New Insight on the Bioactivity of Solanum aethiopicum Linn. Growing in Basilicata Region (Italy): Phytochemical Characterization, Liposomal Incorporation, and Antioxidant Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Extraction
2.3. Total Phenolic Content (TPC)
2.4. DPPH Free Radical Scavenging Test
2.5. Ferric Reducing Antioxidant Power (FRAP)
2.6. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.7. HPLC-DAD Characterization
2.7.1. Phenolic Profile
2.7.2. Carotenoids Profile
2.8. Liposome Preparation and Characterization
2.9. Cell Culture and Treatment with Extracts
2.10. Cell Viability Assay
2.11. Measurement of Intracellular ROS
2.12. Quantitative RT-PCR
2.13. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yields and Total Phenolic Content
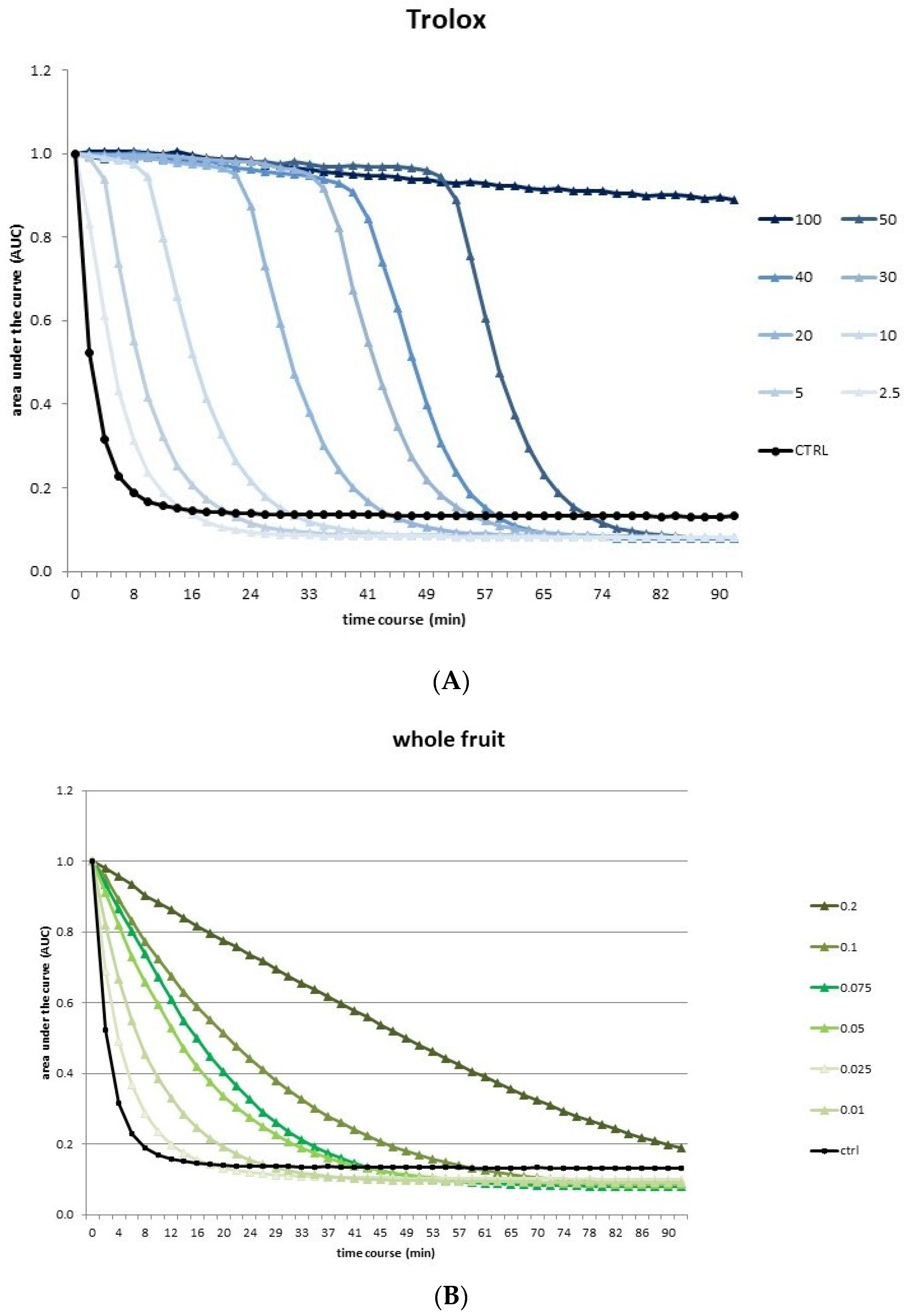
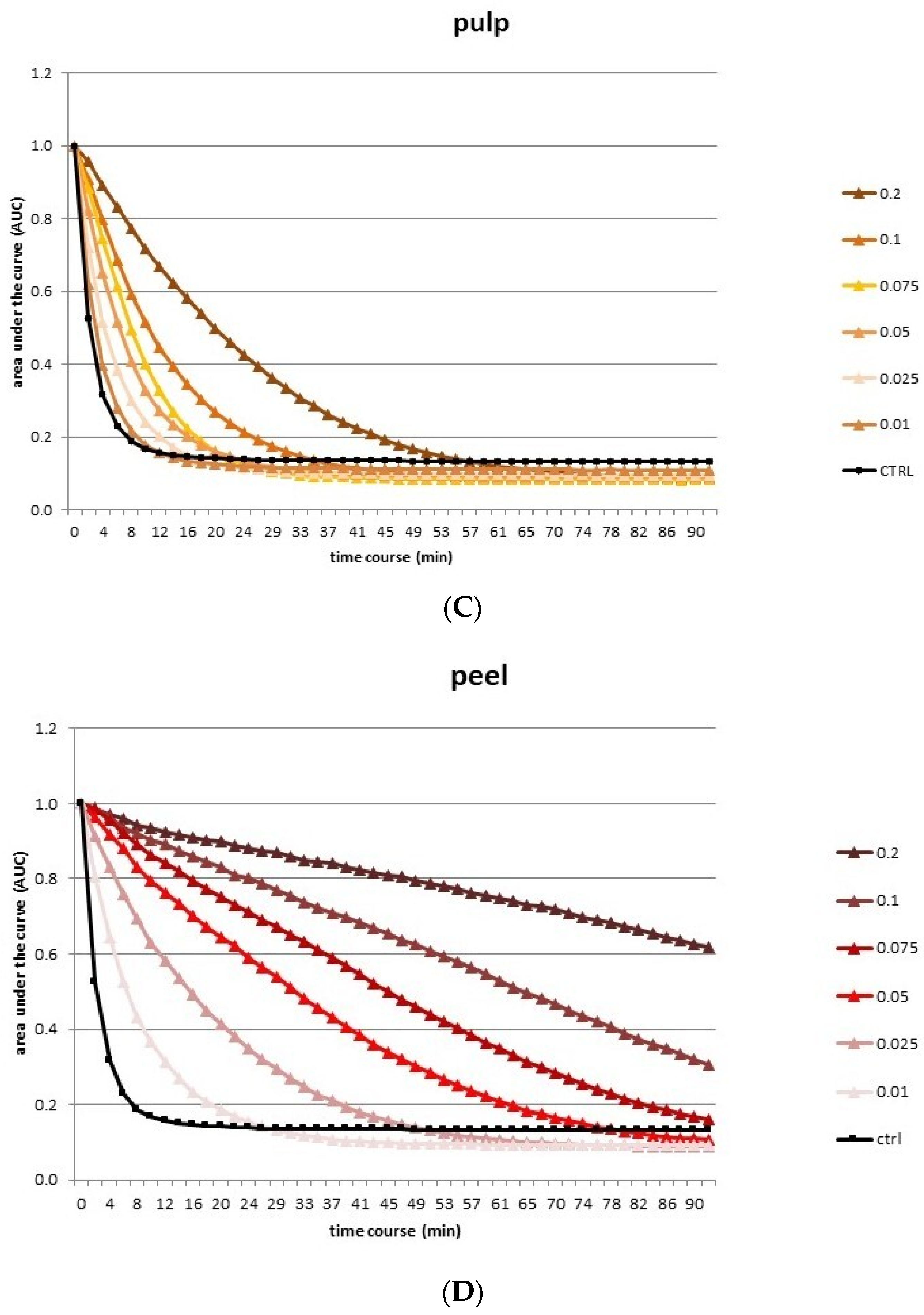
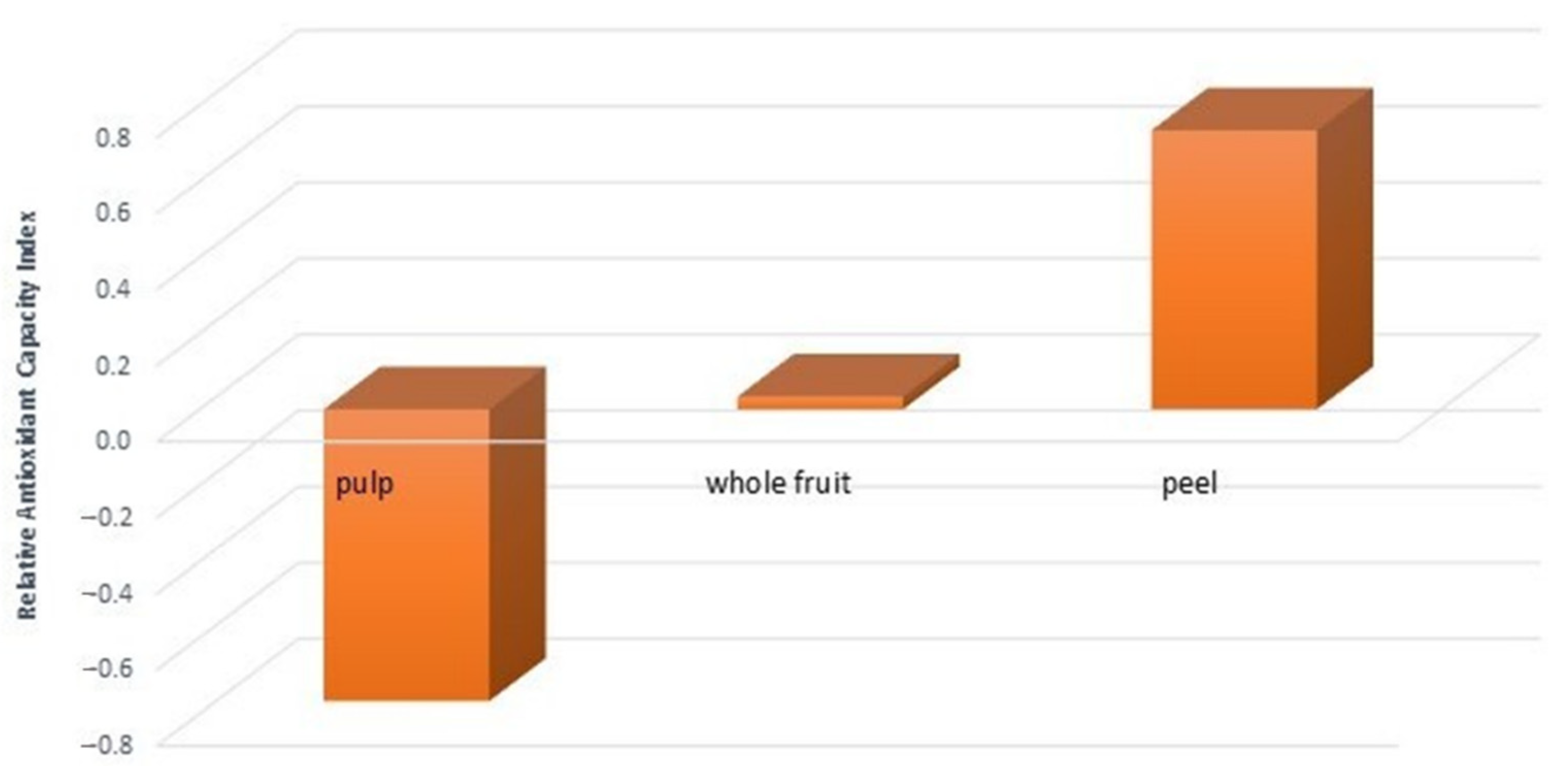
3.2. Antioxidant Activity
3.3. Phytochemical Characterization by HPLC-DAD
3.3.1. Phenolic Profile
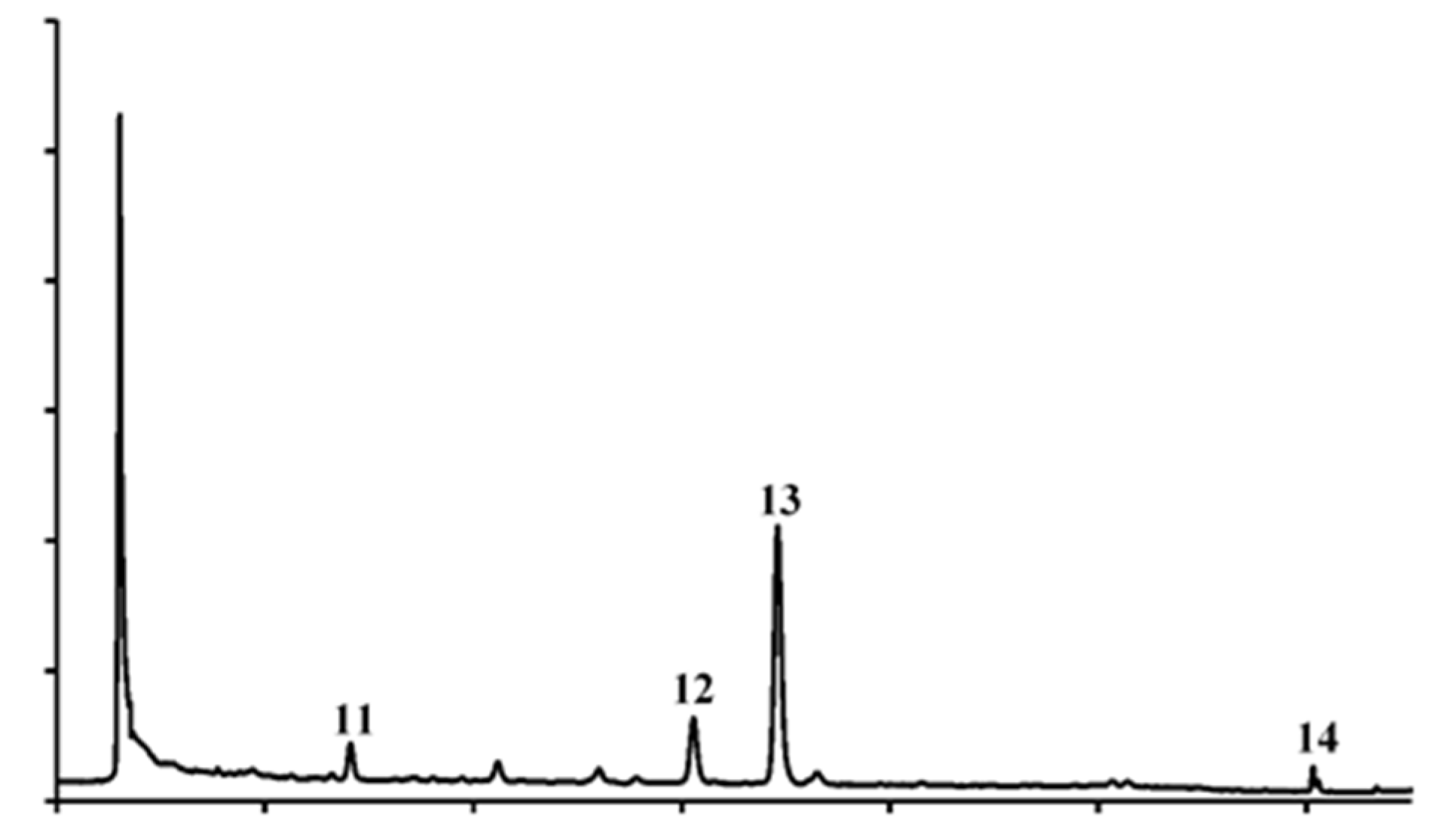
3.3.2. Carotenoids Profile
3.4. Liposome Preparation and Characterization
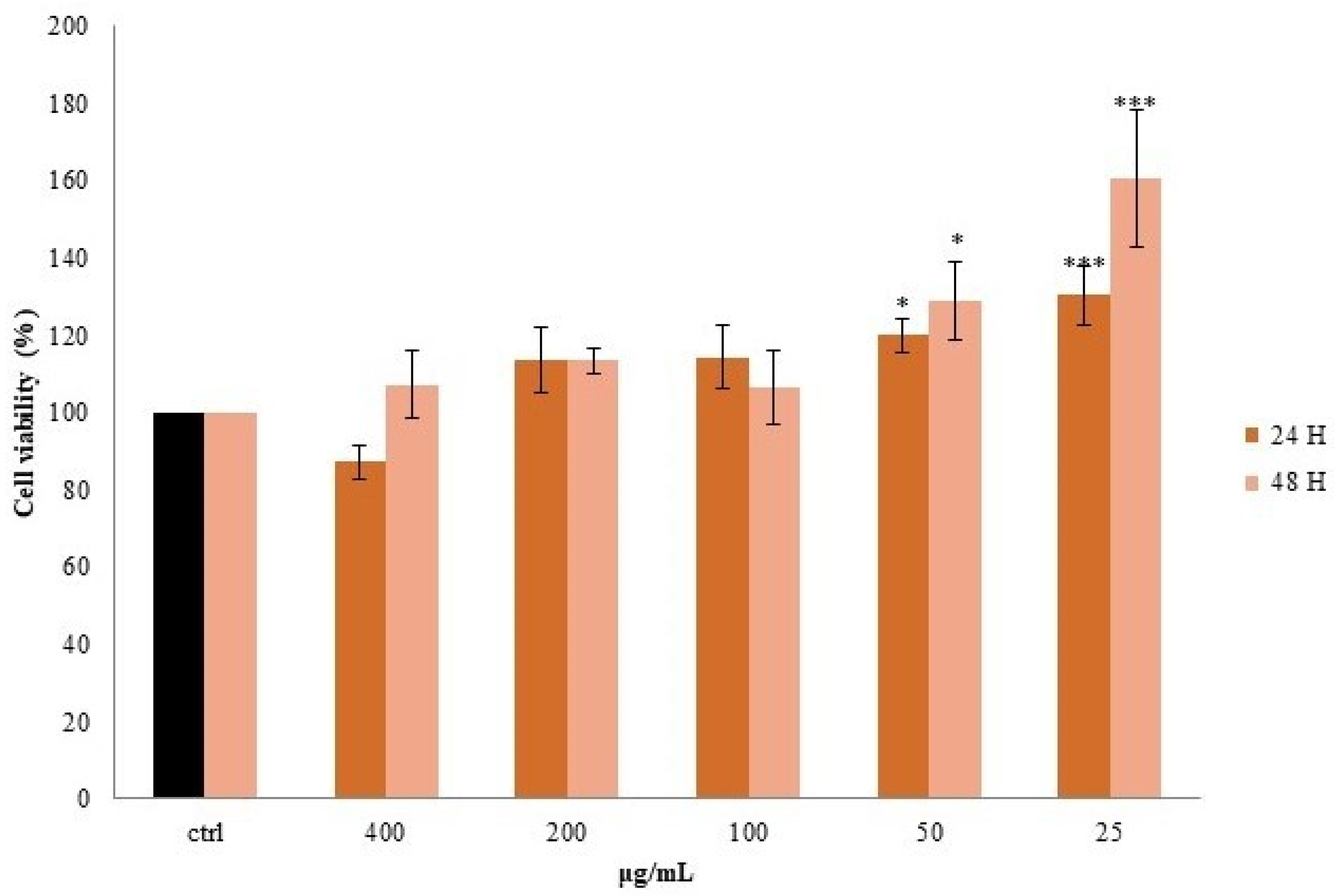
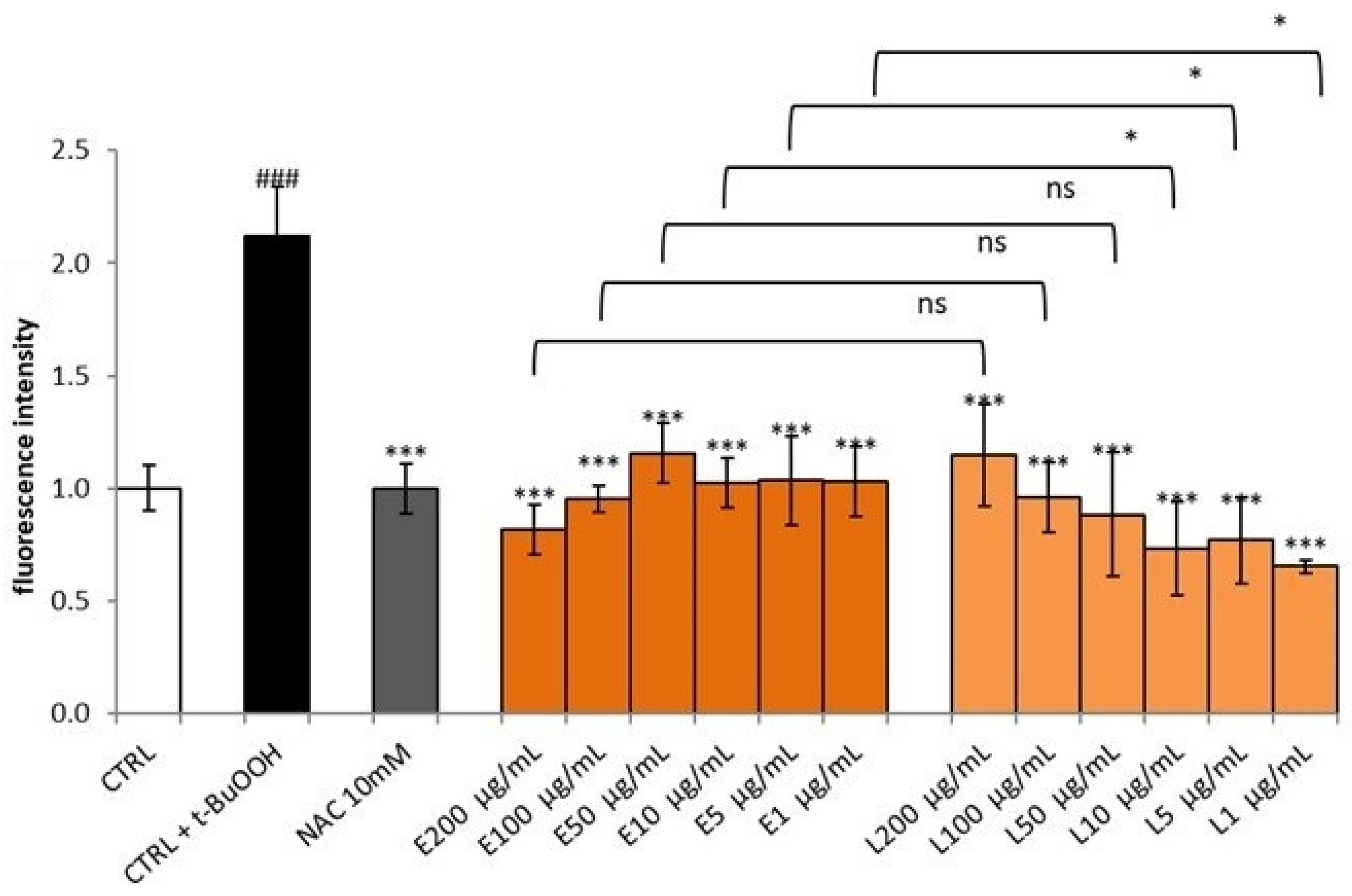
3.5. Effect of S. aethiopicum Peel Extract on Cell Viability and Intracellular ROS
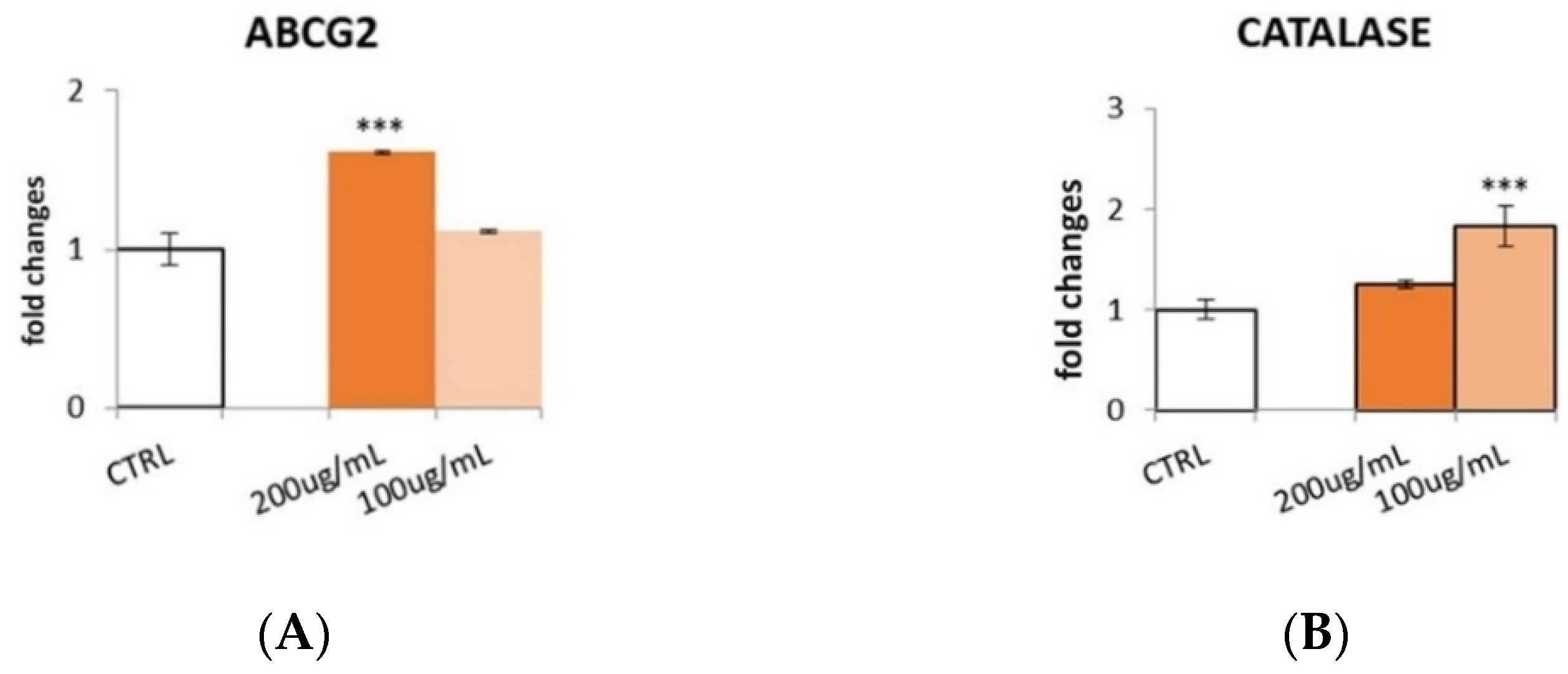
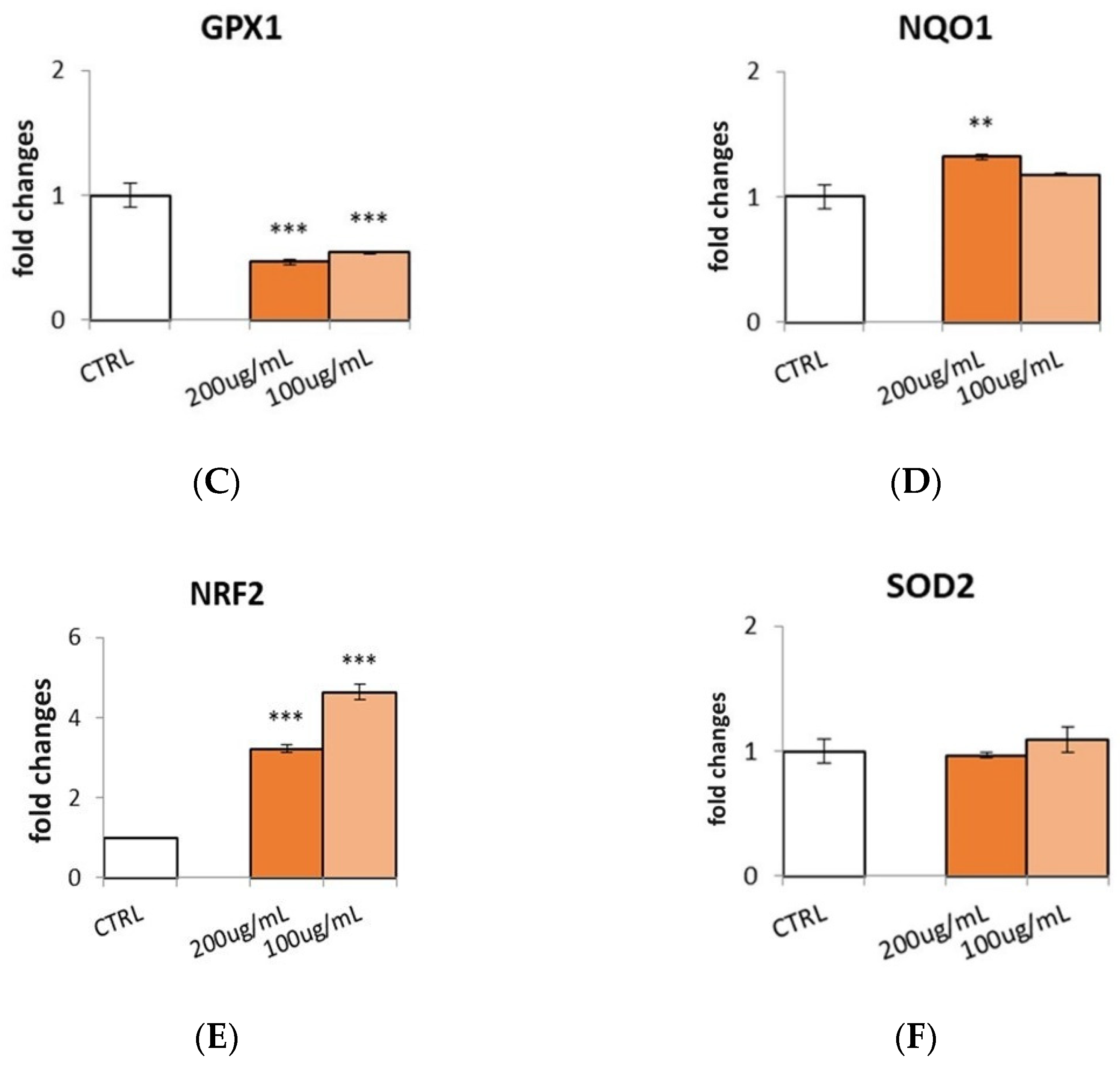
3.6. Effect of S. aethiopicum Peel Extract on Antioxidant Defense Markers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Opoku, K.N.; Bissah, N.A.; Su, T. Solanum aethiopicum: The Nutrient-Rich Vegetable Crop with Great Economic, Genetic Biodiversity and Pharmaceutical Potential. Horticulturae 2021, 7, 126. [Google Scholar] [CrossRef]
