Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials
Abstract
1. Introduction
2. Cancer—The Hidden Pandemics
2.1. Overview of the Current Situation Worldwide
2.2. Colorectal Cancer—An Old Man’s Disease
2.2.1. CRC Epidemiology
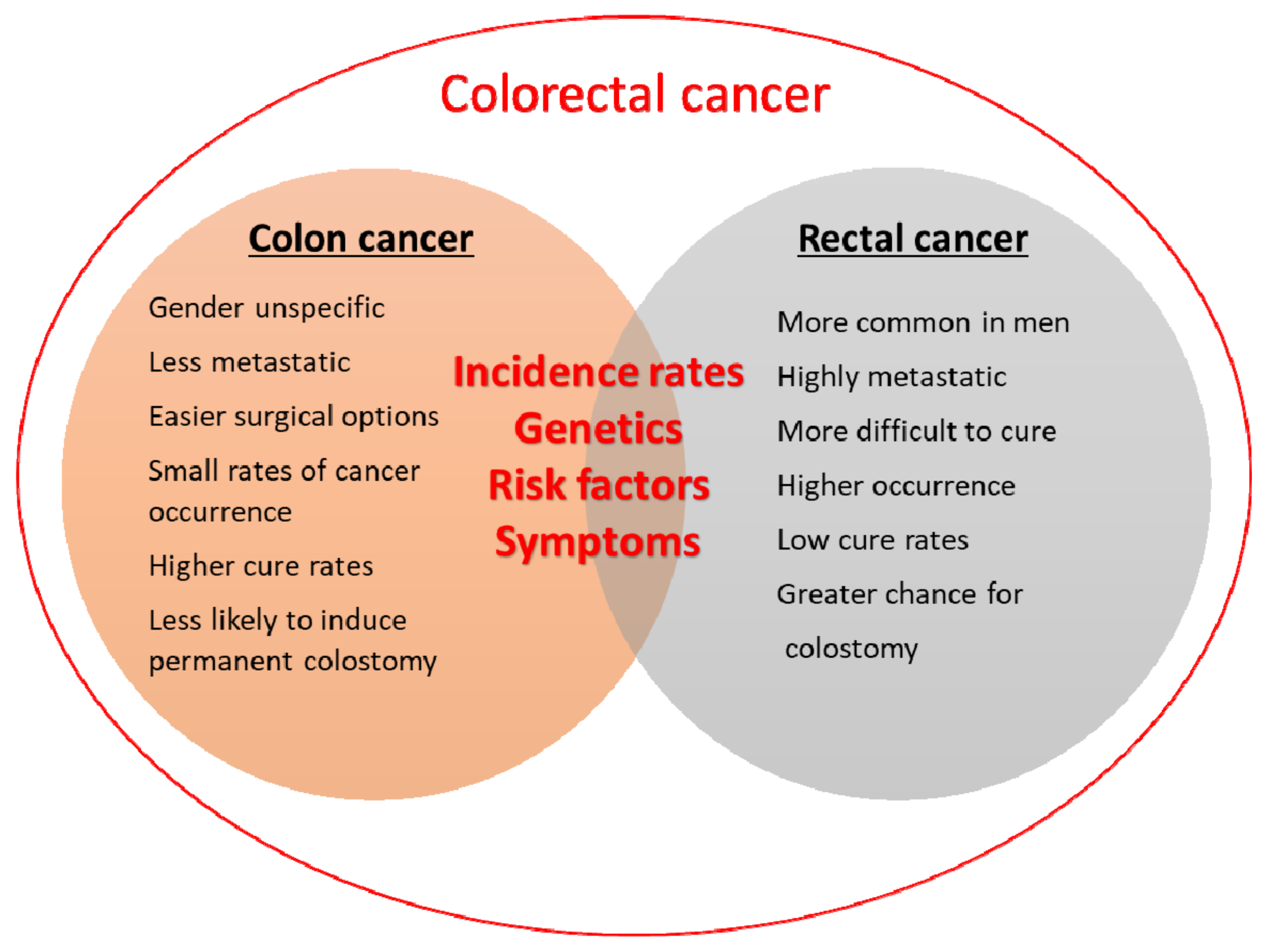
2.2.2. Colon or Colorectal Cancer: What Is the Difference?
2.2.3. Colorectal Carcinogenesis
2.2.4. Stages of CRCs
2.2.5. Current Therapeutic Approaches for CRC
3. Nanomaterials: The New Kids on the Block in Colorectal Cancer Treatment
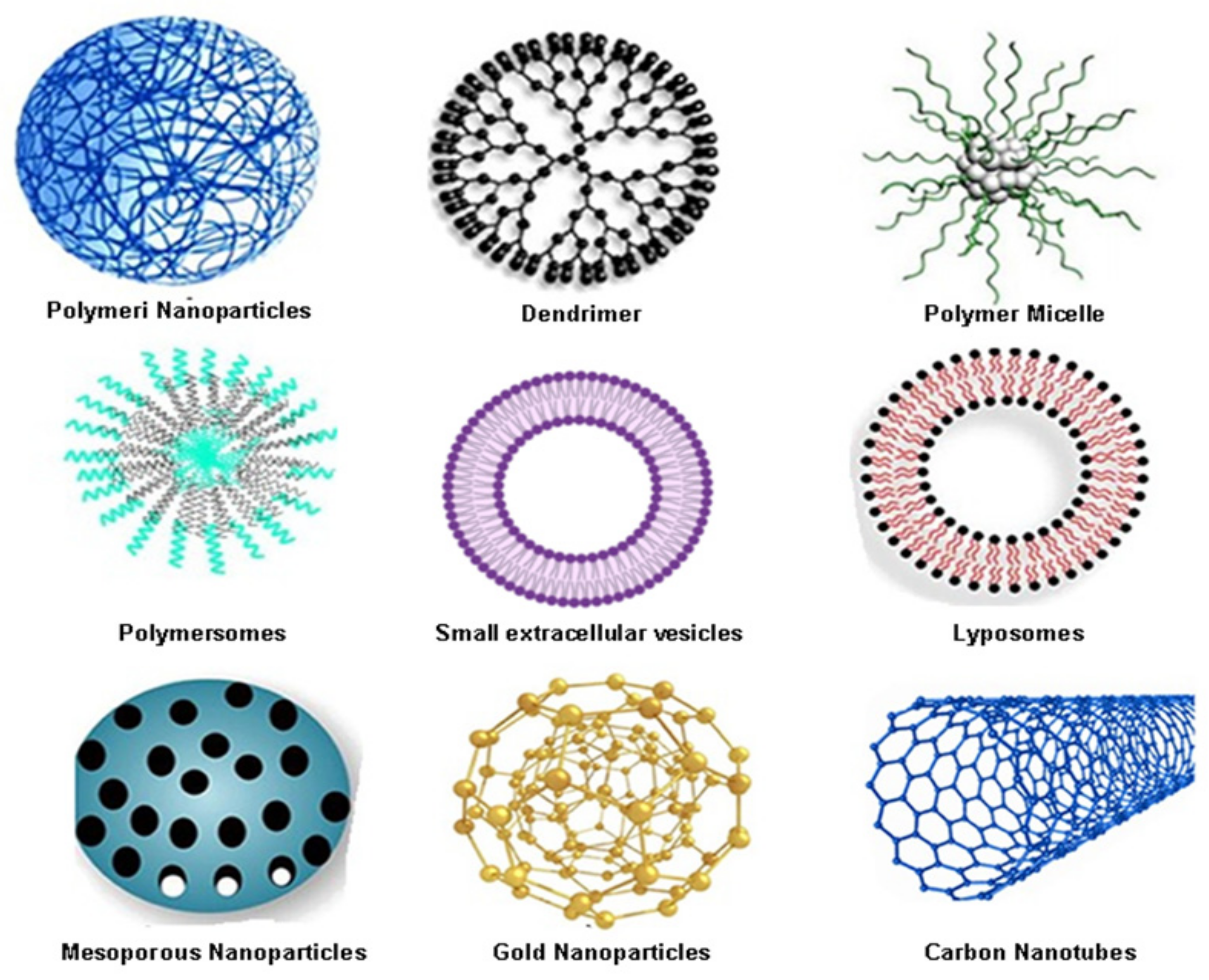
3.1. Organic Nanocarriers for Colorectal Cancer Drug Delivery
3.1.1. Polymeric Nanoparticles (PNPs) as One of the Most Widely Used Organic Nanocarriers in Colorectal Cancer Therapy
3.1.2. Dendrimers as Other Promising Colorectal Cancer Drug Nano-Delivery Systems
3.1.3. Polymeric Micelles (PMs) as Favourable Organic Nanocarriers in Colon Cancer Therapy
3.1.4. Polymeric-Based Nanocarriers as an Emerging Opportunity for Successful Drugs and Nucleic Acids Delivery in Colorectal Cancer
3.1.5. Small Extracellular Vesicles (sEVs)—The Trojan Horse for Many Cancers
3.1.6. Liposomes as Colorectal Therapeutics
3.2. Colorectal Cancer Drug Delivery Based on Inorganic Nanocarriers
3.2.1. Mesoporous Silica Nanoparticles (MSNs) as Carriers of Large Amounts of Biomolecules
3.2.2. Metallic and Magnetic Nanomaterials as Photosensitizers in Colorectal Cancer Treatment
3.2.3. Carbon-Based Nanomaterials
4. Graphene-Based Nanomaterials—An Everlasting Source of Innovations in Biomedicine
4.1. Graphene Oxide Synthesis and Structure, Types, Properties and Applications in Anticancer Treatment
4.1.1. Pristine Graphene Oxide (GO)
4.1.2. Nanographene Oxide (nGO)
4.1.3. Reduced Graphene Oxide (rGO)
4.1.4. Graphene Oxide Nanocomposites
4.2. Graphene Oxide in CRC Treatment Strategies
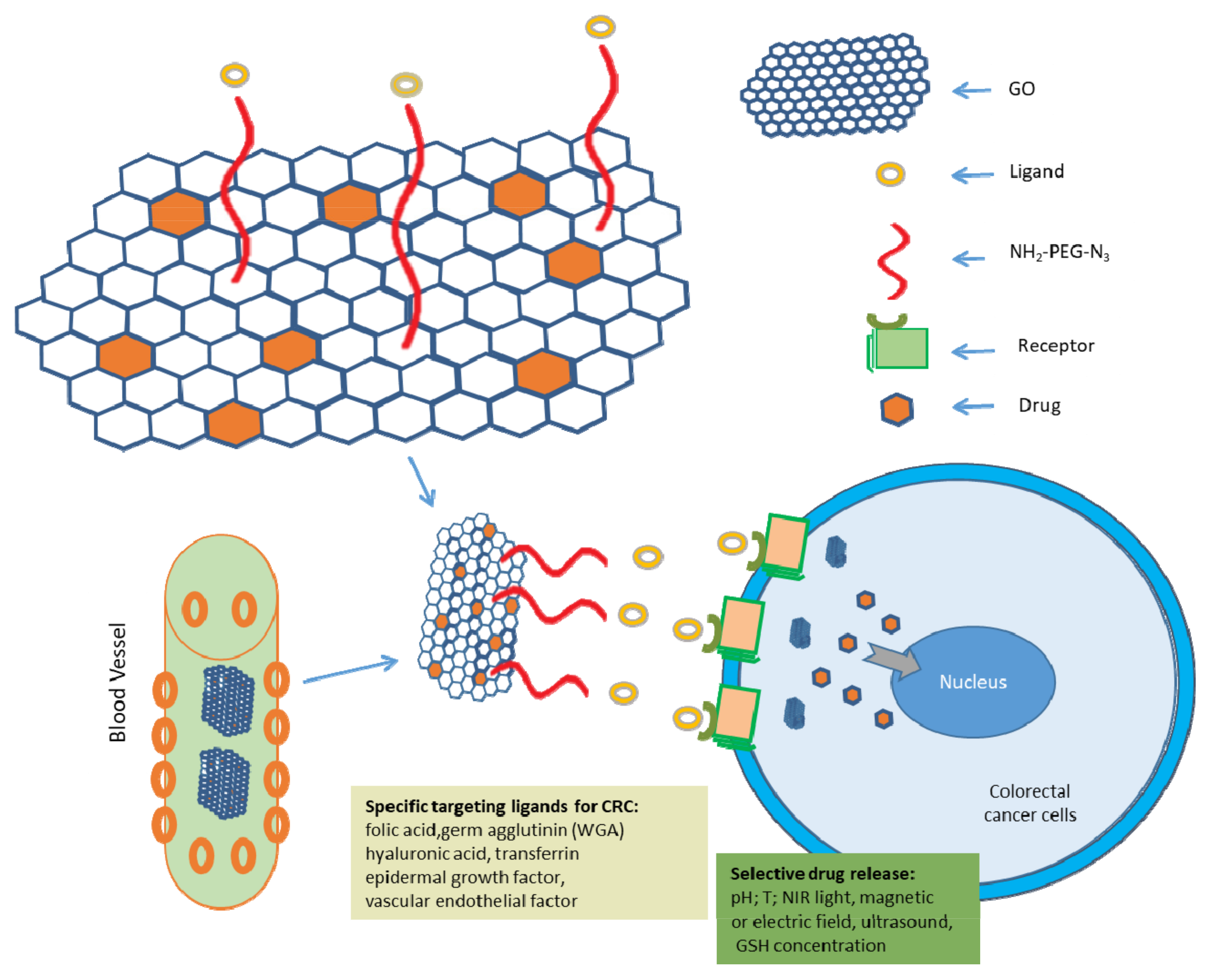
4.2.1. GO as Drug Delivery System in CRC Treatment
4.2.2. Strategies for GO-Based Targeted Therapeutic Approaches for CRC
4.2.3. Photothermal Therapy (PTT) and Photodynamic Therapy (PDT) Are Promising Adjuvant Cancer Therapies
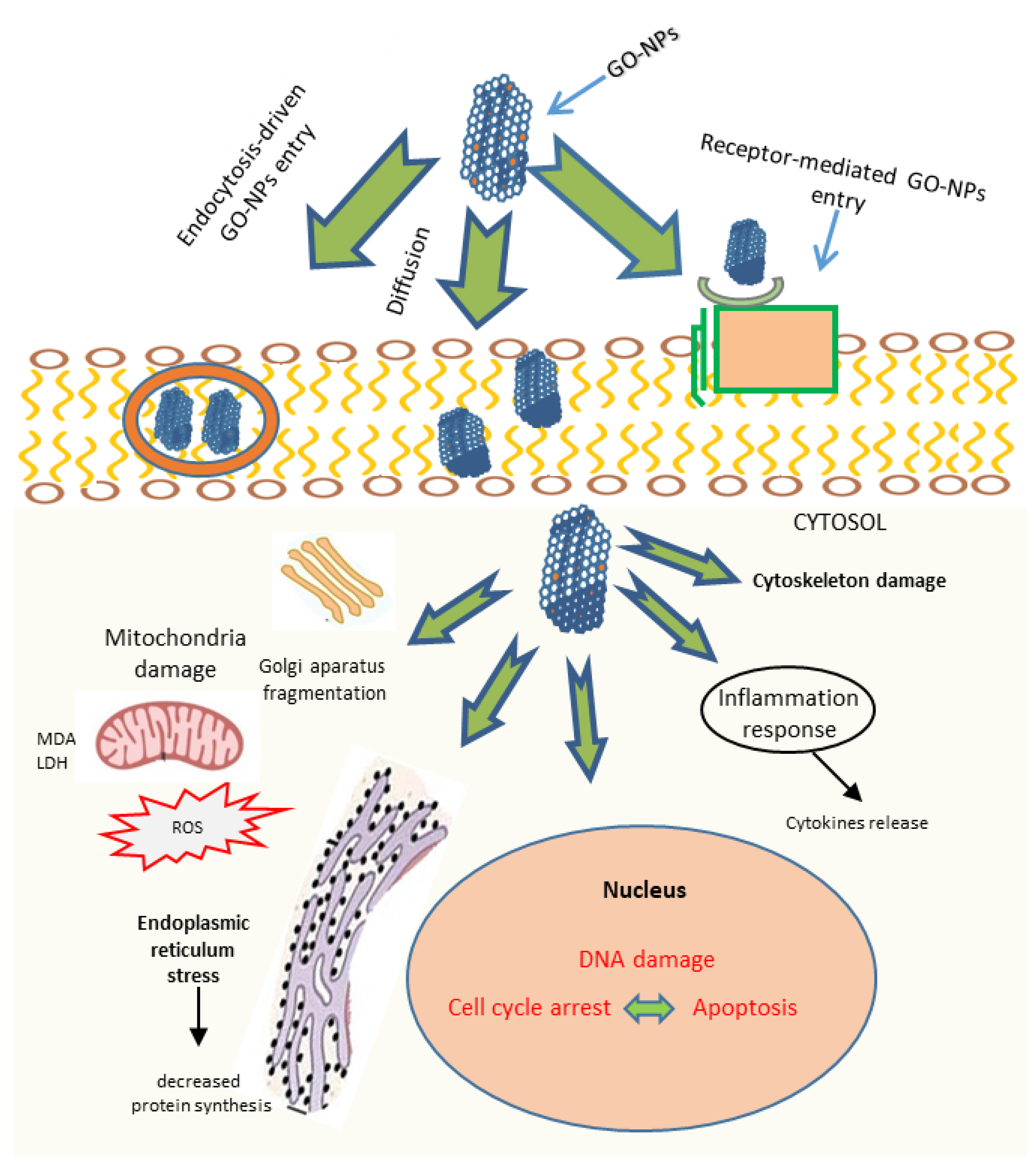
4.3. Direct Killing Effect of Graphene Oxide
4.4. GO as a Chemosensitiser
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Brar, B.; Ranjan, K.; Palria, A.; Kumar, R.; Ghosh, M.; Sihag, S.; Minakshi, P. Nanotechnology in Colorectal Cancer for Precision Diagnosis and Therapy. Front. Nanotechnol. 2021, 3, 699266. [Google Scholar] [CrossRef]
