Abstract
The current studies entail quality by design (QbD)-enabled development of a simple, rapid, precise, accurate, and cost-effective high-performance liquid chromatographic method for estimation of metformin hydrochloride (M-HCl). Design of experiments (DoE) was applied for multivariate optimization of the experimental conditions of the HPLC method. Risk assessment was performed to identify the critical method parameters (CMPs) using Ishikawa diagram. The factor screening studies were performed using a two-factor three-levels design. Two independent factors, buffer pH and mobile phase composition, were used to design mathematical models. Central composite design (CCD) was used to study the response surface methodology and to study in depth the effects of these independent factors, thus evaluating the critical analytical attributes (CAAs), namely, retention time, peak area, and symmetry factor as the parameters of method robustness. Desirability function was used to simultaneously optimize the CAAs. The optimized and predicted data from contour diagram consisted of 0.02 M acetate buffer pH = 3/methanol in a ratio of 70/30 (v/v) as the mobile phase with a flow rate 1 mL/min. The separation was made on a Thermoscientific ODS HypersylTM chromatographic column (250 × 4.6 mm, 5 μm) with oven temperature 35 °C and UV detection at 235 nm. The optimized assay conditions were validated according to ICH guidelines. Hence, the results clearly showed that QbD approach could be successfully applied to optimize HPLC method for estimation of M-HCl. The method was applied both for the evaluation of M-HCl content in tablets, and for in vitro dissolution studies of M-HCl from conventional and prolonged-release tablets.
1. Introduction
Metformin (1,1-dimethylbiguanide or N,N-dimethylimidodicarbonimidic diamide, C4H12ClN5) has a molecular weight of 129.16 and a melting point at 223–226 °C. Metformin hydrochloride (M-HCl, Figure 1) is the hydrochloride salt of the biguanide metformin, used to treat high blood sugar levels that are caused by a type of diabetes mellitus.
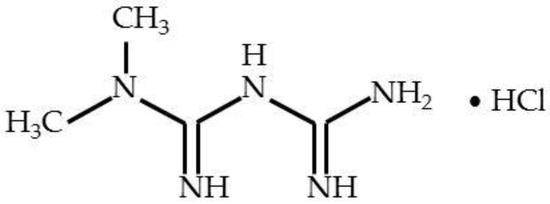
Figure 1.
Chemical structure of metformin hydrochloride (M-HCl).
In the literature, research studies have been identified in which metformin has been linked to a possible reduction in the risk of cancer [1,2], but also to the fact that it also reduces mortality caused by oncological diseases, patients being protected from various types of cancer, such as colon, gastric, breast, endometrial, and glioma [3,4,5,6,7].
Pharmacologic therapy for Type 2 diabetes involves use of metformin as first-line therapy. Metformin hydrochloride acts through three mechanisms; primarily, it reduces hepatic glucose synthesis (inhibits gluconeogenesis and glycogenolysis), at the muscle level, is responsible for increasing insulin sensitivity by intake and using it, but it also delays intestinal glucose absorption [8].
Metformin used alone has been shown to reduce glycosylated hemoglobin [9] by approximately 1.5% and is currently available as 500 mg, 850 mg, and 1000 mg immediate release (IR); extended-release tablets (XR) of 500 mg, 750 mg, and 1000 mg; 500 mg/5 mL liquid formulation, or 500 mg powder sachets [10]. New studies on new pharmaceutical formulations of metformin show major benefits in lipid metabolism and improved glycemic control in patients who have used the prolonged-release form. Reducing the frequency of one tablet per day in the case of metformin XR results in better gastrointestinal tolerability through gradual and controlled release of the active substance, increased compliance with therapy by reducing the number of doses administered per day, but also reducing the number and intensity side effects reported.
In the literature there are presented different methods for metformin hydrochloride analysis in various pharmaceutical formulations, synthetic mixtures, water samples, human plasma, biota, and others, by microgravimetry, electrochemical methods (such as conductometric, voltammetric, and potentiometric), spectrofluorimetry, UV–Vis spectrophotometry, capillary electrophoresis, gas chromatography, and HPLC. The HPLC methods are used for the qualitative identification and quantitative determination of M-HCl alone or in combination with other antidiabetic class, such as sulfonylureas derivatives (i.e., gliclazide, glipizide, glimepiride glibenclamide and tolbutamide); thiazolidinediones (glitazones) (i.e., pioglitazone); alpha-glucosidase inhibitors (i.e., miglitol, acarbose, and voglibose); methylglinides (i.e., nateglinide and repaglinide); dipeptidyl peptidase-4 (DPP-4) inhibitors (i.e., sitagliptin, saxagliptin, vildagliptin, alogliptin, and linagliptin); sodium-glucose co-transporter 2 (SGLT-2) inhibitors (i.e., dapagliflozin, canagliflozin, empagliflozin, ertugliflozin pidolate, and remogliflozin) or with other therapeutic classes of molecules (hypocholesterolemic) [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34].
The quality by design (QbD) approach suggests looking into the quality of the analytical process during the development stage itself. It says that quality should be built into the process design rather than testing final results of analytical process. QbD is defined as a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding based on sound science and quality risk management [35,36]. In alignment with the approach proposed in the draft FDA guidance for process validation [37], a three-stage approach can be applied to method validation: Stage 1. Method Design: define method requirements and conditions and identify critical controls; Stage 2. Method Qualification: confirm that the method is capable of meeting its design intent; Stage 3. Continued Method Verification: gain ongoing assurance to ensure that the method remains in a state of control during routine use.
The QbD approach is more appropriate for application during method development than during method validation, because method validation is a process which demonstrates that the analytical method is appropriate for its intended use. In the development of analytical methods it is frequent practice to implement the principles of QbD. These principles facilitate the scientific and risk-based understanding of major sources of variation. In this way, a high robustness and an improved performance of the analytical methods are obtained.
Regulatory agencies do not define any specific process of analytical QbD; however, a parallel approach can be drawn based on product QbD. Equivalent to process QbD, the outcome of analytical QbD (AQbD) is well understood and fit for intended purpose with robustness throughout the lifecycle. Quality target product profile (QTTP), critical quality attributes (CQA), and design of experiments (DOE) can be interpreted as analytical target profile (ATP), critical method attributes (CMA), such as retention time, peak area, symmetry factor, tailing factor, the resolution between adjacent peaks, plate count, etc., and method operable design region (MODR), respectively [38,39].
Fundamentally, the QbD analytical approach requires the identification of the ATP before considering analytical technology. The next step is to establish the CMAs. An MODR is required for analytical methods during the development phase for a better improvement as well as for a quantitative understanding of the factors that influence the performance of the method. To find high-risk variables that have a critical impact on analytical performance, the aim is to identify critical method parameters (CMPs) such as material attributes, instrument-related aspects, instrument operating parameters, and method parameters, based on risk assessment and factor screening studies, followed by their optimization using appropriate experimental projects to increase method performance. The optimized chromatographic conditions are thus evaluated in terms of the CMAs based on the knowledge obtained in the development stage and the screening studies [35,38,40,41,42].
An important aspect for the development of the HPLC method using the QbD approach using preliminary risk assessment experiments is the choice of CMPs and responses. In addition to the preliminary experiments, the Ishikawa (fish bone) diagram was used to identify and evaluate the CMPs that pose a risk to the performance of the method.
Each analytical technique has different CMPs. In the case of HPLC methods, the CMPs with high risk assessment are those regarding the material, instrumental parameters, mobile phase (buffer type, concentration, and pH, organic modifier, elution method), column characteristics and preparation, and the analyst. In general, for risk identification and assessment, Ishikawa fishbone diagram can be used (Figure 2) [42,43,44,45,46].
