WPI Hydrogels with a Prolonged Drug-Release Profile for Antimicrobial Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
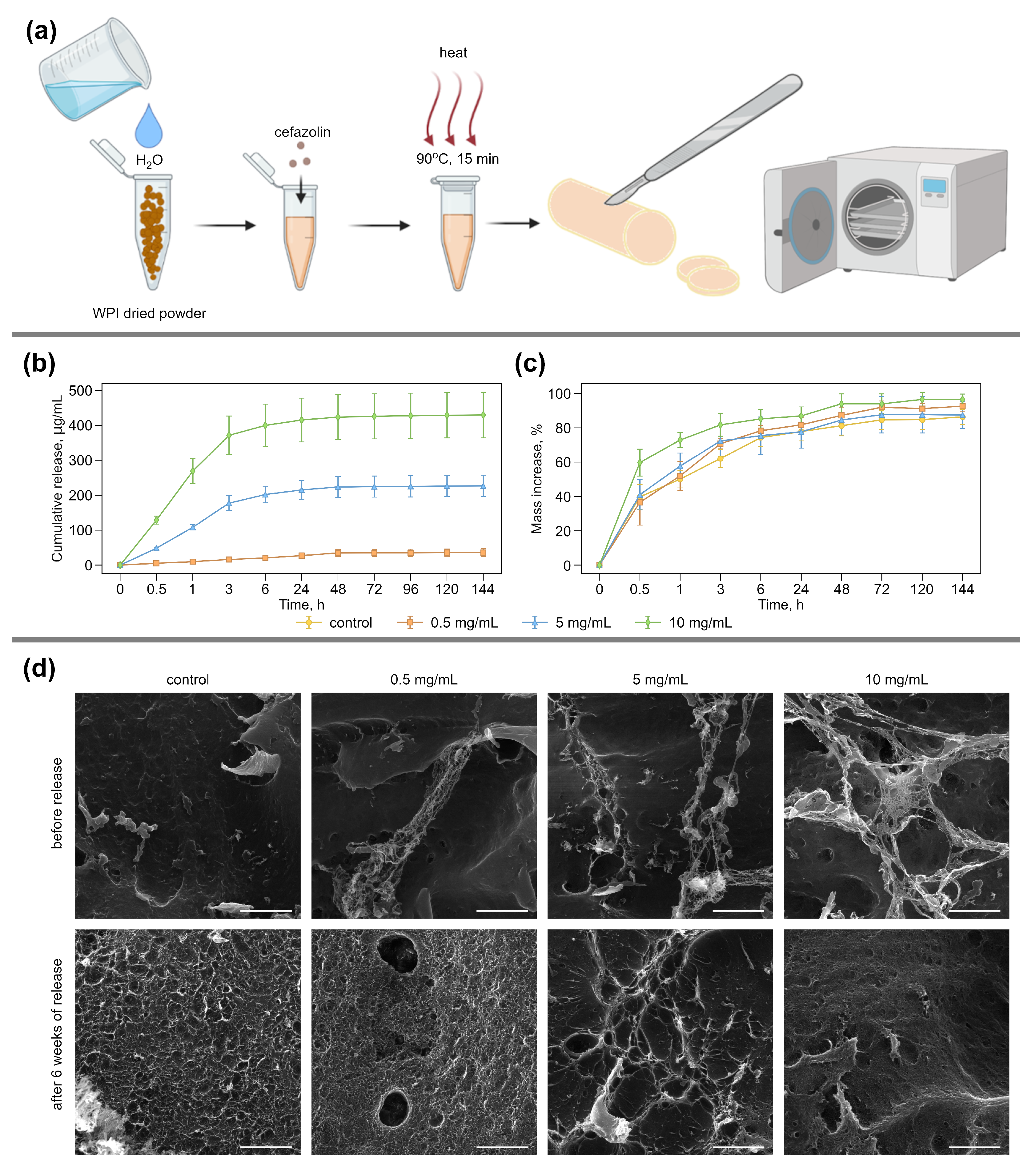
2.2. Fabrication of WPI-Based Hydrogels
2.3. Water Swelling and Cefazolin Release of WPI-Based Hydrogel Samples
2.4. Scanning Electron Microscopy
2.5. In Vitro Study
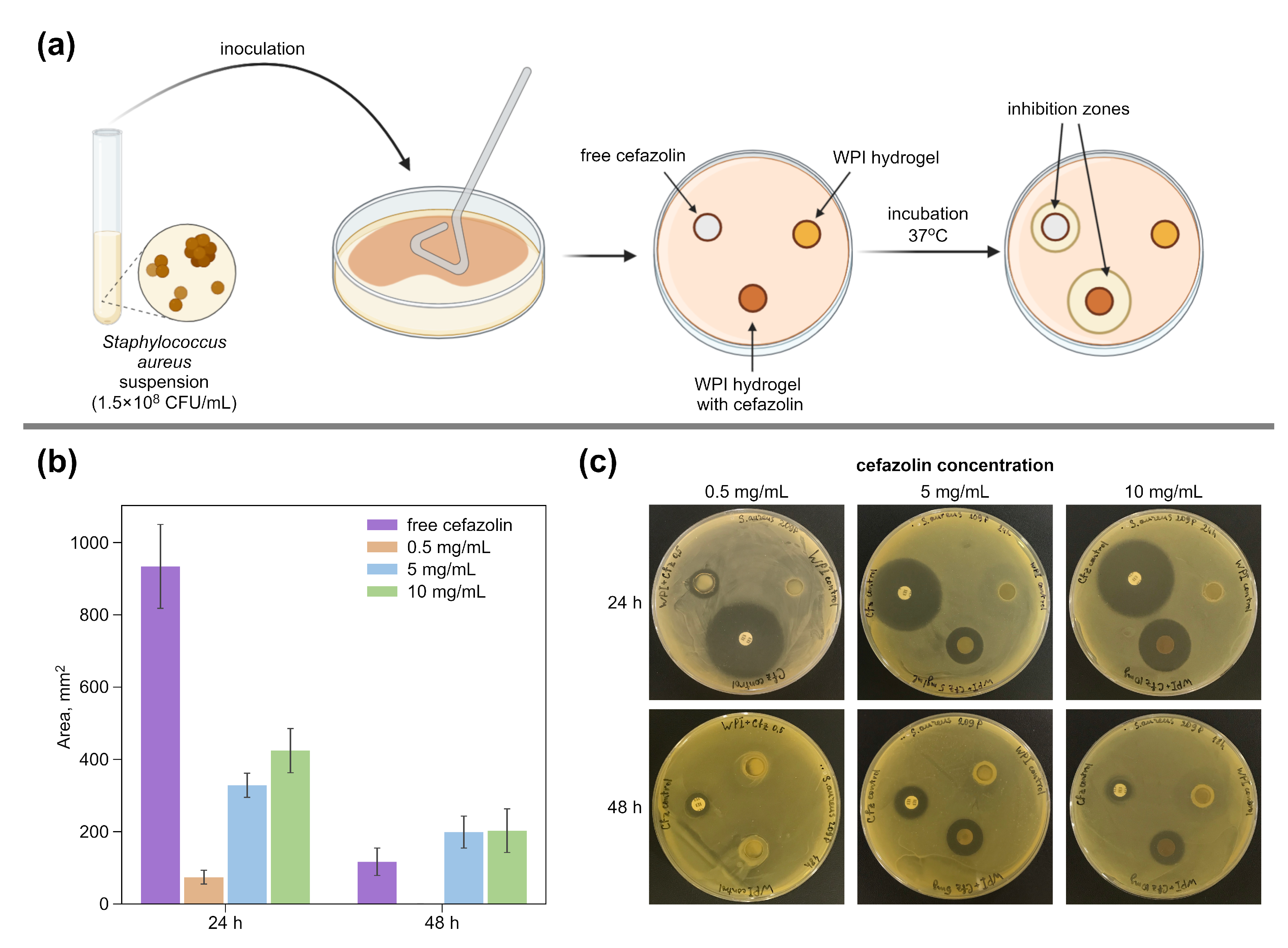
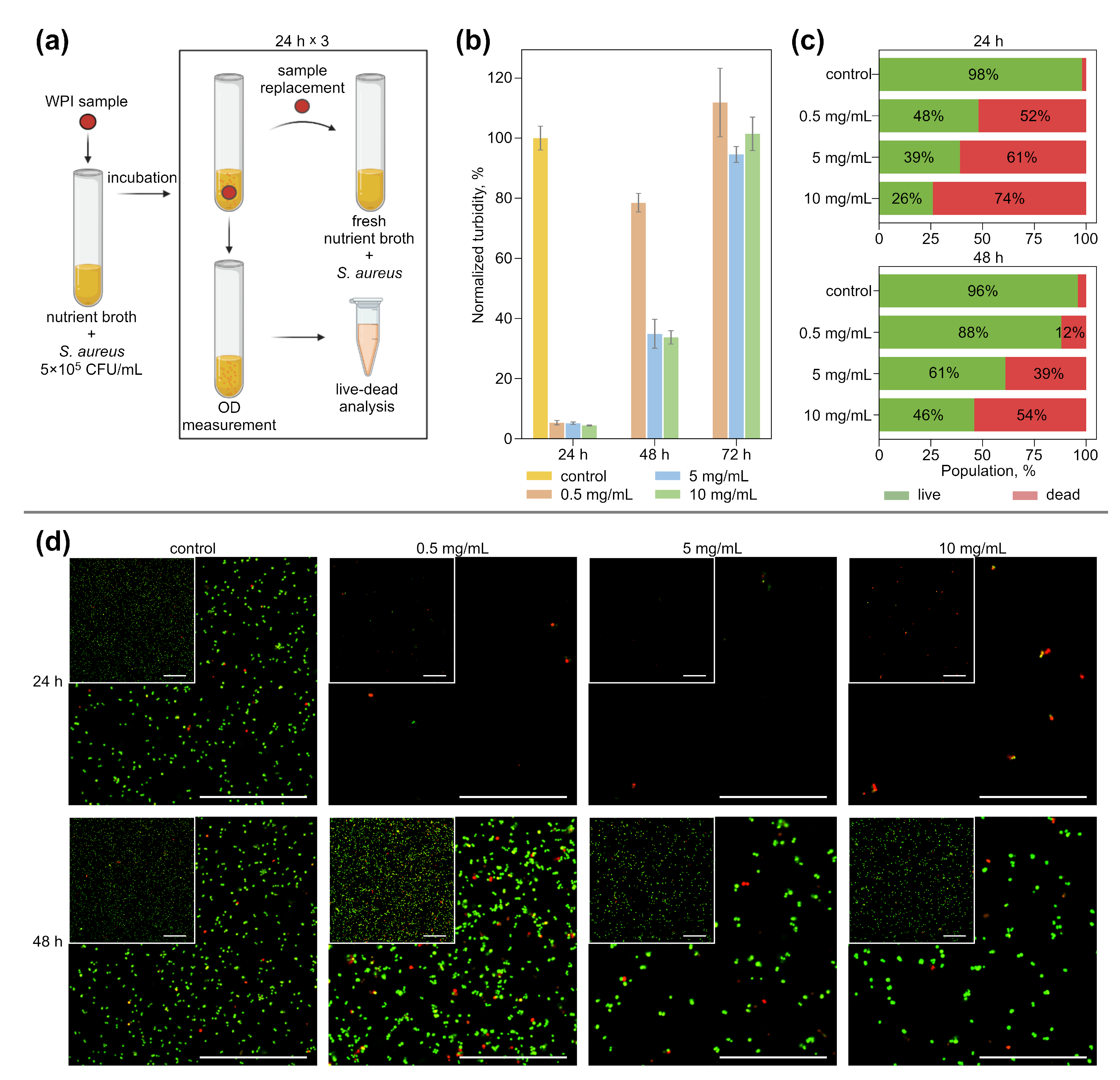
2.5.1. Bacterial Tests
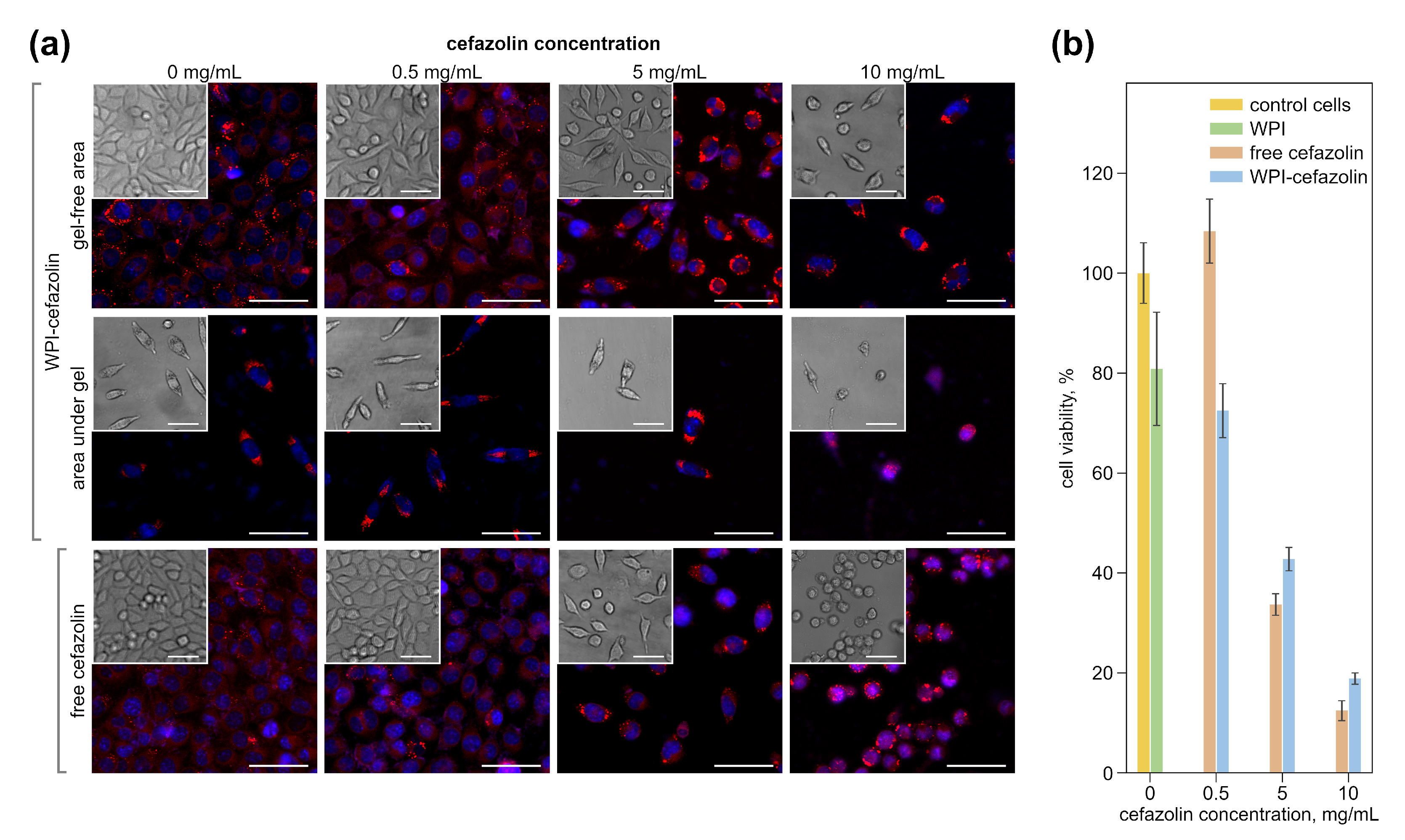
2.5.2. Cell Culturing
2.6. Confocal Laser Scanning Microscopy (CLSM)
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Antibacterial WPI Hydrogels
3.2. Antibacterial Effect
3.3. Impact of Hydrogels with Cefazolin on Eukaryotic Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, B.H.; McElroy, M.J.; Issa, K.; Johnson, A.J.; Bozic, K.J.; Mont, M.A. The Economic Impact of Periprosthetic Infections Following Total Knee Arthroplasty at a Specialized Tertiary-Care Center. J. Arthroplast. 2014, 29, 929–932. [Google Scholar] [CrossRef]
- de Lissovoy, G.; Fraeman, K.; Hutchins, V.; Murphy, D.; Song, D.; Vaughn, B.B. Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am. J. Infect. Control 2009, 37, 387–397. [Google Scholar] [CrossRef]
- Patel, H.; Khoury, H.; Girgenti, D.; Welner, S.; Yu, H. Burden of Surgical Site Infections Associated with Select Spine Operations and Involvement of Staphylococcus aureus. Surg. Infect. 2017, 18, 461–473. [Google Scholar] [CrossRef]
- Bonnevialle, P.; Bonnomet, F.; Philippe, R.; Loubignac, F.; Rubens-Duval, B.; Talbi, A.; Le Gall, C.; Adam, P. Early surgical site infection in adult appendicular skeleton trauma surgery: A multicenter prospective series. Orthop. Traumatol. Surg. Res. 2012, 98, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.G.; Heil, E.L.; Hayes, B.D. Appropriate Antibiotic Therapy. Emerg. Med. Clin. N. Am. 2017, 35, 25–42. [Google Scholar] [CrossRef]