- WHO. Rolling Updates on Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed on 22 April 2021).
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the Evolution of Cancer Treatment Therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed]
- Bousbaa, H. Novel Anticancer Strategies. Pharmaceutics 2021, 13, 275. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Q.; Cao, J.; Gao, Y.; Han, S.; Liang, Y.; Zhang, T.; Song, Y.; Sun, Y. Recent progress of graphene oxide-based multifunctional nanomaterials for cancer treatment. Cancer Nanotechnol. 2021, 12, 18. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Yi, G.; Hong, S.H.; Son, J.; Yoo, J.; Park, C.; Choi, Y.; Koo, H. Recent advances in nanoparticle carriers for photodynamic therapy. Quant. Imaging Med. Surg. 2018, 8, 433–443. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, N.; Staneva, D.; Vasileva, B.; Miloshev, G.; Georgieva, M. Bioactivity of PEGylated Graphene Oxide Nanoparticles Combined with Near-Infrared Laser Irradiation Studied in Colorectal Carcinoma Cells. Nanomaterials 2021, 11, 3061. [Google Scholar] [CrossRef]
- Mukherjee, S.; Liang, L.; Veiseh, O. Recent Advancements of Magnetic Nanomaterials in Cancer Therapy. Pharmaceutics 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-Dimensional Nanomaterials for Biomedical Applications: Emerging Trends and Future Prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.; Lee, H. Enhanced loading efficiency and sustained release of doxorubicin from hyaluronic acid/graphene oxide composite hydrogels by a mussel-inspired catecholamine. J. Nanosci. Nanotechnol. 2014, 14, 7395–7401. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Chen, T. Facile and green reduction of covalently PEGylated nanographene oxide via a ‘water-only’ route for high-efficiency photothermal therapy. Nanoscale Res. Lett. 2014, 9, 86. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.J. Photothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocomposite. Small 2014, 10, 117–126. [Google Scholar] [CrossRef]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The Development and Causes of Cancer. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9963/ (accessed on 22 April 2021).
