Development of Abiraterone Acetate Nanocrystal Tablets to Enhance Oral Bioavailability: Formulation Optimization, Characterization, In Vitro Dissolution and Pharmacokinetic Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
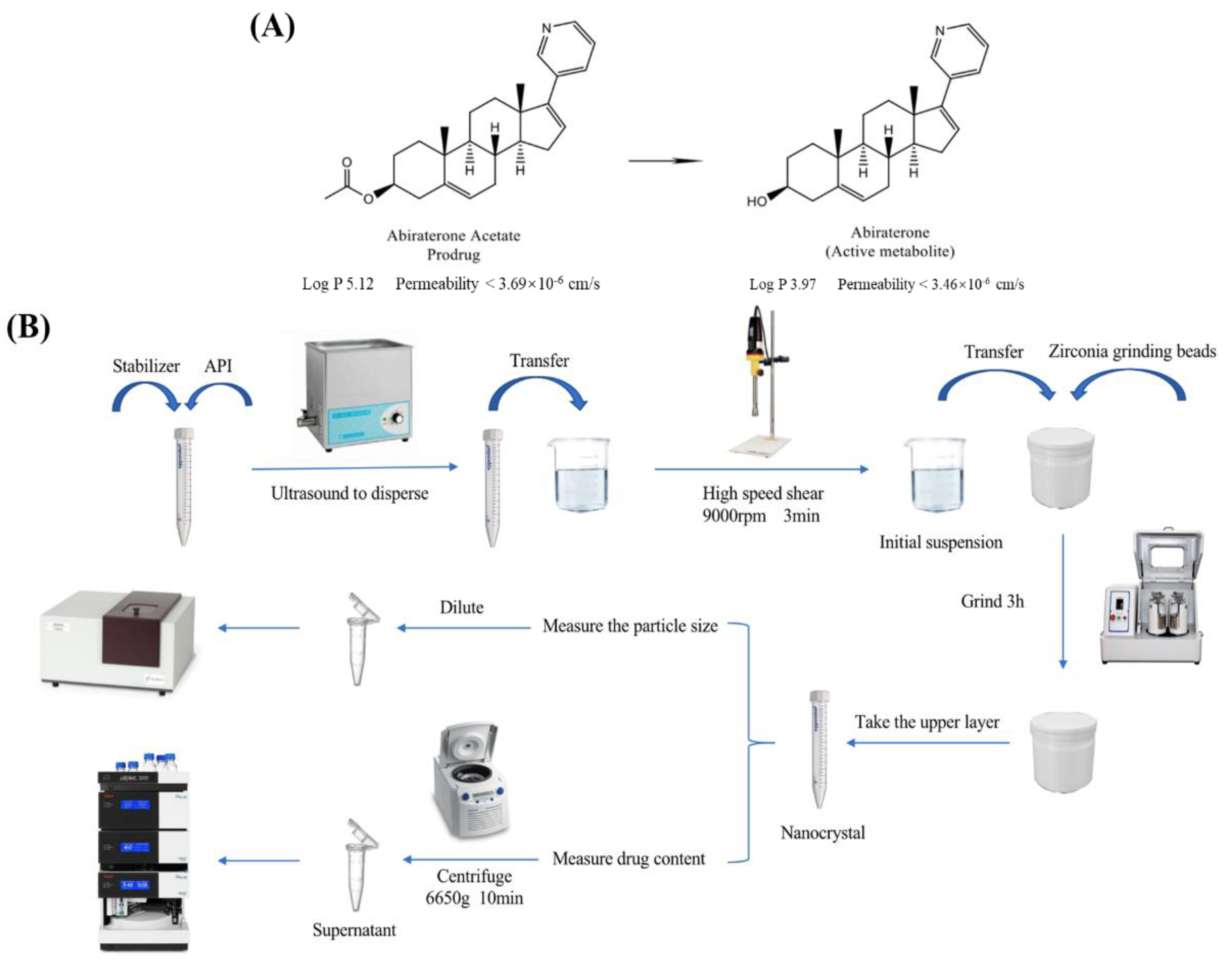
2.2. Preparation of Freeze-Dried Abiraterone Acetate Nanocrystals
2.3. Optimization of Formulation and Preparation Process
2.4. Characterization of Freeze-Dried Abiraterone Acetate Nanocrystals
2.4.1. Transmission Electron Microscopy (TEM)
2.4.2. Particle Size and Zeta Potential
2.4.3. Differential Scanning Calorimetry (DSC)
2.4.4. Powder X-ray Diffraction (XRD)
2.4.5. Determination of Abiraterone Acetate Content
2.5. Preparation of Abiraterone Acetate Nanocrystal Tablets
2.6. In Vitro Release Study
2.7. In Vivo Pharmacokinetic Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Formulation and Preparation Process
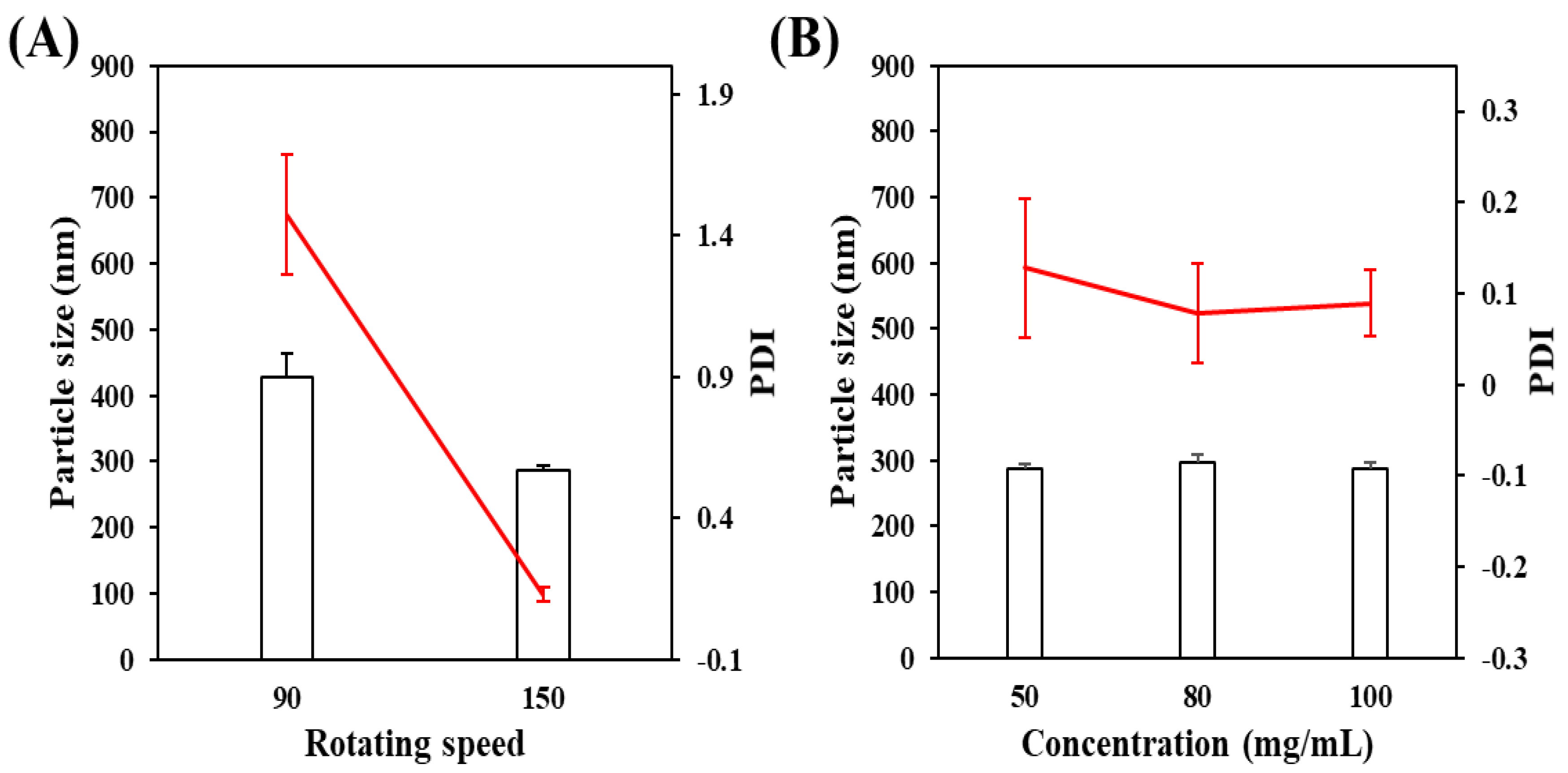
3.1.1. The Mass Ratio of the Stabilizer to Drug
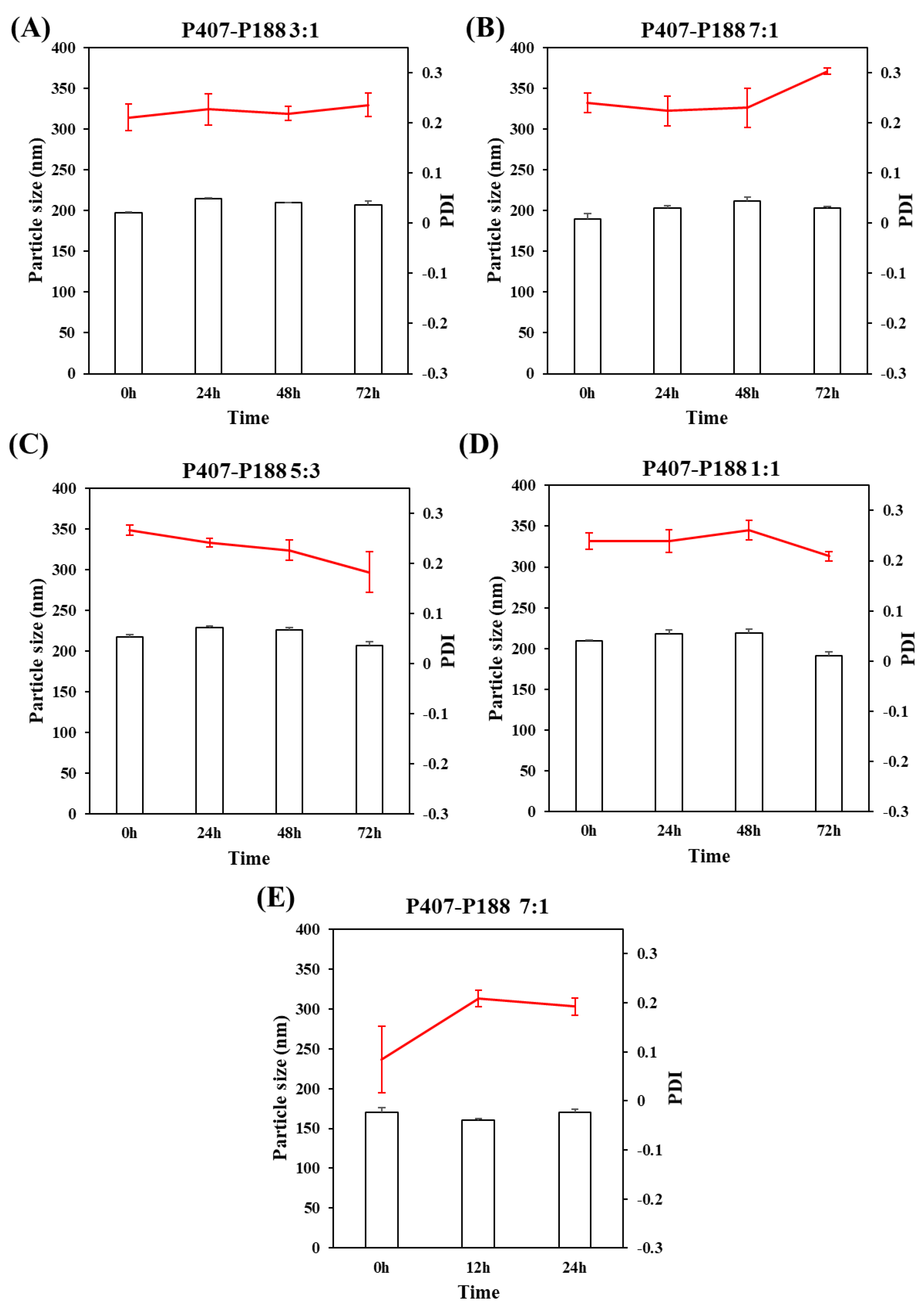
3.1.2. The Type of Stabilizers
3.1.3. Effect on the Rotating Speed of Planetary Ball Mill
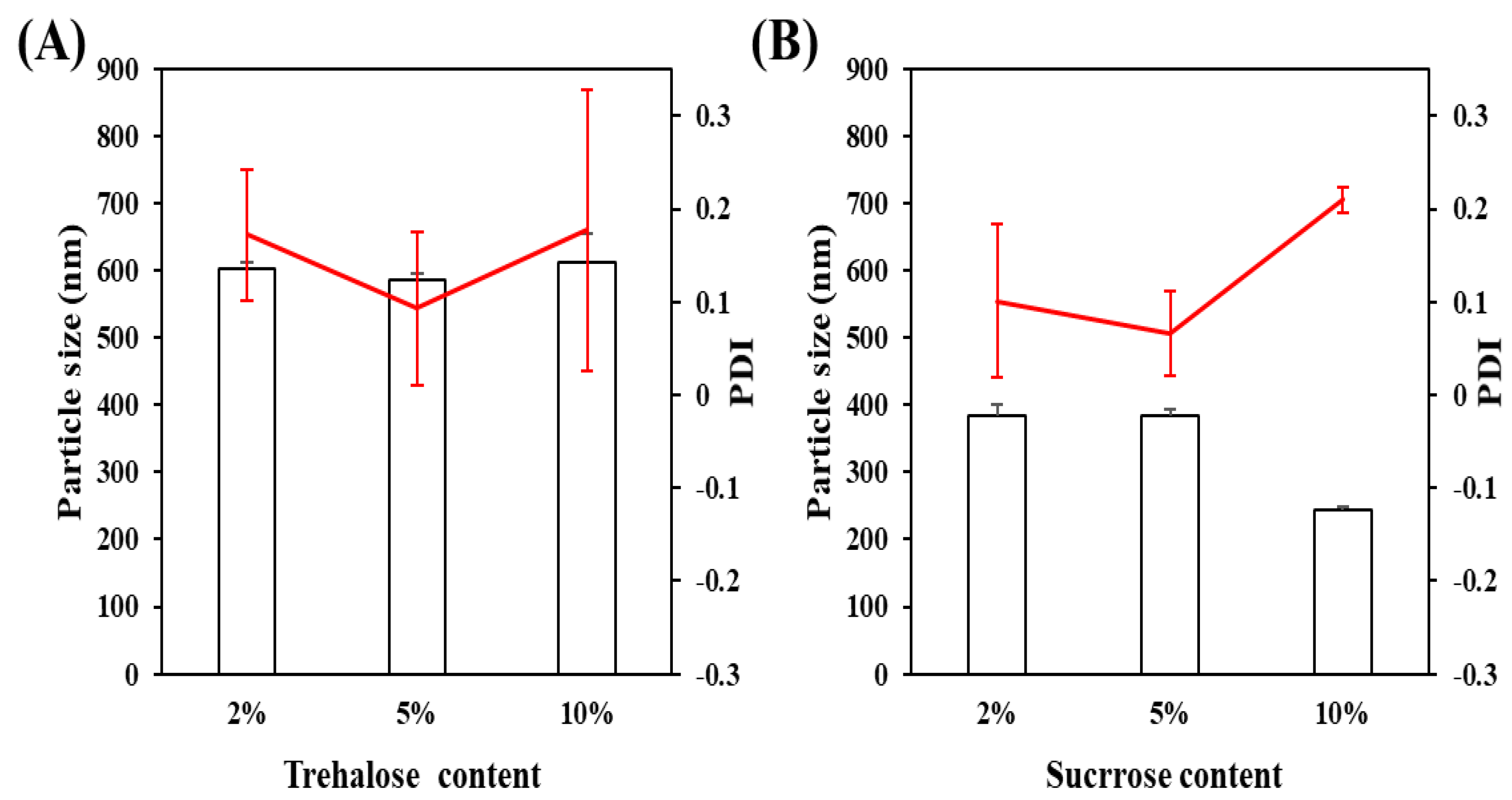
3.1.4. The Type of Lyophilization Protector
3.2. Characterization of Freeze-Dried Abiraterone Acetate Nanocrystals
3.3. Preparation of Abiraterone Acetate Nanocrystal Tablets and In Vitro Release
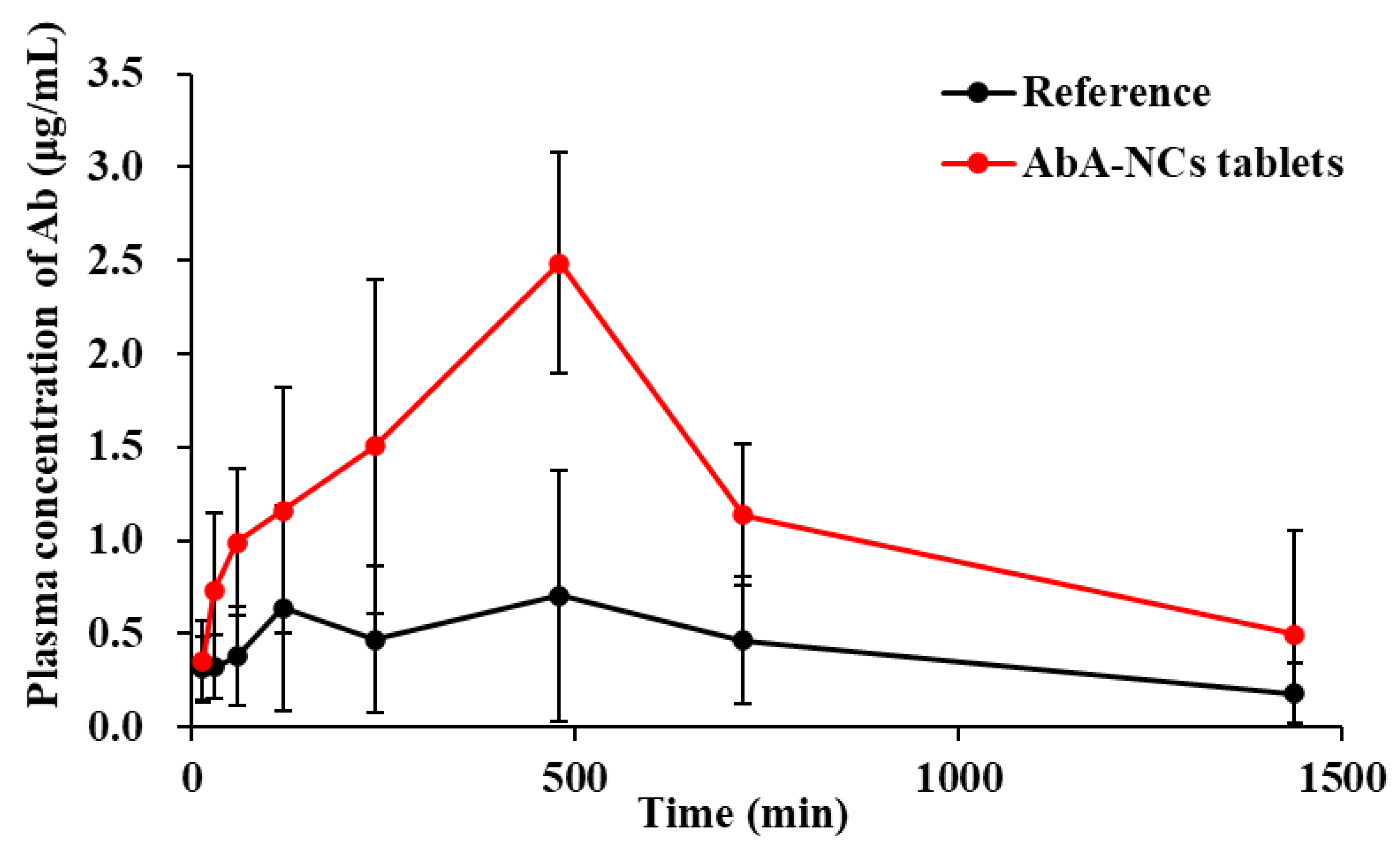
3.4. In Vivo Pharmacokinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowrance, W.T.; Murad, M.H.; Oh, W.K.; Jarrard, D.F.; Resnick, M.J.; Cookson, M.S. Castration-Resistant Prostate Cancer: AUA Guideline Amendment 2018. J. Urol. 2018, 200, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S.; Kyriakopoulos, C.E.; Jarrard, D.F. Newly Diagnosed Metastatic Prostate Cancer: Has the Paradigm Changed? Urol. Clin. 2017, 44, 611–621. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.; Judson, I.; Dowsett, M.; Raynaud, F.; Dearnaley, D.; Mason, M.; Harland, S.; Robbins, A.; Halbert, G.; Nutley, B.; et al. Hormonal Impact of the 17alpha-Hydroxylase/C(17,20)-Lyase Inhibitor Abiraterone Acetate (CB7630) in Patients with Prostate Cancer. Br. J. Cancer 2004, 90, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Ozguroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone Acetate Plus Prednisone in Patients with Newly Diagnosed High-Risk Metastatic Castration-Sensitive Prostate Cancer (LATITUDE): Final overall Survival Analysis of a Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Ozguroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone Plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Kluetz, P.G.; Ning, Y.M.; Maher, V.E.; Zhang, L.; Tang, S.; Ghosh, D.; Aziz, R.; Palmby, T.; Pfuma, E.; Zirkelbach, J.F.; et al. Abiraterone Acetate in Combination with Prednisone for the Treatment of Patients with Metastatic Castration-Resistant Prostate Cancer: U.S. Food and Drug Administration Drug Approval Summary. Clin. Cancer Res. 2013, 19, 6650–6656. [Google Scholar] [CrossRef] [Green Version]
- Geboers, S.; Stappaerts, J.; Mols, R.; Snoeys, J.; Tack, J.; Annaert, P.; Augustijns, P. The Effect of Food on the Intraluminal Behavior of Abiraterone Acetate in Man. J. Pharm. Sci. 2016, 105, 2974–2981. [Google Scholar] [CrossRef] [Green Version]
