pH-Sensitive Liposomes for Enhanced Cellular Uptake and Cytotoxicity of Daunorubicin in Melanoma (B16-BL6) Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liposomes Preparation
2.3. Drug Encapsulation in Liposomes (Active Loading)
2.4. Encapsulation Efficiency (EE%) and Drug Loading (DL%) Measurement
2.5. Determination of Particle Size and Zeta Potential of Liposomal Formulations
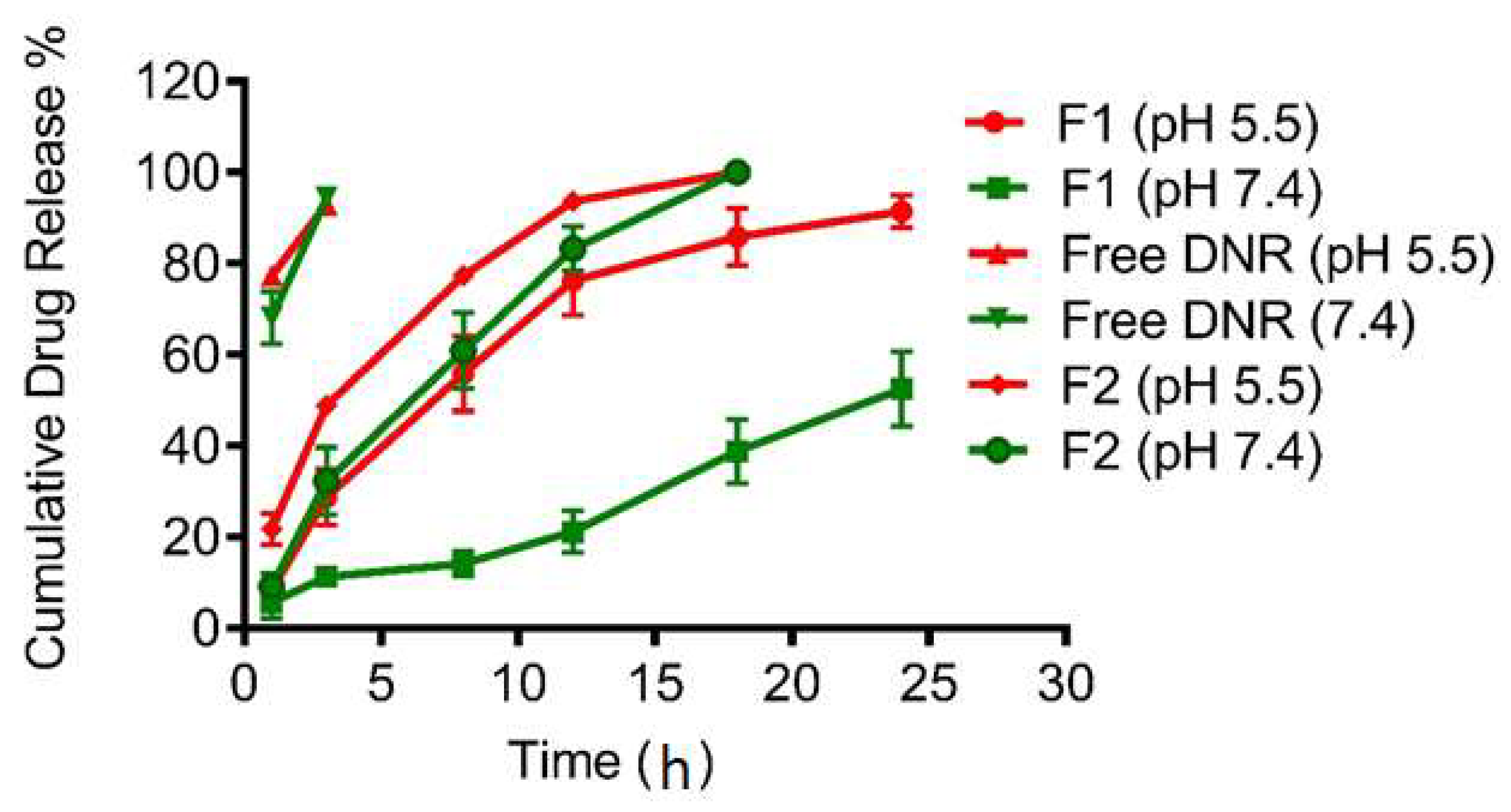
2.6. In Vitro Release Studies
2.7. Stability Studies
2.8. Cell Culture
2.9. Measurement of Cell Viability by MTT Assay
2.10. Cellular Daunorubicin Uptake
2.11. Daunorubicin Retention Studies
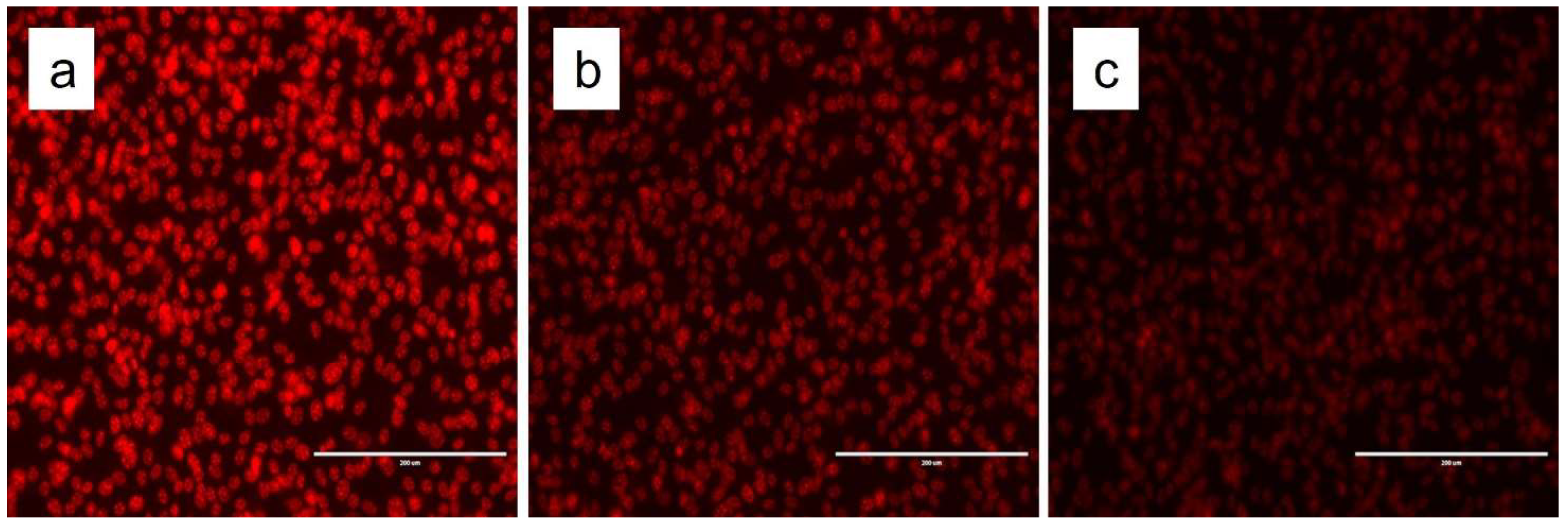
2.12. Fluorescence Microscopy
2.13. Statistical Analysis
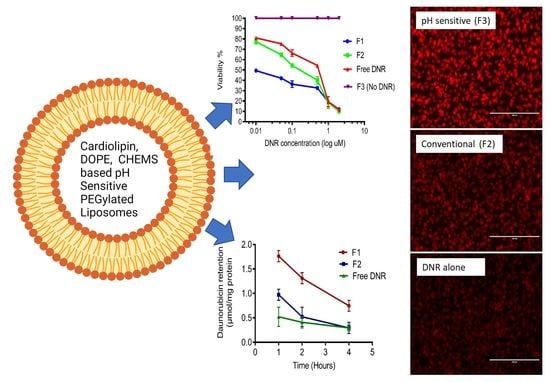
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zbytek, B.; Carlson, J.A.; Granese, J.; Ross, J.; Mihm, M.C., Jr.; Slominski, A. Current concepts of metastasis in melanoma. Expert. Rev. Dermatol. 2008, 3, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheikh, M.S. Melanoma: Molecular Pathogenesis and Therapeutic Management. Mol. Cell. Pharmacol. 2014, 6, 228. [Google Scholar] [PubMed]
- Barker, C.A.; Lee, N.Y. Radiation therapy for cutaneous melanoma. Dermatol. Clin. 2012, 30, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.A.; Maurer, L.H.; O’Donnell, J.; Forcier, R.J.; LeMarbre, P. Combination chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in metastatic melanoma. Cancer Treat. Rep. 1984, 68, 1403–1405. [Google Scholar]
- Hamm, C.; Verma, S.; Petrella, T.; Bak, K.; Charette, M. Melanoma Disease Site Group of Cancer Care Ontario’s Program in Evidence-based, C. Biochemotherapy for the treatment of metastatic malignant melanoma: A systematic review. Cancer Treat. Rev. 2008, 34, 145–156. [Google Scholar] [CrossRef]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Gabizon, A.; Goren, D.; Cohen, R.; Barenholz, Y. Development of liposomal anthracyclines: From basics to clinical applications. J. Control. Release 1998, 53, 275–279. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Sudimack, J.J.; Guo, W.; Tjarks, W.; Lee, R.J. A novel pH-sensitive liposome formulation containing oleyl alcohol. Biochim. Biophys. Acta 2002, 1564, 31–37. [Google Scholar] [CrossRef][Green Version]
- Torchilin, V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007, 9, E128–E147. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell. Int. 2013, 13, 89. [Google Scholar] [CrossRef]
