 2017.
- Thomas, F.; Rochaix, P.; White-Koning, M.; Hennebelle, I.; Sarini, J.; Benlyazid, A.; Malard, L.; Lefebvre, J.L.; Chatelut, E.; Delord, J.P. Population pharmacokinetics of erlotinib and its pharmacokinetic/pharmacodynamic relationships in head and neck squamous cell carcinoma. Eur. J. Cancer 2009, 45, 2316–2323. [Google Scholar] [CrossRef]
- Schmidli, H.; Peng, B.; Riviere, G.J.; Capdeville, R.; Hensley, M.; Gathmann, I.; Bolton, A.E.; Racine-Poon, A.; IRIS Study Group. Population pharmacokinetics of imatinib mesylate in patients with chronic-phase chronic myeloid leukaemia: Results of a phase III study. Br. J. Clin. Pharmacol. 2005, 60, 35–44. [Google Scholar] [CrossRef]
- Rezai, K.; Urien, S.; Isambert, N.; Roche, H.; Dieras, V.; Berille, J.; Bonneterre, J.; Brain, E.; Lokiec, F. Pharmacokinetic evaluation of the vinorelbine-lapatinib combination in the treatment of breast cancer patients. Cancer Chemother. Pharmacol. 2011, 68, 1529–1536. [Google Scholar] [CrossRef]
- Jain, L.; Woo, S.; Gardner, E.R.; Dahut, W.L.; Kohn, E.C.; Kummar, S.; Mould, D.R.; Giaccone, G.; Yarchoan, R.; Venitz, J.; et al. Population pharmacokinetic analysis of sorafenib in patients with solid tumours. Br. J. Clin. Pharmacol. 2011, 72, 294–305. [Google Scholar] [CrossRef]
- Guidance for Industry. Bioanalytical Method Validation. U.S. Department of Health and Human Services. Food and Drug Administration Center for Drug Evaluation and Research (CDER). Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf (accessed on 1 August 2018).
- Validation of Analytical Procedures: Text and Methodology. Ich Topic Q2 (R1). CPMP/ICH/381/95-ICH Q2 (R1). Available online: http://www.ich.org/products/guidelines/quality/quality-single/article/validation-of-analyticalprocedures-text-and-methodology.html (accessed on 1 August 2018).
- Escudero-Ortiz, V.; Pérez-Ruixo, J.J.; Valenzuela, B. Development and validation of a high-performance liquid chromatography ultraviolet method for lapatinib quantification in human plasma. Ther. Drug Monit. 2013, 35, 796–802. [Google Scholar] [CrossRef]
- Escudero-Ortiz, V.; Pérez-Ruixo, J.J.; Valenzuela, B. Development and validation of an HPLC-UV method for sorafenib quantification in human plasma and application to patients with cancer in routine clinical practice. Ther. Drug Monit. 2014, 36, 317–325. [Google Scholar] [CrossRef]
- Beal, S.L.; Sheiner, L.B.; Boeckman, A.J. (Eds.) NONMEN Users Guides (1989–2006); ICON Development Solutions: Ellicott, MA, USA, 1989. [Google Scholar]
- Sureda, M.; Calvo, E.; Mata, J.J.; Escudero-Ortiz, V.; Martinez-Navarro, E.; Catalán, A.; Rebollo, J. Dosage of anti-PD-1 monoclonal antibodies: A cardinal open question. Clin. Transl. Oncol. 2021, 23, 1511–1519. [Google Scholar] [CrossRef]
- Sureda, M.; Mata, J.J.; Catalán, A.; Escudero, V.; Martínez-Navarro, E.; Rebollo, J. Therapeutic drug monitoring of nivolumab in routine clinical practice. A pilot study. Farm. Hosp. 2020, 44, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Chatelut, E.; Hendrikx, J.J.M.A.; Martin, J.; Ciccolini, J.; Moes, D.J.A.R. Unraveling the complexity of therapeutic drug monitoring for monoclonal antibody therapies to individualize dose in oncology. Pharmacol. Res. Perspect. 2021, 9, e00757. [Google Scholar] [CrossRef]
- de Wit, D.; Guchelaar, H.-J.; den Hartigh, J.; Gelderblom, H.; van Erp, N.P. Individualized dosing of tyrosine kinase inhibitors: Are we there yet? Drug Discov. Today 2015, 20, 18–36. [Google Scholar] [CrossRef]