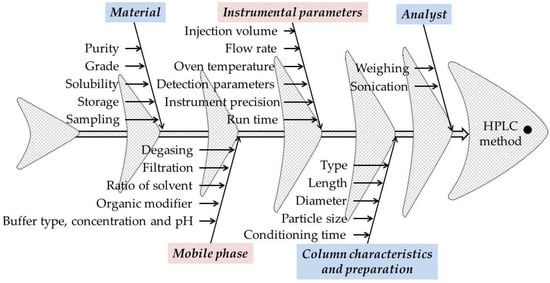
Figure 2.
The Ishikawa (fishbone) diagram to identify potential variables in HPLC method development.
In short, the necessary steps in developing an analytical method with the implementation of QbD principles are as follows: Project initiation → Literature search and initial risk assessment → Identification of ATP, CMAs, risk assessment → Method optimization and method development with DOE → MODR, Control Strategy and Risk Assessment → AQbD method validation → Continuous method monitoring.
With all these in mind, the aim of this work was to go through the necessary steps, namely, the development of an HPLC method for the quantitative determination of M-HCl and its optimization in accordance with QbD principles. The next steps were to validate the method according to ICH Q2 (R1) Guidelines [47] and to apply the optimized and validated HPLC method for the quantitative determination of M-HCl from tablets and for dissolution studies.
2. Materials and Methods
2.1. Chemicals and Reagents
Metformin hydrochloride (97%) was purchased from Sigma Aldrich Chemie Gmbh (Steinheim, Germany), sodium acetate (CH3COONa, ≥99.0%) was obtained from Silal Trading SRL (Bucharest, Romania), sodium phosphate dibasic (Na2HPO4, ≥99.0%) was obtained from Sigma-Aldrich Chemie GmbH (Steinheim, Germany), potassium dihydrogen phosphate (KH2PO4, ≥99.5%) was obtained from Utchim SRL (Râmnicu Vâlcea, Romania), potassium chloride (KCl, ≥99.0%) was obtained from Chemical Company S.A. (Iasi, Romania), orthophosphoric acid (H3PO4, ≥85%) was obtained from Chemical Company S.A. (Iasi, Romania), glacial acetic acid (CH3COOH, 99.84%) was obtained from Chimreactiv SRL (Bucharest, Romania), hydrochloric acid (HCl, ≥37.0%) was obtained from Chemical Company S.A. (Iasi, Romania), sodium hydroxide (NaOH, 98.5%) was purchased from Chemical Company S.A. (Iasi, Romania), gradient grade methanol and acetonitrile (Hiposolv Chromanorm) were obtained from VWR International S.A.S. (Fontenay-sous-Bois, France). Ultrapure water (resistivity 18.2 MΩ·cm) was obtained from a local pharmaceutical company. Industrial M-HCl tablets with a content between 500 mg and 1000 mg were purchased from the local market (pharmacy).
2.2. Equipment
Agilent Technologies 1200 liquid chromatograph was equipped with quaternary pump (type G1311A), diode array detector (DAD type G1315B), degasser (type G1322A), oven column compartment (type G1316A), and Agilent ChemStation 32 software (Rev. B.03.02) (all from Agilent Technologies, Santa Clara, CA, USA). The used chromatographic column was a Thermoscientific ODS Hypersyl TM (250 × 4.6 mm, 5 μm), Lithuania. Other instruments used were pH-meter, inoLAB pH 7110 (Xylem Analytics Germany GmbH, Weilheim, Germany), water bath Biobase (model SY-1L4H, Biobase Biodustry, Shandong, Co., Ltd., Jinan, China), ultrasonic bath Biobase (model UC-40A, Biobase Biodustry, Shandong, Co., Ltd., Jinan, China), analytical balance PIONEER® Analytical OHAUS PX124M (Ohaus Corporation, Parsippany, NJ, USA), dissolution apparatus SR 8 Plus Dissolution Test Station (model 73-100-104, Hanson Research, Chatsworth, CA, USA), microliter™ Syringes, 20 μL Hamilton Bonaduz AG (CH-7402 Bonaduz, Switzerland), Transferpette® Dig. 100–1000 μL (article 704180, Brand GMBH + CO KG, Wertheim, Germany) and Rotilabo®-Mikroliterpipette 0.5–5.0 mL (article TA 26.1, Carl Roth GmbH, Karlsruhe, Germany).
2.3. Preparation of Standard Solutions
Standard solutions were obtained by dissolving 103.1 mg of M-HCl (reference substance, 97%) in 25 mL of ultrapure water (resistivity 18.2 MΩ·cm) and diluting with ultrapure water after complete dissolution to 50 mL to give a solution with a concentration of 2 mg/mL (2000 μg/mL). Any further dilutions for standard solutions were also performed with ultrapure water.
2.4. Selection and Preparation of Mobile Phase
Mobile phases containing methanol, acetonitrile, water, and buffers at different pH were tested in different proportions. Peaks with satisfactory CMAs (retention time, peak area, symmetry factor) were obtained at a flow rate of 1 mL/min with a mobile phase made up of 20 mM acetate buffer (pH 3)/methanol 70/30 (v/v). To prepare a volume of 1 L of mobile phase, a volume of 300 mL methanol is mixed with 700 mL 0.02 M acetate buffer (pH = 3). After the mixture reached room temperature, the mobile phase was filtered through 0.45 μm membrane filters by application of vacuum and sonicated for 15 min before introducing into the system.
2.5. Method Development and Optimization Using QbD Approach
2.5.1. Identification of ATP, CMAs, and Risk Assessment
Identification of ATP was made after literature search and initial risk assessment in which were followed studies that present different methods for determining M-HCl by HPLC, respectively, the implementation of QbD principles in the development of HPLC methods. The selected CMPs were related to a different composition of the mobile phase (i.e., buffer type, buffer pH, organic modifier type, and buffer to organic modifier ratio). For risk identification and assessment, Ishikawa fishbone diagram was used (Figure 2).
2.5.2. Method Development and Optimization
In order to obtain an adequate separation of M-HCl with acceptable values of system suitability parameters (retention time, peak area, and symmetry factor), the chromatograms obtained by HPLC were recorded for various compositions and mobile phase ratios using various buffer solutions (KH2PO4 and CH3COONa acetate buffer with different pH values) and organic modifier (methanol and acetonitrile).
To study the influence of the aqueous phase and of the organic modifier on the separation performance, we first use as a mobile phase a mixture of methanol with water, phosphate buffer, and acetate, respectively (with the same concentration of 0.02 M and pH = 3), and secondly, methanol was replaced with acetonitrile.
To study the influence of the buffer pH and the ratio between the buffer solution and the organic solvent in the composition of the mobile phase, we choose a central composite design (CCD) in which the star points are at the center of each face of the factorial space [46,48,49,50]. This variety requires three levels of each factor (–1, 0, +1). As independent variables (factors selected based on the preliminary analysis), the composition of the mobile phase and the pH of the buffer were chosen. In Table 1 is presented the design matrix with the selected factors at low (–1), medium (0), and high (+1) levels for a number of 11 experimental runs with triplicate tests for the central point (0, 0). The dependent variables were retention time, peak area, and symmetry factor for the proposed independent variables. For the dependent variables, the used constraints were minimum for retention time and maximum for peak area and symmetry factor.

Table 1.
Design matrix as per central composite design (CCD) for optimization of the HPLC method of metformin hydrochloride (M-HCl).
Experimental design (a two-factor, mobile phase composition and pH of buffer solution at three different levels), desirability function, and data analysis calculations were performed by using MATLAB and Statistics Toolbox Release 2020a (The MathWorks, Inc., Natick, MA, USA) software, the best-suited response for second-order polynomial exploring quadratic response surfaces [40,46]:
where A and B are independent variables coded for levels, Y is the measured response associated with each combination of factor level, β0 is the intercept, and β1 to β5 are regression coefficients derived from experimental runs of the observed experimental values of Y. Interaction and quadratic terms, respectively, are represented by the terms AB, A2, and B2. The surface response of dependent variables and the desirability plot were also plotted using MATLAB software.