- Yoneda, T.; Hiasa, M.; Nagata, Y.; Okui, T.; White, F. Contribution of acidic extracellular microenvironment of cancer-colonized bone to bone pain. Biochim. Biophys. Acta 2015, 1848, 2677–2684. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Paliwal, S.R.; Paliwal, R.; Vyas, S.P. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015, 22, 231–242. [Google Scholar] [CrossRef]
- Fattal, E.; Couvreur, P.; Dubernet, C. “Smart” delivery of antisense oligonucleotides by anionic pH-sensitive liposomes. Adv. Drug Deliv. Rev. 2004, 56, 931–946. [Google Scholar] [CrossRef]
- Momekova, D.; Rangelov, S.; Lambov, N. Long-circulating, pH-sensitive liposomes. Methods Mol. Biol. 2010, 605, 527–544. [Google Scholar] [CrossRef]
- Cullis, P.R.; de Kruijff, B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta 1979, 559, 399–420. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; De Benedictis, V.; Ruggiero, F.M.; Petrosillo, G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta 2014, 1837, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Ye, C.; McCain, K.; Greenberg, M.L. The Role of Cardiolipin in Cardiovascular Health. Biomed. Res. Int. 2015, 2015, 891707. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Modak, A.; Nangia, S.; Daman, T.H.; Gunsel, U.; Robinson, V.L.; Mokranjac, D.; May, E.R.; Alder, N.N. Cardiolipin mediates membrane and channel interactions of the mitochondrial TIM23 protein import complex receptor Tim50. Sci. Adv. 2017, 3, e1700532. [Google Scholar] [CrossRef] [PubMed]
- Unsay, J.D.; Cosentino, K.; Subburaj, Y.; Garcia-Saez, A.J. Cardiolipin effects on membrane structure and dynamics. Langmuir 2013, 29, 15878–15887. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, W.J.; de Kruijff, B.; Verkleij, A.J.; de Gier, J.; van Deenen, L.L. Ca2+-induced isotropic motion and phosphatidylcholine flip-flop in phosphatidylcholine-cardiolipin bilayers. Biochim. Biophys. Acta 1980, 598, 554–560. [Google Scholar] [CrossRef]
- Sennato, S.; Bordi, F.; Cametti, C.; Coluzza, C.; Desideri, A.; Rufini, S. Evidence of domain formation in cardiolipin-glycerophospholipid mixed monolayers. A thermodynamic and AFM study. J. Phys. Chem. B 2005, 109, 15950–15957. [Google Scholar] [CrossRef]