- Gala, U.; Miller, D.; Williams, R.O. Improved Dissolution and Pharmacokinetics of Abiraterone through KinetiSol® Enabled Amorphous Solid Dispersions. Pharmaceutics 2020, 12, 357. [Google Scholar] [CrossRef] [Green Version]
- Chi, K.N.; Spratlin, J.; Kollmannsberger, C.; North, S.; Pankras, C.; Gonzalez, M.; Bernard, A.; Stieltjes, H.; Peng, L.; Jiao, J.; et al. Food Effects on Abiraterone Pharmacokinetics in Healthy Subjects and Patients with Metastatic Castration-Resistant Prostate Cancer. J. Clin. Pharmacol. 2015, 55, 1406–1414. [Google Scholar] [CrossRef]
- Stappaerts, J.; Geboers, S.; Snoeys, J.; Brouwers, J.; Tack, J.; Annaert, P.; Augustijns, P. Rapid Conversion of the Ester Prodrug Abiraterone Acetate Results in Intestinal Supersaturation and Enhanced Absorption of Abiraterone: In Vitro, Rat in Situ and Human in Vivo Studies. Eur. J. Pharm. Biopharm. 2015, 90, 1–7. [Google Scholar] [CrossRef]
- Goldwater, R.; Hussaini, A.; Bosch, B.; Nemeth, P. Comparison of a Novel Formulation of Abiraterone Acetate Vs. the Originator Formulation in Healthy Male Subjects: Two Randomized, Open-Label, Crossover Studies. Clin. Pharmacokinet. 2017, 56, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Lubtow, M.M.; Marciniak, H.; Schmiedel, A.; Roos, M.; Lambert, C.; Luxenhofer, R. Ultra-High to Ultra-Low Drug-Loaded Micelles: Probing Host-Guest Interactions by Fluorescence Spectroscopy. Chemistry 2019, 25, 12601–12610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boel, E.; Smeets, A.; Vergaelen, M.; De la Rosa, V.R.; Hoogenboom, R.; Van den Mooter, G. Comparative Study of the Potential of Poly(2-Ethyl-2-Oxazoline) as Carrier in the Formulation of Amorphous Solid Dispersions of Poorly Soluble Drugs. Eur. J. Pharm. Biopharm. 