Utility of Downstream Biomarkers to Assess and Optimize Intranasal Delivery of Oxytocin
Abstract
1. Introduction
1.1. Intranasal Delivery Compared with Other Noninvasive Methods (e.g., Oral, Transdermal)
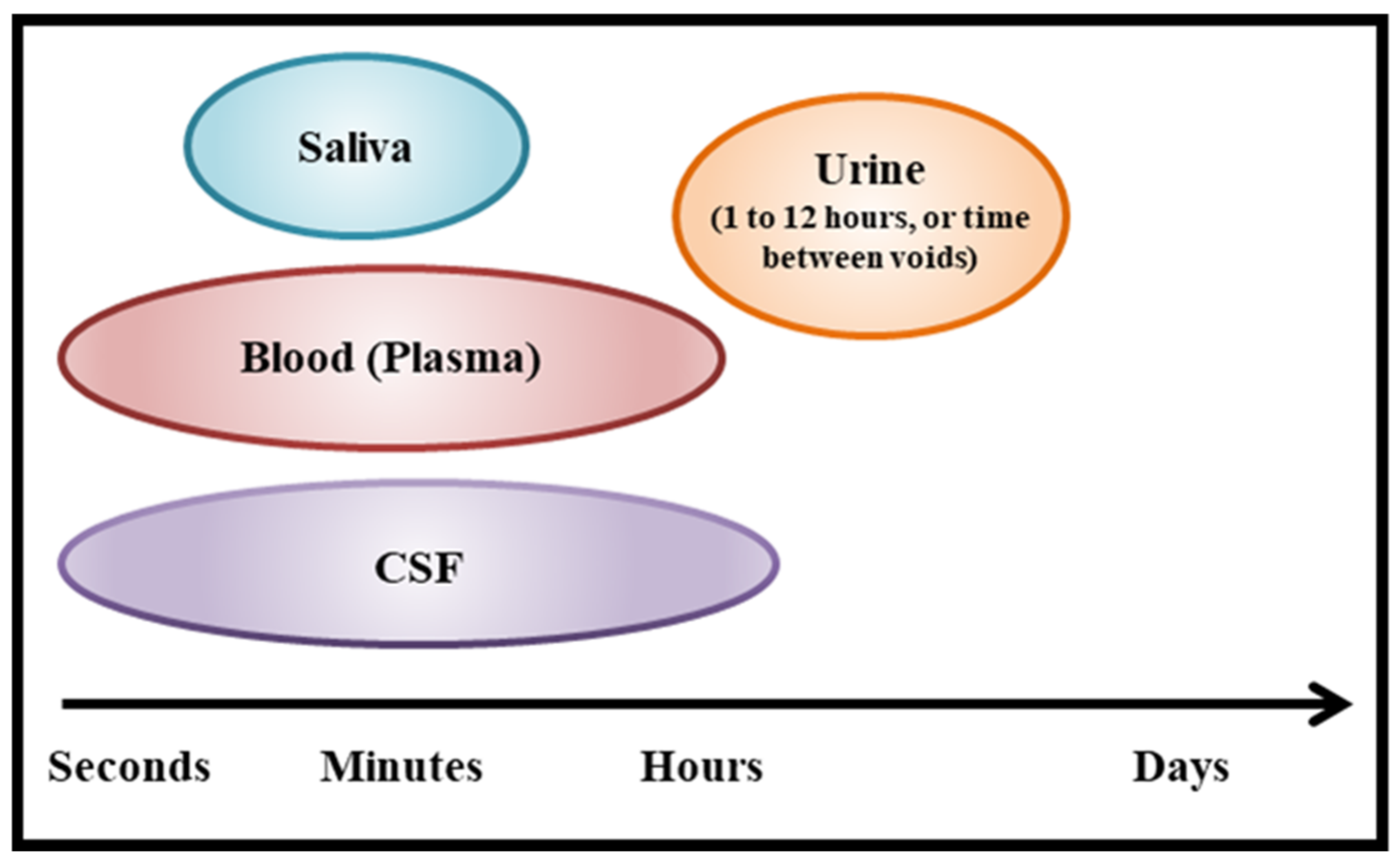
1.2. Biomarkers to Determine Effects of Intranasal Delivery
2. Materials and Methods
2.1. Participants
2.2. Design
2.3. Drug
2.4. Procedure
2.5. General Protocols: Urine and Plasma Oxytocin Levels
2.5.1. Urine
2.5.2. Plasma (ASD Participants Only)
2.6. Data Analyses
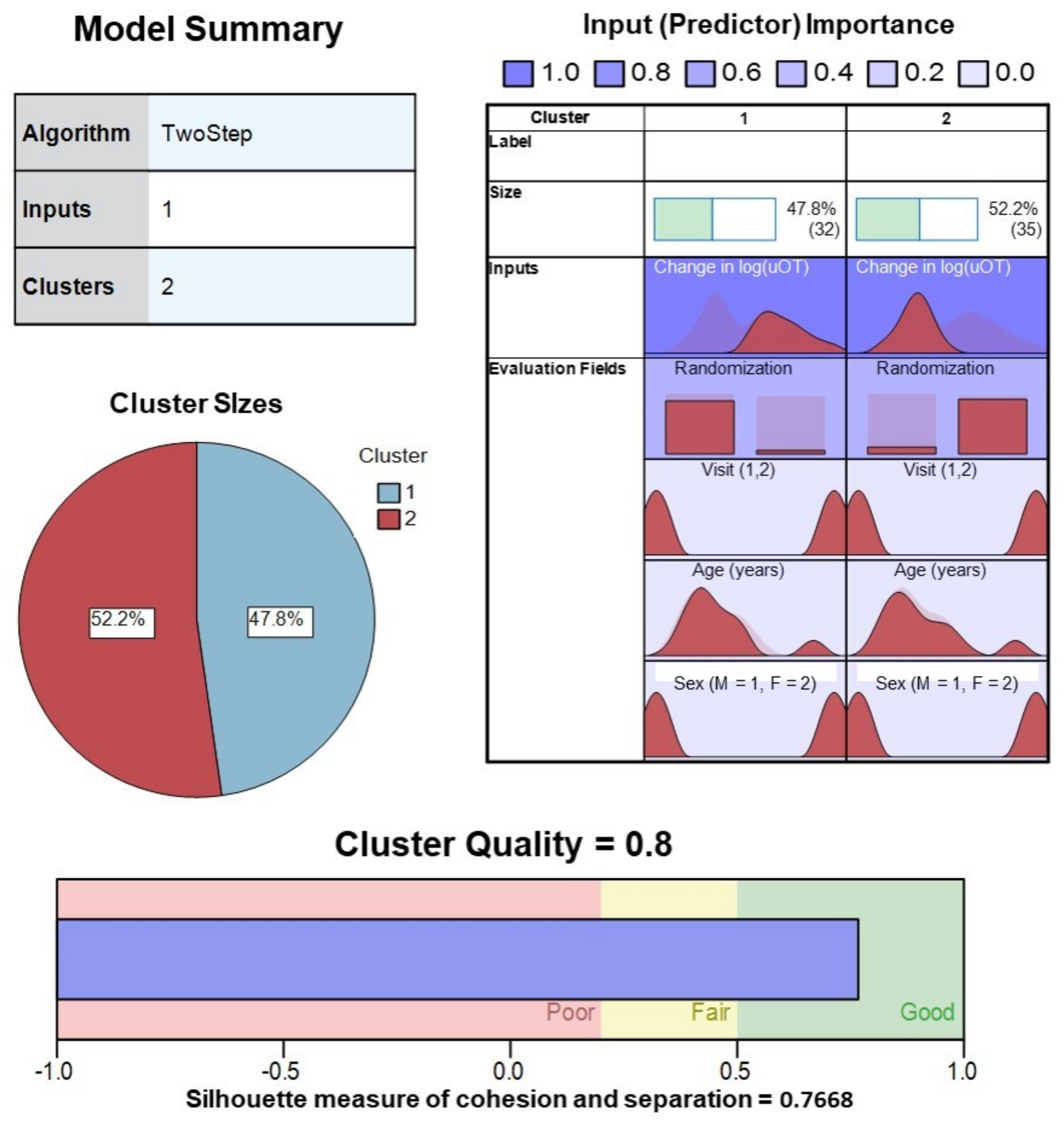
2.7. Cluster Analyses
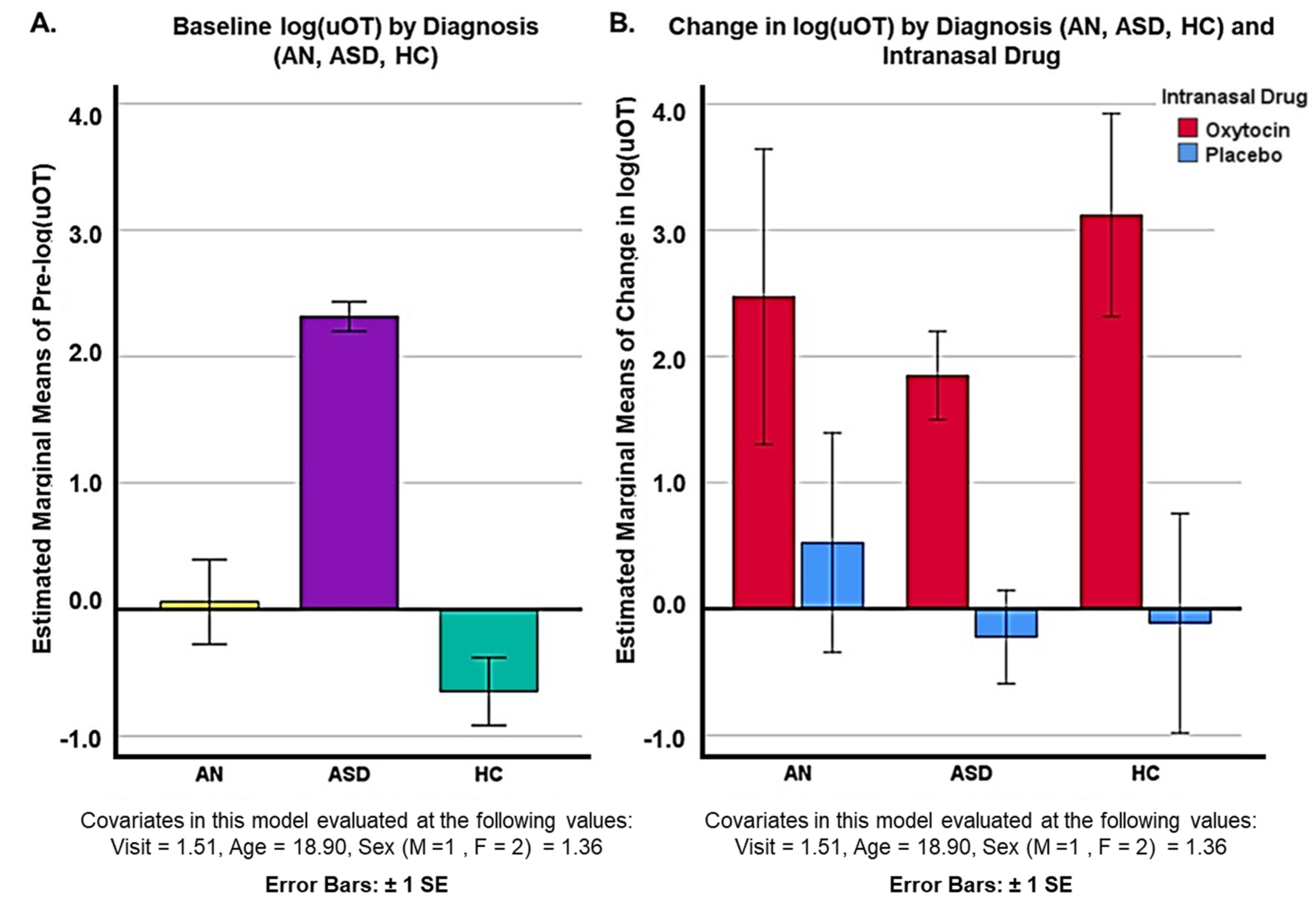
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veronesi, M.C.; Kubek, D.J.; Kubek, M.J. Intranasal Delivery of Neuropeptides. Methods Mol. Biol. 2011, 789, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.; Ludwig, M. Intranasal Oxytocin: Myths and Delusions. Biol. Psychiatry 2016, 79, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.M.; Sagar, A.; Levin-Decanini, T.; Liu, W.; Carter, C.S.; Jacob, S. Oxytocin and Vasopressin Systems in Genetic Syndromes and Neurodevelopmental Disorders. Brain Res. 2014, 1580, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Lischke, A.; Grace, S.; Scheele, D.; Ma, Y.; Becker, B. Advances in the Field of Intranasal Oxytocin Research: Lessons Learned and Future Directions for Clinical Research. Mol. Psychiatry 2021, 26, 80–91. [Google Scholar] [CrossRef]
- Rubin, L.H.; Li, S.; Yao, L.; Keedy, S.K.; Reilly, J.L.; Hill, S.K.; Bishop, J.R.; Sue Carter, C.; Pournajafi-Nazarloo, H.; Drogos, L.L.; et al. Peripheral Oxytocin and Vasopressin Modulates Regional Brain Activity Differently in Men and Women with Schizophrenia. Schizophr. Res. 2018, 202, 173–179. [Google Scholar] [CrossRef]
- Russell, J.; Maguire, S.; Hunt, G.E.; Kesby, A.; Suraev, A.; Stuart, J.; Booth, J.; McGregor, I.S. Intranasal Oxytocin in the Treatment of Anorexia Nervosa: Randomized Controlled Trial during Re-Feeding. Psychoneuroendocrinology 2018, 87, 83–92. [Google Scholar] [CrossRef]
- Pallister, E.; Waller, G. Anxiety in the Eating Disorders: Understanding the Overlap. Clin. Psychol. Rev. 2008, 28, 366–386. [Google Scholar] [CrossRef]
- Westwood, H.; Tchanturia, K. Autism Spectrum Disorder in Anorexia Nervosa: An Updated Literature Review. Curr. Psychiatry Rep. 2017, 19, 41. [Google Scholar] [CrossRef]
- Cardoso, C.; Kingdon, D.; Ellenbogen, M.A. A Meta-Analytic Review of the Impact of Intranasal Oxytocin Administration on Cortisol Concentrations during Laboratory Tasks: Moderation by Method and Mental Health. Psychoneuroendocrinology 2014, 49, 161–170. [Google Scholar] [CrossRef]
