Population Pharmacokinetics of Busulfan and Its Metabolite Sulfolane in Patients with Myelofibrosis Undergoing Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Dosing, Pharmacokinetic Sampling and Quantification
2.3. Genotyping
2.4. PopPK Analysis
2.5. Covariate Model
2.6. Model Evaluation
3. Results
3.1. Patients and Data
3.2. Base Model
3.3. Covariate Model
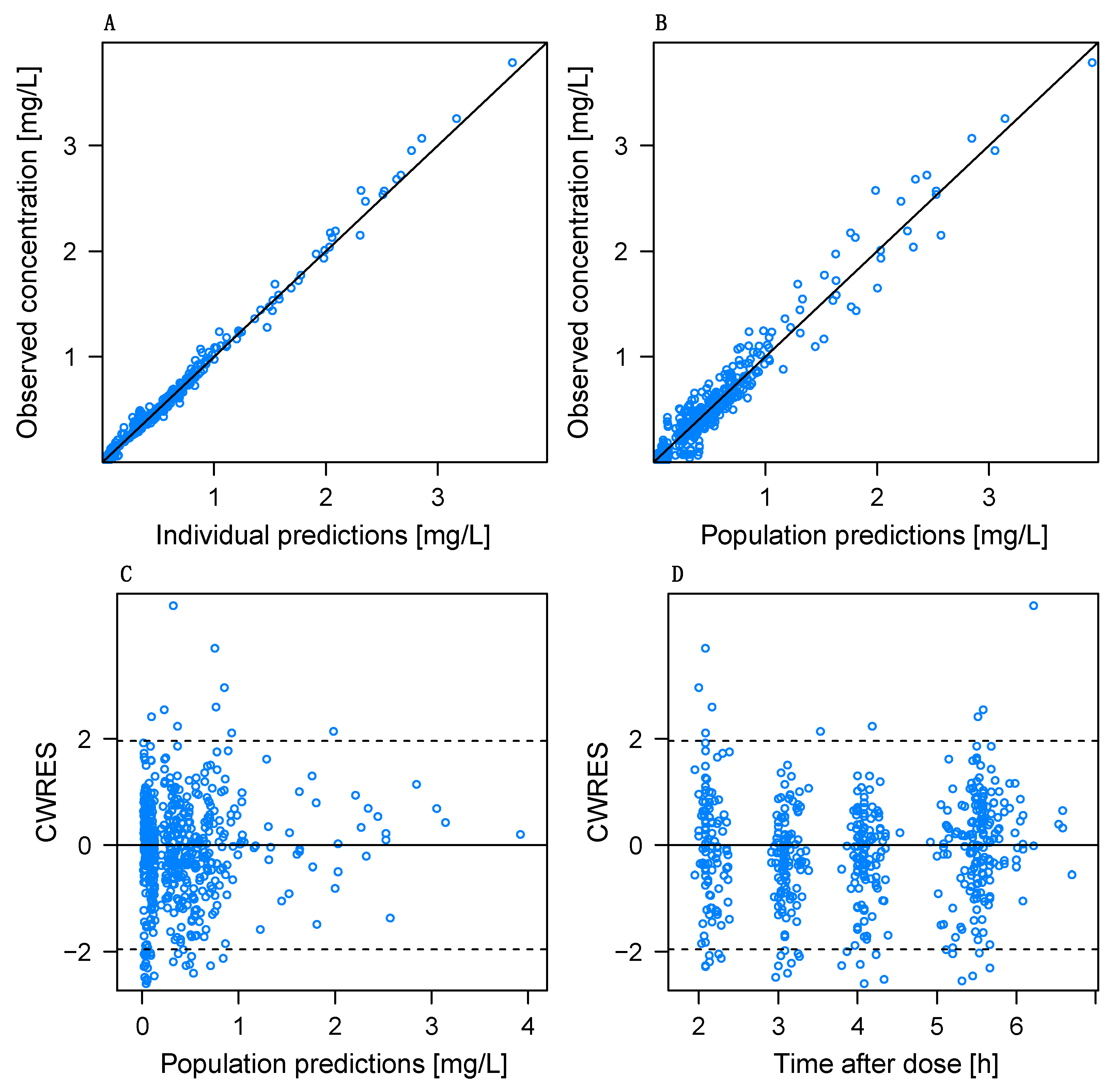
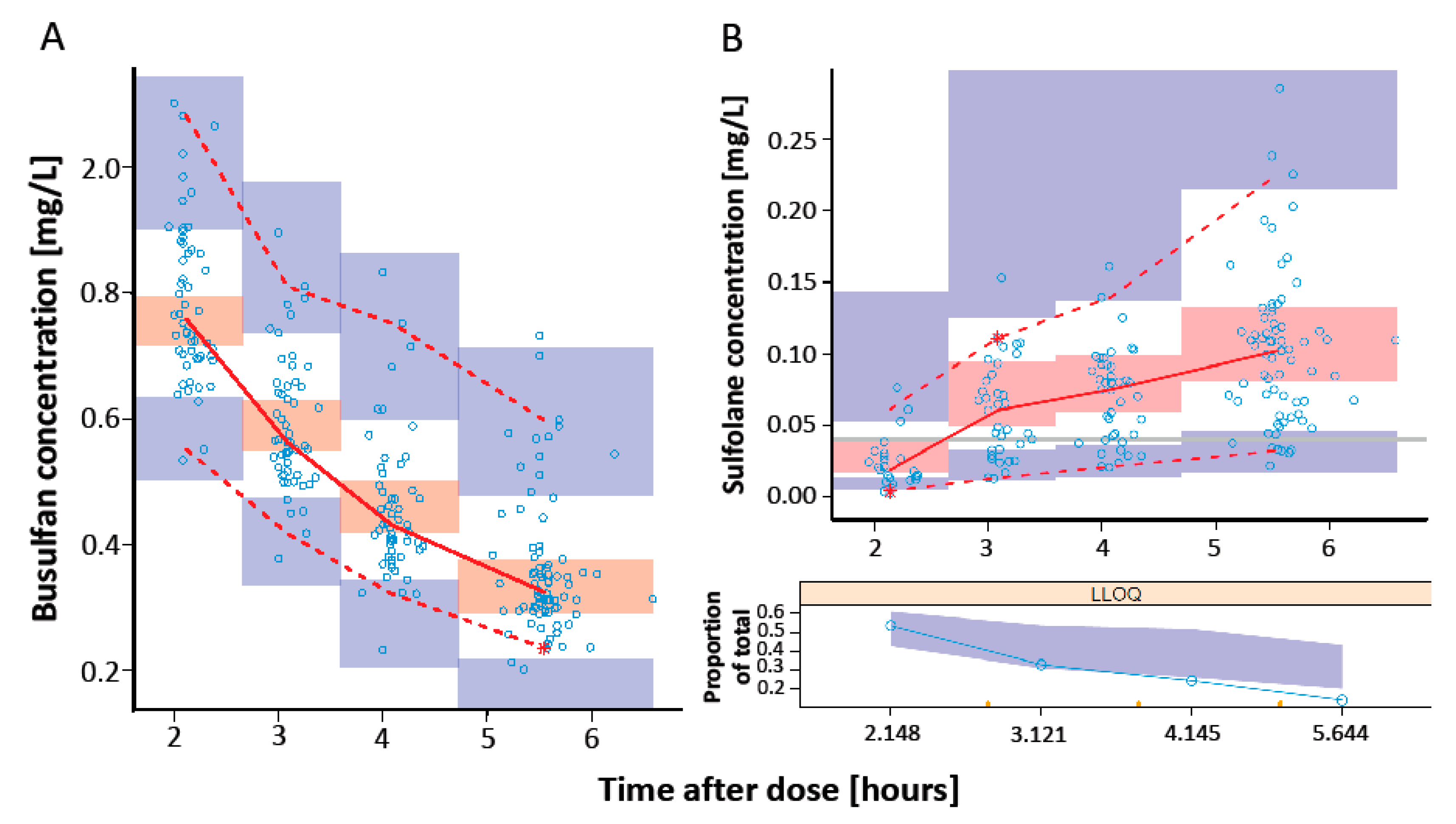
3.4. Model Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farhadfar, N.; Cerquozzi, S.; Patnaik, M.; Tefferi, A. Allogeneic Hematopoietic Stem-Cell Transplantation for Myelofibrosis: A Practical Review. J. Oncol. Pract. 2016, 12, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Kralovics, R. From Janus kinase 2 to calreticulin: The clinically relevant genomic landscape of myeloproliferative neoplasms. Blood 2014, 123, 3714–3719. [Google Scholar] [CrossRef] [PubMed]
- Rumi, E.; Pietra, D.; Pascutto, C.; Guglielmelli, P.; Martínez-Trillos, A.; Casetti, I.; Colomer, D.; Pieri, L.; Pratcorona, M.; Rotunno, G.; et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood 2014, 124, 1062–1069. [Google Scholar] [CrossRef]
- McLornan, D.P.; Malpassuti, V.; Lippinkhof-Kozijn, A.; Potter, V.; Beelen, D.; Bunjes, D.; Sengeloev, V.; Radujkovic, A.; Passweg, J.; Chalandon, Y.; et al. Outcome of allogeneic haemato-poietic stem cell transplantation in myeloproliferative neoplasm, unclassifiable: A retrospective study by the Chronic Malignancies Working Party of the EBMT. Br. J. Haematol. 2020, 190, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Holler, E.; Kobbe, G.; Bornhäuser, M.; Schwerdtfeger, R.; Baurmann, H.; Nagler, A.; Bethge, W.; Stelljes, M.; Uharek, L.; et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: A prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood 2009, 114, 5264–5270. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Kunze, K.L.; Temkit, M.; Partain, D.K.; Patnaik, M.S.; Slack, J.L.; Khera, N.; Hogan, W.J.; Roy, V.; Noel, P.; et al. Comparison of reduced intensity conditioning regimens used in patients undergoing hematopoietic stem cell transplantation for myelofibrosis. Bone Marrow Transplant. 