- Sunseri, F.; Polignano, G.; Alba, V.; Lotti, C.; Bisignano, V.; Mennella, G.; Drsquo, A.; Bacchi, M.; Riccardi, P.; Fiore, M. Genetic diversity and characterization of African eggplant germplasm collection. Afr. J. Plant Sci. 2010, 4, 231–241. [Google Scholar]
- De Cristofaro, A.; De Maria, S.; Rivelli, A.R. Indagine conoscitiva della melanzana rossa di Rotonda (Solanum aethiopicicum L.) nel Parco Nazionale del Pollino. In Proceedings of the Natura 2000 in Basilicata: Percorsi di Contaminazione tra Natura, Scienza, Arte e cultura Dei Luoghi, Aliano, Italy, 4–6 April 2014. [Google Scholar]
- Nwanna, E.E.; Adebayo, A.A.; Ademosun, A.O.; Oboh, G. Phenolic distribution, antioxidant activity, and enzyme inhibitory properties of eggplant (Solanum aethiopicum) cultivated in two different locations within Nigeria. J. Food Biochem. 2019, 43, e12797. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Mbondo, N.N.; Owino, W.O.; Ambuko, J.; Sila, D.N. Effect of drying methods on the retention of bioactive compounds in African eggplant. Food Sci. Nutr. 2018, 6, 814–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Mata, M.-C.; Yokoyama, W.E.; Hong, Y.-J.; Prohens, J. α-Solasonine and α-solamargine contents of gboma (Solanum macrocarpon L.) and scarlet (Solanum aethiopicum L.) eggplants. J. Agric. Food Chem. 2010, 58, 5502–5508. [Google Scholar] [CrossRef]
- Cordenunsi, B.R.; Oliveira do Nascimento, J.R.; Genovese, M.I.; Lajolo, F.M. Influence of cultivar on quality parameters and chemical composition of strawberry fruits grown in Brazil. J. Agric. Food Chem. 2002, 50, 2581–2586. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G. A critical appraisal of solubility enhancement techniques of polyphenols. J. Pharm. 2014, 2014, 180845. [Google Scholar] [CrossRef] [Green Version]