- Zunino, F.; Giuliani, F.; Savi, G.; Dasdia, T.; Gambetta, R. Anti-tumor activity of daunorubicin linked to poly-L-aspartic acid. Int. J. Cancer 1982, 30, 465–470. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Hortobagyi, G.N. Anthracyclines in the treatment of cancer. An overview. Drugs 1997, 54, 1–7. [Google Scholar] [CrossRef]
- Rafiyath, S.M.; Rasul, M.; Lee, B.; Wei, G.; Lamba, G.; Liu, D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: A meta-analysis. Exp. Hematol. Oncol. 2012, 1, 10. [Google Scholar] [CrossRef]
- Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Mayer, L.D.; Tai, L.C.; Bally, M.B.; Mitilenes, G.N.; Ginsberg, R.S.; Cullis, P.R. Characterization of liposomal systems containing doxorubicin entrapped in response to pH gradients. Biochim. Biophys. Acta 1990, 1025, 143–151. [Google Scholar] [CrossRef]
- Johnston, M.J.; Edwards, K.; Karlsson, G.; Cullis, P.R. Influence of drug-to-lipid ratio on drug release properties and liposome integrity in liposomal doxorubicin formulations. J. Liposome Res. 2008, 18, 145–157. [Google Scholar] [CrossRef]
- Haran, G.; Cohen, R.; Bar, L.K.; Barenholz, Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1993, 1151, 201–215. [Google Scholar] [CrossRef]
- Wei, X.; Shamrakov, D.; Nudelman, S.; Peretz-Damari, S.; Nativ-Roth, E.; Regev, O.; Barenholz, Y. Cardinal role of intraliposome doxorubicin-sulfate nanorod crystal in doxil properties and performance. ACS Omega 2018, 3, 2508–2517. [Google Scholar] [CrossRef]
- Plourde, K.; Derbali, R.M.; Desrosiers, A.; Dubath, C.; Vallee-Belisle, A.; Leblond, J. Aptamer-based liposomes improve specific drug loading and release. J. Control. Release 2017, 251, 82–91. [Google Scholar] [CrossRef]
- Hood, R.R.; Vreeland, W.N.; DeVoe, D.L. Microfluidic remote loading for rapid single-step liposomal drug preparation. Lab A Chip 2014, 14, 3359–3367. [Google Scholar] [CrossRef]
- Deamer, D.W.; Prince, R.C.; Crofts, A.R. The response of fluorescent amines to pH gradients across liposome membranes. Biochim. Biophys. Acta 1972, 274, 323–335. [Google Scholar] [CrossRef]