- Guastella, A.J.; Gray, K.M.; Rinehart, N.J.; Alvares, G.A.; Tonge, B.J.; Hickie, I.B.; Keating, C.M.; Cacciotti-Saija, C.; Einfeld, S.L. The Effects of a Course of Intranasal Oxytocin on Social Behaviors in Youth Diagnosed with Autism Spectrum Disorders: A Randomized Controlled Trial. J. Child Psychol. Psychiatry 2015, 56, 444–452. [Google Scholar] [CrossRef]
- Yatawara, C.J.; Einfeld, S.L.; Hickie, I.B.; Davenport, T.A.; Guastella, A.J. The Effect of Oxytocin Nasal Spray on Social Interaction Deficits Observed in Young Children with Autism: A Randomized Clinical Crossover Trial. Mol. Psychiatry 2016, 21, 1225–1231. [Google Scholar] [CrossRef]
- Parker, K.J.; Oztan, O.; Libove, R.A.; Sumiyoshi, R.D.; Jackson, L.P.; Karhson, D.S.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. Intranasal Oxytocin Treatment for Social Deficits and Biomarkers of Response in Children with Autism. Proc. Natl. Acad. Sci. USA 2017, 114, 8119–8124. [Google Scholar] [CrossRef]
- Domes, G.; Heinrichs, M.; Kumbier, E.; Grossmann, A.; Hauenstein, K.; Herpertz, S.C. Effects of Intranasal Oxytocin on the Neural Basis of Face Processing in Autism Spectrum Disorder. Biol. Psychiatry 2013, 74, 164–171. [Google Scholar] [CrossRef]
- Sikich, L.; Kolevzon, A.; King, B.H.; McDougle, C.J.; Sanders, K.B.; Kim, S.-J.; Spanos, M.; Chandrasekhar, T.; Trelles, M.D.P.; Rockhill, C.M.; et al. Intranasal Oxytocin in Children and Adolescents with Autism Spectrum Disorder. N. Engl. J. Med. 2021, 385, 1462–1473. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Ebstein, R.P.; Yu, R. Intranasal Oxytocin in the Treatment of Autism Spectrum Disorders: A Multilevel Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 122, 18–27. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/home (accessed on 15 April 2022).
- Lee, M.R.; Wehring, H.J.; McMahon, R.P.; Linthicum, J.; Cascella, N.; Liu, F.; Bellack, A.; Buchanan, R.W.; Strauss, G.P.; Contoreggi, C.; et al. Effects of Adjunctive Intranasal Oxytocin on Olfactory Identification and Clinical Symptoms in Schizophrenia: Results from a Randomized Double Blind Placebo Controlled Pilot Study. Schizophr. Res. 2013, 145, 110–115. [Google Scholar] [CrossRef][Green Version]
- Shin, N.Y.; Park, H.Y.; Jung, W.H.; Park, J.W.; Yun, J.-Y.; Jang, J.H.; Kim, S.N.; Han, H.J.; Kim, S.-Y.; Kang, D.-H.; et al. Effects of Oxytocin on Neural Response to Facial Expressions in Patients with Schizophrenia. Neuropsychopharmacology 2015, 40, 2286. [Google Scholar] [CrossRef]