- Conte, R.; Calarco, A.; Napoletano, A.; Valentino, A.; Margarucci, S.; Di Cristo, F.; Di Salle, A.; Peluso, G. Polyphenols nanoencapsulation for therapeutic applications. J. Biomol. Res. Ther 2016, 5, 1–13. [Google Scholar]
- Vassallo, A.; Armentano, M.F.; Miglionico, R.; Caddeo, C.; Chirollo, C.; Gualtieri, M.J.; Ostuni, A.; Bisaccia, F.; Faraone, I.; Milella, L. Hura crepitans L. extract: Phytochemical characterization, antioxidant activity, and nanoformulation. Pharmaceutics 2020, 12, 553. [Google Scholar] [CrossRef]
- Braca, A.; Sinisgalli, C.; De Leo, M.; Muscatello, B.; Cioni, P.L.; Milella, L.; Ostuni, A.; Giani, S.; Sanogo, R. Phytochemical profile, antioxidant and antidiabetic activities of Adansonia digitata L.(Baobab) from Mali, as a source of health-promoting compounds. Molecules 2018, 23, 3104. [Google Scholar] [CrossRef] [Green Version]
- Faraone, I.; Rai, D.K.; Russo, D.; Chiummiento, L.; Fernandez, E.; Choudhary, A.; Milella, L. Antioxidant, antidiabetic, and anticholinesterase activities and phytochemical profile of Azorella glabra Wedd. Plants 2019, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Sinisgalli, C.; Faraone, I.; Vassallo, A.; Caddeo, C.; Bisaccia, F.; Armentano, M.F.; Milella, L.; Ostuni, A. Phytochemical profile of Capsicum annuum L. cv Senise, incorporation into liposomes, and evaluation of cellular antioxidant activity. Antioxidants 2020, 9, 428. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Valentão, P.; Pereira, J.A.; Silva, B.M.; Tavares, F.; Andrade, P.B. Ficus carica L: Metabolic and biological screening. Food Chem. Toxicol. 2009, 47, 2841–2846. [Google Scholar] [CrossRef]