- Rao, N.; Ziran, B.H.; Lipsky, B.A. Treating Osteomyelitis: Antibiotics and Surgery. Plast. Reconstr. Surg. 2011, 127, 177S–187S. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.; Bitterman, R.; Shofty, B.; Paul, M.; Neuberger, A. Management of post-neurosurgical meningitis: Narrative review. Clin. Microbiol. Infect. 2017, 23, 621–628. [Google Scholar] [CrossRef]
- Chen, A.F.; Parvizi, J. Antibiotic-Loaded Bone Cement and Periprosthetic Joint Infection. J. Long-Term Eff. Med. Implant. 2014, 24, 89–97. [Google Scholar] [CrossRef]
- Simon, D.; Kumar, K.; Sivadasan, S.; Varma, H.; Balan, A. Hydroxyapatite carriers as drug eluting agents—An In Vitro analysis. Indian J. Dent. Res. 2020, 31, 481. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, T.A.G.; Arts, J.J.; Geurts, J.A.P. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Mordovina, E.A.; Plastun, V.O.; Abdurashitov, A.S.; Proshin, P.I.; Raikova, S.V.; Bratashov, D.N.; Inozemtseva, O.A.; Goryacheva, I.Y.; Sukhorukov, G.B.; Sindeeva, O.A. “Smart” Polylactic Acid Films with Ceftriaxone Loaded Microchamber Arrays for Personalized Antibiotic Therapy. Pharmaceutics 2021, 14, 42. [Google Scholar] [CrossRef]
- Hettwer, W.H.; Horstmann, P.F.; Hovgaard, T.B.; Grum-Scwensen, T.A.; Petersen, M.M. Low Infection Rate after Tumor Hip Arthroplasty for Metastatic Bone Disease in a Cohort Treated with Extended Antibiotic Prophylaxis. Adv. Orthop. 2015, 2015, 428986. [Google Scholar] [CrossRef][Green Version]
- Azab, M.; Allen, M.; Daniels, J. Evaluation of a silver-impregnated coating to inhibit colonization of orthopaedic implants by biofilm forming methicillin-resistant Staphylococcus pseudintermedius. Vet. Comp. Orthop. Traumatol. 2016, 29, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.C.; Foxall-Smith, M.; Roberton, A.; Beswick, A.; Kieser, D.C.; Whitehouse, M.R. The use of silver coating in hip megaprostheses: A systematic review. HIP Int. 2019, 29, 7–20. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Ostojić, S.; Pavlović, M.; Živić, M.; Filipović, Z.; Gorjanović, S.; Hranisavljević, S.; Dojčinović, M. Processing of whey from dairy industry waste. Environ. Chem. Lett. 2005, 3, 29–32. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Keppler, J.K.; Vandrovcová, M.; Plencner, M.; Beranová, J.; Feuereisen, M.; Parakhonskiy, B.V.; Svenskaya, Y.; Atkin, V.; Ivanova, A.; et al. Enhancement of Biomimetic Enzymatic Mineralization of Gellan Gum Polysaccharide Hydrogels by Plant-Derived Gallotannins. Int. J. Mol. Sci. 2020, 21, 2315. [Google Scholar] [CrossRef]
- Pitarresi, G.; Palumbo, F.S.; Calascibetta, F.; Fiorica, C.; Di Stefano, M.; Giammona, G. Medicated hydrogels of hyaluronic acid derivatives for use in orthopedic field. Int. J. Pharm. 2013, 449, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.; McLaren, A.; Calara, F.; Vernon, B.; McLemore, R. Local Gentamicin Delivery From Resorbable Viscous Hydrogels Is Therapeutically Effective. Clin. Orthop. Relat. Res. 2015, 473, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Boot, W.; Dimas, K.; Malizos, K.; Hänsch, G.M.; Stuyck, J.; Gawlitta, D.; Romanò, C.L. Does Implant Coating With Antibacterial-Loaded Hydrogel Reduce Bacterial Colonization and Biofilm Formation in Vitro? Clin. Orthop. Relat. Res. 2014, 472, 3311–3323. [Google Scholar] [CrossRef] [PubMed]
- Gaetano, G.; Giuseppe, P.; Salvatore, P.F.; Susanna, M.; Sara, S.; Luca, R.C. Hyaluronic-Based Antibacterial Hydrogel Coating for Implantable Biomaterials in Orthopedics and Trauma: From Basic Research to Clinical Applications. In Hydrogels; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Romanò, C.L.; Malizos, K.; Capuano, N.; Mezzoprete, R.; D’Arienzo, M.; Der, C.V.; Scarponi, S.; Drago, L. Does an Antibiotic-Loaded Hydrogel Coating Reduce Early Post-Surgical Infection After Joint Arthroplasty? J. Bone Jt. Infect. 2016, 1, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Aicale, R.; Oliva, F.; Maffulli, N.; Maffulli, N. Defensive Antibacterial Coating (DAC®) for prevention of infection in ACL reconstruction: A feasibility study. Muscle Ligaments Tendons J. 2020, 10, 151. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Physicochemical and Emulsifying Properties of Whey Protein Isolate (WPI)–Dextran Conjugates Produced in Aqueous Solution. J. Agric. Food Chem. 2010, 58, 2988–2994. [Google Scholar] [CrossRef] [PubMed]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Llamas-Unzueta, R.; Suárez, M.; Fernández, A.; Díaz, R.; Montes-Morán, M.A.; Menéndez, J.A. Whey-Derived Porous Carbon Scaffolds for Bone Tissue Engineering. Biomedicines 2021, 9, 1091. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ko, S.; Xiao, L. Use of whey proteins for encapsulation and controlled delivery applications. J. Food Eng. 2007, 83, 31–40. [Google Scholar] [CrossRef]
- Douglas, T.E.; Vandrovcová, M.; Kročilová, N.; Keppler, J.K.; Zárubová, J.; Skirtach, A.G.; Bačáková, L. Application of whey protein isolate in bone regeneration: Effects on growth and osteogenic differentiation of bone-forming cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Dziadek, M.; Douglas, T.E.; Dziadek, K.; Zagrajczuk, B.; Serafim, A.; Stancu, I.C.; Cholewa-Kowalska, K. Novel whey protein isolate-based highly porous scaffolds modified with therapeutic ion-releasing bioactive glasses. Mater. Lett. 2020, 261, 127115. [Google Scholar] [CrossRef]
- Serfert, Y.; Lamprecht, C.; Tan, C.P.; Keppler, J.; Appel, E.; Rossier-Miranda, F.; Schroen, K.; Boom, R.; Gorb, S.; Selhuber-Unkel, C.; et al. Characterisation and use of β-lactoglobulin fibrils for microencapsulation of lipophilic ingredients and oxidative stability thereof. J. Food Eng. 2014, 143, 53–61. [Google Scholar] [CrossRef]
- Keppler, J.K.; Martin, D.; Garamus, V.M.; Berton-Carabin, C.; Nipoti, E.; Coenye, T.; Schwarz, K. Functionality of whey proteins covalently modified by allyl isothiocyanate. Part 1 physicochemical and antibacterial properties of native and modified whey proteins at pH 2 to 7. Food Hydrocoll. 2017, 65, 130–143. [Google Scholar] [CrossRef]