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer in Europe. Available online: https://www.euronews.com/next/2021/12/01/cancer-in-europe-the-devastating-impact-of-covid-on-diagnosis-and-treatment-country-by-cou (accessed on 2 December 2021).
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Al-Azri, M.H. Delays in Cancer Diagnosis During the Era of the Coronavirus Disease 2019 Pandemic. Sultan Qaboos Univ. Med. J. [SQUMJ] 2021, 21, 341–343. [Google Scholar] [CrossRef]
- Skovlund, C.W.; Friis, S.; Christensen, J.; Nilbert, M.C.; Mørch, L.S. Drop in cancer diagnosis during the COVID-19 pandemic in Denmark: Assessment of impact during 2020. Acta Oncol. 2022, 61, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Neal, R.D.; Duffy, S.R.G.; Scott, S.E.; Whitaker, K.L.; Brain, K. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: The view from primary care. Lancet Oncol. 2020, 21, 748–750. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Chen, Z.; Niles, J.; Fesko, Y. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA Netw. Open 2020, 3, e2017267. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, N.; Baldin, P.; Eynde, M.V.D. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: What is the future beyond deficient mismatch-repair tumours? Gastroenterol. Rep. 2020, 8, 11–24. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Araghi, M.; Soerjomataram, I.; Bardot, A.; Ferlay, J.; Cabasag, C.J.; Morrison, D.S.; De, P.; Tervonen, H.; Walsh, P.M.; Bucher, O.; et al. Changes in colorectal cancer incidence in seven high-income countries: A population-based study. Lancet Gastroenterol. Hepatol. 2019, 4, 511–518. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Connell, L.C.; Mota, J.M.; Braghiroli, M.I.; Hoff, P.M. The Rising Incidence of Younger Patients With Colorectal Cancer: Questions About Screening, Biology, and Treatment. Curr. Treat. Options Oncol. 2017, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and Familial Colon Cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef]
- Colorectal Cancer Risk Factors and Prevention. Available online: https://www.cancer.net/ (accessed on 1 January 2021).
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol. Rev. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Murphy, N.; Moreno, V.; Hughes, D.J.; Vodicka, L.; Vodicka, P.; Aglago, E.K.; Gunter, M.J.; Jenab, M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Asp. Med. 2019, 69, 2–9. [Google Scholar] [CrossRef]
- Bours, M.J.; Beijer, S.; Winkels, R.M.; van Duijnhoven, F.J.; Mols, F.; Breedveld-Peters, J.J.; Kampman, E.; Weijenberg, M.P.; van de Poll-Franse, L.V. Dietary changes and dietary supplement use, and underlying motives for these habits reported by colorectal cancer survivors of the Patient Reported Outcomes Following Initial Treatment and Long-Term Evaluation of Survivorship (PROFILES) registry. Br. J. Nutr. 2015, 114, 286–296. [Google Scholar] [CrossRef]
- How Colon and Rectal Cancer Differ? Available online: https://healthblog.uofmhealth.org/cancer-care/how-colon-and-rectal-cancer-differ (accessed on 21 March 2019).
- What Is Colorectal Cancer? Available online: https://www.cancer.org/cancer/colon-rectal-cancer/about/what-is-colorectal-cancer.html (accessed on 29 June 2020).
- Colorectal and Colon Cancer. Available online: https://my.clevelandclinic.org/health/diseases/14501-colorectal-colon-cancer (accessed on 22 April 2020).
- Colon and Rectal Cancer-Whats Difference? Available online: https://www.webmd.com/colorectal-cancer/colon-rectal-cancer-whats-difference (accessed on 17 July 2020).
- Differences between Colon Cancer and Rectal Cancer. Available online: https://www.ccalliance.org/blog/patient-support/differences-colon-cancer-rectal-cancer/ (accessed on 11 June 2017).
- Anatomy of the Colon. Available online: https://www.kenhub.com/en/library/anatomy/the-colon (accessed on 16 March 2022).
- Anatomy of the Rectum. Available online: https://www.kenhub.com/en/library/anatomy/the-rectum (accessed on 16 March 2022).
- Datta, K.; Suman, S.; Kumar, S.; Fornace, A. Colorectal Carcinogenesis, Radiation Quality, and the Ubiquitin-Proteasome Pathway. J. Cancer 2016, 7, 174–183. [Google Scholar] [CrossRef]
- Jass, J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007, 50, 113–130. [Google Scholar] [CrossRef]
- Molinari, C.; Marisi, G.; Passardi, A.; Matteucci, L.; De Maio, G.; Ulivi, P. Heterogeneity in Colorectal Cancer: A Challenge for Personalized Medicine? Int. J. Mol. Sci. 2018, 19, 3733. [Google Scholar] [CrossRef]
- Yamagishi, H.; Kuroda, H.; Imai, Y.; Hiraishi, H. Molecular pathogenesis of sporadic colorectal cancers. Chin. J. Cancer 2016, 35, 4. [Google Scholar] [CrossRef]
- Pancione, M.; Remo, A.; Colantuoni, V. Genetic and Epigenetic Events Generate Multiple Pathways in Colorectal Cancer Progression. Pathol. Res. Int. 2012, 2012, 509348. [Google Scholar] [CrossRef]
- Aoki, T.; Takeda, S.; Yanagisawa, A.; Kato, Y.; Ajioka, Y.; Watanabe, H.; Kudo, S.; Nakamura, Y. APC and p53 mutations in de novo colorectal adenocarcinomas. Hum. Mutat. 1994, 3, 342–346. [Google Scholar] [CrossRef]
- Beckman, R.A.; Loeb, L.A. Genetic instability in cancer: Theory and experiment. Semin. Cancer Biol. 2005, 15, 423–435. [Google Scholar] [CrossRef]
- Rustgi, A.K. The genetics of hereditary colon cancer. Genes Dev. 2007, 21, 2525–2538. [Google Scholar] [CrossRef]
- Tarik, K.; Ghias, K. Colorectal cancer carcinogenesis: A review of mechanisms. Cancer Biol. Med. 2016, 13, 120–135. [Google Scholar] [CrossRef]
- Pino, M.S.; Chung, D.C. The Chromosomal Instability Pathway in Colon Cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef]
- Grady, W.M.; Carethers, J.M. Genomic and Epigenetic Instability in Colorectal Cancer Pathogenesis. Gastroenterology 2008, 135, 1079–1099. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087.e3. [Google Scholar] [CrossRef]
- Toyota, M.; Ahuja, N.; Ohe-Toyota, M.; Herman, J.G.; Baylin, S.B.; Issa, J.-P. CpG island methylator phenotype in colorectal cancer. Proc. Natl. Acad. Sci. USA 1999, 96, 8681–8686. [Google Scholar] [CrossRef]
- Boland, C.R.; Komarova, N.L.; Goel, A. Chromosomal instability and cancer: Not just one CINgle mechanism. Gut 2009, 58, 163–164. [Google Scholar] [CrossRef]
- Wong, J.J.-L.; Hawkins, N.J.; Ward, R.L.; Hitchins, M.P. Methylation of the 3p22 region encompassing MLH1 is representative of the CpG island methylator phenotype in colorectal cancer. Mod. Pathol. 2011, 24, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Yamada, H.Y. Genomic Instability and Colon Carcinogenesis: From the Perspective of Genes. Front. Oncol. 2013, 3, 130. [Google Scholar] [CrossRef]
- Colorectal Cancer Stages. Available online: https://www.cancercenter.com (accessed on 16 March 2022).
- Young, P.E.; Womeldorph, C.M.; Johnson, E.K.; Maykel, J.A.; Brucher, B.; Stojadinovic, A.; Avital, I.; Nissan, A.; Steele, S.R. Early Detection of Colorectal Cancer Recurrence in Patients Undergoing Surgery with Curative Intent: Current Status and Challenges. J. Cancer 2014, 5, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Ablation and Embolization for Colorectal Cancer. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/treating/ablation-embolization.html (accessed on 29 June 2020).