- Amaro, H.M.; Fernandes, F.; Valentão, P.; Andrade, P.B.; Sousa-Pinto, I.; Malcata, F.X.; Guedes, A.C. Effect of solvent system on extractability of lipidic components of Scenedesmus obliquus (M2-1) and Gloeothece sp. on antioxidant scavenging capacity thereof. Mar. Drugs 2015, 13, 6453–6471. [Google Scholar] [CrossRef] [Green Version]
- Armentano, M.F.; Caterino, M.; Miglionico, R.; Ostuni, A.; Pace, M.C.; Cozzolino, F.; Monti, M.; Milella, L.; Carmosino, M.; Pucci, P. New insights on the functional role of URG7 in the cellular response to ER stress. Biol. Cell 2018, 110, 147–158. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Fatmawati, S.; Asri, A.W. The effect of ethanol concentrations as the extraction solvent on antioxidant activity of Katuk (Sauropus androgynus (L.) Merr.) leaves extracts. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surakarta, Indonesia, 24–25 August 2021; p. 012060. [Google Scholar]
- Khatoon, U.; Sharma, L.; Dubey, R. Assessment of bioactive compounds, antioxidative activity and quantification of phenols through HPLC in solanum species. Ethno Med. 2018, 12, 87–95. [Google Scholar]
- Nakitto, A.M.S.; Byaruhanga, Y.B.; Wagner, A.E.; Muyonga, J.H. Morphological characteristics, bioactive compounds content, and antioxidant activity of different accessions of African eggplant (Solanum anguivi Lam.). J. Appl. Bot. Food Qual. 2021, 94, 220–228. [Google Scholar]
- Russo, D.; Malafronte, N.; Frescura, D.; Imbrenda, G.; Faraone, I.; Milella, L.; Fernandez, E.; De Tommasi, N.J.N.P.R. Antioxidant activities and quali-quantitative analysis of different Smallanthus sonchifolius [(Poepp. and Endl.) H. Robinson] landrace extracts. Nat. Prod. Res. 2015, 29, 1673–1677. [Google Scholar] [CrossRef]