2019, 144, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirri, D.; Landini, I.; Massai, L.; Mini, E.; Maestrelli, F.; Messori, L. Cyclodextrin Inclusion Complexes of Auranofin and Its Iodido Analog: A Chemical and Biological Study. Pharmaceutics 2021, 13, 727. [Google Scholar] [CrossRef]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Chmiel, K.; Kurek, M.; Gawlak, K.; Odrobinska, J.; Paluch, M.; Jachowicz, R. The Self-Assembly Phenomenon of Poloxamers and its Effect on the Dissolution of a Poorly Soluble Drug from Solid Dispersions obtained by Solvent Methods. Pharmaceutics 2019, 11, 130. [Google Scholar] [CrossRef] [Green Version]
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability Enhancement of Poorly Water-Soluble Drugs Via Nanocomposites: Formulation(-)Processing Aspects and Challenges. Pharmaceutics 2018, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Solymosi, T.; Otvos, Z.; Angi, R.; Ordasi, B.; Jordan, T.; Semsey, S.; Molnar, L.; Ranky, S.; Filipcsei, G.; Heltovics, G.; et al. Development of an Abiraterone Acetate Formulation with Improved Oral Bioavailability Guided by Absorption Modeling Based on in Vitro Dissolution and Permeability Measurements. Int. J. Pharm. 2017, 532, 427–434. [Google Scholar] [CrossRef]
- Sun, B.; Yeo, Y. Nanocrystals for the Parenteral Delivery of Poorly Water-Soluble Drugs. Curr. Opin. Solid State Mater. Sci. 2012, 16, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Fahr, A.; Liu, X. Drug Delivery Strategies for Poorly Water-Soluble Drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef]