- Rabe, R.; Hempel, U.; Martocq, L.; Keppler, J.K.; Aveyard, J.; Douglas, T.E.L. Dairy-Inspired Coatings for Bone Implants from Whey Protein Isolate-Derived Self-Assembled Fibrils. Int. J. Mol. Sci. 2020, 21, 5544. [Google Scholar] [CrossRef] [PubMed]
- Ozel, B.; Cikrikci, S.; Aydin, O.; Oztop, M.H. Polysaccharide blended whey protein isolate-(WPI) hydrogels: A physicochemical and controlled release study. Food Hydrocoll. 2017, 71, 35–46. [Google Scholar] [CrossRef]
- Abdel-Salam, B.K.A.H. Modulatory Effect of Whey Proteins in Some Cytokines Involved in Wound Healing in Male Diabetic Albino Rats. Inflammation 2014, 37, 1616–1622. [Google Scholar] [CrossRef]
- Wijayanti, H.B.; Bansal, N.; Deeth, H.C. Stability of Whey Proteins during Thermal Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1235–1251. [Google Scholar] [CrossRef]
- Carson, M.; Keppler, J.K.; Brackman, G.; Dawood, D.; Vandrovcova, M.; Fawzy El-Sayed, K.; Coenye, T.; Schwarz, K.; Clarke, S.A.; Skirtach, A.G.; et al. Whey Protein Complexes with Green Tea Polyphenols: Antimicrobial, Osteoblast-Stimulatory, and Antioxidant Activities. Cells Tissues Organs 2018, 206, 106–118. [Google Scholar] [CrossRef]
- Gomide, R.A.C.; Oliveira, A.C.S.; Luvizaro, L.B.; Yoshida, M.I.; Oliveira, C.R.; Borges, S.V. Biopolymeric films based on whey protein isolate/lignin microparticles for waste recovery. J. Food Process Eng. 2021, 44, e13596. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Dias, M.V.; Borges, S.V.; Costa, A.L.R.; Silva, E.K.; Medeiros, É.A.A.; Soares, N.d.F.F. Development of whey protein isolate bio-nanocomposites: Effect of montmorillonite and citric acid on structural, thermal, morphological and mechanical properties. Food Hydrocoll. 2015, 48, 179–188. [Google Scholar] [CrossRef]
- Dziadek, M.; Charuza, K.; Kudlackova, R.; Aveyard, J.; D’Sa, R.; Serafim, A.; Stancu, I.C.; Iovu, H.; Kerns, J.G.; Allinson, S.; et al. Modification of heat-induced whey protein isolate hydrogel with highly bioactive glass particles results in promising biomaterial for bone tissue engineering. Mater. Des. 2021, 205, 109749. [Google Scholar] [CrossRef]