- Joye, I.; Haustermans, K. Early and Late Toxicity of Radiotherapy for Rectal Cancer. In Early Gastrointestinal Cancers II: Rectal Cancer; Otto, F., Lutz, M.P., Eds.; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Chu, E. An Update on the Current and Emerging Targeted Agents in Metastatic Colorectal Cancer. Clin. Color. Cancer 2012, 11, 1–13. [Google Scholar] [CrossRef]
- Jayakumar, J.; Mohammed, Z.H.; Jayaprakash, B.U.; Ramzi, M.M.; Bhat, A.A. Recent Developments in Nanomedicine. In Treatment Options for Colorectal Cancer, in Modern Technology: Present and Future of Cancer; Macha, M.A., Ed.; OMICS Group eBooks: San Mateo, CA, USA, 2015. [Google Scholar]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Buddolla, V.; Lee, K. Recent insights into nanotechnology development for detection and treatment of colorectal cancer. Int. J. Nanomed. 2016, 11, 2491–2504. [Google Scholar] [CrossRef][Green Version]
- Cisterna, B.A.; Kamaly, N.; Choi, W.I.; Tavakkoli, A.; Farokhzad, O.C.; Vilos, C. Targeted nanoparticles for colorectal cancer. Nanomedicine 2016, 11, 2443–2456. [Google Scholar] [CrossRef]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef]
- Messersmith, W.A. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 599–601. [Google Scholar]
- Brown, K.G.M.; Solomon, M.J.; Mahon, K.; O’Shannassy, S. Management of colorectal cancer. BMJ 2019, 366, l4561. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.; Vila, M.; Portolés, M.-T.; Vallet-Regí, M.; Grácio, J.; Marques, P.A.A.P. Nano-Graphene Oxide: A Potential Multifunctional Platform for Cancer Therapy. Adv. Health Mater. 2013, 2, 1072–1090. [Google Scholar] [CrossRef] [PubMed]
- Barui, S.; Cauda, V. Multimodal Decorations of Mesoporous Silica Nanoparticles for Improved Cancer Therapy. Pharmaceutics 2020, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Martinho, N.; Damgé, C.; Reis, C.P. Recent Advances in Drug Delivery Systems. J. Biomater. Nanobiotechnol. 2011, 2, 510–526. [Google Scholar] [CrossRef]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G.; Ryman, B.E. Liposomes as carriers of enzymes or drugs: A new approach to the treatment of storage diseases. Biochem. J. 1971, 124, 58P. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.; Neves, A.; Pires, B.; Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Chen, Z.G.; Shin, D.M. Advances of Cancer Therapy by Nanotechnology. Cancer Res. Treat. 2009, 41, 1. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Sah, H.; Thoma, L.A.; Desu, H.R.; Sah, E.; Wood, G.C. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int. J. Nanomed. 2013, 8, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.A.; Kartal-Hodzic, A.; Oksanen, T.; Ismael, H.R.; Afouna, M.M.; Yliperttula, M.; Samy, A.M.; Viitala, T. Factorial design formulation optimization and in vitro characterization of curcumin-loaded PLGA nanoparticles for colon delivery. J. Drug Deliv. Sci. Technol. 2016, 32, 10–20. [Google Scholar] [CrossRef]
- Tummala, S.; Satish Kumar, M.N.; Prakash, A. Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm. J. 2015, 23, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Essa, S.; Daoud, J.; Lafleur, M.; Martel, S.; Tabrizian, M. SN-38 active loading in poly(lactic-co-glycolic acid) nanoparticles and assessment of their anticancer properties on COLO-205 human colon adenocarcinoma cells. J. Microencapsul. 2015, 32, 784–793. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Shi, C.; Guo, D.; Xu, G.; Wang, L.; Bodman, A.; Luo, J. Fine-Tuning Vitamin E-Containing Telodendrimers for Efficient Delivery of Gambogic Acid in Colon Cancer Treatment. Mol. Pharm. 2015, 12, 1216–1229. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ficker, M.; Christensen, J.B.; Trohopoulos, P.N.; Moghimi, S.M. Dendrimers in Medicine: Therapeutic Concepts and Pharmaceutical Challenges. Bioconjugate Chem. 2015, 26, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.-S.; Lou, P.-J.; Peng, C.-L.; Pai, C.-L.; Yen, W.-N.; Huang, M.-Y.; Young, T.-H.; Shieh, M.-J. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J. Control. Release 2007, 122, 39–46. [Google Scholar] [CrossRef]
- Malik, N.; Evagorou, E.G.; Duncan, R. Dendrimer-platinate: A novel approach to cancer chemotherapy. Anti-Cancer Drugs 1999, 10, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.X.; Du, B.; Lu, Z.R. In vitro release of 5-fluorouracil with cyclic core dendritic polymer. J. Control. Release 1999, 57, 249–257. [Google Scholar] [CrossRef]
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Fréchet, J.M.J.; Dy, E.E.; Szoka, F.C. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654. [Google Scholar] [CrossRef] [PubMed]
- Tunki, L.; Kulhari, H.; Sistla, R.; Pooja, D. 5-Dendrimer-Based Targeted Drug Delivery. In Pharmaceutical Applications of Dendrimers; Chauhan, A., Kulhari, H., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–129. ISBN 978-0-12-814527-2. [Google Scholar]
- Marzbali, M.Y.; Khosroushahi, A.Y. Polymeric micelles as mighty nanocarriers for cancer gene therapy: A review. Cancer Chemother. Pharmacol. 2017, 9, 637–649. [Google Scholar] [CrossRef]
- Bhadra, D.; Bhadra, S.; Jain, S.; Jain, N. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharm. 2003, 257, 111–124. [Google Scholar] [CrossRef]
- Amin, M.C.I.M.; Butt, A.M.; Amjad, M.W.; Kesharwani, P. Polymeric micelles for drug targeting and delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Academic Press: Cambridge, MA, USA; pp. 167–202. [CrossRef]
- Ameli, H.; Alizadeh, N. Targeted delivery of capecitabine to colon cancer cells using nano polymeric micelles based on beta cyclodextrin. RSC Adv. 2022, 12, 4681–4691. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Prasher, P.; Aljabali, A.A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D.K.; Tambuwala, M.; Dua, K.; et al. Emerging era of “somes”: Polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kang, S.-W.; Bae, Y.H. Polymersome Formation from AB2 Type 3-Miktoarm Star Copolymers. Macromolecules 2009, 42, 7456–7464. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, L. Polymersomes: Preparation and Characterization. Pharm. Nanotechnol. 2019, 2000, 247–265. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Alibolandi, M.; Shahriari, M.; Ramezani, M. Smart Polymersomes as Intelligent Nanomedicines in Cancer Treatment. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Academic Press: Amsterdam, The Netherlands; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 343–371. [Google Scholar] [CrossRef]
- Pegoraro, C.; Cecchin, D.; Gracia, L.S.; Warren, N.; Madsen, J.; Armes, S.P.; Lewis, A.; MacNeil, S.; Battaglia, G. Enhanced drug delivery to melanoma cells using PMPC-PDPA polymersomes. Cancer Lett. 2013, 334, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. Synthesis of hyaluronic acid-based polymersomes for doxorubicin delivery to metastatic breast cancer. Int. J. Pharm. 2019, 572, 118835. [Google Scholar] [CrossRef]
- Qin, H.; Jiang, Y.; Zhang, J.; Deng, C.; Zhong, Z. Oncoprotein Inhibitor Rigosertib Loaded in ApoE-Targeted Smart Polymersomes Reveals High Safety and Potency against Human Glioblastoma in Mice. Mol. Pharm. 2019, 16, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhang, Q.; Cai, Y.; Duan, S.; Chen, S.; Lv, N.; Jin, T.; Chen, Y.; Yuan, W. PEG–PCL–DEX polymersome–protamine vector as an efficient gene delivery system via PEG-guided self-assembly. Nanomedicine 2014, 9, 1193–1207. [Google Scholar] [CrossRef]
- Kim, H.-O.; Lim, J.-W.; Choi, J.; Lee, H.; Son, H.Y.; Kim, J.; Park, G.; Chun, H.; Song, D.; Huh, Y.-M.; et al. Anchored protease-activatable polymersomes for molecular diagnostics of metastatic cancer cells. J. Mater. Chem. B 2017, 5, 9571–9578. [Google Scholar] [CrossRef]
- Petersen, M.A.; Hillmyer, M.A.; Kokkoli, E. Bioresorbable Polymersomes for Targeted Delivery of Cisplatin. Bioconjugate Chem. 2013, 24, 533–543. [Google Scholar] [CrossRef]
- Discher, D.E.; Ahmed, F. Annual Review of Biomedical Engineering. Polymerosomes 2006, 8, 323–341. [Google Scholar]
- He, X.; Zhong, X.; Hu, Z.; Zhao, S.; Wei, P.; Li, D. An insight into small extracellular vesicles: Their roles in colorectal cancer progression and potential clinical applications. Clin. Transl. Med. 2020, 10, e249. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, D.; Ma, X.; Wang, J.; Hou, W.; Zhang, W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed. Pharmacother. 2020, 128, 110237. [Google Scholar] [CrossRef] [PubMed]
- Hartman, Z.C.; Wei, J.; Glass, O.K.; Guo, H.; Lei, G.; Yang, X.-Y.; Osada, T.; Hobeika, A.; Delcayre, A.; Le Pecq, J.-B.; et al. Increasing vaccine potency through exosome antigen targeting. Vaccine 2011, 29, 9361–9367. [Google Scholar] [CrossRef]
- André, F.; Chaput, N.; Schartz, N.E.C.; Flament, C.; Aubert, N.; Bernard, J.; Lemonnier, F.; Raposo, G.; Escudier, B.; Hsu, D.-H.; et al. Exosomes as Potent Cell-Free Peptide-Based Vaccine. I. Dendritic Cell-Derived Exosomes Transfer Functional MHC Class I/Peptide Complexes to Dendritic Cells. J. Immunol. 2004, 172, 2126–2136. [Google Scholar] [CrossRef]
- Romagnoli, G.G.; Zelante, B.B.; Toniolo, P.A.; Migliori, I.K.; Barbuto, J.A.M. Dendritic Cell-Derived Exosomes may be a Tool for Cancer Immunotherapy by Converting Tumor Cells into Immunogenic Targets. Front. Immunol. 2014, 5, 692. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; Van Solinge, W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053. [Google Scholar] [CrossRef]
- Suntres, Z.E. Liposomal Antioxidants for Protection against Oxidant-Induced Damage. J. Toxicol. 2011, 2011, 152474. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27. [Google Scholar] [CrossRef]
- Abreu, A.S.; Castanheira, E.M.; Queiroz, M.-J.R.; Ferreira, P.M.; Vale-Silva, L.A.; Pinto, E. Nanoliposomes for encapsulation and delivery of the potential antitumoral methyl 6-methoxy-3-(4-methoxyphenyl)-1H-indole-2-carboxylate. Nanoscale Res. Lett. 2011, 6, 482. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release Off. J. Control Release Soc. 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Hennink, W.E.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, H.; Yu, M.; Fang, J. Targeted Delivery of Doxorubicin Using a Colorectal Cancer-Specific ssDNA Aptamer. Anat. Rec. 2014, 297, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Stratton, B.; Engel, A.; Deng, W. Liposome technologies towards colorectal cancer therapeutics. Acta Biomater. 2021, 127, 24–40. [Google Scholar] [CrossRef]
- Clinical Trials Database: NCT00361842. Available online: https://clinicaltrials.gov/ct2/show/NCT00361842 (accessed on 2 July 2021).