- Straubinger, R.M. pH-sensitive liposomes for delivery of macromolecules into cytoplasm of cultured cells. Methods Enzym. 1993, 221, 361–376. [Google Scholar]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Nicholas, A.R.; Scott, M.J.; Kennedy, N.I.; Jones, M.N. Effect of grafted polyethylene glycol (PEG) on the size, encapsulation efficiency and permeability of vesicles. Biochim. Biophys. Acta 2000, 1463, 167–178. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim. Biophys. Acta 2009, 1788, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Nag, O.K.; Yadav, V.R.; Hedrick, A.; Awasthi, V. Post-modification of preformed liposomes with novel non-phospholipid poly(ethylene glycol)-conjugated hexadecylcarbamoylmethyl hexadecanoic acid for enhanced circulation persistence in vivo. Int. J. Pharm. 2013, 446, 119–129. [Google Scholar] [CrossRef]
- Rothkopf, C.; Fahr, A.; Fricker, G.; Scherphof, G.L.; Kamps, J.A. Uptake of phosphatidylserine-containing liposomes by liver sinusoidal endothelial cells in the serum-free perfused rat liver. Biochim. Biophys. Acta 2005, 1668, 10–16. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Nag, O.K.; Awasthi, V. Surface engineering of liposomes for stealth behavior. Pharmaceutics 2013, 5, 542–569. [Google Scholar] [CrossRef]
- Heidarli, E.; Dadashzadeh, S.; Haeri, A. State of the Art of Stimuli-Responsive Liposomes for Cancer Therapy. Iran. J. Pharm. Res. 2017, 16, 1273–1304. [Google Scholar]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef]
- Litzinger, D.C.; Huang, L. Phosphatidylethanolamine liposomes: Drug delivery, gene transfer and immunodiagnostic applications. Biochim. Biophys. Acta 1992, 1113, 201–227. [Google Scholar] [CrossRef]
- Ishida, T.; Okada, Y.; Kobayashi, T.; Kiwada, H. Development of pH-sensitive liposomes that efficiently retain encapsulated doxorubicin (DXR) in blood. Int. J. Pharm. 2006, 309, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Slepushkin, V.A.; Simoes, S.; Dazin, P.; Newman, M.S.; Guo, L.S.; Pedroso de Lima, M.C.; Duzgunes, N. Sterically stabilized pH-sensitive liposomes. Intracellular delivery of aqueous contents and prolonged circulation in vivo. J. Biol. Chem. 1997, 272, 2382–2388. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Ddos, S.; Lopes, S.C.; Franco, M.S.; Oliveira, M.C. pH-sensitive liposomes for drug delivery in cancer treatment. Ther. Deliv. 2013, 4, 1099–1123. [Google Scholar] [CrossRef] [PubMed]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Rivera, E. Liposomal anthracyclines in metastatic breast cancer: Clinical update. Oncologist 2003, 8, 3–9. [Google Scholar] [CrossRef]