- Killion, J.A.; Kehoe, S.; Geever, L.M.; Devine, D.M.; Sheehan, E.; Boyd, D.; Higginbotham, C.L. Hydrogel/bioactive glass composites for bone regeneration applications: Synthesis and characterisation. Mater. Sci. Eng. C 2013, 33, 4203–4212. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Liu, F.; Ren, F.; Zhao, G.; Leng, X. Fabrication and characterization of TiO2/whey protein isolate nanocomposite film. Food Hydrocoll. 2011, 25, 1098–1104. [Google Scholar] [CrossRef]
- Giano, M.C.; Ibrahim, Z.; Medina, S.H.; Sarhane, K.A.; Christensen, J.M.; Yamada, Y.; Brandacher, G.; Schneider, J.P. Injectable bioadhesive hydrogels with innate antibacterial properties. Nat. Commun. 2014, 5, 4095. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Fan, D. Conductive, adaptive, multifunctional hydrogel combined with electrical stimulation for deep wound repair. Chem. Eng. J. 2021, 421, 129578. [Google Scholar] [CrossRef]
- Overstreet, D.J.; Badha, V.S.; Heffernan, J.M.; Childers, E.P.; Moore, R.C.; Vernon, B.L.; McLaren, A.C. Temperature-responsive PNDJ hydrogels provide high and sustained antimicrobial concentrations in surgical sites. Drug Deliv. Transl. Res. 2019, 9, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Gilbert, V.; Wang, H.; Subirade, M. In vivo evaluation of whey protein-based biofilms as scaffolds for cutaneous cell cultures and biomedical applications. Biomed. Mater. 2007, 2, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Waitz, J.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; National Committee for Clinical Laboratory Standards: Tokyo, Japan, 1990. [Google Scholar]
- LIVE/DEAD BacLight Bacterial Viability Kit Protocol. Available online: https://www.thermofisher.com/ru/ru/home/references/protocols/cell-and-tissue-analysis/protocols/live-dead-baclight-bacterial-viability-protocol.html (accessed on 25 April 2022).