- Lei, S.; Chien, P.-Y.; Sheikh, S.; Zhang, A.; Ali, S.; Ahmad, I. Enhanced therapeutic efficacy of a novel liposome-based formulation of SN-38 against human tumor models in SCID mice. Anti-Cancer Drugs 2004, 15, 773–778. [Google Scholar] [CrossRef]
- ClinicalTrials.gov, Identifier: NCT00311610. Available online: https://clinicaltrials.gov/ct2/show/NCT00311610 (accessed on 29 June 2016).
- Chen, K.-J.; Chaung, E.-Y.; Wey, S.-P.; Lin, K.-J.; Cheng, F.; Lin, C.-C.; Liu, H.-L.; Tseng, H.-W.; Liu, C.-P.; Wei, M.-C.; et al. Hyperthermia-Mediated Local Drug Delivery by a Bubble-Generating Liposomal System for Tumor-Specific Chemotherapy. ACS Nano 2014, 8, 5105–5115. [Google Scholar] [CrossRef] [PubMed]
- Celsion. Phase 2 Study of Thermodox as Adjuvant Therapy with Thermal Ablation (RFA) in Treatment of Metastatic Colorectal Cancer(mCRC) (ABLATE). 2011. Available online: https://clinicaltrials.gov/ct2/show/NCT01464593 (accessed on 29 June 2016).
- Yang, C.; Liu, H.Z.; Fu, Z.X.; Lu, W.D. Oxaliplatin long-circulating liposomes improved therapeutic index of colorectal carcinoma. BMC Biotechnol. 2011, 11, 21. [Google Scholar] [CrossRef]
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007, 6, 1276–1282. [Google Scholar] [CrossRef]
- Cay, O.; Kruskal, J.B.; Nasser, I.; Thomas, P.; Clouse, M.E. Liver metastases from colorectal cancer: Drug delivery with liposome-encapsulated doxorubicin. Radiology 1997, 205, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Stang, J.; Haynes, M.; Carson, P.; Moghaddam, M. A Preclinical System Prototype for Focused Microwave Thermal Therapy of the Breast. IEEE Trans. Biomed. Eng. 2012, 59, 2431–2438. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Gupta, S.; Gnanadhas, D.P.; Ramamurthy, P.C.; Chakravortty, D.; Raichur, A.M. Protamine-Capped Mesoporous Silica Nanoparticles for Biologically Triggered Drug Release. Part. Part. Syst. Charact. 2013, 31, 449–458. [Google Scholar] [CrossRef]
- Yu, M.; Jambhrunkar, S.; Thorn, P.; Chen, J.; Gu, W.; Yu, C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef]
- Gidding, C.E.; Kellie, S.J.; Kamps, W.A.; de Graaf, S.S. Vincristine Revisited. Crit. Rev. Oncol. Hematol. 1999, 29, 267–287. [Google Scholar] [CrossRef]
- Hanafi-Bojd, M.Y.; Jaafari, M.R.; Ramezanian, N.; Xue, M.; Amin, M.; Shahtahmassebi, N.; Malaekeh-Nikouei, B. Surface functionalized mesoporous silica nanoparticles as an effective carrier for epirubicin delivery to cancer cells. Eur. J. Pharm. Biopharm. 2015, 89, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Bretin, L.; Pinon, A.; Bouramtane, S.; Ouk, C.; Richard, L.; Perrin, M.-L.; Chaunavel, A.; Carrion, C.; Bregier, F.; Sol, V.; et al. Photodynamic Therapy Activity of New Porphyrin-Xylan-Coated Silica Nanoparticles in Human Colorectal Cancer. Cancers 2019, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Advances in Colorectal Cancer Research. Nanotechnology to Improve Early Detection and Treatment of Colorectal Cancer. 2016. Available online: https://www.nih.gov/research-training/nanotechnology-improve-doneearly-detection-treatment-colorectal-cancer (accessed on 15 March 2016).
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Liu, T.-Y.; Chan, T.-Y.; Tsai, S.-C.; Hardiansyah, A.; Huang, L.-Y.; Yang, M.-C.; Lu, R.-H.; Jiang, J.-K.; Yang, C.-Y.; et al. Magnetically triggered nanovehicles for controlled drug release as a colorectal cancer therapy. Colloids Surf. B Biointerfaces 2016, 140, 567–573. [Google Scholar] [CrossRef]
- Esmaelbeygi, E.; Khoei, S.; Khoee, S.; Eynali, S. Role of iron oxide core of polymeric nanoparticles in the thermosensitivity of colon cancer cell line HT-29. Int. J. Hyperth. 2015, 31, 489–497. [Google Scholar] [CrossRef]
- Feng, S.-T.; Li, J.; Luo, Y.; Yin, T.; Cai, H.; Wang, Y.; Dong, Z.; Shuai, X.; Li, Z.-P. pH-Sensitive Nanomicelles for Controlled and Efficient Drug Delivery to Human Colorectal Carcinoma LoVo Cells. PLoS ONE 2014, 9, e100732. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J. Drug Deliv. 2014, 2014, 670815. [Google Scholar] [CrossRef]
- Hampel, S.; Kunze, D.; Haase, D.; Krämer, K.; Rauschenbach, M.; Ritschel, M.; Leonhardt, A.; Thomas, J.; Oswald, S.; Hoffmann, V.; et al. Carbon nanotubes filled with a chemotherapeutic agent: A nanocarrier mediates inhibition of tumor cell growth. Nanomedicine 2008, 3, 175–182. [Google Scholar] [CrossRef]
- Lee, Y.; Geckeler, K.E. Cellular Interactions of a Water-Soluble Supramolecular Polymer Complex of Carbon Nanotubes with Human Epithelial Colorectal Adenocarcinoma Cells. Macromol. Biosci. 2012, 12, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Peng, Z.; Liao, S.; Li, P.; Li, S. Design of microencapsulated carbon nanotube-based microspheres and its application in colon targeted drug delivery. Drug Deliv. 2014, 21, 101–109. [Google Scholar] [CrossRef]
- Levi-Polyachenko, N.H.; Merkel, E.J.; Jones, B.T.; Carroll, D.L.; Stewart, I.J.H. Rapid Photothermal Intracellular Drug Delivery Using Multiwalled Carbon Nanotubes. Mol. Pharm. 2009, 6, 1092–1099. [Google Scholar] [CrossRef]
- Zakaria, A.B.; Picaud, F.; Rattier, T.; Pudlo, M.; Dufour, F.; Saviot, L.; Chassagnon, R.; Lherminier, J.; Gharbi, T.; Micheau, O.; et al. Nanovectorization of TRAIL with Single Wall Carbon Nanotubes Enhances Tumor Cell Killing. Nano Lett. 2015, 15, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Sahoo, N.G.; Bao, H.; Pan, Y.; Li, L. Functionalized Graphene Oxide as Nanocarrier for Loading and Delivery of Ellagic Acid. Curr. Med. Chem. 2011, 18, 4503–4512. [Google Scholar] [CrossRef] [PubMed]
- Magne, T.M.; Vieira, T.D.O.; Alencar, L.M.R.; Junior, F.F.M.; Gemini-Piperni, S.; Carneiro, S.V.; Fechine, L.M.U.D.; Freire, R.M.; Golokhvast, K.; Metrangolo, P.; et al. Graphene and its derivatives: Understanding the main chemical and medicinal chemistry roles for biomedical applications. J. Nanostruct. Chem. 2021, 1–35. [Google Scholar] [CrossRef]
- Banerjee, A.N. Graphene and its derivatives as biomedical materials: Future prospects and challenges. Interface Focus 2018, 8, 20170056. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Holst, R. Über die säurenatur und die methylierung von graphitoxyd. Ber. Der Dtsch. Chem. Ges. (A B Ser.) 1939, 72, 754–771. [Google Scholar] [CrossRef]
- Ruess, G. Über das graphitoxyhydroxyd (graphitoxyd). Mon. Chem. Verwandte Teile And. Wiss. 1947, 76, 381–417. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the Local Chemical Structure of Graphene Oxide and Reduced Graphene Oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Todd, A.D.; Bielawski, C.W. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014, 43, 5288–5301. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Eigler, S.; Hirsch, A. Chemistry with Graphene and Graphene Oxide-Challenges for Synthetic Chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef] [PubMed]
- Dimiev, A.; Kosynkin, D.V.; Alemany, L.B.; Chaguine, P.; Tour, J.M. Pristine Graphite Oxide. J. Am. Chem. Soc. 2012, 134, 2815–2822. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Rourke, J.P.; Pandey, P.A.; Moore, J.J.; Bates, M.; Kinloch, I.A.; Young, R.J.; Wilson, N.R. The Real Graphene Oxide Revealed: Stripping the Oxidative Debris from the Graphene-like Sheets. Angew. Chem. Int. Ed. 2011, 50, 3173–3177. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Shi, G. Functional Composite Materials Based on Chemically Converted Graphene. Adv. Mater. 2011, 23, 1089–1115. [Google Scholar] [CrossRef]
- Brodie, B.C. On the atomic weight of graphite. Proc. R. Soc. Lond. 1860, 149, 249–259. [Google Scholar] [CrossRef]
- Hummers, W.S.H.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Poh, H.L.; Šaněk, F.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Pumera, M. Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 2012, 4, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.; Jose, G.; Lu, Y.-J.; Chen, J.-P. Functionalized Reduced Graphene Oxide as a Versatile Tool for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 2989. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Covalent chemistry on graphene. Chem. Soc. Rev. 2013, 42, 3222–3233. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Liu, J.; Zhai, G. Recent Developments of Phototherapy Based on Graphene Family Nanomaterials. Curr. Med. Chem. 2017, 24, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Ju, D.T.; Chang, C.F.; Muralidhar Reddy, P.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. BioMedicine 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Colorectal Cancer: Types of Treatment. Available online: https://www.cancer.net/cancer-types/colorectal-cancer/types-treatment (accessed on 1 January 2021).