2019, 54, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Popat, U.; Mehta, R.S.; Bassett, R.; Kongtim, P.; Chen, J.; Alousi, A.M.; Anderlini, P.; Ciurea, S.; Hosing, C.; Jones, R.B.; et al. Optimizing the Conditioning Regimen for Hematopoietic Cell Transplant in Myelofibrosis: Long-Term Results of a Prospective Phase II Clinical Trial. Biol. Blood Marrow Transplant. 2020, 26, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Andersson, B.S.; Thall, P.F.; Valdez, B.C.; Milton, D.R.; Alatrash, G.; Chen, J.; Gulbis, A.; Chu, D.; Martinez, C.; Parmar, S.; et al. Fludarabine with pharmacokinetically guided IV busulfan is superior to fixed-dose delivery in pretransplant conditioning of AML/MDS patients. Bone Marrow Transplant. 2016, 52, 580–587. [Google Scholar] [CrossRef]
- Palmer, J.; McCune, J.S.; Perales, M.-A.; Marks, D.; Bubalo, J.; Mohty, M.; Wingard, J.R.; Paci, A.; Hassan, M.; Bredeson, C.; et al. Personalizing Busulfan-Based Conditioning: Considerations from the American Society for Blood and Marrow Transplantation Practice Guidelines Committee. Biol. Blood Marrow. Transplant. 2016, 22, 1915–1925. [Google Scholar] [CrossRef]
- Perkins, J.; Field, T.; Kim, J.; Kharfan-Dabaja, M.A.; Ayala, E.; Pérez, L.; Fernandez, H.; Fancher, K.; Tate, C.; Shaw, L.M.; et al. Pharmacokinetic targeting of i.v. BU with fludarabine as conditioning before hematopoietic cell transplant: The effect of first-dose area under the concentration time curve on transplant-related outcomes. Bone Marrow Transplant. 2010, 46, 1418–1425. [Google Scholar] [CrossRef][Green Version]
- Bartelink, I.H.; Lalmohamed, A.; van Reij, E.M.L.; Dvorak, C.; Savic, R.M.; Zwaveling, J.; Bredius, R.G.M.; Egberts, T.; Bierings, M.; Kletzel, M.; et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: A multicentre, retrospective cohort analysis. Lancet Haematol. 2016, 3, e526–e536. [Google Scholar] [CrossRef]
- McCune, J.S.; Holmberg, L.A. Busulfan in hematopoietic stem cell transplant setting. Expert Opin. Drug Metab. Toxicol. 2009, 5, 957–969. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.S.; Bemer, M.J.; Barrett, J.S.; Baker, K.S.; Gamis, A.S.; Holford, N. Busulfan in Infant to Adult Hematopoietic Cell Transplant Recipients: A Population Pharmacokinetic Model for Initial and Bayesian Dose Personalization. Clin. Cancer Res. 2013, 20, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, I.H.; Boelens, J.J.; Bredius, R.G.M.; Egberts, A.C.G.; Wang, C.; Bierings, M.B.; Shaw, P.J.; Nath, C.E.; Hempel, G.; Zwaveling, J.; et al. Body Weight-Dependent Pharmacoki-netics of Busulfan in Paediatric Haematopoietic Stem Cell Transplantation Patients: Towards Individualized Dosing. Clin. Pharmacokinet. 2012, 51, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Trame, M.N.; Bergstrand, M.; Karlsson, M.O.; Boos, J.; Hempel, G. Population Pharmacokinetics of Busulfan in Children: In-creased Evidence for Body Surface Area and Allometric Body Weight Dosing of Busulfan in Children. Clin. Cancer Res. 2011, 17, 6867–6877. [Google Scholar] [CrossRef]
- Long-Boyle, J.; Savic, R.; Yan, S.; Bartelink, I.; Musick, L.; French, D.; Law, J.; Horn, B.; Cowan, M.J.; Dvorak, C.C. Population Pharmacokinetics of Busulfan in Pediatric and Young Adult Patients Undergoing Hematopoietic Cell Transplant: A Model-Based Dosing Algorithm for Personalized Therapy and Implementation into Routine Clinical Use. Ther. Drug Monit. 2016, 26, 236. [Google Scholar] [CrossRef]
- Takahashi, T.; Illamola, S.M.; Jennissen, C.A.; Long, S.E.; Lund, T.C.; Orchard, P.J.; Gupta, A.O.; Long-Boyle, J.R. Busulfan dose Recommendation in Inherited Metabolic Disorders: Population Pharmacokinetic Analysis. Transplant. Cell. Ther. 2021, 28, 104. [Google Scholar] [CrossRef]
- Essmann, S.; Dadkhah, A.; Janson, D.; Wolschke, C.; Ayuk, F.; Kröger, N.M.; Langebrake, C. Iron Chelation with Deferasirox Increases Busulfan AUC During Conditioning Chemotherapy Prior to Allogeneic Stem Cell Transplantation. Transplant. Cell. Ther. 2021, 28, 115.e1–115.e5. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, H.J.; Lee, S.H.; Yu, K.-S.; Kim, N.H.; Yuk, Y.J.; Jang, M.K.; Han, E.J.; Kim, H.; Song, S.H.; et al. Highly Variable Pharmacokinetics of Once-Daily Intravenous Busulfan When Combined with Fludarabine in Pediatric Patients: Phase I Clinical Study for Determination of Optimal Once-Daily Busulfan Dose Using Pharmacokinetic Modeling. Biol. Blood Marrow Transplant. 2012, 18, 944–950. [Google Scholar] [CrossRef]