- Van Zuiden, M.; Frijling, J.L.; Nawijn, L.; Koch, S.B.J.; Goslings, J.C.; Luitse, J.S.; Biesheuvel, T.H.; Honig, A.; Veltman, D.J.; Olff, M. Intranasal Oxytocin to Prevent Posttraumatic Stress Disorder Symptoms: A Randomized Controlled Trial in Emergency Department Patients. Biol. Psychiatry 2017, 81, 1030–1040. [Google Scholar] [CrossRef]
- Lischke, A.; Herpertz, S.C.; Berger, C.; Domes, G.; Gamer, M. Divergent Effects of Oxytocin on (para-)limbic Reactivity to Emotional and Neutral Scenes in Females with and without Borderline Personality Disorder. Soc. Cogn. Affect. Neurosci. 2017, 12, 1783–1792. [Google Scholar] [CrossRef]
- Labuschagne, I.; Phan, K.L.; Wood, A.; Angstadt, M.; Chua, P.; Heinrichs, M.; Stout, J.C.; Nathan, P.J. Oxytocin Attenuates Amygdala Reactivity to Fear in Generalized Social Anxiety Disorder. Neuropsychopharmacology 2010, 35, 2403–2413. [Google Scholar] [CrossRef]
- Plessow, F.; Marengi, D.A.; Perry, S.K.; Felicione, J.M.; Franklin, R.; Holmes, T.M.; Holsen, L.M.; Makris, N.; Deckersbach, T.; Lawson, E.A. Effects of Intranasal Oxytocin on the Blood Oxygenation Level-Dependent Signal in Food Motivation and Cognitive Control Pathways in Overweight and Obese Men. Neuropsychopharmacology 2018, 43, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M.F.; Martin-Santos, R.; Osório, F.L. Oxytocin Effects on the Cognition of Women with Postpartum Depression: A Randomized, Placebo-Controlled Clinical Trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110098. [Google Scholar] [CrossRef]
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal Drug Delivery: Opportunities and Toxicologic Challenges during Drug Development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef] [PubMed]
- Mathias, N.R.; Hussain, M.A. Non-Invasive Systemic Drug Delivery: Developability Considerations for Alternate Routes of Administration. J. Pharm. Sci. 2010, 99, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of Intranasal Delivery Route of Drug Administration for Brain Targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, L.M.; Togias, A. Allergic Rhinitis. N. Engl. J. Med. 2015, 372, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.; Merkus, H.M.; Nauman, J.; Bruschi, F.; Foidart, J.M.; Calaf, J. Randomized Comparison of Intranasal and Transdermal Estradiol. Obstet. Gynecol. 2000, 96, 906–912. [Google Scholar] [CrossRef]
- Lee, J.; Brock, G.; Barkin, J.; Bryson, N.; Gronski, M.A.; Ormsby, R. The My-T Study: Patient Satisfaction and Preference Comparing Topical and Nasal Testosterone Therapies. Can. Urol. Assoc. J. 2019, 13, 384–389. [Google Scholar] [CrossRef]
- Hanson, L.R.; Frey, W.H., 2nd. Intranasal Delivery Bypasses the Blood-Brain Barrier to Target Therapeutic Agents to the Central Nervous System and Treat Neurodegenerative Disease. BMC Neurosci. 2008, 9, S5. [Google Scholar] [CrossRef]