- Al-Ani, L.A.; Kadir, F.A.; Hashim, N.M.; Julkapli, N.M.; Seyfoddin, A.; Lu, J.; AlSaadi, M.A.; Yehye, W.A. The impact of curcumin-graphene based nanoformulation on cellular interaction and redox-activated apoptosis: An in vitro colon cancer study. Heliyon 2020, 6, e05360. [Google Scholar] [CrossRef] [PubMed]
- Dhanavel, S.; Revathy, T.A.; Sivaranjani, T.; Sivakumar, K.; Palani, P.; Narayanan, V.; Stephen, A. 5-Fluorouracil and curcumin co-encapsulated chitosan/reduced graphene oxide nanocomposites against human colon cancer cell lines. Polym. Bull. 2020, 77, 213–233. [Google Scholar] [CrossRef]
- Wu, S.-Y.; An, S.S.A.; Hulme, J. Current applications of graphene oxide in nanomedicine. Int. J. Nanomed. 2015, 10, 9–24. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-Efficiency Loading and Controlled Release of Doxorubicin Hydrochloride on Graphene Oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Depan, D.; Shah, J.; Misra, R. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Wang, X.L.; Shi, G.Q. A pH-sensitive graphene oxide composite hydrogel. Chem. Commun. 2010, 46, 2376–2378. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.-V.; Shim, K.; Thi, T.-T.V.; Kook, J.-K.; An, S.S.A.; Lee, S.-W. Targeted and controlled drug delivery by multifunctional mesoporous silica nanoparticles with internal fluorescent conjugates and external polydopamine and graphene oxide layers. Acta Biomater. 2018, 74, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Mauro, N.; Pitarresi, G.; Scialabba, C.; Palumbo, F.S.; Giammona, G. Double-Network-Structured Graphene Oxide-Containing Nanogels as Photothermal Agents for the Treatment of Colorectal Cancer. Biomacromolecules 2017, 18, 1010–1018. [Google Scholar] [CrossRef]
- Hou, L.; Shi, Y.; Jiang, G.; Liu, W.; Han, H.; Feng, Q.; Ren, J.; Yuan, Y.; Wang, Y.; Shi, J.; et al. Smart nanocomposite hydrogels based on azo crosslinked graphene oxide for oral colon-specific drug delivery. Nanotechnology 2016, 27, 315105. [Google Scholar] [CrossRef]
- Dawidczyk, C.M.; Russell, L.; Searson, P.C. Nanomedicines for cancer therapy: State-of-the-art and limitations to pre-clinical studies that hinder future developments. Front. Chem. 2014, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Roger, E.; Lautram, N.; Benoît, J.-P.; Passirani, C. The rise and rise of stealth nanocarriers for cancer therapy: Passive versus active targeting. Nanomedicine 2010, 5, 1415–1433. [Google Scholar] [CrossRef] [PubMed]
- Dill, K.; Lin, M.; Poteras, C.; Fraser, C.; Hafeman, D.; Owicki, J.; Olson, J. Antibody-Antigen Binding Constants Determined in Solution-Phase with the Threshold Membrane-Capture System: Binding Constants for Antifluorescein, Anti-Saxitoxin, and Anti-Ricin Antibodies. Anal. Biochem. 1994, 217, 128–138. [Google Scholar] [CrossRef]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef]
- Tiwari, A.; Saraf, S.; Jain, A.; Panda, P.K.; Verma, A.; Jain, S.K. Basics to advances in nanotherapy of colorectal cancer. Drug Deliv. Transl. Res. 2020, 10, 319–338. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Termsarasab, U.; Park, J.-H.; Lee, S.Y.; Ko, S.-H.; Shim, J.-S.; Chung, S.-J.; Cho, H.-J.; Kim, D.-D. Dual CD44 and folate receptor-targeted nanoparticles for cancer diagnosis and anticancer drug delivery. J. Control. Release 2016, 236, 38–46. [Google Scholar] [CrossRef]
- Jiang, W.; Mo, F.; Jin, X.; Chen, L.; Xu, L.J.; Guo, L.; Fu, F. Tumor-Targeting Photothermal Heating-Responsive Nanoplatform Based on Reduced Graphene Oxide/Mesoporous Silica/Hyaluronic Acid Nanocomposite for Enhanced Photodynamic Therapy. Adv. Mater. Interfaces 2017, 4, 1700425. [Google Scholar] [CrossRef]
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials 2013, 34, 6239–6248. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Casalongue, H.S.; Vinh, D.; Dai, H. Ultrasmall Reduced Graphene Oxide with High Near-Infrared Absorbance for Photothermal Therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Abdolahad, M.; Janmaleki, M.; Mohajerzadeh, S.; Akhavan, O.; Abbasi, S. Polyphenols attached graphene nanosheets for high efficiency NIR mediated photodestruction of cancer cells. Mater. Sci. Eng. C 2013, 33, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Liu, Y.; Zhu, X.; Wang, X.; Liu, L.; Sun, H.; Wang, C.; Kong, D.; Ma, G. Nanoscale Reduced Graphene Oxide-Mediated Photothermal Therapy Together with IDO Inhibition and PD-L1 Blockade Synergistically Promote Antitumor Immunity. ACS Appl. Mater. Interfaces 2018, 11, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; He, F.; Yu, C.; Liang, X.; Liang, D.; Ma, L.; Zhang, Q.; Lv, J.; Wu, J. Advances on graphene-based nanomaterials for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 764–780. [Google Scholar] [CrossRef] [PubMed]
- Zaharie-Butucel, D.; Potara, M.; Suarasan, S.; Licarete, E.; Astilean, S. Efficient combined near-infrared-triggered therapy: Phototherapy over chemotherapy in chitosan-reduced graphene oxide-IR820 dye-doxorubicin nanoplatforms. J. Colloid Interface Sci. 2019, 552, 218–229. [Google Scholar] [CrossRef]
- Bera, S.; Ghosh, S.; Ali, A.; Pal, M.; Chakrabarti, P. Inhibition of microtubule assembly and cytotoxic effect of graphene oxide on human colorectal carcinoma cell HCT116. Arch. Biochem. Biophys. 2021, 708, 108940. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, M.; Vasileva, B.; Speranza, G.; Wang, D.; Stoyanov, K.; Draganova-Filipova, M.; Zagorchev, P.; Sarafian, V.; Miloshev, G.; Krasteva, N. Amination of Graphene Oxide Leads to Increased Cytotoxicity in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2020, 21, 2427. [Google Scholar] [CrossRef]
- Krasteva, N.; Keremidarska-Markova, M.; Hristova-Panusheva, K.; Andreeva, T.; Speranza, G.; Wang, D.; Draganova-Filipova, M.; Miloshev, G.; Georgieva, M. Aminated Graphene Oxide as a Potential New Therapy for Colorectal Cancer. Oxidative Med. Cell. Longev. 2019, 2019, 3738980. [Google Scholar] [CrossRef] [PubMed]
- Keremidarska-Markova, M.; Hristova-Panusheva, K.; Andreeva, T.; Speranza, G.; Wang, D.; Krasteva, N. Cytotoxicity Evaluation of Ammonia-Modified Graphene Oxide Particles in Lung Cancer Cells and Embryonic Stem Cells. Adv. Condens. Matter Phys. 2018, 2018, 9571828. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, B.; Zheng, J.-J.; Yu, M.; Zhou, T.; Zhao, K.; Jia, Y.; Gao, X.; Chen, C.; Wei, T. The inhibition of migration and invasion of cancer cells by graphene via the impairment of mitochondrial respiration. Biomaterials 2014, 35, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Y.; Meng, C.-L.; Lin, K.-C.; Tuan, H.-Y.; Yang, H.-J.; Chen, C.-L.; Li, K.-C.; Chiang, C.-S.; Hu, Y.-C. Graphene oxide as a chemosensitizer: Diverted autophagic flux, enhanced nuclear import, elevated necrosis and improved antitumor effects. Biomaterials 2015, 40, 12–22. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, M.; Gao, M.; Zhang, Z.; Xu, Y.; Xia, T.; Liu, S. Graphene Oxide Induced Perturbation to Plasma Membrane and Cytoskeletal Meshwork Sensitize Cancer Cells to Chemotherapeutic Agents. ACS Nano 2017, 11, 2637–2651. [Google Scholar] [CrossRef]
- Hao, S.; Wang, B.; Wang, Y.; Xu, Y. Enteric-coated sustained-release nanoparticles by coaxial electrospray: Preparation, characterization, and in vitro evaluation. J. Nanoparticle Res. 2014, 16, 2204. [Google Scholar] [CrossRef]
- Tummala, S.; Kuppusamy, G.; Kumar, M.N.S.; Praveen, T.K.; Wadhwani, A. 5-Fluorouracil enteric-coated nanoparticles for improved apoptotic activity and therapeutic index in treating colorectal cancer. Drug Deliv. 2015, 23, 2902–2910. [Google Scholar] [CrossRef]
- Hosny, K.M. Alendronate Sodium as Enteric Coated Solid Lipid Nanoparticles; Preparation, Optimization, and In Vivo Evaluation to Enhance Its Oral Bioavailability. PLoS ONE 2016, 11, e0154926. [Google Scholar] [CrossRef]
- Amini-Fazl, M.S.; Mohammadi, R.; Kheiri, K. 5-Fluorouracil loaded chitosan/polyacrylic acid/Fe3O4 magnetic nanocomposite hydrogel as a potential anticancer drug delivery system. Int. J. Biol. Macromol. 2019, 132, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Wathoni, N.; Ny Nguyen, A.; Rusdin, A.; Umar, A.K.; Mohammed, A.F.A.; Motoyama, K.; Joni, I.M.; Muchtaridi, M. Enteric-Coated Strategies in Colorectal Cancer Nanoparticle Drug Delivery System. Drug Des. Dev. Ther. 2020, 14, 4387–4405. [Google Scholar] [CrossRef]