- Marsit, H.; Philippe, M.; Neely, M.; Rushing, T.; Bertrand, Y.; Ducher, M.; Leclerc, V.; Guitton, J.; Bleyzac, N.; Goutelle, S. Intra-individual Pharmacokinetic Variability of Intravenous Busulfan in Hematopoietic Stem Cell-Transplanted Children. Clin. Pharmacokinet. 2020, 59, 1049–1061. [Google Scholar] [CrossRef]
- Lawson, R.; Staatz, C.E.; Fraser, C.J.; Hennig, S. Review of the Pharmacokinetics and Pharmacodynamics of Intravenous Busulfan in Paediatric Patients. Clin. Pharmacokinet. 2020, 60, 17–51. [Google Scholar] [CrossRef] [PubMed]
- Zwaveling, J.; Press, R.R.; Bredius, R.G.M.; van Derstraaten, T.R.J.H.M.; Hartigh, J.D.; Bartelink, I.H.; Boelens, J.J.; Guchelaar, H.-J. Glutathione S-transferase Polymorphisms Are Not Associated with Population Pharmacokinetic Parameters of Busulfan in Pediatric Patients. Ther. Drug Monit. 2008, 30, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Kim, M.G.; Han, N.; Kim, T.; Ji, E.; Park, S.; Kim, I.-W.; Oh, J.M. Population pharmacokinetics and pharmacodynamics of busulfan with GSTA1 polymorphisms in patients undergoing allogeneic hematopoietic stem cell transplantation. Pharmacogenomics 2015, 16, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Curtis, P.H.-D.; Uppugunduri, C.R.S.; Rezgui, M.A.; Nava, T.; Mlakar, V.; Lesne, L.; Theoret, Y.; Chalandon, Y.; Dupuis, L.L.; et al. GSTA1 diplotypes affect busulfan clearance and toxicity in children undergoing allogeneic hematopoietic stem cell transplantation: A multicenter study. Oncotarget 2017, 8, 90852–90867. [Google Scholar] [CrossRef]
- Nava, T.; Kassir, N.; Rezgui, M.A.; Uppugunduri, C.R.S.; Huezo-Diaz Curtis, P.; Duval, M.; Theoret, Y.; Daudt, L.E.; Litalien, C.; Ansari, M.; et al. Incorporation of GSTA1 genetic variations into a population pharmacokinetic model for IV busulfan in paediatric hematopoietic stem cell transplantation: GSTA1-based busulfan population pharmacokinetic model in children. Br. J. Clin. Pharmacol. 2018, 84, 1494–1504. [Google Scholar] [CrossRef]
- Hassine, K.B.; Nava, T.; Théoret, Y.; Nath, C.E.; Daali, Y.; Kassir, N.; Lewis, V.; Bredius, R.G.M.; Shaw, P.J.; Shaw, P.J.; et al. Precision dosing of intravenous busulfan in pediatric hematopoietic stem cell transplantation: Results from a multicenter population pharmacokinetic study. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 1043–1056. [Google Scholar] [CrossRef]