- Nwanna, E.; Ibukun, E.; Oboh, G.; Ademosun, A.; Boligon, A.; Athayde, M. HPLC-DAD analysis and in-vitro property of polyphenols extracts from (Solanum Aethiopium) Fruits on α-amylase, α-glucosidase and angiotensin-1-converting enzyme activities. Int. J. Biomed. Sci. IJBS 2014, 10, 272–281. [Google Scholar]
- Olszowy-Tomczyk, M. How to express the antioxidant properties of substances properly? Chem. Pap. 2021, 75, 6157–6167. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.; Villano, D.; Troncoso, A.; Garcia-Parrilla, M. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Sun, T.; Tanumihardjo, S. An integrated approach to evaluate food antioxidant capacity. J. Food Sci. 2007, 72, R159–R165. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Ferreira, M.A. Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and jam: Antioxidant activity. J. Agric. Food Chem. 2004, 52, 4705–4712. [Google Scholar] [CrossRef]
- Ma, C.; Whitaker, B.D.; Kennelly, E.J. New 5-O-caffeoylquinic acid derivatives in fruit of the wild eggplant relative Solanum viarum. J. Agric. Food Chem. 2010, 58, 11036–11042. [Google Scholar] [CrossRef]
- Plazas, M.; Prohens, J.; Cuñat, A.N.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Andújar, I. Reducing capacity, chlorogenic acid content and biological activity in a collection of scarlet (Solanum aethiopicum) and gboma (S. macrocarpon) eggplants. Int. J. Mol. Sci. 2014, 15, 17221–17241. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Mibei, E.K.; Ambuko, J.; Giovannoni, J.J.; Onyango, A.N.; Owino, W.O. Carotenoid profiling of the leaves of selected African eggplant accessions subjected to drought stress. Food Sci. Nutr. 2017, 5, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Akanitapichat, P.; Phraibung, K.; Nuchklang, K.; Prompitakkul, S. Antioxidant and hepatoprotective activities of five eggplant varieties. Food Chem. Toxicol. 2010, 48, 3017–3021. [Google Scholar] [CrossRef]