- Akinc, A.; Battaglia, G. Exploiting Endocytosis for Nanomedicines. Cold Spring Harb. Perspect. Biol. 2013, 5, a016980. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, F.; Deng, T.; Zhu, S.; Liu, W.; Zhong, H.; Yu, H.; Luo, R.; Deng, Z. Eudragit S100-Coated Chitosan Nanoparticles Co-loading Tat for Enhanced Oral Colon Absorption of Insulin. AAPS PharmSciTech 2017, 18, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-H.; Kim, J.-E.; Lee, Y.-N.; Noh, S.M.; An, S.-W.; Cho, H.-J.; Kim, D.-D. Chitosan-Based Hybrid Nanocomplex for siRNA Delivery and Its Application for Cancer Therapy. Pharm. Res. 2014, 31, 3323–3334. [Google Scholar] [CrossRef]
- Hoang, B.; Ernsting, M.J.; Murakami, M.; Undzys, E.; Li, S.-D. Docetaxel–carboxymethylcellulose nanoparticles display enhanced anti-tumor activity in murine models of castration-resistant prostate cancer. Int. J. Pharm. 2014, 471, 224–233. [Google Scholar] [CrossRef]
- Vilos, C.; Morales, F.A.; Solar, P.; Herrera, N.S.; Nilo, F.D.G.; Aguayo, D.A.; Mendoza, H.L.; Comer, J.; Bravo, M.L.; Gonzalez, P.A.; et al. Paclitaxel-PHBV nanoparticles and their toxicity to endometrial and primary ovarian cancer cells. Biomaterials 2013, 34, 4098–4108. [Google Scholar] [CrossRef]
- Cao, B.; Yang, M.; Zhu, Y.; Qu, X.; Mao, C. Stem Cells Loaded with Nanoparticles as a Drug Carrier for In Vivo Breast Cancer Therapy. Adv. Mater. 2014, 26, 4627–4631. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, M.; Shao, S.; Huang, W.; Yang, F.; Chen, W.; Zhang, S.; Xia, G.; Gao, Z. Small-sized polymeric micelles incorporating docetaxel suppress distant metastases in the clinically-relevant 4T1 mouse breast cancer model. BMC Cancer 2014, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Z.; Lin, J.; Zhang, Y.; Lv, S.; Song, W.; Huang, Y.; Chen, X. Synergistic Antitumor Effects of Doxorubicin-Loaded Carboxymethyl Cellulose Nanoparticle in Combination with Endostar for Effective Treatment of Non-Small-Cell Lung Cancer. Adv. Health Mater. 2014, 3, 1877–1888. [Google Scholar] [CrossRef]
- Emami, J.; Pourmashhadi, A.; Sadeghi, H.; Varshosaz, J.; Hamishehkar, H. Formulation and optimization of celecoxib-loaded PLGA nanoparticles by the Taguchi design and their In vitro cytotoxicity for lung cancer therapy. Pharm. Dev. Technol. 2015, 20, 791–800. [Google Scholar] [CrossRef]
- Wang, B.; Guo, H.; Xu, H.; Chen, Y.; Zhao, G.; Yu, H. The Role of Graphene Oxide Nanocarriers in Treating Gliomas. Front. Oncol. 2022, 12, 736177. [Google Scholar] [CrossRef]
- Chong, Y.; Ma, Y.; Shen, H.; Tu, X.; Zhou, X.; Xu, J.; Dai, J.; Fan, S.; Zhang, Z. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 2014, 35, 5041–5048. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, S.; Sastry, M.; Panjikar, S.; Raman, R.S. Graphene and Graphene Oxide as a Support for Biomolecules in the Development of Biosensors. Nanotechnol. Sci. Appl. 2021, 14, 197–220. [Google Scholar] [CrossRef]
- Rasoulzadeh, M.; Namazi, H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.K.; Thakur, M.; Bahadur, R.; Kaku, T.; Prabhuraj, R.S.; Ninawe, A.; Srivastava, R. Preparation of graphene oxide-graphene quantum dots hybrid and its application in cancer theranostics. Mater. Sci. Eng. C 2019, 103, 109774. [Google Scholar] [CrossRef]
- Gupta, N.; Rai, D.B.; Jangid, A.K.; Kulhari, H. A Review of Theranostics Applications and Toxicities of Carbon Nanomaterials. Curr. Drug Metab. 2019, 20, 506–532. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Lan, Y.-H.; Chuang, C.-C.; Lu, W.-T.; Chan, L.-Y.; Hsu, P.-W.; Chen, J.-P. Injectable Thermo-Sensitive Chitosan Hydrogel Containing CPT-11-Loaded EGFR-Targeted Graphene Oxide and SLP2 shRNA for Localized Drug/Gene Delivery in Glioblastoma Therapy. Int. J. Mol. Sci. 2020, 21, 7111. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-Functionalized Graphene Oxide as a Nanocarrier for Drug and Gene Delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Iravani, S.; Varma, R.S. Graphene and graphene oxide with anticancer applications: Challenges and future perspectives. MedComm 2022, 3, e118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Li, X.; Shi, J.; Jiang, Z.; Zhang, C.Y. Graphene-based nanomaterials for cancer therapy and anti-infections. Bioact. Mater. 2022, 14, 335–349. [Google Scholar] [CrossRef]
- Cui, G.; Wu, J.; Lin, J.; Liu, W.; Chen, P.; Yu, M.; Zhou, D.; Yao, G. Graphene-based nanomaterials for breast cancer treatment: Promising therapeutic strategies. J. Nanobiotechnol. 2021, 19, 211. [Google Scholar] [CrossRef]
- Durán, M.; Durán, N.; Fávaro, W.J. In vivo nanotoxicological profile of graphene oxide. J. Phys. Conf. Ser. 2017, 838, 012026. [Google Scholar] [CrossRef]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.-L.; Wong, W.-Y.; Bian, Z.; Chui, C.-H.; Gambari, R. Recent advances in green nanoparticulate systems for drug delivery: Efficient delivery and safety concern. Nanomedicine 2017, 12, 357–385. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20–37. [Google Scholar] [CrossRef]

| Colorectal Cancer (CRC) Staging and Treatment Approaches | ||
|---|---|---|
| TNM | (T) How far the tumour has grown through the colon/rectal wall (N) The presence of cancer in lymph modes (M) Whether cancer metastasised (spread) to the other parts of the body apart from the colon/rectum | |
| Stages/Types of treatment | Stage 0 Local treatments: surgical resection only | The earliest stage of colorectal cancer, known also as carcinoma “in situ” or intramucosal carcinoma, meaning the cancer is in the mucosa (moist tissue lining the colon), and it is not grown through the colon/rectum wall. |
| Stage I Local treatments: surgical resection of the malignant polyp or surgical procedure/partial colectomy of tumour and local lymph nodes | Cancer is still in the inner lining but has grown through the mucosa (second (T1) or third (T2) layer) of the colon and invaded the muscle layer. It has not spread to nearby lymph nodes (N0) or distant sites (M0). | |
| Stage II Local and systematic treatments: surgical resection without chemotherapy; chemotherapy (5-FU, leucovorin, oxaliplatin, or capecitabine) | Cancer has grown beyond the wall of the colon or rectum (T3) and is attached to or has grown into other nearby tissues or organs (T4b). It has not spread to nearby lymph nodes (N0) or distant sites (M0). | |
| Stage III Local, systematic, and combined treatments:surgery followed by adjuvant chemotherapy, including FOLFOX (5-FU, leucovorin, and oxaliplatin) or CapeOx (capecitabine and oxaliplatin) Surgery, Neoadjuvant chemotherapy, and Radiation—for some advanced colon cancers, radiation therapy and/or chemotherapy—for people who are not healthy enough for surgery | Cancer has spread from the colon/rectum to nearby lymph nodes (N2a), or there are small tumour deposits in the fat around the colon/rectum. It has not spread to distant sites (M0). | |
| Stage IV Local, systematic, and combined treatments: Radiotherapy; chemotherapy with FOLFOIRI; FOLFIRI (5-FU, LV, and irinotecan); FOLFOX; CAPIRI (capecitabine and irinotecan); CAPOX; 5-FU with LV; irinotecan; capecitabine and Trifluridine plus Tipiracil (Lonsurf); immunotherapy (Pembrolizumab (Keytruda) or Nivolumab (Opdivo); targeted therapies; palliative surgery/stenting; radiofrequency ablation; radioembolization | Cancer has metastasised to distant sites (N2a) and has been carried through the lymph and blood systems to distant parts of the body. The most likely organs to develop metastasis from colorectal cancer are the lungs and liver. | |
| Nanoparticles Type | Advantages | Disadvantages |
|---|---|---|
| Organic nanoparticles | ||
| Polymeric nanoparticles | Biocompatible and biodegradable; ability to entrap both hydrophilic and hydrophobic drugs; easy to modify; controlled drug release; protect the drug from metabolic degradation; prolonged residence time—bio-adhesive properties; good tissue penetration; easy manipulation; stability of drug; delivery of a higher concentration of drug to the desired location; easy merged into other activities associated to a drug delivery | Burst effect; limited drug loading capacity; high cost; low cell affinity; toxicity of degradation products; nondegradable polymers tend to accumulate in tissue; promote allergic reaction; in vivo metabolism and elimination is not elucidated; rapid clearance out of the abdominal cavity; toxic, reactive residues, unreacted monomers increase the risk of chemical reactions and the formation of unwanted oligomers |