Y = β0 + β1A + β2B + β3AB + β4A2 + β5B2
2.5.3. Risk Assessment and Control Strategy
The optimized method is checked by the CMAs to see if the method is efficient and operational throughout its life. Thus, for robustness and ruggedness studies, the parameters and performance of the method were evaluated in several deliberately obtained circumstances (different reagents, analysts, and days). The robustness of the method was determined by making some minor changes in the CMPs (source of methanol, mobile phase flow, and pH of the buffer solution) and the ruggedness was determined by changing the analyst and the days when analyses were performed. The relative standard deviation (RSD%) acceptance limits for retention time, peak area, and symmetry factor must be less than 2%.
Following the development of the method, a control strategy was implemented for the development of which the ATP was established, i.e., a planned set of controls of some parameters to ensure that both the quality of the results obtained and the method performance fall within the established ATP.
2.6. Method Validation
The HPLC method for M-HCl was validated in terms of linearity, limit of detection (LOD), limit of quantification (LOQ), precision, and accuracy according to ICH Q2 (R1) Guidelines [47].
The linearity was determined by preparing a calibration curve for 19 standard solutions with concentrations ranging from 10 to 2000 μg/mL. Each solution was analyzed in triplicate; from the obtained chromatograms, the peak areas were determined by integration and used to generate the calibration curve using the corresponding concentration of M-HCl. The equation of regression line was determined using the least squares method and mathematical estimates of the degree of linearity (correlation coefficient—r, coefficient of determination—r2, the slope and the intercept with ordinate of the regression line), using regression function in Excel.
The detection limit (LOD) and quantification limit (LOQ) were calculated using the equations Signal/Noise > 3 and Signal/Noise > 10 for LOD and LOQ respectively, where signal is the peak area for the signal and noise is the peak area for noise. These limits are calculated using the equation of the regression curve obtained in linearity study:
The precision of the developed HPLC method was evaluated in terms of repeatability (intra-day precision) and intermediate precision (inter-day precision). For determination of repeatability, we evaluated in triplicate, on the same day, three solutions of M-HCl at three concentrations (700, 1000, and 1300 μg/mL). Similarly, for the determination of intermediate precision, the above-prepared solutions were analyzed on three consecutive days. The repeatability and intermediate precision of the method were presented as RSD%.
The accuracy of the method has been determined by application of the analytical procedure to recovery studies using the standard addition method. In this, formerly evaluated sample solutions consisting of a known amount of M-HCl were spiked with three different concentration levels. Briefly, to 0.6 mL of 1000 μg/mL M-HCl solution, 0.4, 0.7, and 1.0 mL of 2000 μg/mL M-HCl and 1.0 mL, 0.7 mL, and 0.4 mL of water were added to obtain a final volume of 2 mL. In this way, solutions with concentrations of 700, 1000, and 1300 μg/mL M-HCl were obtained. The accuracy was expressed in terms of percent recovery for final concentrations. The accuracy of the method was also studied at lower concentrations, in this case being calculated in terms of percent relative error (RE%) using the following equation:
where CM and CR are the measured and real concentration, respectively.
2.7. Stability Studies for M-HCl Solutions
For the stability study over time of M-HCl solutions, we prepared solutions at concentrations of 1000 and 2000 µg/mL. These solutions were analyzed initially and then after 24 and 48 h, the solutions were stored in the refrigerator, at room temperature, and in a water bath at 37 °C. In all cases, recovery of M-HCl was calculated.
2.8. Determination of M-HCl Content in Tablets
Initially, the average weight of one tablet (Mm) was determined according to the provisions of the European Pharmacopoeia, 10th edition [51], for a number of 20 tablets, then the 20 tablets were crushed into powder form. Samples of formulated M-HCl (tablets) were prepared by dissolving a quantity of the powdered tablets equivalent to 500 mg of M-HCl in 500 mL ultrapure water to obtain a concentration of M-HCl of 1000 µg/mL. The obtained solution was filtered through 13 mm polytetrafluoroethylene (PTFE) filters, 0.45 µm, analyzed in the method conditions, and the percent content (C%) of M-HCl was calculated using the following equation:
where Mm is average weight of a tablet calculated for 20 tablets (in g), A is the declared content (in mg), a is the quantity of tablet powder (in g), PA is the peak area, and Int and S are the intercept and the slope of the regression line, respectively.
2.9. Dissolution Studies
In vitro dissolution tests were performed according to the specifications of the “2.9.3. Dissolution Test for Solid Pharmaceutical Forms” and “5.17. Recommendations on methods for dosage forms testing” of the European Pharmacopoeia, 10th edition [51].
The dissolution tests were performed at 37 ± 0.5 °C, using apparatus 2 (paddle apparatus). Two dissolution media were prepared for dissolution studies: for simulated gastric fluid with pH = 1.2 (3.7 g KCl, 7.5 mL concentrated HCl, and distilled water up to 1000 g) and simulated intestinal fluid pH = 6.8 (6.8 g KH2PO4, 22.4 mL 1M NaOH solution, and distilled water up to 1000 g). The pH value of the dissolution media was checked with a pH-meter and, if necessary, the solution was adjusted with concentrated HCl or 1 M NaOH solution, as appropriate. The test sample (tablet) was placed on the bottom of the cylindrical vessel, after which the air bubbles were removed from the surface of the test sample; the apparatus was started, and the rotational speed was adjusted. The dissolution test for conventional release tablets (tablets and film-coated tablets, samples named CP-2, CP-3, and CP-4) was performed for 2 h under the following working conditions: dissolution medium 500 mL solution for simulated gastric fluid with pH = 1.2; temperature 37 ± 0.5 °C; 60 rpm; duration 2 h. In the case of prolonged-release tablets, named CP-1 and CP-5, the dissolution test was performed for a period of 24 h, using specific working conditions, as follows: first 2 h, simulated gastric fluid pH = 1.2; temperature 37 ± 0.5 °C; 60 rpm. The dissolution medium was replaced with simulated intestinal fluid pH = 6.8; temperature 37 ± 0.5 °C; 60 rpm. A total of 2 mL of medium was collected; after each sampling, volume was kept constant in the cylindrical vessel by replacing with the same volume of fresh dissolution medium at 37 °C. In the case of conventional release tablets, we took samples at the following intervals: 5, 10, 15, 30, 45, 60, 90, and 120 min; for prolonged-release tablets, we took samples on time: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, and 24 h. Samples were collected at the declared time from the distance between the surface of the dissolution medium and the paddle, but also at least 10 mm from the wall of the vessel, then filtered using nylon filters (0.45 μm; diameter 25 mm). The samples were analyzed by the described method. If the area of the peak corresponding to M-HCl was greater than that obtained for standard M-HCl (1300 μg/mL), the solution was diluted twice and reanalyzed, from where a dilution factor (DF) appeared in the equation used for calculations. The dissolution test was performed on six samples (tablets).
The following equations were used to determine the amount of M-HCl released:
where
- C% = percentage release in the dissolution medium;
- CI% = percentage concentration calculated for the first sampling;
- CII% = percentage concentration calculated in the 2 mL taken previously;
- DF = dilution factor (1 or 2);
- PA = peak area (mAU·min);
- Int and S = intercept and slope of the regression line respectively;
- A = declared content (mg);
- tx = current sampling time;
- tx − 1 = previous sampling time.
At simulated gastric fluid with pH = 1.2, Equation (5) was used for the first sample taken, at the time of 5 min (conventional release tablets) and 1 h (prolonged-release tablets); for the following samples, Equation (7) was used. In the case of prolonged-release tablets, the dissolution medium was replaced with simulated intestinal fluid pH = 6.8, so that Equation (5) was used for the three hour sample, then Equation (7) for subsequent sampling. The values obtained were added with the final concentration obtained at medium pH = 1.2 (simulated gastric fluid).