- Yu, H.; Steeghs, N.; Nijenhuis, C.M.; Schellens, J.H.; Beijnen, J.H.; Huitema, A.D. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: Focus on the pharmacokinetic targets. Clin. Pharmacokinet. 2014, 53, 305–325. [Google Scholar] [CrossRef]
- Lucas, C.J.; Martin, J.H. Pharmacokinetic-Guided Dosing of New Oral Cancer Agents. J. Clin. Pharmacol. 2017, 57 (Suppl. S10), 78–98. [Google Scholar] [CrossRef]
- Lankheet, N.A.; Knapen, L.M.; Schellens, J.H.; Beijnen, J.H.; Steeghs, N.; Huitema, A.D. Plasma concentrations of tyrosine kinase inhibitors imatinib, erlotinib, and sunitinib in routine clinical outpatient cancer care. Ther. Drug Monit. 2014, 36, 326–334. [Google Scholar] [CrossRef]
- Hidalgo, M.; Siu, L.L.; Nemunaitis, J.; Rizzo, J.; Hammond, L.A.; Takimoto, C.; Eckhardt, S.G.; Tolcher, A.; Britten, C.D.; Denis, L.; et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J. Clin. Oncol. 2001, 19, 3267–3279. [Google Scholar] [CrossRef]
- Erlotinib Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/tarceva-epar-product-information_en.pdf (accessed on 15 June 2018).
- Lu, J.F.; Eppler, S.M.; Wolf, J.; Hamilton, M.; Rakhit, A.; Bruno, R.; Lum, B.L. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin. Pharmacol. Ther. 2006, 80, 136–145. [Google Scholar] [CrossRef]
- Rudin, C.M.; Liu, W.; Desai, A.; Karrison, T.; Jiang, X.; Janisch, L.; Das, S.; Ramirez, J.; Poonkuzhali, B.; Schuetz, E.; et al. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J. Clin. Oncol. 2008, 26, 1119–1127. [Google Scholar] [CrossRef]
- Catalán-Latorre, A.; Sureda, M.; Brugarolas-Masllorens, A.; Escudero-Ortiz, V. Therapeutic Drug Monitoring of Erlotinib in Non-Small Cell Lung Carcinoma: A Case Study. Ther. Drug Monit. 2021, 43, 447–450. [Google Scholar] [CrossRef]
- Tiseo, M.; Andreoli, R.; Gelsomino, F.; Mozzoni, P.; Azzoni, C.; Bartolotti, M.; Bortesi, B.; Goldoni, M.; Silini, E.M.; De Palma, G.; et al. Correlation between erlotinib pharmacokinetics, cutaneous toxicity and clinical outcomes in patients with advanced non-small cell lung cancer (NSCLC). Lung Cancer 2014, 83, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Motoshima, K.; Nakamura, Y.; Sano, K.; Ikegami, Y.; Ikeda, T.; Mizoguchi, K.; Takemoto, S.; Fukuda, M.; Nagashima, S.; Iida, T.; et al. Phase II trial of erlotinib in patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations: Additive analysis of pharmacokinetics. Cancer Chemother. Pharmacol. 2013, 72, 1299–1304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soulieres, D.; Senzer, N.N.; Vokes, E.E.; Hidalgo, M.; Agarwala, S.S.; Siu, L.L. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J. Clin. Oncol. 2004, 22, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wacker, B.; Nagrani, T.; Weinberg, J.; Witt, K.; Clark, G.; Cagnoni, P.J. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin. Cancer Res. 2007, 13, 3913–3921. [Google Scholar] [CrossRef] [PubMed]
- Steffens, M.; Paul, T.; Hichert, V.; Scholl, C.; von Mallek, D.; Stelzer, C.; Sörgel, F.; Reiser, B.; Schumann, C.; Rüdiger, S.; et al. Dosing to rash?—The role of erlotinib metabolic ratio from patient serum in the search of predictive biomarkers for EGFR inhibitor-mediated skin rash. Eur. J. Cancer 2016, 55, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Mita, A.C.; Papadopoulos, K.; de Jonge, M.J.; Schwartz, G.; Verweij, J.; Mita, M.M.; Ricart, A.; Chu, Q.S.; Tolcher, A.W.; Wood, L.; et al. Erlotinib ‘dosing-to-rash’: A phase II intrapatient dose escalation and pharmacologic study of erlotinib in previously treated advanced non-small cell lung cancer. Br. J. Cancer 2011, 105, 938–944. [Google Scholar] [CrossRef]