- Quintana, D.S.; Westlye, L.T.; Smerud, K.T.; Mahmoud, R.A.; Andreassen, O.A.; Djupesland, P.G. Saliva Oxytocin Measures Do Not Reflect Peripheral Plasma Concentrations after Intranasal Oxytocin Administration in Men. Horm. Behav. 2018, 102, 85–92. [Google Scholar] [CrossRef]
- Martins, D.; Gabay, A.S.; Mehta, M.; Paloyelis, Y. Salivary and Plasmatic Oxytocin Are Not Reliable Trait Markers of the Physiology of the Oxytocin System in Humans. Elife 2020, 9, e62456. [Google Scholar] [CrossRef]
- Jasim, H.; Carlsson, A.; Hedenberg-Magnusson, B.; Ghafouri, B.; Ernberg, M. Saliva as a Medium to Detect and Measure Biomarkers Related to Pain. Sci. Rep. 2018, 8, 3220. [Google Scholar] [CrossRef]
- Weisman, O.; Zagoory-Sharon, O.; Feldman, R. Intranasal Oxytocin Administration Is Reflected in Human Saliva. Psychoneuroendocrinology 2012, 37, 1582–1586. [Google Scholar] [CrossRef]
- Hoffman, E.R.; Brownley, K.A.; Hamer, R.M.; Bulik, C.M. Plasma, Salivary, and Urinary Oxytocin in Anorexia Nervosa: A Pilot Study. Eat. Behav. 2012, 13, 256–259. [Google Scholar] [CrossRef]
- Feldman, R.; Gordon, I.; Zagoory-Sharon, O. Maternal and Paternal Plasma, Salivary, and Urinary Oxytocin and Parent-Infant Synchrony: Considering Stress and Affiliation Components of Human Bonding. Dev. Sci. 2011, 14, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Hamada, H.; Kikusui, T.; Mogi, K.; Nagasawa, M.; Mitsui, S.; Higuchi, T.; Hasegawa, T.; Hiraki, K. Urinary Oxytocin Positively Correlates with Performance in Facial Visual Search in Unmarried Males, without Specific Reaction to Infant Face. Front. Neurosci. 2014, 8, 217. [Google Scholar] [CrossRef]
- Seltzer, L.J.; Ziegler, T.E.; Pollak, S.D. Social Vocalizations Can Release Oxytocin in Humans. Proc. Biol. Sci. 2010, 277, 2661–2666. [Google Scholar] [CrossRef]
- Carter, C.S.; Pournajafi-Nazarloo, H.; Kramer, K.M.; Ziegler, T.E.; White-Traut, R.; Bello, D.; Schwertz, D. Oxytocin: Behavioral Associations and Potential as a Salivary Biomarker. Ann. N.Y. Acad. Sci. 2007, 1098, 312–322. [Google Scholar] [CrossRef]
- Parker, K.J.; Garner, J.P.; Libove, R.A.; Hyde, S.A.; Hornbeak, K.B.; Carson, D.S.; Liao, C.-P.; Phillips, J.M.; Hallmayer, J.F.; Hardan, A.Y. Plasma Oxytocin Concentrations and OXTR Polymorphisms Predict Social Impairments in Children with and without Autism Spectrum Disorder. Proc. Natl. Acad. Sci. USA 2014, 111, 12258–12263. [Google Scholar] [CrossRef]
- American Psychiatric Association. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision (DSM-IV-TR); American Psychiatric Association: Arlington, VA, USA, 2000. [Google Scholar]