3. Results and Discussions
3.1. Method Development and Optimization Using QbD Approach
3.1.1. Identification of ATP, CMAs, and Risk Assessment
For the assay and dissolution study of M-HCl from tablets using the HPLC technique with UV detection, the desired ATP was to obtain a retention time of less than 10 min, and the area and symmetry of the peak to be as large as possible. The CMAs requirements are to use a simple mobile phase (buffer/organic modifier) with isocratic elution for an aqueous sample with a concentration around 1000 μg/mL using a C18 chromatographic column with UV detection with a minimum retention time, with maximum peak area and symmetry of the corresponding peak. A last, but not least, important attribute is that the HPLC separation method can also be used for mass spectrometry (MS) detection.
For HPLC analysis, most research studies report the use of a non-polar stationary phase (C18) in different chromatographic columns (lengths between 50 and 250 mm, inner diameter of 3.9 or 4.6 mm, and particle size of 4 or 5 μm) and a mobile phase that consists of a mixture of two or three solvents (e.g., acetonitrile, methanol, water, phosphate buffer, ammonium buffer, tetrahydrofuran), with aqueous phase having a pH = 2.8–7.0. The ratio of mobile phases differs greatly, for example, the organic modifier being used in a proportion of 5–65%. The flow rate of the mobile phase was generally 1 mL/min, with extreme values of 0.5 mL/min and 1.7 mL/min. The detection was also different (by mass spectrometry or by spectrophotometry in UV). For most research studies, the detection was performed in UV, the wavelength used being between 221–270 nm, most of which were between 220 and 235 nm) [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34].
Regarding the CMPs, from Ishikawa diagram and preliminary experiments that were conducted, CMPs selected for the further study with high risk assessment that can cause variability are the instrument precision and the mobile phase (buffer type, buffer pH, type of organic modifier, and the ratio between the buffer and the organic modifier). Different compositions of the mobile phase (i.e., buffer type, buffer pH, type of organic modifier, and the ratio between the buffer and the organic modifier) were used to study the influence of the mobile phase on the results. To study the precision of the instrument, after the final separation conditions were established, the same solution was analyzed six times and the results were calculated as RSD% of the peak area. The obtained value was less than 2% (see Section 3.2.3).
3.1.2. Method Development and Optimization
Once the CMPs were identified, the next step was to optimize them in terms of the CMAs. As shown before, in order to better understand the performance of the method and to identify the independent CMPs and their effect on the dependent variables, various preliminary experiments were performed by trial and error.
Effect of Chromatographic Factors on Responses
Initially, a mobile phase formed by water/methanol (80/20 v/v) was tried; the peak was observed to be asymmetric. The further mobile phase tried was 0.02 M KH2PO4 (pH = 3) or 0.02 M CH3COONa buffers (pH = 3)/methanol (80/20, v/v). It was observed that the improvement of peak shape and symmetry was achieved by adjusting the buffer pH. In both cases, the retention times are close to each other, but when the acetate buffer is used, the peak area increases by about 8% and the peak height decreases by about 7.5%, which leads to an increase in detection sensitivity.
Taking into account the fact that one of the purposes of the method is to be able to be also used for mass spectrometry (MS) detection, we chose to use acetate instead of phosphate buffer.
Next, the nature of the organic modifier in the composition of the mobile phase was studied. For this, we replaced the methanol in the mobile phase with acetonitrile, with the percentage and pH of the buffer solution being kept constant (70% and pH = 3, respectively). In this case, there is no significant change in retention time and peak symmetry, but it is observed that a hypochromic effect occurs, decreasing the height and area of the peak, which leads to a decrease in detection sensitivity. As a conclusion, we kept methanol in the mobile phase composition.
Evaluation of Experimental Results and Selection of Final Method Conditions
For the study of the influence of the pH value of the buffer solution and of the ratio between buffer solution and the organic solvent in the composition of the mobile phase, we chose a face CCD. Using the CCD approach, these method conditions were assessed. At the first step, the conditions for retention time, peak area, and symmetry factor were evaluated. For M-HCl, this led to distinct chromatographic conditions. The acceptable value falls within those regions where deliberate variations in the parameters of the method do not affect the quality of HPLC separation. On the basis of the factor screening studies, selection of the CMPs actually affecting the method performance was optimized using a two-factor CCD at three equidistant levels, i.e., low (−1), intermediate (0), and high (+1) levels. Table 2 summarizes the design matrix as per the CCD. The same standard concentration was used for all experimental runs, which were analyzed for method CMAs, i.e., retention time, peak area, height, tailing, and symmetry.

Table 2.
Design matrix as per the CCD for optimization of parameters for analysis of M-HCl.
As an example, Figure 3 shows the chromatograms obtained for a mobile phase composition consisting of (a) 0.02 M acetate buffer (pH = 3)/methanol in a ratio of 70/30, 80/20, and 90/10, v/v, and (b) 0.02 M acetate buffer (pH = 3, 4, and 5)/methanol in a ratio of 70:30, v/v.
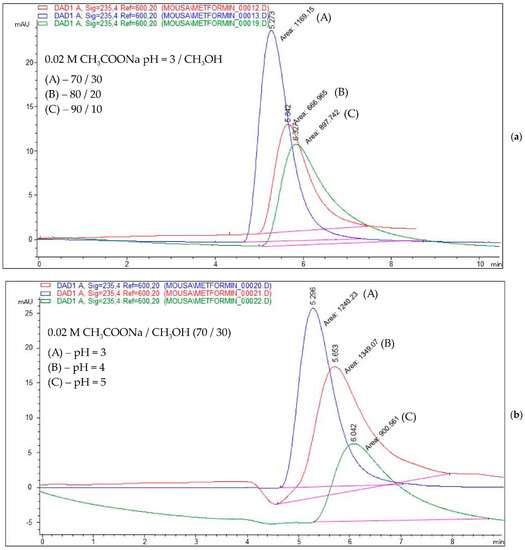
Figure 3.
Overlayed chromatograms for a mobile phase consisting of a mixture of (a) 0.02 M acetate buffer (pH = 3)/methanol in a ratio of 70/30, 80/20, and 90/10, respectively, v/v; (b) 0.02 M acetate buffer (pH = 3, 4, and 5, respectively)/methanol in a ratio of 70/30, v/v.
Design Space
The response surface study type, a CCD with 11 runs, was used. The proposed CCD experimental design was applied and the evaluation of mobile phase composition and pH of buffer was performed against the three responses, retention time, peak area, and symmetry factor; the results are summarized in Table 2 and Figure 4, respectively.
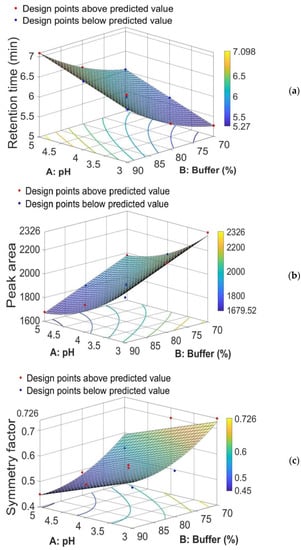
Figure 4.
Three-dimensional (3D) response surface plot for (a) retention time, (b) peak area, and (c) symmetry factor, showing effect of % of buffer and pH in the mobile phase.
After calculation of a second-order polynomial exploring quadratic response surfaces, in the models below, we have retained only those coefficients β that are significant at 95% confidence level. The results are presented in Table 3.

Table 3.
Statistical calculation of a second-order polynomial exploring quadratic response surfaces with equation Y = β0 + β1A + β2B + β3AB + β4A2 + β5B2.