- Fan, M.; Geng, S.; Liu, Y.; Wang, J.; Wang, Y.; Zhong, J.; Yan, Z.; Yu, L. Nanocrystal Technology as a Strategy to Improve Drug Bioavailability and Antitumor Efficacy for the Cancer Treatment. Curr. Pharm. Des. 2018, 24, 2416–2424. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X. Drug Nanocrystals: In Vivo Performances. J. Control. Release 2012, 160, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Malamatari, M.; Taylor, K.M.G.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical Nanocrystals: Production by Wet Milling and Applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Sharma, O.P.; Mehta, T. Nanocrystal: A Novel Approach to Overcome Skin Barriers for Improved Topical Drug Delivery. Expert Opin. Drug Deliv. 2018, 15, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Pooja, D.; Ravuri, H.G.; Gunukula, A.; Kulhari, H.; Sistla, R. Fabrication of Surfactant-Stabilized Nanosuspension of Naringenin to Surpass its Poor Physiochemical Properties and Low Oral Bioavailability. Phytomedicine 2018, 40, 48–54. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Q.; Niu, B.; Wu, B.; Zhao, Y.; Quan, G.; Pan, X.; Wu, C. Comparative Studies on Amphotericin B Nanosuspensions Prepared by a High Pressure Homogenization Method and an Antisolvent Precipitation Method. Colloids Surf. B Biointerfaces 2018, 172, 372–379. [Google Scholar] [CrossRef]
- Pawar, V.K.; Singh, Y.; Meher, J.G.; Gupta, S.; Chourasia, M.K. Engineered Nanocrystal Technology: In-Vivo Fate, Targeting and Applications in Drug Delivery. J. Control. Release 2014, 183, 51–66. [Google Scholar] [CrossRef]