- Pérez-Soler, R.; Chachoua, A.; Hammond, L.A.; Rowinsky, E.K.; Huberman, M.; Karp, D.; Rigas, J.; Clark, G.M.; Santabárbara, P.; Bonomi, P. Determinants of tumor response and survival with erlotinib in patients with non—small-cell lung cancer. J. Clin. Oncol. 2004, 22, 3238–3247. [Google Scholar] [CrossRef]
- Ling, J.; Johnson, K.A.; Miao, Z.; Rakhit, A.; Pantze, M.P.; Hamilton, M.; Lum, B.L.; Prakash, C. Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab. Dispos. 2006, 34, 420–426. [Google Scholar] [CrossRef]
- Eechoute, K.; Fransson, M.N.; Reyners, A.K.; de Jong, F.A.; Sparreboom, A.; van der Graaf, W.T.; Friberg, L.E.; Schiavon, G.; Wiemer, E.A.; Verweij, J.; et al. A long-term prospective population pharmacokinetic study on imatinib plasma concentrations in GIST patients. Clin. Cancer Res. 2012, 18, 5780–5787. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.; Jarvis, B. Imatinib. Drugs 2001, 61, 1765–1774; discussion 1775–1776. [Google Scholar] [CrossRef]
- Demetri, G.D.; Wang, Y.; Wehrle, E.; Racine, A.; Nikolova, Z.; Blanke, C.D.; Joensuu, H.; von Mehren, M. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J. Clin. Oncol. 2009, 27, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Druker, B.J.; Guilhot, F.; O’Brien, S.G.; Riviere, G.J.; Krahnke, T.; Gathmann, I.; Wang, Y.; IRIS (International Randomized Interferon vs STI571) Study Group. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: A subanalysis of the IRIS study. Blood 2008, 111, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Guilhot, F.; Hughes, T.P.; Cortes, J.; Druker, B.J.; Baccarani, M.; Gathmann, I.; Hayes, M.; Granvil, C.; Wang, Y. Plasma exposure of imatinib and its correlation with clinical response in the Tyrosine Kinase Inhibitor Optimization and Selectivity Trial. Haematologica 2012, 97, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Lapatinib Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/tyverb-epar-product-information_en.pdf (accessed on 15 June 2018).
- Midgley, R.S.; Kerr, D.J.; Flaherty, K.T.; Stevenson, J.P.; Pratap, S.E.; Koch, K.M.; Smith, D.A.; Versola, M.; Fleming, R.A.; Ward, C.; et al. A phase I and pharmacokinetic study of lapatinib in combination with infusional 5-fluorouracil, leucovorin and irinotecan. Ann. Oncol. 2007, 18, 2025–2029. [Google Scholar] [CrossRef]
- Burris, H.A., 3rd; Hurwitz, H.I.; Dees, E.C.; Dowlati, A.; Blackwell, K.L.; O’Neil, B.; Marcom, P.K.; Ellis, M.J.; Overmoyer, B.; Jones, S.F.; et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J. Clin. Oncol. 2005, 23, 5305–5313. [Google Scholar] [CrossRef]
- Nakagawa, K.; Minami, H.; Kanezaki, M.; Mukaiyama, A.; Minamide, Y.; Uejima, H.; Kurata, T.; Nogami, T.; Kawada, K.; Mukai, H.; et al. Phase I dose-escalation and pharmacokinetic trial of lapatinib (GW572016), a selective oral dual inhibitor of ErbB-1 and -2 tyrosine kinases, in Japanese patients with solid tumors. Jpn. J. Clin. Oncol. 2009, 39, 116–123. [Google Scholar] [CrossRef][Green Version]
- Sorafenib Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/nexavar-epar-product-information_en.pdf (accessed on 15 June 2018).