- Meerloo, J.V.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture; Springer: Berlin/Heidelberg, Germany, 2011; pp. 237–245. [Google Scholar]
- Yadav, D.; Sandeep, K.; Pandey, D.; Dutta, R.K. Liposomes for drug delivery. J. Biotechnol. Biomater. 2017, 7, 276. [Google Scholar] [CrossRef]
- Giglio, F.; Castiglione Morelli, M.A.; Matera, I.; Sinisgalli, C.; Rossano, R.; Ostuni, A. Muscari comosum L. Bulb Extracts Modulate Oxidative Stress and Redox Signaling in HepG2 Cells. Molecules 2021, 26, 416. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Callaghan, D.; Xiong, H.; Yang, Z.; Huang, P.; Zhang, W. Abcg2 deficiency augments oxidative stress and cognitive deficits in Tg-SwDI transgenic mice. J. Neurochem. 2012, 122, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Wasik, U.; Milkiewicz, M.; Kempinska-Podhorodecka, A.; Milkiewicz, P. Protection against oxidative stress mediated by the Nrf2/Keap1 axis is impaired in Primary Biliary Cholangitis. Sci. Rep. 2017, 7, 44769. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Pan, J.; Ren, D.; Cheng, Y.; Fan, P.; Lou, H. Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food Chem. Toxicol. 2008, 46, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679. [Google Scholar] [CrossRef]
- Khan, M.M.; Ahmad, A.; Ishrat, T.; Khuwaja, G.; Srivastawa, P.; Khan, M.B.; Raza, S.S.; Javed, H.; Vaibhav, K.; Khan, A. Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res. 2009, 1292, 123–135. [Google Scholar] [CrossRef]
- Alía, M.; Mateos, R.; Ramos, S.; Lecumberri, E.; Bravo, L.; Goya, L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur. J. Nutr. 2006, 45, 19–28. [Google Scholar] [CrossRef]