- Kratz, F.; Warnecke, A.; Scheuermann, K.; Stockmar, C.; Schwab, J.; Lazar, P.; Druckes, P.; Esser, N.; Drevs, J.; Rognan, D.; et al. Probing the cysteine-34 position of endogenous serum albumin with thiol-binding doxorubicin derivatives. Improved efficacy of an acid-sensitive doxorubicin derivative with specific albumin-binding properties compared to that of the parent compound. J. Med. Chem. 2002, 45, 5523–5533. [Google Scholar] [CrossRef]
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Frechet, J.M.; Dy, E.E.; Szoka, F.C. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654. [Google Scholar] [CrossRef]
- Fukushima, T.; Ueda, T.; Uchida, M.; Nakamura, T. Action mechanism of idarubicin (4-demethoxydaunorubicin) as compared with daunorubicin in leukemic cells. Int. J. Hematol. 1993, 57, 121–130. [Google Scholar]
- Speelmans, G.; Staffhorst, R.W.; de Kruijff, B.; de Wolf, F.A. Transport studies of doxorubicin in model membranes indicate a difference in passive diffusion across and binding at the outer and inner leaflets of the plasma membrane. Biochemistry 1994, 33, 13761–13768. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Srinivasan, S.; Tang, Y.; Manchanda, R.; Nagesetti, A.; Fernandez-Fernandez, A.; Mc Goron, A.J. Comparing cellular uptake and cytotoxicity of targeted drug carriers in cancer cell lines with different drug resistance mechanisms. Nanomedicine 2011, 7, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Xue, H.Y.; Babakhanian, K.; Wu, X.Y. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J. Pharm. Exp. Ther. 2006, 317, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Na, K.; Song, S.C.; Lee, J.; Kuh, H.J. The distribution and retention of paclitaxel and doxorubicin in multicellular layer cultures. Oncol. Rep. 2012, 27, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Wielinga, P.R.; Westerhoff, H.V.; Lankelma, J. The relative importance of passive and P-glycoprotein mediated anthracycline efflux from multidrug-resistant cells. Eur. J. Biochem. 2000, 267, 649–657. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Edwards, K.; Johnsson, M.; Karlsson, G.; Silvander, M. Effect of polyethyleneglycol-phospholipids on aggregate structure in preparations of small unilamellar liposomes. Biophys. J. 1997, 73, 258–266. [Google Scholar] [CrossRef]
- Li, X.; Hirsh, D.J.; Cabral-Lilly, D.; Zirkel, A.; Gruner, S.M.; Janoff, A.S.; Perkins, W.R. Doxorubicin physical state in solution and inside liposomes loaded via a pH gradient. Biochim. Biophys. Acta 1998, 1415, 23–40. [Google Scholar] [CrossRef]