- Anagnostou, E.; Soorya, L.; Brian, J.; Dupuis, A.; Mankad, D.; Smile, S.; Jacob, S. Intranasal Oxytocin in the Treatment of Autism Spectrum Disorders: A Review of Literature and Early Safety and Efficacy Data in Youth. Brain Res. 2014, 1580, 188–198. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. Structured Clinical Interview for DSM-5—Research Version (SCID-5-RV, Version 1.0. 0); American Psychiatric Association: Arlington, VA, USA, 2015; pp. 1–94. [Google Scholar]
- United States Department of Health and Human Services. Protection of Human Subjects: Title 45, Code of Federal Regulations, Part 46; Revised on 18 June 1991; Department of Health and Human Services: Washington, DC, USA, 1992. [Google Scholar]
- MacDonald, E.; Dadds, M.R.; Brennan, J.L.; Williams, K.; Levy, F.; Cauchi, A.J. A Review of Safety, Side-Effects and Subjective Reactions to Intranasal Oxytocin in Human Research. Psychoneuroendocrinology 2011, 36, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, M.G.; Francis, S.M.; Lee, R.; de Wit, H.; Jacob, S. Plasma Oxytocin Concentrations Following MDMA or Intranasal Oxytocin in Humans. Psychoneuroendocrinology 2014, 46, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Andari, E.; Duhamel, J.-R.; Zalla, T.; Herbrecht, E.; Leboyer, M.; Sirigu, A. Promoting Social Behavior with Oxytocin in High-Functioning Autism Spectrum Disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 4389–4394. [Google Scholar] [CrossRef]
- Ziegler, T.E.; Scheffler, G.; Snowdon, C.T. The Relationship of Cortisol Levels to Social Environment and Reproductive Functioning in Female Cotton-Top Tamarins, Saguinus Oedipus. Horm. Behav. 1995, 29, 407–424. [Google Scholar] [CrossRef]
- Bick, J.; Dozier, M. Mothers’ and Children’s Concentrations of Oxytocin Following Close, Physical Interactions with Biological and Non-Biological Children. Dev. Psychobiol. 2010, 52, 100–107. [Google Scholar] [CrossRef]
- Gray, P.B.; Parkin, J.C.; Samms-Vaughan, M.E. Hormonal Correlates of Human Paternal Interactions: A Hospital-Based Investigation in Urban Jamaica. Horm. Behav. 2007, 52, 499–507. [Google Scholar] [CrossRef]
- Fries, A.B.W.; Wismer Fries, A.B.; Ziegler, T.E.; Kurian, J.R.; Jacoris, S.; Pollak, S.D. Early Experience in Humans Is Associated with Changes in Neuropeptides Critical for Regulating Social Behavior. Proc. Natl. Acad. Sci. USA 2005, 102, 17237–17240. [Google Scholar] [CrossRef]
- Yuen, K.W.; Garner, J.P.; Carson, D.S.; Keller, J.; Lembke, A.; Hyde, S.A.; Kenna, H.A.; Tennakoon, L.; Schatzberg, A.F.; Parker, K.J. Plasma Oxytocin Concentrations Are Lower in Depressed vs. Healthy Control Women and Are Independent of Cortisol. J. Psychiatr. Res. 2014, 51, 30–36. [Google Scholar] [CrossRef]