| Extraction Yield (%) | TPC 1 (mg GAE/g) | DPPH 2 (mg TE/g) | ORAC 3 (μmol TE/kg) | FRAP 4 (mg TE/g) | |
|---|---|---|---|---|---|
| PEEL | 5.82 | 20.94 ± 0.83 a | 16.96 ± 0.57 a | 4477.22 ± 330.64 a | 21.52 ± 0.58 a |
| PULP | 4.72 | 6.38 ± 0.19 c | 4.73 ± 0.14 c | 700.37 ± 125.56 c | 6.30 ± 0.12 c |
| WHOLE FRUIT | 5.65 | 9.41 ± 0.17 b | 8.03 ± 0.16 c | 1565.31 ± 374.65 b | 9.80 ± 0.58 b |
| Peak | Compound | Peel | Pulp | Whole Fruit |
|---|---|---|---|---|
| 1 | 4-O-Caffeoylquinic acid | – | Nq | 56.23 ± 1.33 |
| 2 | 5-O-Caffeoylquinic acid | 1639.82 ± 117.50 | 1278.22 ± 129.33 | 1722.19 ± 36.66 |
| 3 | Eriodictyol-7-O-glucoside | 221.21 ± 12.18 | – | – |
| 4 | Naringenin-7-O-glucoside | 1270.99 ± 115.80 | – | 206.86 ± 7.74 |
| 5 | Eriodictyol | 106.26 ± 4.868 | – | Nq |
| 6 | Quercetin-3-O-rutinoside | 4807.21 ± 543.00 | – | 639.15 ± 29.77 |
| 7 | 4,5-di-O-Caffeoylquinic acid | Nq | 53.22 ± 3.18 | 91.37 ± 3.77 |
| 8 | Kaempferol-3-O-glucoside | 712.85 ± 75.17 | – | 83.58 ± 4.23 |
| 9 | Kaempferol-3-O-rutinoside | 2320.18 ± 245.93 | – | 265.78 ± 10.38 |
| 10 | Naringenin | 5047.89 ± 509.23 | – | 558.78 ± 31.56 |
| TOTAL | 16126.40 ± 1623.48 | 1331.44 ± 132.50 | 3623.55 ± 106.19 |
| Peak | Compound | Peel | Pulp | Whole Fruit |
|---|---|---|---|---|
| 11 | Lutein | 10.84 ± 0.80 | – | Nq |
| 12 | α-Carotene | 13.61 ± 0.56 | – | Nq |
| 13 | β-Carotene | 933.80 ± 1.83 | – | Nq |
| 14 | Lycopene | 95.06 ± 1.83 | – | – |
| TOTAL | 1053.30 ± 45.16 | – |
| Mean Diameter (nm) | P.I. | Zeta Potential (mV) | |
|---|---|---|---|
| Empty liposomes | 80 ± 6.4 | 0.27 ± 0.01 | −16 ± 3.6 |