From the equation for the retention time (Rt), that is, Rt = 10.209 + 0.38833 × A − 0.19623 × B + 0.0015567 × B2, we see that the coefficient of A is positive (+0.38833). We can interpret this coefficient as follows: if we keep B fixed, then an increase/decrease in A by one unit will determine an increase/decrease in retention time by 0.38833 units. On the other hand, the coefficient of B is negative (−0.19623), but this coefficient can no longer be interpreted as we did before, because the term B2 also appears in the formula. We can only say that, for a fixed value of A and the range of B between 70 and 90, retention time is an increasing function of B. This means that a decrease in B will determine a decrease in retention time.
From the equation for the peak area (Pa), that is, Pa = 4979.6 − 736.18 × A − 15.384 × B + 70.254 × A2, we see that the coefficient of B is negative (−15.384). We can interpret this coefficient as follows: if we keep A fixed, then an increase/decrease in B by one unit will determine a decrease/increase in peak area by 15.384 units. We also see that the coefficient of A is negative (−736.18), but this coefficient can no longer be interpreted as we did for B, because the term A2 also appears in the equation. We can only say that, for a fixed value of B and the range of A between 3 and 5, peak area is a decreasing function of A. This means that a decrease in A will determine an increase in peak area. In other words, if we intersect the peak area response surface by the plane of equation B = c (here, 70 ≤ c ≤ 90), then the curve of intersection represents a decreasing function of A. Therefore, a decrease in A will determine an increase in peak area.
Similarly, from the equation for symmetry factor (Sf), that is, Sf = 3.876 − 0.0667 × A − 0.0692 × B + 0.00038667 × B2, we see that the coefficient of A is negative (−0.0667). We can interpret this coefficient as follows: if we suppose that B is fixed, then an increase/decrease in A by one unit will determine a decrease/increase in symmetry factor by 0.0667 units. The coefficient of B is negative (−0.0692), but this coefficient can no longer be interpreted as we did for A, because the term B2 also appears in the equation. We can only say that, for a fixed value of A and the range of B between 70 and 90, symmetry factor is a decreasing function of B, and, thus, a decrease in B will determine an increase in symmetry factor.
In conclusion, from Figure 4a–c and the equations for the retention time, peak area, and symmetry factor, we can conclude that if both the pH value (code factor A) and the percentage of sodium acetate buffer (code factor B) in the mobile phase composition decrease, then the value of the retention time decreases simultaneously with the increase in peak area and the increase in symmetry factor for the corresponding M-HCl peak.
Optimized Chromatographic Conditions
The search for the optimal solution was performed by numerical optimization by “trading off” various CAAs to achieve the desired objectives, i.e., maximization of peak area and symmetry factor and minimization of retention time, to obtain the desirability function close to 1. The optimized solution showed that the mobile phase composition containing a mixture of 0.02 M acetate buffer (pH = 3)/methanol in a ratio of 70/30 (v/v) yielded desirability close to 1.0, along with all the CAAs in the desired ranges. The optimized values for pH and buffer content in the mobile phase and predicted responses are shown in Table 4. The 3D desirability plot is presented in Figure 5.

Table 4.
Obtained solution for optimized formulation.
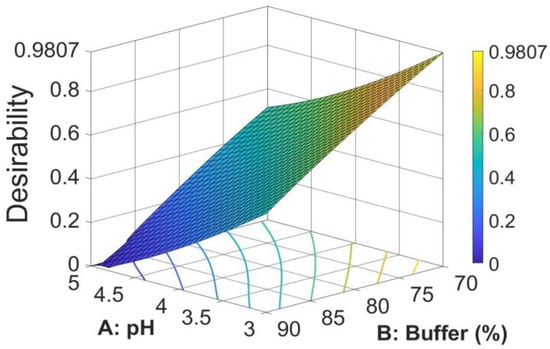
Figure 5.
3D surface plot for desirability for optimal formulation.
Thus, the optimal mobile phase consists of a mixture of 0.02 M acetate buffer (pH = 3)/methanol in a ratio of 70/30, v/v, conditions in which the method is faster and has a higher sensitivity. The final chromatographic conditions for M-HCl are shown in Table 5.

Table 5.
Final chromatographic conditions.
As a comment, if a higher percentage of organic modifier is used (for example, for a mobile phase consisting of 0.02 M CH3COONa (pH = 3)/CH3OH in a ratio of 60/40, the method is faster (retention time decreases) and slightly more sensitive (peak area increases). However, we opted for a 70/30 ratio for three reasons: (i) at a percentage lower than 70% of the buffer solution in the composition of the mobile phase, a negative peak appears in front of the corresponding peak of M-HCl, which makes the integration difficult, and the precision of the peak integration decreases; (ii) reducing costs (by reducing the amount of methanol used; and (iii) reducing pollution (by disposing of as little organic solvent as waste).
3.1.3. Risk Assessment and Control Strategy
For robustness and ruggedness studies, a solution of M-HCl with a concentration of 1000 μg/mL was used. The robustness study was performed by deliberately changing the value of CMPs (methanol from another source, mobile phase flow between 0.9 and 1.1 mL/min, and pH of the buffer solution between 2.8 and 3.2). For the ruggedness study, the determinations were made on different days by another analyst. In both cases, the values obtained for the retention time, peak area, and symmetry factor, respectively, had RSD values less than 2%.
As a control strategy, we planned a set of controls of some parameters to ensure that both the quality of the results obtained and the method performance fall within the established ATP (preparation and storage conditions of samples, measurements performed, and doubling of control operations).
3.2. Method Validation
3.2.1. Linearity
Linearity of the proposed method was evaluated according to the ICH guidelines [47]. A number of three sets of working solutions were prepared for the study of linearity over the concentration range 10–2000 μg/mL. Each of these samples was analyzed under the mentioned conditions and, from the chromatograms obtained, the area of the peaks corresponding to M-HCl was determined. M-HCl showed linearity in the concentration range of 10–2000 μg/mL (r2 = 0.9999). The regression equation obtained was PA = 2.4266 × C − 56.361, where PA is peak area and C is concentration of M-HCl in μg/mL.
3.2.2. Limit of Detection (LOD) and Limit of Quantification (LOQ)
Using the peak area for noise (1.51212) and Equation (2), the detection and quantification limits were calculated:
LOD = (3 × 1.51212 + 56.361)/2.4266 = 25.09 μg/mL
LOQ = (10 × 1.51212 + 56.361)/2.4266 = 29.46 μg/mL
3.2.3. Precision and Accuracy
To examine the system precision, the same solution containing M-HCl at the concentration of interest (1000 μg/mL) was injected six times to obtain as many chromatograms. From the chromatograms obtained, the peak areas were measured, calculating the mean value, the SD, and the RSD%. The obtained RSD% value is less than 2% (Table 6).

Table 6.
System precision.
The repeatability and the intermediate precision were evaluated by injecting three different concentrations (700, 1000, and 1300 μg/mL) of M-HCl. For repeatability (intra-day) determination, sets of three replicates of the three concentrations were analyzed on the same day. For intermediate precision (inter-day), three replicates were analyzed on three different days. In both cases, the RSD% value is less than 2%, indicating that the method was precise.
In order to estimate the accuracy, this was determined by performing the recovery experiments for three different solutions at three concentration levels (700, 1000, and 1300 μg/mL), and the final recovery was calculated. The mean recovery is 99.75% in the range 98.12–100.93%.
The precision and the accuracy of the method are presented in Table 7.

Table 7.
Precision and accuracy of the assay method of M-HCl by HPLC.
Because, in addition to the quantitative determination of M-HCl from tablets, the application of the method to dissolution studies is also considered, the accuracy was also studied at lower concentrations, being calculated in terms of percent relative error (RE%) using Equation (3). Keeping in account the fact that the calculated value for LOQ is 29.46 μg/mL, the accuracy was thus calculated for the range 40–2000 μg/mL, with the mean values of three replicates (Table 8). Since the mean recovery is 99.12% in the range 96.37–101.04%, results show that the method is accurate over the entire measured range. Analyzing the data presented in Table 8, it is observed that the mean percent error (%RE) calculated with Equation (3) is 0.88%, and recovery values lower than 97% are obtained in the case of concentrations lower than 80 μg/mL, which represents less than 8% of the concentration of interest (1000 μg/mL) and, as a result, presents an acceptable variation in the case of dissolution studies.