| Ingredients | F1 | F2 * | F3 # |
|---|---|---|---|
| DOPE | 40 | - | 40 |
| CHEMS | 30 | 30 | |
| DSPE-mPEG (2000) | 5 | - | 5 |
| DSPC | - | 10 | - |
| Cholesterol | - | 5 | - |
| CL | 17 | - | 17 |
| SA | 8 | - | 8 |
| DNR:lipid ratio | 1:5 | 1:10:5 | - |
| DNR Liposomal Formulation | Encapsulation Efficiency % | Drug Loading (%) | Particle Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|---|---|
| F1 | 95.0 ± 3.7 | 15.8 ± 0.6 | 94.0 ± 3.7 | 0.16 ± 0.03 | −39.1 ± 3.1 |
| F2 | 94.0 ± 0.5 | 15.8 ± 0.4 | 83.0 ± 3.1 | 0.18 ± 0.07 | −5.0 ± 1.8 |
| F1 | F2 | |||
|---|---|---|---|---|
| Formulation | Initial | 1 month | Initial | 1 month |
| Size Particle Size (nm) | 94.0 ± 3.7 | 112.3 ± 4.2 | 83.0 ± 3.1 | 86.0 ± 3.2 |
| PI | 0.16 ± 0.03 | 0.18 ± 0.01 | 0.18 ± 0.07 | 0.23 ± 0.07 |
| EE % | 95.0 ± 3.7 | 86.0 ± 4.4 | 94.0 ± 0.5 | 92.0 ± 2.7 |
| DL % | 15.8 ± 0.6 | 14.7 ± 0.8 | 15.8 ± 0.4 | 16.6 ± 0.3 |
| Zeta Potential (mV) | −39.1 ± 3.1 | −36.4 ± 3.9 | −5.0 ± 1.8 | −4.0 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrbyawi, H.; Poudel, I.; Annaji, M.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. pH-Sensitive Liposomes for Enhanced Cellular Uptake and Cytotoxicity of Daunorubicin in Melanoma (B16-BL6) Cell Lines. Pharmaceutics 2022, 14, 1128. https://doi.org/10.3390/pharmaceutics14061128
Alrbyawi H, Poudel I, Annaji M, Boddu SHS, Arnold RD, Tiwari AK, Babu RJ. pH-Sensitive Liposomes for Enhanced Cellular Uptake and Cytotoxicity of Daunorubicin in Melanoma (B16-BL6) Cell Lines. Pharmaceutics. 2022; 14(6):1128. https://doi.org/10.3390/pharmaceutics14061128
Chicago/Turabian StyleAlrbyawi, Hamad, Ishwor Poudel, Manjusha Annaji, Sai H. S. Boddu, Robert D. Arnold, Amit K. Tiwari, and R. Jayachandra Babu. 2022. "pH-Sensitive Liposomes for Enhanced Cellular Uptake and Cytotoxicity of Daunorubicin in Melanoma (B16-BL6) Cell Lines" Pharmaceutics 14, no. 6: 1128. https://doi.org/10.3390/pharmaceutics14061128
APA StyleAlrbyawi, H., Poudel, I., Annaji, M., Boddu, S. H. S., Arnold, R. D., Tiwari, A. K., & Babu, R. J. (2022). pH-Sensitive Liposomes for Enhanced Cellular Uptake and Cytotoxicity of Daunorubicin in Melanoma (B16-BL6) Cell Lines. Pharmaceutics, 14(6), 1128. https://doi.org/10.3390/pharmaceutics14061128