| S. aethiopicum extract liposomes | 77 ± 6.7 | 0.26 ± 0.03 | −20 ± 4.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraone, I.; Lela, L.; Ponticelli, M.; Gorgoglione, D.; De Biasio, F.; Valentão, P.; Andrade, P.B.; Vassallo, A.; Caddeo, C.; Falabella, R.; et al. New Insight on the Bioactivity of Solanum aethiopicum Linn. Growing in Basilicata Region (Italy): Phytochemical Characterization, Liposomal Incorporation, and Antioxidant Effects. Pharmaceutics 2022, 14, 1168. https://doi.org/10.3390/pharmaceutics14061168
Faraone I, Lela L, Ponticelli M, Gorgoglione D, De Biasio F, Valentão P, Andrade PB, Vassallo A, Caddeo C, Falabella R, et al. New Insight on the Bioactivity of Solanum aethiopicum Linn. Growing in Basilicata Region (Italy): Phytochemical Characterization, Liposomal Incorporation, and Antioxidant Effects. Pharmaceutics. 2022; 14(6):1168. https://doi.org/10.3390/pharmaceutics14061168
Chicago/Turabian StyleFaraone, Immacolata, Ludovica Lela, Maria Ponticelli, Domenico Gorgoglione, Filomena De Biasio, Patricia Valentão, Paula B. Andrade, Antonio Vassallo, Carla Caddeo, Roberto Falabella, and et al. 2022. "New Insight on the Bioactivity of Solanum aethiopicum Linn. Growing in Basilicata Region (Italy): Phytochemical Characterization, Liposomal Incorporation, and Antioxidant Effects" Pharmaceutics 14, no. 6: 1168. https://doi.org/10.3390/pharmaceutics14061168
APA StyleFaraone, I., Lela, L., Ponticelli, M., Gorgoglione, D., De Biasio, F., Valentão, P., Andrade, P. B., Vassallo, A., Caddeo, C., Falabella, R., Ostuni, A., & Milella, L. (2022). New Insight on the Bioactivity of Solanum aethiopicum Linn. Growing in Basilicata Region (Italy): Phytochemical Characterization, Liposomal Incorporation, and Antioxidant Effects. Pharmaceutics, 14(6), 1168. https://doi.org/10.3390/pharmaceutics14061168