- Sarnes, A.; Kovalainen, M.; Häkkinen, M.R.; Laaksonen, T.; Laru, J.; Kiesvaara, J.; Ilkka, J.; Oksala, O.; Rönkkö, S.; Järvinen, K. Nanocrystal-Based Per-Oral Itraconazole Delivery: Superior in Vitro Dissolution Enhancement Versus Sporanox® is Not Realized in in Vivo Drug Absorption. J. Control. Release 2014, 180, 109–116. [Google Scholar] [CrossRef]
- McGuckin, M.B.; Wang, J.; Ghanma, R.; Qin, N.; Palma, S.D.; Donnelly, R.F.; Paredes, A.J. Nanocrystals as a Master Key to Deliver Hydrophobic Drugs Via Multiple Administration Routes. J. Control. Release 2022, 345, 334–353. [Google Scholar] [CrossRef]
- Gao, W.; Lee, D.; Meng, Z.; Li, T. Exploring Intracellular Fate of Drug Nanocrystals with Crystal-Integrated and Environment-Sensitive Fluorophores. J. Control. Release 2017, 267, 214–222. [Google Scholar] [CrossRef]
- Jarvis, M.; Krishnan, V.; Mitragotri, S. Nanocrystals: A Perspective on Translational Research and Clinical Studies. Bioeng. Transl. Med. 2019, 4, 5–16. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Gigliobianco, M.R.; Pellitteri, R.; Santonocito, D.; Carbone, C.; Di Martino, P.; Puglisi, G.; Musumeci, T. Optimization of Curcumin Nanocrystals as Promising Strategy for Nose-to-Brain Delivery Application. Pharmaceutics 2020, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Latif, R.; Makar, R.R.; Hosni, E.A.; El Gazayerly, O.N. The Potential of Intranasal Delivery of Nanocrystals in Powder Form on the Improvement of Zaleplon Performance: In-Vitro, in-Vivo Assessment. Drug Dev. Ind. Pharm. 2021, 47, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Gala, U.H.; Miller, D.A.; Su, Y.; Spangenberg, A.; Williams, R.O.B., III. The Effect of Drug Loading on the Properties of Abiraterone–hydroxypropyl Beta Cyclodextrin Solid Dispersions Processed by Solvent Free KinetiSol® Technology. Eur. J. Pharm. Biopharm. 