- Bertrand, J.; Laffont, C.M.; Mentré, F.; Chenel, M.; Comets, E. Development of a Complex Parent-Metabolite Joint Population Pharmacokinetic Model. AAPS J. 2011, 13, 390–404. [Google Scholar] [CrossRef]
- Uppugunduri, C.R.S.; Rezgui, M.A.; Diaz, P.H.; Tyagi, A.K.; Rousseau, J.; Daali, Y.; Duval, M.; Bittencourt, H.; Krajinovic, M.; Ansari, M. The association of cytochrome P450 genetic polymorphisms with sulfolane formation and the efficacy of a busulfan-based conditioning regimen in pediatric patients undergoing hematopoietic stem cell transplantation. Pharm. J. 2013, 14, 263–271. [Google Scholar] [CrossRef]
- Passamonti, F.; Cervantes, F.; Vannucchi, A.M.; Morra, E.; Rumi, E.; Pereira, A.; Guglielmelli, P.; Pungolino, E.; Caramella, M.; Maffioli, M.; et al. A dynamic prognostic model to predict survival in primary myelofibrosis: A study by the IWG-MRT (International Working Group for Myeloproliferative Neo-plasms Research and Treatment). Blood 2010, 115, 1703–1708. [Google Scholar] [CrossRef]
- Passamonti, F.; Giorgino, T.; Mora, B.; Guglielmelli, P.; Rumi, E.; Maffioli, M.; Rambaldi, A.; Caramella, M.; Komrokji, R.; Gotlib, J.; et al. A clinical-molecular prognostic model to predict survival in patients with post polycythemia vera and post essential thrombocythemia myelofibrosis. Leukemia 2017, 31, 2726–2731. [Google Scholar] [CrossRef]
- Gagelmann, N.; Eikema, D.-J.; de Wreede, L.C.; Koster, L.; Wolschke, C.; Arnold, R.; Kanz, L.; McQuaker, G.; Marchand, T.; Socié, G.; et al. Comparison of Dynamic International Prognostic Scoring System and MYelofibrosis SECondary to PV and ET Prognostic Model for Prediction of Outcome in Polycythemia Vera and Essential Thrombocythemia Myelofibrosis after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, e204–e208. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.; Shen, D.D.; Shireman, L.; Phillips, B. Bioanalytical Method: Tetrahydrothiophene-1-Oxide and Sulfolane and 3-Hydroxysulfolane in Plasma; Report No.: BAM217; National Cancer Institute, University of Washington School of Pharmacy: Seattle, WA, USA, 2017.