| Dendrimers | Lower polydispersity index; the outer surface of dendrimer has multiple functional groups; they can be designed and synthesised for a specific application | High cost of synthesis process; non-specific toxicity; low loading capacity |
| Polymeric micelles | Prolonged retention time; easily synthesis; can be coupled with targeting ligands to increase accessibility to tumour sites, reduce the side effects; ability to control drug dissemination over a long period | Increased systemic toxicity |
| Polymersomes | Chemical versatility; an ability for controlled release and improved cellular uptake of anti-cancer molecules; low toxicity | Toxicity risk of polymers or metabolites; Polymer aggregation; hydration may be a challenge |
| Small extracellular vesicles | Biocompatible; safe degradation products; non-toxic; non-immunogenic; possibility for cell targeting | Low water solubility |
| Liposomes | Increase the efficacy and therapeutic index of drugs; biocompatible and completely biodegradable; low toxicity; flexible; improved pharmacokinetics of cargo; able to entrap both hydrophilic and hydrophobic drugs; controlled release protects the drug from metabolic degradation prolonged residence time—precorneal and vitreous; decreased the exposure of sensitive tissue to toxic drugs | Poor stability; could crystallise after prolonged storage conditions; difficult to prepare and sterilise; high cost; poor or moderate drug loading capacity; immunogenicity; low solubility; short half-time; leakage and fusion of encapsulated drug/molecules |
| Inorganic nanoparticles | ||
| Mesoporous silica nanoparticles | High drug and genes loading capacity; tuneable pore size; large surface area; biocompatible and biodegradable; controlled porosity; versatility; non-toxic; easy endocytosis, and resistance to heat and pH | Expensive; not enough information about cytotoxicity, biodistribution, biocompatibility’ low stability formation of aggregates, haemolysis |
| Metallic and magnetic nanomaterials | Easy preparation and functionalisation; large surface area; multimodal application; high surface area; multiple forms (spherical, nanorod, triangles); biocompatibility; tuneable size; easy functionalisation excellent biodegradability in vivo; no leakage of encapsulated drugs | Low stability and storage; not enough information about uptake, biocompatibility, and low cytotoxicity in vivo |
| Carbon-based nanomaterials | ||
| Carbon nanotubes | Water-soluble; multifunctional; less toxic; biocompatibility; biodegradability; able to entrap both hydrophilic and hydrophobic drugs; high loading capacity; a high number of possibilities for surface modification; high surface area, needle-like structure, heat conductivity, and chemical stability | Expensive to produce; low degradation; not enough in vivo studies |
| Type of Nanoparticles | Delivered Anticancer Drug/Active Compound | Type of CRC Cells/Tumour | Highlights of the Study | References |
| PGLA NPs | Curcumin | HT-29 | Increased cellular uptake than pure curcumin | [77] |
| PGLA NPs | SN-38 | COLO-205 | Improved solubility, stability, and cellular uptake; reduced cell proliferation and death | [79] |
| PEG-Telodendrimers | Vitamin E, Cholic acid, Gambogic acid (GA) | HT-29 | A superior in vitro cytotoxic activity compared to the free drug | [82] |
| PEG-PES- dendrimers | PES-Polyester Doxorubicin | C-26; BALBc mice with s.c. C-26 tumours | Dendrimer–DOX was 10 times less toxic than free DOX towards C-26 cells after 72 h-exposure nine-fold higher tumour uptake than i.v. administered freeDOX at 48 h. Incomplete tumour regression and 100% survival of the mice over the 60-day experiment. Enhanced blood circulation time, reduced toxicity and less accumulation of drugs prevent the development of Dox-unresponsive C-26 tumour | [87] |
| PR_b-PEO-PMCL and PR_b-PEO-PMCL polymersomes | Cisplatin | DLD-1 overexpressing α5β1 integrin | Increased delivery efficacy to DLD cells; Decreased colon cancer cell viability specific targeting to α5β1 overexpressing cancer cells | [101] |
| HA-MSNs | Doxorubicin | HCT-116 | Increased cellular uptake of DOX-HA-MSNs conjugate; enhanced cytotoxicity in cancer cells | [117] |
| MPVA-AP1 | Doxorubicin, Vitamin B12, Curcumin | CT26-IL4R | Excellent selectivity and targeting toward CT26-IL4R cells; rapid and controlled drug release upon treatment with a high-frequency magnetic field | [122] |
| PLGA-coated iron NPs | 5-fluorouracil (5-FU) | HT-29 | Greater DNA damage in an HT-29 colon tumour cell line as compared with hyperthermia | [123] |
| PTX-SPIO-PEALCa micelles | Paclitaxel, Super-paramagnetic iron oxide (SPIO) | CRCLoVo | Inhibition of CRC LoVo cell growth | [124] |
| PDM-SWCNTs | Paclitaxel | Caco-2, HT-29 | Enhanced anticancer effects in Caco-2 and HT-29 cells compared to paclitaxel alone; induced disruption of tight junctions in Caco-2 cells | [128] |
| MWCNTs | Oxaliplatin, Mitomycin C induced by infrared light rays | RKO, HCT 116 | Increased cell membrane permeability due to hyperthermia; reduction in cancer cell viability | [130] |
| TRAIL -SWCNTs | carcinoma cell lines | Ten times increased cell death in comparison with alone TRAIL | [130] |
| Nanocarrier | Functionalisation Agent/Drug | Cancer Cell Line | Type of Study | Highlights of the Study | Reference |
|---|---|---|---|---|---|
| Chemotherapy | |||||
| CS-(NaPO4)3/rGO | Chitosan (CS), 5-FU, Curcumin | HT-29 | in vitro | Higher entrapment efficiency (>90%); Increased cytotoxicity, IC50 of 23.8μg/mL for dual-drug-loaded nanocomposite; Synergistic cytotoxicity | [186] |
| NGO-PEG | SN38 | HCT-116 | in vitro | IC50 of ~6 nM ~1000-fold more potent than CPT-11 and similar to that of free SN38 | [15] |
| GO-F38, GO-T80 and GO-MD | Ellagic acid (EA) | HT29 | in vitro | Increased antitumour activity | [161] |
| AuNPs-rGO | Curcumin | HT-29, SW-948 | in vitro | Increased ROS and cytotoxicity | [185] |
| GO–N=N–GO/PVA | Curcumin | mice | in vivo | Improved bioavailability of curcumin and preferential accumulation in the colon | [193] |
| Phototherapy | |||||
| GT-rGO sheets | Green tea | SW48, HT29 | in vitro | 20% higher photothermal destruction of the high metastatic SW48 cancer cells than that of the low metastatic HT29 cells | [179,204] |
| Phototherapy/Chemotherapy | |||||
| rGO-PDA@MS/HA | Mesoporous silica (MS), Hyaluronic acid (HA), Polydopamine (PDA), Ce6 | HT-29, HCT-116 | in vitro | [200] | |
| GO-HA-Asp | Irinotecan, Hyaluronic acid/Polyaspartamide | HCT 116 | in vitro | Synergistic hyperthermic/cytotoxic effect | [192] |
| Chit-rGO-IR-820 | DOX, Chitosan, IR-820 | C26 | in vitro | Synergistic anticancer activity (chemotherapy, PTT and PDT) | [207] |
| Phototherapy/Immunotherapy | |||||
| PEG-rGO-FA-IDOi | IDO inhibitor (IDOi), Folic acid, PEG | CT26 | in vitro, in vivo | Synergistically immune/PTT antitumour response | [205] |
| Direct Killing/Chemosensitizing effect | |||||
| GO | HCT116 | in vitro | Disrupted microtubule assembly; Arrested cells in the S phase with increased accumulation in Sub-G1 population of cell cycle; induces apoptosis by generating reactive oxygen species (ROS) in a dose- and time-dependent manner | [208] | |
| GO, GO-NH2 | C26 | in vitro | Induced ROS production and blocked cell cycle in the G0-G1 | [210] | |
| GO | HCT116 | in vitro | Inhibited migration and invasion of cancer cells by inhibiting the activities of electron transport chain (ETC) complexes present in mitochondria; reduced ATP synthesis; inhibited F-actin cytoskeleton assembly | [212] | |
| GO | CT26, mice | in vitro, in vivo | Induced Toll-like receptors (TLRs) responses and autophagy | [213] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasteva, N.; Georgieva, M. Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials. Pharmaceutics 2022, 14, 1213. https://doi.org/10.3390/pharmaceutics14061213
Krasteva N, Georgieva M. Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials. Pharmaceutics. 2022; 14(6):1213. https://doi.org/10.3390/pharmaceutics14061213
Chicago/Turabian StyleKrasteva, Natalia, and Milena Georgieva. 2022. "Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials" Pharmaceutics 14, no. 6: 1213. https://doi.org/10.3390/pharmaceutics14061213
APA StyleKrasteva, N., & Georgieva, M. (2022). Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials. Pharmaceutics, 14(6), 1213. https://doi.org/10.3390/pharmaceutics14061213