2021, 165, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.B.; Meola, T.R.; Thomas, N.; Prestidge, C.A. Oral Formulation Strategies to Improve the Bioavailability and Mitigate the Food Effect of Abiraterone Acetate. Int. J. Pharm. 2020, 577, 119069. [Google Scholar] [CrossRef]
- Beck, C.; Sievens-Figueroa, L.; Gärtner, K.; Jerez-Rozo, J.I.; Romañach, R.J.; Bilgili, E.; Davé, R.N. Effects of Stabilizers on Particle Redispersion and Dissolution from Polymer Strip Films Containing Liquid Antisolvent Precipitated Griseofulvin Particles. Powder Technol. 2013, 236, 37–51. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Sacha, G.A.; Nail, S.L. Thermal Analysis of Frozen Solutions: Multiple Glass Transitions in Amorphous Systems. J. Pharm. Sci. 2009, 98, 3397–3405. [Google Scholar] [CrossRef]
- Fu, X.; Xu, S.; Li, Z.; Chen, K.; Fan, H.; Wang, Y.; Xie, Z.; Kou, L.; Zhang, S. Enhanced Intramuscular Bioavailability of Cannabidiol using Nanocrystals: Formulation, in Vitro Appraisal, and Pharmacokinetics. AAPS PharmSciTech 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Li, X.M.; Huang, Q.; Chen, W.; Liu, R.; Chen, B.; Wei, P. Study on the Release of Fenofibrate Nanosuspension in Vitro and its Correlation with in Situ Intestinal and in Vivo Absorption Kinetics in Rats. Drug Dev. Ind. Pharm. 2014, 40, 972–979. [Google Scholar] [CrossRef]




| Element | Weight (mg) |
|---|---|
| freeze-dried abiraterone acetate nanocrystals | 301.2 |
| lactose | 198.65 |
| microcrystalline cellulose | 118.62 |
| croscarmellose sodium | 42.9 |
| povidone K30 | 35.75 |
| colloidal silicon dioxide | 7.15 |
| magnesium stearate | 10.73 |