- Seltzer, L.J.; Ziegler, T.E. Non-Invasive Measurement of Small Peptides in the Common Marmoset (Callithrix jacchus): A Radiolabeled Clearance Study and Endogenous Excretion under Varying Social Conditions. Horm. Behav. 2007, 51, 436–442. [Google Scholar] [CrossRef]
- Snowdon, C.T.; Pieper, B.A.; Boe, C.Y.; Cronin, K.A.; Kurian, A.V.; Ziegler, T.E. Variation in Oxytocin Is Related to Variation in Affiliative Behavior in Monogamous, Pairbonded Tamarins. Horm. Behav. 2010, 58, 614–618. [Google Scholar] [CrossRef]
- Valstad, M.; Alvares, G.A.; Egknud, M.; Matziorinis, A.M.; Andreassen, O.A.; Westlye, L.T.; Quintana, D.S. The Correlation between Central and Peripheral Oxytocin Concentrations: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2017, 78, 117–124. [Google Scholar] [CrossRef]
- Hammock, E.; Veenstra-VanderWeele, J.; Yan, Z.; Kerr, T.M.; Morris, M.; Anderson, G.M.; Sue Carter, C.; Cook, E.H.; Jacob, S. Examining Autism Spectrum Disorders by Biomarkers: Example From the Oxytocin and Serotonin Systems. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 712–721. [Google Scholar] [CrossRef]
- McCullough, M.E.; Churchland, P.S.; Mendez, A.J. Problems with Measuring Peripheral Oxytocin: Can the Data on Oxytocin and Human Behavior Be Trusted? Neurosci. Biobehav. Rev. 2013, 37, 1485–1492. [Google Scholar] [CrossRef]
- Francis, S.M.; Kirkpatrick, M.G.; de Wit, H.; Jacob, S. Urinary and Plasma Oxytocin Changes in Response to MDMA or Intranasal Oxytocin Administration. Psychoneuroendocrinology 2016, 74, 92–100. [Google Scholar] [CrossRef]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing Neuropeptides: A Transnasal Approach to the Human Brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef]
- Striepens, N.; Kendrick, K.M.; Hanking, V.; Landgraf, R.; Wüllner, U.; Maier, W.; Hurlemann, R. Elevated Cerebrospinal Fluid and Blood Concentrations of Oxytocin Following Its Intranasal Administration in Humans. Sci. Rep. 2013, 3, 3440. [Google Scholar] [CrossRef]
- Neumann, I.D.; Landgraf, R. Balance of Brain Oxytocin and Vasopressin: Implications for Anxiety, Depression, and Social Behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Higashida, H. RAGE Regulates Oxytocin Transport into the Brain. Commun. Biol. 2020, 3, 70. [Google Scholar] [CrossRef]
- Parker, K.J.; Oztan, O.; Libove, R.A.; Mohsin, N.; Karhson, D.S.; Sumiyoshi, R.D.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. A Randomized Placebo-Controlled Pilot Trial Shows That Intranasal Vasopressin Improves Social Deficits in Children with Autism. Sci. Transl. Med. 2019, 11, 7356. [Google Scholar] [CrossRef]