Table 8.
Accuracy of the method in the range 40–2000 μg/mL.
3.3. Stability Studies for M-HCl Solutions
Solutions with concentration of 1000 and 2000 μg/mL, respectively, were analyzed under the method conditions at intervals of 0, 24, and 48 h after preparation, with the solutions being stored in refrigerator, at room temperature, and in a water bath at 37 °C. By integrating the obtained chromatograms, the area of the peaks (Table 9) corresponding to M-HCl were determined, then, using the equation of the regression line, the concentration in M-HCl was calculated.

Table 9.
Experimental results obtained in the study of the stability over time of M-HCl solutions with concentrations of 1000 and 2000 µg/mL.
From this study, it is found that as the temperature at which the aqueous M-HCl solution is stored is increased, its stability is maintained. Thus, for the concentration of 1000 μg/mL, the recovery decreases by 0.19, 0.3, and 0.35% in 24 h and by 0.58, 1.03, and 1.61%; in the case of the concentration of 2000 μg/mL, the recovery decreases by 0.17, 0.28, and 0.37% in 24 h and by 0.88, 1.19, and 1.59% if the solution is stored in the refrigerator, at room temperature, or in a water bath, respectively. The best stability is the solution stored in the refrigerator, and as the storage temperature increases, the stability decreases. In conclusion, the method can be applied to determine the M-HCl content of tablets, with the samples being able to be analyzed within a reasonable time. In the case of dissolution studies, due to the large number of samples, the samples collected at different time intervals are stored in the refrigerator and are analyzed chromatographically in a maximum of 24 h.
3.4. Determination of M-HCl Content in Tablets
Under the method conditions, the peak of M-HCl has a retention time around of 5.27 min. Confirmation of the identification of the corresponding M-HCl peak was performed by comparing the retention time of the M-HCl peak in the sample chromatogram with that in the chromatogram of a standard. To increase the quality of the identification, the absorption spectrum measured at the apex of the peak from the sample chromatogram was compared with the standard spectrum of M-HCl stored in the spectra library.
In order to determine the presence of compounds with similar retention times (co-eluents as impurities or excipients), the study of the spectral purity of the chromatographic peak was performed. From this study, it is found that no co-eluents appear, the purity of the peak being higher than 99%.
The experimental results obtained by this method of recovery of M-HCl from different types of tablets are presented in Table 10. The percent content in tablets is calculated using Equation (4).

Table 10.
Experimental data obtained from the study of the M-HCl content of tablets.
Analyzing the data in Table 10, it is found that all the pharmaceutical products analyzed have a percentage content of M-HCl in the range 97.78–99.20%, for a permissible deviation of ±5%.
3.5. Dissolution Studies
Conventional-release tablets (850 mg tablets and 1000 mg film-coated tablets) were used in the dissolution test for tablets, but also for modified-release tablets (500 mg and 1000 mg prolonged-release tablets).
In the case of conventional release tablets, at the dissolution test performed at simulated gastric fluid with pH = 1.2, there is a minimum release for M-HCl for CP-2 (63.85%), CP-3 (33.8%), and for CP-4 (47.97%) at the first sampling at 5 min. After 30 min, all three formulas of conventional release tablets released over 80% of the declared amount of active substance (Figure 6), which confirms the inclusion of the formulations analyzed in the conventional-release solid dosage forms category according to “2.9.3. Dissolution test for solid dosage forms” and “5.17. Recommendations on methods for dosage forms testing” from European Pharmacopoeia, 10th edition [51]. At the end of the dissolution test, after 2 h, a release of over 98% was registered for all the studied formulations (Figure 6).
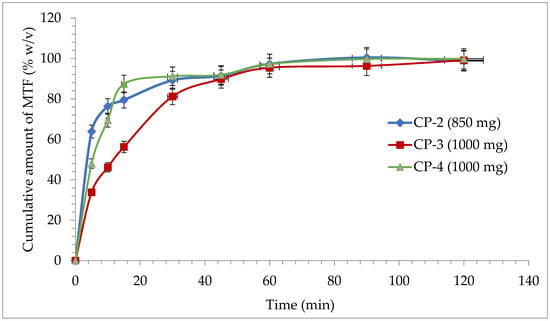
Figure 6.
Release profile of M-HCl from conventional tablets.
In the case of prolonged-release tablets with 500 mg and 1000 mg M-HCl, it is observed that at the first sampling, simulated gastric fluid with pH = 1.2 has a release of M-HCl from the CP-1 sample of 41.74% and in the case of the CP-5 sample of 23.57%, which corresponds to the acceptance criteria of the European Pharmacopoeia, 10th edition [51], by preventing the occurrence of “dose dumping”. After 2 h, in the simulated gastric fluid with pH = 1.2, formulation CP-1 has a maximum release of 49.71%, respectively, 35.96% in CP-5 (Figure 7).
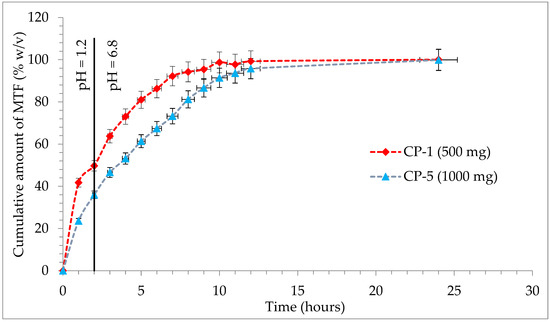
Figure 7.
Release profile of M-HCl from prolonged-release tablets.
The second specification point of the dissolution test will be defined by the release of approximately 50% of M-HCl from prolonged-release tablets. In the case of the first studied formulation, a much faster release profile is observed, so that the second point is achieved after 2 h in simulated gastric fluid with pH = 1.2 (CP-1: 49.71%), unlike formulation CP-5, which released 53.17% of M-HCl at 4 h in simulated intestinal fluid, pH = 6.8. The final specification point stipulated in the conditions of European Pharmacopoeia is the assurance of complete release, which is understood to be at least 80% achieved in a simulated intestinal fluid pH = 6.8. For the CP-1 test, this point is reached after 5 h (81%), with a maximum release of 100.04% at the end of the test; for the CP-5 81.21% is released after 8 h, with a maximum 99.87% after 24 h (Figure 7). The two samples analyzed are compliant and fall into the release profile for extended-release dosage forms, according to “2.9.3. Dissolution test for solid dosage forms” and “5.17. Recommendations on methods for dosage forms testing” from European Pharmacopoeia, 10th edition [51].
4. Conclusions
The paper describes the development of an HPLC method for the determination of M-HCl by the QbD approach using a central composite design by studying the interrelationships of two factors regarding the mobile phase (the pH of aqueous phase and the ratio between acetate buffer and methanol) at three different levels. The optimized CMPs were found after calculations of second-order polynomial exploring quadratic response surfaces for retention time, peak area, and symmetry factor as functions of buffer pH and ratio between buffer and methanol. The optimized solution showed the mobile phase composition containing a mixture of 0.02 M acetate buffer (pH = 3)/methanol in a ratio of 70/30 (v/v), a case for which the desirability function was very close to 1 (desirability function = 0.9807).
The optimized method was fully validated according to ICH Q2 (R1) Guidelines. The method showed good linearity (r2 = 0.9999) in the studied range (10–2000 μg/mL) with good detection and quantification limits (LOD = 25.09 μg/mL and LOQ = 29.46 μg/mL, respectively), good precision (RSD < 2%) and accuracy (mean recovery = 99.75%) in the range 700–1300 μg/mL, and a mean percent error of 1.07% in the range of 40–2000 μg/mL. From the obtained results, it can be concluded that the M-HCl solutions are stable enough at room temperature 24 h after preparations. If the samples were analyzed after more than 24 h, it is recommended that they be kept in a refrigerator.