- Back, S.A.; Khan, R.; Gan, X.; Rosenberg, P.A.; Volpe, J.J. A new Alamar Blue viability assay to rapidly quantify oligodendrocyte death. J. Neurosci. Methods 1999, 91, 47–54. [Google Scholar] [CrossRef]
- Mayorova, O.A.; Jolly, B.C.N.; Verkhovskii, R.A.; Plastun, V.O.; Sindeeva, O.A.; Douglas, T.E.L. pH-Sensitive Dairy-Derived Hydrogels with a Prolonged Drug Release Profile for Cancer Treatment. Materials 2021, 14, 749. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Kudlackova, R.; Zima, A.; Slosarczyk, A.; Ziabka, M.; Jelen, P.; Shkarina, S.; Cecilia, A.; Zuber, M.; Baumbach, T.; et al. Novel multicomponent organic–inorganic WPI/gelatin/CaP hydrogel composites for bone tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 2479–2491. [Google Scholar] [CrossRef]
- Platania, V.; Douglas, T.E.; Zubko, M.K.; Ward, D.; Pietryga, K.; Chatzinikolaidou, M. Phloroglucinol-enhanced whey protein isolate hydrogels with antimicrobial activity for tissue engineering. Mater. Sci. Eng. C 2021, 129, 112412. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, A.S.; George, P.A.; Cooper-White, J.J. Directing osteogenic and myogenic differentiation of MSCs: Interplay of stiffness and adhesive ligand presentation. Am. J. Physiol.-Cell Physiol. 2008, 295, C1037–C1044. [Google Scholar] [CrossRef]
- Hajebi, S.; Mohammadi Nasr, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Bioresorbable composite polymeric materials for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 926–940. [Google Scholar] [CrossRef]
- Radisavljevic, A.; Stojanovic, D.B.; Perisic, S.; Djokic, V.; Radojevic, V.; Rajilic-Stojanovic, M.; Uskokovic, P.S. Cefazolin-loaded polycaprolactone fibers produced via different electrospinning methods: Characterization, drug release and antibacterial effect. Eur. J. Pharm. Sci. 2018, 124, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Traub, W.H.; Leonhard, B. Heat stability of the antimicrobial activity of sixty-two antibacterial agents. J. Antimicrob. Chemother. 1995, 35, 149–154. [Google Scholar] [CrossRef]
- Thamthaweechok, N.; Tiengrim, S.; Thamlikitkul, V. Heat Stability of Antibiotics Commonly Used in Food Animals and Agriculture in Thailand. J. Med. Assoc. Thail. 2018, 101, 863–867. [Google Scholar]
- Timaeva, O.; Pashkin, I.; Mulakov, S.; Kuzmicheva, G.; Konarev, P.; Terekhova, R.; Sadovskaya, N.; Czakkel, O.; Prevost, S. Synthesis and physico-chemical properties of poly(N-vinyl pyrrolidone)-based hydrogels with titania nanoparticles. J. Mater. Sci. 2020, 55, 3005–3021. [Google Scholar] [CrossRef]
- Anastasova, E.I.; Ivanovski, V.; Fakhardo, A.F.; Lepeshkin, A.I.; Omar, S.; Drozdov, A.S.; Vinogradov, V.V. A pure magnetite hydrogel: Synthesis, properties and possible applications. Soft Matter 2017, 13, 8651–8660. [Google Scholar] [CrossRef] [PubMed]
- Barros, W. Solvent self-diffusion dependence on the swelling degree of a hydrogel. Phys. Rev. E 2019, 99, 052501. [Google Scholar] [CrossRef]
- Hellebois, T.; Gaiani, C.; Cambier, S.; Noo, A.; Soukoulis, C. Exploration of the co-structuring and stabilising role of flaxseed gum in whey protein isolate based cryo-hydrogels. Carbohydr. Polym. 2022, 289, 119424. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Emam-Djomeh, Z. Characterization of hydrogels formed by non-toxic chemical cross-linking of mixed nanofibrillated/heat-denatured whey proteins. J. Iran. Chem. Soc. 2019, 16, 2731–2741. [Google Scholar] [CrossRef]
- de Oliveira, A.C.S.; Ugucioni, J.C.; da Rocha, R.A.; Borges, S.V. Development of whey protein isolate/polyaniline smart packaging: Morphological, structural, thermal, and electrical properties. J. Appl. Polym. Sci. 2019, 136, 47316. [Google Scholar] [CrossRef]