- Colloca, L.; Pine, D.S.; Ernst, M.; Miller, F.G.; Grillon, C. Vasopressin Boosts Placebo Analgesic Effects in Women: A Randomized Trial. Biol. Psychiatry 2016, 79, 794–802. [Google Scholar] [CrossRef]
- Yang, J.; Lu, L.; Wang, H.-C.; Zhan, H.-Q.; Hai, G.-F.; Pan, Y.-J.; Lv, Q.-Q.; Wang, D.-X.; Wu, Y.-Q.; Li, R.-R.; et al. Effect of Intranasal Arginine Vasopressin on Human Headache. Peptides 2012, 38, 100–104. [Google Scholar] [CrossRef]
- Yang, F.-J.; Ma, L.; Yang, J.; Zhu, Z.-L.; Wang, C.-H. Intranasal Vasopressin Relieves Orthopedic Pain After Surgery. Pain Manag. Nurs. 2019, 20, 126–132. [Google Scholar] [CrossRef]
- Zhuang, Q.; Zheng, X.; Becker, B.; Lei, W.; Xu, X.; Kendrick, K.M. Intranasal Vasopressin like Oxytocin Increases Social Attention by Influencing Top-down Control, but Additionally Enhances Bottom-up Control. Psychoneuroendocrinology 2021, 133, 105412. [Google Scholar] [CrossRef]
- Kaya, E.; Sahin, F.K.; Köken, G.; Köse, M.; Cevrioglu, A.S. Acute Effect of Intranasal Estrogen on Cerebral and Cerebellar Perfusion in Postmenopausal Women. Maturitas 2008, 59, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Panay, N.; Toth, K.; Pelissier, C.; Studd, J. Dose-Ranging Studies of a Novel Intranasal Estrogen Replacement Therapy. Maturitas 2001, 38, S15–S22. [Google Scholar] [CrossRef]
- Kurdoglu, M.; Yildirim, M.; Kurdoglu, Z.; Erdem, A.; Erdem, M.; Bilgihan, A.; Goktas, B. Cardiovascular Risk Assessment with Oxidised LDL Measurement in Postmenopausal Women Receiving Intranasal Estrogen Replacement Therapy. Gynecol. Endocrinol. 2011, 27, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Fréchou, M.; Zhang, S.; Liere, P.; Delespierre, B.; Soyed, N.; Pianos, A.; Schumacher, M.; Mattern, C.; Guennoun, R. Intranasal Delivery of Progesterone after Transient Ischemic Stroke Decreases Mortality and Provides Neuroprotection. Neuropharmacology 2015, 97, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Schüssler, P.; Kluge, M.; Adamczyk, M.; Beitinger, M.E.; Beitinger, P.; Bleifuss, A.; Cordeiro, S.; Mattern, C.; Uhr, M.; Wetter, T.C.; et al. Sleep after Intranasal Progesterone vs. Zolpidem and Placebo in Postmenopausal Women—A Randomized, Double-Blind Cross over Study. Psychoneuroendocrinology 2018, 92, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Fréchou, M.; Zhu, X.; Liere, P.; Pianos, A.; Schumacher, M.; Mattern, C.; Guennoun, R. Dose-Dependent and Long-Term Cerebroprotective Effects of Intranasal Delivery of Progesterone after Ischemic Stroke in Male Mice. Neuropharmacology 2020, 170, 108038. [Google Scholar] [CrossRef] [PubMed]