- Minami, H.; Kawada, K.; Ebi, H.; Kitagawa, K.; Kim, Y.I.; Araki, K.; Mukai, H.; Tahara, M.; Nakajima, H.; Nakajima, K. Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors. Cancer Sci. 2008, 99, 1492–1498. [Google Scholar] [CrossRef]
- Blanchet, B.; Billemont, B.; Cramard, J.; Benichou, A.S.; Chhun, S.; Harcouet, L.; Ropert, S.; Dauphin, A.; Goldwasser, F.; Tod, M. Validation of an HPLC-UV method for sorafenib determination in human plasma and application to cancer patients in routine clinical practice. J. Pharm. Biomed. Anal. 2009, 49, 1109–1114. [Google Scholar] [CrossRef]
- Lathia, C.; Lettieri, J.; Cihon, F.; Gallentine, M.; Radtke, M.; Sundaresan, P. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother. Pharmacol. 2006, 57, 685–692. [Google Scholar] [CrossRef]
- Pécuchet, N.; Lebbe, C.; Mir, O.; Billemont, B.; Blanchet, B.; Franck, N.; Viguier, M.; Coriat, R.; Tod, M.; Avril, M.F.; et al. Sorafenib in advanced melanoma: A critical role for pharmacokinetics? Br. J. Cancer 2012, 107, 455–461. [Google Scholar] [CrossRef]
- Fukudo, M.; Ito, T.; Mizuno, T.; Shinsako, K.; Hatano, E.; Uemoto, S.; Kamba, T.; Yamasaki, T.; Ogawa, O.; Seno, H.; et al. Exposure-toxicity relationship of sorafenib in Japanese patients with renal cell carcinoma and hepatocellular carcinoma. Clin. Pharmacokinet. 2014, 53, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Boudou-Rouquette, P.; Ropert, S.; Mir, O.; Coriat, R.; Billemont, B.; Tod, M.; Cabanes, L.; Franck, N.; Blanchet, B.; Goldwasser, F. Variability of sorafenib toxicity and exposure over time: A pharmacokinetic/pharmacodynamic analysis. Oncologist 2012, 17, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
| Drug | Sampling Times |
|---|---|
| Erlotinib Imatinib Lapatinib | Before drug administration and then at 1, 2, 4 and 6 h after drug administration |
| Sorafenib | Before drug administration and then at 1, 3, 6 and 8 h after drug administration |
| Baseline Characteristics | Treatment | |||
|---|---|---|---|---|
| Erlotinib | Lapatinib | Imatinib | Sorafenib | |
| Number of Patients in Treatment | 22 | 16 | 9 | 11 |
| Number of total cycles monitored | 55 | 35 | 22 | 29 |
| Cycles monitored by patient (n, %) | ||||
| 1 cycle | 8 (38.1) | 9 (56.3) | 3 (33.3) | 7 (63.6) |
| 2 cycles | 6 (28.6) | 4 (25.0) | 2 (22.2) | 1 (9.1) |
| 3 cycles | 3 (13.6) | 2 (12.5) | 2 (22.2) | 1 (9.1) |
| 4 cycles | 2 (9.5) | -- | 1 (11.1) | 1 (9.1) |
| >5 cycles | 3 (14.3) | 1 (6.3) | 1 (11.1) | 1 (9.1) |
| Gender (n. %) | ||||
| Male | 14 (63.6) | 4 (25.0) | 3 (33.3) | 4 (36.4) |
| Female | 8 (36.4) | 12 (75.0) | 6 (66.7) | 7 (63.6) |
| Age (mean (SD). years) | 63.0 (12.3) | 54.5 (12.6) | 50.8 (16.4) | 57.1 (15.6) |
| Weight (mean (SD). Kg) | 80.2 (14.6) | 69.7 (11.0) | 78.50 (18.2) | 71.2 (13.3) |
| Size (mean (SD). cm) | 169.7 (9.3) | 163.8 (8.4) | 168 (11.5) | 166.6 (12.2) |
| Body surface area (mean (SD). m2) | 1.9 (0.2) | 1.7 (0.1) | 1.87 (0.3) | 1.8 (0.2) |