- Gupta, D.; Kocot, M.; Tryba, A.M.; Serafim, A.; Stancu, I.C.; Jaegermann, Z.; Pamuła, E.; Reilly, G.C.; Douglas, T.E. Novel naturally derived whey protein isolate and aragonite biocomposite hydrogels have potential for bone regeneration. Mater. Des. 2020, 188, 108408. [Google Scholar] [CrossRef]
- Jo, A.; Ding, T.; Ahn, J. Synergistic antimicrobial activity of bacteriophages and antibiotics against Staphylococcus aureus. Food Sci. Biotechnol. 2016, 25, 935–940. [Google Scholar] [CrossRef]
- Li, J.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Evaluation of Ultrasound-Induced Damage to Escherichia coli and Staphylococcus aureus by Flow Cytometry and Transmission Electron Microscopy. Appl. Environ. Microbiol. 2016, 82, 1828–1837. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Zhang, X.; Wu, H.; Zou, Y.; Li, P.; Sun, C.; Xu, W.; Liu, F.; Wang, D. Class III bacteriocin Helveticin-M causes sublethal damage on target cells through impairment of cell wall and membrane. J. Ind. Microbiol. Biotechnol. 2018, 45, 213–227. [Google Scholar] [CrossRef]
- Duewelhenke, N.; Krut, O.; Eysel, P. Influence on Mitochondria and Cytotoxicity of Different Antibiotics Administered in High Concentrations on Primary Human Osteoblasts and Cell Lines. Antimicrob. Agents Chemother. 2007, 51, 54–63. [Google Scholar] [CrossRef]
- Savcı, A.; Koçpınar, E.F.; Budak, H.; Çiftci, M.; Şişecioğlu, M. The Effects of Amoxicillin, Cefazolin, and Gentamicin Antibiotics on the Antioxidant System in Mouse Heart Tissues. Protein Pept. Lett. 2020, 27, 614–622. [Google Scholar] [CrossRef]
- Park, E.S.; Maniar, M.; Shah, J.C. Biodegradable polyanhydride devices of cefazolin sodium, bupivacaine, and taxol for local drug delivery: Preparation, and kinetics and mechanism of in vitro release. J. Control. Release 1998, 52, 179–189. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Q. Investigation of the cytotoxicity of food-grade nanoemulsions in Caco-2 cell monolayers and HepG2 cells. Food Chem. 2013, 141, 29–33. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.; Surampalli, R. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair Regen. 2017, 25, 260–269. [Google Scholar] [CrossRef]
- Akatov, V.; Lezhnev, E.; Vexler, A.; Kublik, L. Low pH value of pericellular medium as a factor limiting cell proliferation in dense cultures. Exp. Cell Res. 1985, 160, 412–418. [Google Scholar] [CrossRef]
- Fellenz, M.P.; Gerweck, L.E. Influence of Extracellular pH on Intracellular pH and Cell Energy Status: Relationship to Hyperthermic Sensitivity. Radiat. Res. 1988, 116, 305. [Google Scholar] [CrossRef]
- Flinck, M.; Kramer, S.H.; Pedersen, S.F. Roles of pH in control of cell proliferation. Acta Physiol. 2018, 223, e13068. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plastun, V.O.; Prikhozhdenko, E.S.; Gusliakova, O.I.; Raikova, S.V.; Douglas, T.E.L.; Sindeeva, O.A.; Mayorova, O.A. WPI Hydrogels with a Prolonged Drug-Release Profile for Antimicrobial Therapy. Pharmaceutics 2022, 14, 1199. https://doi.org/10.3390/pharmaceutics14061199
Plastun VO, Prikhozhdenko ES, Gusliakova OI, Raikova SV, Douglas TEL, Sindeeva OA, Mayorova OA. WPI Hydrogels with a Prolonged Drug-Release Profile for Antimicrobial Therapy. Pharmaceutics. 2022; 14(6):1199. https://doi.org/10.3390/pharmaceutics14061199
Chicago/Turabian StylePlastun, Valentina O., Ekaterina S. Prikhozhdenko, Olga I. Gusliakova, Svetlana V. Raikova, Timothy E. L. Douglas, Olga A. Sindeeva, and Oksana A. Mayorova. 2022. "WPI Hydrogels with a Prolonged Drug-Release Profile for Antimicrobial Therapy" Pharmaceutics 14, no. 6: 1199. https://doi.org/10.3390/pharmaceutics14061199
APA StylePlastun, V. O., Prikhozhdenko, E. S., Gusliakova, O. I., Raikova, S. V., Douglas, T. E. L., Sindeeva, O. A., & Mayorova, O. A. (2022). WPI Hydrogels with a Prolonged Drug-Release Profile for Antimicrobial Therapy. Pharmaceutics, 14(6), 1199. https://doi.org/10.3390/pharmaceutics14061199