- Luberti, F.R.; Reside, T.-L.; Bonin, P.L.; Carré, J.M. Development of a Single-Dose Intranasal Testosterone Administration Paradigm for Use in Men and Women. Horm. Behav. 2021, 136, 105046. [Google Scholar] [CrossRef]
- Laron, Z.; Frenkel, J.; Deghenghi, R.; Anin, S.; Klinger, B.; Silbergeld, A. Intranasal Administration of the GHRP Hexarelin Accelerates Growth in Short Children. Clin. Endocrinol. 1995, 43, 631–635. [Google Scholar] [CrossRef]
- Rönnberg, L.; Koskimies, A.; Laatikainen, T.; Ranta, T.; Saastamoinen, J. Efficacy of Gonadotropin-Releasing Hormone Agonist (buserelin) in the Treatment of Endometriosis. Acta Obstet. Gynecol. Scand. 1989, 68, 49–53. [Google Scholar] [CrossRef]
- Benedict, C.; Frey, W.H., 2nd; Schiöth, H.B.; Schultes, B.; Born, J.; Hallschmid, M. Intranasal Insulin as a Therapeutic Option in the Treatment of Cognitive Impairments. Exp. Gerontol. 2011, 46, 112–115. [Google Scholar] [CrossRef]
- Freiherr, J.; Hallschmid, M.; Frey, W.H.; Brünner, Y.F.; Chapman, C.D.; Hölscher, C.; Craft, S.; De Felice, F.G.; Benedict, C. Intranasal Insulin as a Treatment for Alzheimer’s Disease: A Review of Basic Research and Clinical Evidence. CNS Drugs 2013, 27, 505–514. [Google Scholar] [CrossRef]
- Reger, M.A.; Watson, G.S.; Frey, W.H., 2nd; Baker, L.D.; Cholerton, B.; Keeling, M.L.; Belongia, D.A.; Fishel, M.A.; Plymate, S.R.; Schellenberg, G.D.; et al. Effects of Intranasal Insulin on Cognition in Memory-Impaired Older Adults: Modulation by APOE Genotype. Neurobiol. Aging 2006, 27, 451–458. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DuBois, M.; Tseng, A.; Francis, S.M.; Haynos, A.F.; Peterson, C.B.; Jacob, S. Utility of Downstream Biomarkers to Assess and Optimize Intranasal Delivery of Oxytocin. Pharmaceutics 2022, 14, 1178. https://doi.org/10.3390/pharmaceutics14061178
DuBois M, Tseng A, Francis SM, Haynos AF, Peterson CB, Jacob S. Utility of Downstream Biomarkers to Assess and Optimize Intranasal Delivery of Oxytocin. Pharmaceutics. 2022; 14(6):1178. https://doi.org/10.3390/pharmaceutics14061178
Chicago/Turabian StyleDuBois, Megan, Angela Tseng, Sunday M. Francis, Ann F. Haynos, Carol B. Peterson, and Suma Jacob. 2022. "Utility of Downstream Biomarkers to Assess and Optimize Intranasal Delivery of Oxytocin" Pharmaceutics 14, no. 6: 1178. https://doi.org/10.3390/pharmaceutics14061178
APA StyleDuBois, M., Tseng, A., Francis, S. M., Haynos, A. F., Peterson, C. B., & Jacob, S. (2022). Utility of Downstream Biomarkers to Assess and Optimize Intranasal Delivery of Oxytocin. Pharmaceutics, 14(6), 1178. https://doi.org/10.3390/pharmaceutics14061178