The optimized and validated method was used for the M-HCL assay from tablets and for dissolution studies. In the case of assay studies, all the pharmaceutical products analyzed have a percentage content of M-HCl in the range 97.78–99.20%, for a permissible deviation of ±5%. In the case of dissolution studies, for conventional-release tablets, after 2 h a release of over 98% was registered for all the studied formulations. In the case of prolonged-release tablets, the two samples analyzed are compliant and fall into the release profile for extended-release dosage forms, according to “2.9.3. Dissolution test for solid dosage forms” and “5.17. Recommendations on methods for dosage forms testing” from European Pharmacopoeia, 10th edition.
Author Contributions
Conceptualization, L.O. and A.F.S.; methodology, A.F.S. and L.O.; software, I.S.; validation, M.S., A.F.S. and M.H.; formal analysis, A.F.S. and I.S.; investigation, M.S., M.H., A.B. and M.S.C.; resources, M.S., L.O. and A.F.S.; data curation, M.S. and A.F.S.; writing—original draft preparation, M.S., A.B. and M.S.C.; writing—review and editing, A.F.S., I.S. and L.O.; visualization, M.S. and A.F.S.; supervision, L.O. and A.F.S.; project administration, L.O.; funding acquisition, L.O., A.F.S., M.S., A.B. and M.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grigore T. Popa University of Medicine and Pharmacy Iasi, Romania, grant no. 10307/29.06.2020.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AQbD: analytical quality by design; ATP: analytical target profile; CAA: critical analytical attribute; CCD: central composite design; CMA: critical method attribute; CMP: critical method parameter; CQA: critical quality attribute; DAD: diode array detector; DoE: design of experiments; FDA: Food and Drug Administration; ICH: International Conference on Harmonization; IR: immediate release; LOD: limit of detection; LOQ: limit of quantification; M-HCl: metformin hydrochloride; MODR: method operable design region; MS: mass spectrometry; QbD: quality by design; QTTP: quality target product profile; RE%: percent relative error; RSD%: relative standard deviation; XR: extended-release.
References
- Aljofan, M.; Riethmacher, D. Anticancer activity of metformin: A systematic review of the literature. Future Sci. OA 2019, 5, FSO410. [Google Scholar] [CrossRef]
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef]
- Sena, P.; Mancini, S.; Benincasa, M.; Mariani, F.; Palumbo, C.; Roncucci, L. Metformin Induces Apoptosis and Alters Cellular Responses to Oxidative Stress in Ht29 Colon Cancer Cells: Preliminary Findings. Int. J. Mol. Sci. 2018, 19, 1478. [Google Scholar] [CrossRef]
- Tseng, C.-H. The Relationship between Diabetes Mellitus and Gastric Cancer and the Potential Benefits of Metformin: An Extensive Review of the Literature. Biomolecules 2021, 11, 1022. [Google Scholar] [CrossRef]
- Samuel, S.M.; Varghese, E.; Kubatka, P.; Triggle, C.R.; Büsselberg, D. Metformin: The Answer to Cancer in a Flower? Current Knowledge and Future Prospects of Metformin as an Anti-Cancer Agent in Breast Cancer. Biomolecules 2019, 9, 846. [Google Scholar] [CrossRef]
- Lee, T.Y.; Martinez-Outschoorn, U.E.; Schilder, R.J.; Kim, C.H.; Richard, S.D.; Rosenblum, N.G.; Johnson, J.M. Metformin as a therapeutic target in endometrial cancers. Front. Oncol. 2018, 8, 341. [Google Scholar] [CrossRef]
- Mazurek, M.; Litak, J.; Kamieniak, P.; Kulesza, B.; Jonak, K.; Baj, J.; Grochowski, C. Metformin as Potential Therapy for High-Grade Glioma. Cancers 2020, 12, 210. [Google Scholar] [CrossRef]
- Sha’at, M.; Ghiciuc, C.M.; Bujor, A.; Timofte, D.V.; Ștefanache, A.; Ochiuz, L. Literature review of oral treatment of type 2 diabetes mellitus. Med. Surg. J. 2020, 124, 482–495. [Google Scholar]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Development and validation of a new analytical HPLC method for simultaneous determination of the antidiabetic drugs, metformin and gliclazide. J. Food Drug. Anal. 2019, 27, 315–322. [Google Scholar] [CrossRef]
- Gite, S.; Patravale, V. Validation of RP-HPLC Method and Stress Degradation for the Combination of Metformin HCl, Atorvastatin Calcium and Glimepiride: Application to Nanoparticles. J. Chromatogr. Sci. 2015, 53, 1654–1662. [Google Scholar] [CrossRef]
- Sebaiy, M.M.; El-Adl, S.M.; Baraka, M.M.; Hassan, A.A. Rapid RP-HPLC method for simultaneous estimation of metformin, pioglitazone, and glimepiride in human plasma. Acta Chromatogr. 2020, 32, 16–21. [Google Scholar] [CrossRef]
- Kant, R.; Bodla, R.B.; Kapoor, G.; Bhutani, R. Optimization of a single HPLC-PDA method for quantifying metformin, gliclazide, pioglitazone, dapagliflozin, empagliflozin, saxagliptin, linagliptin and teneligliptin using central composite design. Bioorg. Chem. 2019, 91, 103111. [Google Scholar] [CrossRef]
- Sufiyan, A.; Ansari, S.; Rageeb, U.; Mohammed, I.; Rashid, A. Development and validation of RP-HPLC method for simultaneous estimation of metformin and miglitol in bulk and dosage form. Asian J. Pharm. R. 2017, 7, 139–147. [Google Scholar] [CrossRef]
- Attimarad, M.V.; Nair, A.B.; Aldhubaib, B.E. Development of liquid chromatographic method for the simultaneous determination of metformin and miglitol in human plasma: Application to pharmacokinetic studies. J. Iran. Chem. Soc. 2015, 12, 1629–1636. [Google Scholar] [CrossRef]
- Raja, A.M.; Dhanalaxmi, J.; Banji, D.; Rao, K.N.V.; Selva Kumar, D. Stability indicating RP-HPLC method development and validation of simultaneous estimation of metformin and acarbose in bulk and pharmaceutical formulation. Asian J. Res. Biol. Pharm. Sci. 2015, 3, 66–77. [Google Scholar]
- Shende, M.A.; Budde, B.R. Novel RP-HPLC method development and validation for simultaneous estimation of metformin, voglibose and pioglitazone in bulk and triple fixed drug combinations pharmaceutical dosage form. J. Drug Deliv. Ther. 2019, 9, 30–37. [Google Scholar] [CrossRef]
- Chengalva, P.; Parameswari, A.S.; Aruna, G. Development and validation of RP-HPLC method for metformin hydrochloride and nateglinide in bulk and combined dosage form. Int. J. Pharm. Pharm. Sci. 2016, 8, 267–271. [Google Scholar]
- Wairkar, S.; Gaud, R. Co-Amorphous Combination of Nateglinide-Metformin Hydrochloride for Dissolution Enhancement. AAPS PharmSciTech 2016, 17, 673–681. [Google Scholar] [CrossRef]
- Aslan, S.S.; Yilmaz, B. Derivative spectrophotometric and isocratic high performance liquid chromatographic methods for simultaneous determination of repaglinide and metformin hydrochloride in pharmaceutical preparations. Am. J. Anal. Chem. 2017, 8, 541–552. [Google Scholar] [CrossRef][Green Version]
- Mahrouse, M.A.; Lamie, N.T. Experimental design methodology for optimization and robustness determination in ion pair RP-HPLC method development: Application for the simultaneous determination of metformin hydrochloride, alogliptin benzoate and repaglinide in tablets. Microchem. J. 2019, 147, 691–706. [Google Scholar] [CrossRef]