- Coles, B.F.; Morel, F.; Rauch, C.; Huber, W.W.; Yang, M.; Teitel, C.H.; Green, B.; Lang, N.; Kadlubar, F.F. Effect of polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and GSTA2 expression. Pharmacogenetics 2001, 11, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; van Benten, M.; Beijnen, J.H.; Schellens, J.H.; Huitema, A.D. Piraña and PCluster: A modeling environment and cluster infrastructure for NONMEM. Comput. Methods Programs Biomed. 2011, 101, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Bonate, P.L. Pharmacokinetic-Pharmacodynamic Modeling and Simulation, 2nd ed.; Springer: Boston, MA, USA, 2011. [Google Scholar] [CrossRef]
- Savic, R.M.; Karlsson, M.O. Importance of Shrinkage in Empirical Bayes Estimates for Diagnostics: Problems and Solutions. AAPS J. 2009, 11, 558–569. [Google Scholar] [CrossRef]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-Corrected Visual Predictive Checks for Diagnosing Non-linear Mixed-Effects Models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef]
- Dosne, A.-G.; Bergstrand, M.; Harling, K.; Karlsson, M.O. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J. Pharmacokinet. Pharmacodyn. 2016, 43, 583–596. [Google Scholar] [CrossRef]
- Ms, A.K.; Funaki, T.; Kim, S. Population Pharmacokinetic Analysis of Busulfan in Japanese Pediatric and Adult HCT Patients. J. Clin. Pharmacol. 2018, 58, 1196–1204. [Google Scholar] [CrossRef]
- Neroutsos, E.; Nalda-Molina, R.; Paisiou, A.; Zisaki, K.; Goussetis, E.; Spyridonidis, A.; Kitra, V.; Grafakos, S.; Valsami, G.; Dokoumetzidis, A. Development of a Population Pharmacokinetic Model of Busulfan in Children and Evaluation of Different Sampling Schedules for Precision Dosing. Pharmaceutics 2022, 14, 647. [Google Scholar] [CrossRef]
- Wong, K.M.; Atenafu, E.G.; Kim, D.; Kuruvilla, J.; Lipton, J.H.; Messner, H.; Gupta, V. Incidence and Risk Factors for Early Hepato-toxicity and Its Impact on Survival in Patients with Myelofibrosis Undergoing Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2012, 18, 1589–1599. [Google Scholar] [CrossRef][Green Version]
- Broeker, A.; Nardecchia, M.; Klinker, K.; Derendorf, H.; Day, R.; Marriott, D.; Carland, J.; Stocker, S.; Wicha, S. Towards precision dosing of vancomycin: A systematic evaluation of pharmacometric models for Bayesian forecasting. Clin. Microbiol. Infect. 2019, 25, 1286.e1–1286.e7. [Google Scholar] [CrossRef]

| Patient Characteristics (n = 37) | Median [IQR] or n (%) |
|---|---|
| Age [years] | 60 [53.5–65.5] |
| Sex [female/male] | 19 (51.4)/18 (48.6) |
| Weight [kg] | 75 [64.05–88.25] |
| Height [cm] | 174 [168–181] |
| BSA [m2] | 1.84 [1.75–2.07] |
| Diagnosis | |
| PMF | 18 (48.7) |
| Post-ET MF | 9 (24.3) |
| Post-PV MF | 10 (27) |
| Dosing regime | |
| Q6H | 30 (81) |
| Q24H | 7 (19) |
| DIPSS/MYSEC | |
| Intermediate-1 | 2 (5)/1 (3) |
| Intermediate-2 | 15 (40)/14 (38) |
| High Risk | 1 (3)/4 (11) |
| Mutation | |
| JAK2 | 26 (70.3) |
| CALR | 7 (18.9) |