| Parameters | Zytiga® Reference Tablet | Abiraterone Acetate Nanocrystal Tablets |
|---|---|---|
| Tmax (min) | 480 | 480 |
| Cmax (µg/mL) | 0.71 ± 0.71 | 2.49 ± 0.77 ** |
| AUC0→t (µg/mL × min) | 629.84 ± 594.86 | 1763.03 ± 830.94 * |
| AUCinf (µg/mL × min) | 760.08 ± 594.13 | 2081.27 ± 1292.88 * |
| Frel (%) | 100 | 279.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, Y.; Xu, P.; Shen, Y.; Tang, B.; Wang, Q. Development of Abiraterone Acetate Nanocrystal Tablets to Enhance Oral Bioavailability: Formulation Optimization, Characterization, In Vitro Dissolution and Pharmacokinetic Evaluation. Pharmaceutics 2022, 14, 1134. https://doi.org/10.3390/pharmaceutics14061134
Liu Y, Li Y, Xu P, Shen Y, Tang B, Wang Q. Development of Abiraterone Acetate Nanocrystal Tablets to Enhance Oral Bioavailability: Formulation Optimization, Characterization, In Vitro Dissolution and Pharmacokinetic Evaluation. Pharmaceutics. 2022; 14(6):1134. https://doi.org/10.3390/pharmaceutics14061134
Chicago/Turabian StyleLiu, Yuanfen, Yuqi Li, Pengcheng Xu, Yan Shen, Baoqiang Tang, and Qiyue Wang. 2022. "Development of Abiraterone Acetate Nanocrystal Tablets to Enhance Oral Bioavailability: Formulation Optimization, Characterization, In Vitro Dissolution and Pharmacokinetic Evaluation" Pharmaceutics 14, no. 6: 1134. https://doi.org/10.3390/pharmaceutics14061134
APA StyleLiu, Y., Li, Y., Xu, P., Shen, Y., Tang, B., & Wang, Q. (2022). Development of Abiraterone Acetate Nanocrystal Tablets to Enhance Oral Bioavailability: Formulation Optimization, Characterization, In Vitro Dissolution and Pharmacokinetic Evaluation. Pharmaceutics, 14(6), 1134. https://doi.org/10.3390/pharmaceutics14061134