- Raza, A.; Murtaza, S.H.; Hanif, S.; Iqbal, J.; Ali, I.; Aftab, T.; Shakir, R.; Bedar, R.; Ali Syed, M. Validation of a rapid and economical RP-HPLC method for simultaneous determination of metformin hydrochloride and sitagliptin phosphate monohydrate: Greenness evaluation using AGREE score. Pak. J. Pharm. Sci. 2022, 35, 15–21. [Google Scholar]
- Balamurugan, K.; Kirtimaya, M. Quality by design based development and validation of RP-HPLC method for simultaneous estimation of sitagliptin and metformin in bulk and pharmaceutical dosage forms. Int. J. Pharm. Investig. 2020, 10, 512–518. [Google Scholar] [CrossRef]
- Prasad, P.B.N.; Satyanaryana, K.; Krishnamohan, G. Development and validation of a method for simultaneous determination of metformin and saxagliptin in a formulation by RP-HPLC. Am. J. Anal. Chem. 2015, 6, 841–850. [Google Scholar] [CrossRef]
- Shakoor, A.; Ahmed, M.; Ikram, R.; Hussain, S.; Tahir, A.; Jan, B.M.; Adnan, A. Stability-indicating RP-HPLC method for simultaneous determination of metformin hydrochloride and vildagliptin in tablet and biological samples. Acta Chromtogr. 2020, 32, 39–43. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Reddy, P.S.; Narayanan, K.L.; Bhosale, P.N. Development of RP-HPLC, Stability Indicating Method for Degradation Products of Linagliptin in Presence of Metformin HCl by Applying 2 Level Factorial Design; and Identification of Impurity-VII, VIII and IX and Synthesis of Impurity-VII. Sci. Pharm. 2017, 85, 25. [Google Scholar] [CrossRef]
- Kumari, K.S.; Bandhakavi, S. Development and validation of stability-indicating RP-HPLC method for the simultaneous determination of ertugliflozin pidolate and metformin hydrochloride in bulk and tablets. Future J. Pharm. Sci. 2020, 6, 66. [Google Scholar] [CrossRef]
- Attimarad, M.; Elgorashe, R.E.E.; Subramaniam, R.; Islam, M.M.; Venugopala, K.N.; Nagaraja, S.; Balgoname, A.A. Development and Validation of Rapid RP-HPLC and Green Second-Derivative UV Spectroscopic Methods for Simultaneous Quantification of Metformin and Remogliflozin in Formulation Using Experimental Design. Separations 2020, 7, 59. [Google Scholar] [CrossRef]
- Al-Rimawi, F. Development and validation of an analytical method for metformin hydrochloride and its related compound (1-cyanoguanidine) in tablet formulations by HPLC-UV. Talanta 2009, 79, 1368–1371. [Google Scholar] [CrossRef]
- Kumar, D.; Srivastava, S.; Ramakrishna, R.; Dixit, R.K. Development and validation RP-HPLC-PDA method for the determination of metformin in bulk and dosage form. World J. Pharm. Pharm. Sci. 2014, 3, 745–757. [Google Scholar]
- Madhukar, A.; Prince, A.; Vijay Kumar, R.; Sanjeeva, Y.; Jagadeeshwar, K.; Raghupratap, D. Simple and sensitive analytical method development and validation of metformin hydrochloride by RP-HPLC. Int. J. Pharm. Pharm. Sci. 2011, 3, 117–120. [Google Scholar]
- Ahmed, R. A simple and convenient method for the simultaneous in vitro study of metformin and glimepiride tablets. Pak. J. Pharm. Sci. 2014, 27, 1939–1943. [Google Scholar]
- Mohamed, D.; Elshahed, M.S.; Nasr, T.; Aboutaleb, N.; Zakaria, O. Novel LC–MS/MS method for analysis of metformin and canagliflozin in human plasma: Application to a pharmacokinetic study. BMC Chem. 2019, 13, 82. [Google Scholar] [CrossRef]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Considerations (ICH) Guideline Q8 (R2) on Pharmaceutical Development. Step 5. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-11.pdf (accessed on 19 August 2021).
- Godfrey, A.B.; Kenett, R.S.; Joseph, M. Juran, a perspective on past contributions and future impact. Qual. Reliab. Eng. Int. 2007, 23, 653–663. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration. “Guidance for Industry Process Validation: General Principles and Practices”, January 2011, Current Good Manufacturing Practices (CGMP) Revision 1. Available online: https://www.fda.gov/files/drugs/published/Process-Validation--General-Principles-and-Practices.pdf (accessed on 20 August 2021).
- Kumar, N.; Sangeetha, D. Analytical method development by using QbD—An emerging approach for robust analytical method development. J. Pharm. Sci. Res. 2020, 12, 1298–1305. [Google Scholar]
- Raman, N.V.V.S.S.; Mallu, U.R.; Bapatu, H.R. Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing. J. Chem. 2015, 2015, 435129. [Google Scholar] [CrossRef]
- Sandhu, P.S.; Beg, S.; Katare, O.P.; Singh, B. QbD-Driven Development and Validation of a HPLC Method for Estimation of Tamoxifen Citrate with Improved Performance. J. Chromatogr. Sci. 2016, 54, 1373–1384. [Google Scholar] [CrossRef]
- Palakurthi, A.K.; Dongala, T.; Katakam, L.N.R. QbD based development of HPLC method for simultaneous quantification of Telmisartan and Hydrochlorothiazide impurities in tablets dosage form. Pract. Lab. Med. 2020, 21, e00169. [Google Scholar] [CrossRef]
- Jadhav, M.L.; Tambe, S.R. Implementation of QbD Approach to the Analytical Method Development and Validation for the Estimation of Propafenone Hydrochloride in Tablet Dosage Form. Chromatogr. Res. Int. 2013, 2013, 676501. [Google Scholar] [CrossRef]
- Saha, C.; Gupta, N.V.; Chandan, R.S. Development and validation of a UPLC-MS method for determination of atazanavir sulfate by the “analytical quality by design” approach. Acta Pharm. 2020, 70, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Șpac, A.F.; Miftode, A.M.; Asaftei, I.V.; Sandu, I. Quality by Design (QbD) approach to develop and validate a HPLC method for piroxicam from serum. Rev. Chim.-Buchar. 2018, 69, 2167–2171. [Google Scholar] [CrossRef]
- Veerubhotla, K.; Walker, R.B. Development and Validation of a Stability-indicating RP-HPLC Method Using Quality by Design for Estimating Captopril. Indian J. Pharm. Sci. 2019, 81, 45–56. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, T.; Patel, A.; Kaur, R.; Tripathi, S.K.; Katare, O.P.; Singh, B. QbD-steered development and validation of an RP-HPLC method for quantification of ferulic acid: Rational application of chemometric tools. J. Chromatogr. B 2020, 1155, 122300. [Google Scholar] [CrossRef]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Topic Q 2 (R1). Validation of Analytical Procedures: Text and Methodology. Step 5. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 19 August 2021).
- Bhattacharya, S. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. In Response Surface Methodology in Engineering Science; Kayaroganam, P., Ed.; IntechOpen: London, UK, 2021; p. 270. [Google Scholar]
- Jain, A.; Beg, S.; Saini, S.; Sharma, T.; Katare, O.P.; Singh, B. Application of chemometric approach for QbD Enabled development and validation of an RP-HPLC method for estimation of methotrexate. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 502–512. [Google Scholar] [CrossRef]
- Lestari, A.D.; Rohman, A.; Martono, S. Central Composite Design Applied in HPLC Optimization for Analysis of Tartrazine and Auramine O in Powder Drinks. Int. J. Appl. Pharm. 2019, 11, 211–215. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines and HealthCare. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).