| MPL | 1 (3) |
| TET2 | 9 (24.3) |
| ASXL1 | 13 (35.1) |
| Blood chemistry, serum levels | |
| AST [U/L] | 21 [15.5–31.5] |
| ALT [U/L] | 21 [18.5–47.5] |
| De Ritis Ratio | 0.76 [0.58–1.07] |
| Albumin [g/L] | 37.8 [34.6–41.4] |
| Alkaline Phosphatase [U/L] | 85 [63–115] |
| Bilirubin [mg/dL] | 0.6 [0.5–0.8] |
| Fibroscan [kPa] | 5.6 [4.8–7] |
| Missing Data | 14 (37.8) |
| GSTA1 52G > A | 28 (75.7) |
| GSTM1 Deletion | 19 (51.35) |
| Mucositis Grade 1/2/3/4 | 10 (27)/14 (38)/1 (2.7)/1 (2.7) |
| aGvHD Grade 1/2/3 | 10 (27)/7 (19)/4 (11) |
| cGvHD Grade 1/2/3 | 12 (32)/6 (16)/1 (2.7) |
| SOS | 2 (5.4) |
| Relapse | 2 (5.4) |
| Death | 5 (13.5) |
| Parameters | Final Model | SIR (M/m = 5000/1000) | ||
|---|---|---|---|---|
| Estimate | RSE (%) | Shrinkage (%) | 95% CI | |
| CLBu [L/h] | 16.3 | 3.6 | - | 15.18–17.35 |
| VBu [L] | 61.5 | 2 | - | 59.37–63.78 |
| CLSu [L/h] | 1.61 | 37 | - | 0.84–2.24 |
| VSu [L] | 48.8 | 35.2 | - | 30.75–78.46 |
| MF | 0.0704 | 28.6 | - | 0.0463–0.1029 |
| COV_VBu_TBW [kg] | 0.854 | 11.6 | - | 0.665–1.059 |
| COV_CLSu_GSTA1 | 1.43 | 43.6 | - | 0.63–2.40 |
| IIV CLBu [CV%] | 21.5 | 14.8 | 2 | 16.4–27.4 |
| IIV VBu [CV%] | 10 | 12 | 18 | 7.2–12.1 |
| IIV CLSu [CV%] | 112.8 | 26.1 | 22 | 80.3–206.2 |
| IIV VSu [CV%] | 77.6 | 14.2 | 18 | 59.2–106.4 |
| IOV CLBu [CV%] | 7.6 | 13.5 | 39 | 5.6–9.1 |
| Prop. σBu [CV%] | 7.1 | 12.8 | 14 | 6.3–8.2 |
| Prop. σSu [CV%] | 36.2 | 7.2 | 12 | 32.6–40.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadkhah, A.; Wicha, S.G.; Kröger, N.; Müller, A.; Pfaffendorf, C.; Riedner, M.; Badbaran, A.; Fehse, B.; Langebrake, C. Population Pharmacokinetics of Busulfan and Its Metabolite Sulfolane in Patients with Myelofibrosis Undergoing Hematopoietic Stem Cell Transplantation. Pharmaceutics 2022, 14, 1145. https://doi.org/10.3390/pharmaceutics14061145
Dadkhah A, Wicha SG, Kröger N, Müller A, Pfaffendorf C, Riedner M, Badbaran A, Fehse B, Langebrake C. Population Pharmacokinetics of Busulfan and Its Metabolite Sulfolane in Patients with Myelofibrosis Undergoing Hematopoietic Stem Cell Transplantation. Pharmaceutics. 2022; 14(6):1145. https://doi.org/10.3390/pharmaceutics14061145
Chicago/Turabian StyleDadkhah, Adrin, Sebastian Georg Wicha, Nicolaus Kröger, Alexander Müller, Christoph Pfaffendorf, Maria Riedner, Anita Badbaran, Boris Fehse, and Claudia Langebrake. 2022. "Population Pharmacokinetics of Busulfan and Its Metabolite Sulfolane in Patients with Myelofibrosis Undergoing Hematopoietic Stem Cell Transplantation" Pharmaceutics 14, no. 6: 1145. https://doi.org/10.3390/pharmaceutics14061145
APA StyleDadkhah, A., Wicha, S. G., Kröger, N., Müller, A., Pfaffendorf, C., Riedner, M., Badbaran, A., Fehse, B., & Langebrake, C. (2022). Population Pharmacokinetics of Busulfan and Its Metabolite Sulfolane in Patients with Myelofibrosis Undergoing Hematopoietic Stem Cell Transplantation. Pharmaceutics, 14(6), 1145. https://doi.org/10.3390/pharmaceutics14061145