| Metastasis (number of patients. %) | 19 (86.4) | 15 (93.8) | 5 (55.6) | 9 (81.8) |
| Previous lines of treatment (range) | 1.64 (0–7) | 2.4 (1–6) | 0.56 (0–2) | 1.91 (0–5) |
| Drug | Progression-Free Survival (Months) | Overall Survival (Months) | ||||
|---|---|---|---|---|---|---|
| Median | SE | CI 95% | Median | SE | CI 95% | |
| Erlotinib | 8 | 4.7 | 0.0–17.1 | 32 | 31.6 | 0.0–93.9 |
| Imatinib | 28 | 11.9 | 4.6–54.4 | 90 | 29.9 | 31.3–148.0 |
| Lapatinib | 8 | 3.0 | 2.1–13.9 | 46 | 18.9 | 9.0–83.0 |
| Sorafenib | 9 | 3.4 | 2.3–15.6 | 9 | 5.1 | 0.1–19.1 |
| Characteristic | Erlotinib | Imatinib | Lapatinib | Sorafenib | ||||
|---|---|---|---|---|---|---|---|---|
| Patients with toxicity [n. (%)] | 9 (45.4) | 5 (55.5) | 12 (75.0) | 7 (63.3) | ||||
| Toxicity [n. (%)] | RI G1 | 9 (45.4) | Anemia G2 | 2 (22.2) | RI G1 | 6 (37.5) | Abdominal pain | 2 (18.2) |
| Skin rash G1 | 5 (22.7) | RI G1 | 2 (22.2) | Anemia G1 | 3 (18.7) | IR G1 | 2 (18.2) | |
| Anemia G1 | 4 (18.1) | Fatigue G1 | 1 (11.1) | Diarrhea G1 | 2 (12.5) | Anemia G1 | 2 (18.2) | |
| RI G2 | 4 (18.1) | Anemia G1 | 1 (11.1) | Pain | 2 (12.5) | Diarrea G2 | 1 (9.1) | |
| Skin rash G2 | 3 (13.6) | RI G2 | 1 (11.1) | Anemia G2 | 2 (12.5) | Anemia G2 | 1 (9.1) | |
| Skin rash G3 | 3 (13.6) | Neutr. G3 | 2 (12.5) | Thromb. G1 | 1 (9.1) | |||
| Diarrhea G2 | 3 (13.6) | RI G2 | 2 (12.5) | |||||
| Fatigue G2 | 2 (9.1) | Skin rash G1 | 1 (6.2) | |||||
| Anemia G2 | 2 (9.1) | Skin rash G3 | 1 (6.2) | |||||
| Mucositis G3 | 1 (4.5) | Diarrhea G2 | 1 (6.2) | |||||
| Thromb. G3 | 1 (4.5) | Diarrhea G3 | 1 (6.2) | |||||
| Mucositis G3 | 1 (6.2) | |||||||
| Anemia G3 | 1 (6.2) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero-Ortiz, V.; Domínguez-Leñero, V.; Catalán-Latorre, A.; Rebollo-Liceaga, J.; Sureda, M. Relevance of Therapeutic Drug Monitoring of Tyrosine Kinase Inhibitors in Routine Clinical Practice: A Pilot Study. Pharmaceutics 2022, 14, 1216. https://doi.org/10.3390/pharmaceutics14061216
Escudero-Ortiz V, Domínguez-Leñero V, Catalán-Latorre A, Rebollo-Liceaga J, Sureda M. Relevance of Therapeutic Drug Monitoring of Tyrosine Kinase Inhibitors in Routine Clinical Practice: A Pilot Study. Pharmaceutics. 2022; 14(6):1216. https://doi.org/10.3390/pharmaceutics14061216
Chicago/Turabian StyleEscudero-Ortiz, Vanesa, Vanessa Domínguez-Leñero, Ana Catalán-Latorre, Joseba Rebollo-Liceaga, and Manuel Sureda. 2022. "Relevance of Therapeutic Drug Monitoring of Tyrosine Kinase Inhibitors in Routine Clinical Practice: A Pilot Study" Pharmaceutics 14, no. 6: 1216. https://doi.org/10.3390/pharmaceutics14061216
APA StyleEscudero-Ortiz, V., Domínguez-Leñero, V., Catalán-Latorre, A., Rebollo-Liceaga, J., & Sureda, M. (2022). Relevance of Therapeutic Drug Monitoring of Tyrosine Kinase Inhibitors in Routine Clinical Practice: A Pilot Study. Pharmaceutics, 14(6), 1216. https://doi.org/10.3390/pharmaceutics14061216








