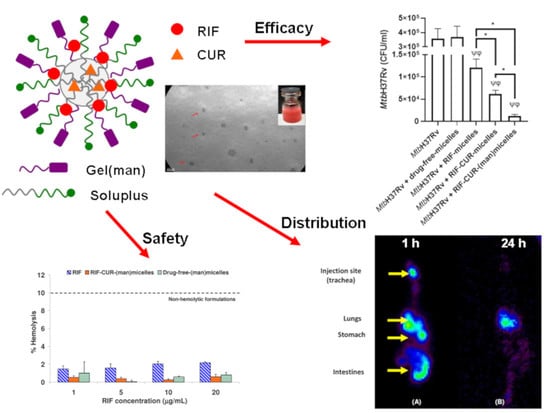
Inhalable Mannosylated Rifampicin–Curcumin Co-Loaded Nanomicelles with Enhanced In Vitro Antimicrobial Efficacy for an Optimized Pulmonary Tuberculosis Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gelatin/Mannose Formulation and Characterization
2.3. Measurement of the Critical Micellar Concentration (CMC)
2.4. Micellar Preparation and Drug Encapsulation
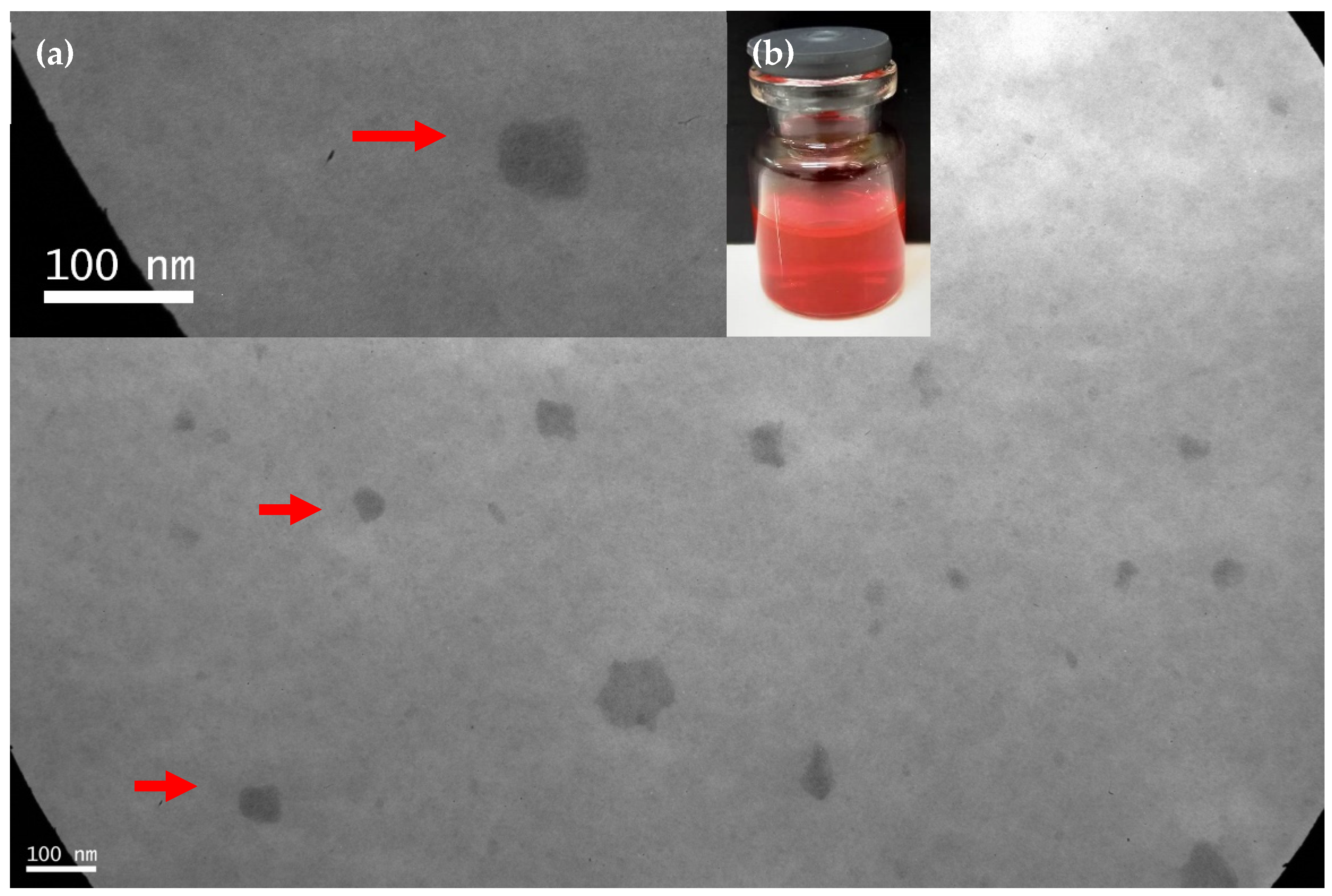
2.5. Measurement of Micellar Size and Morphological Characterization
2.6. In Vitro Antioxidant Capacity Assays
2.6.1. DPPH Colorimetric Assay
2.6.2. ABTS Colorimetric Assay
2.7. In Vitro Nebulization Studies
2.8. Stability of the Micellar Systems in Simulated Interstitial Lung Fluid
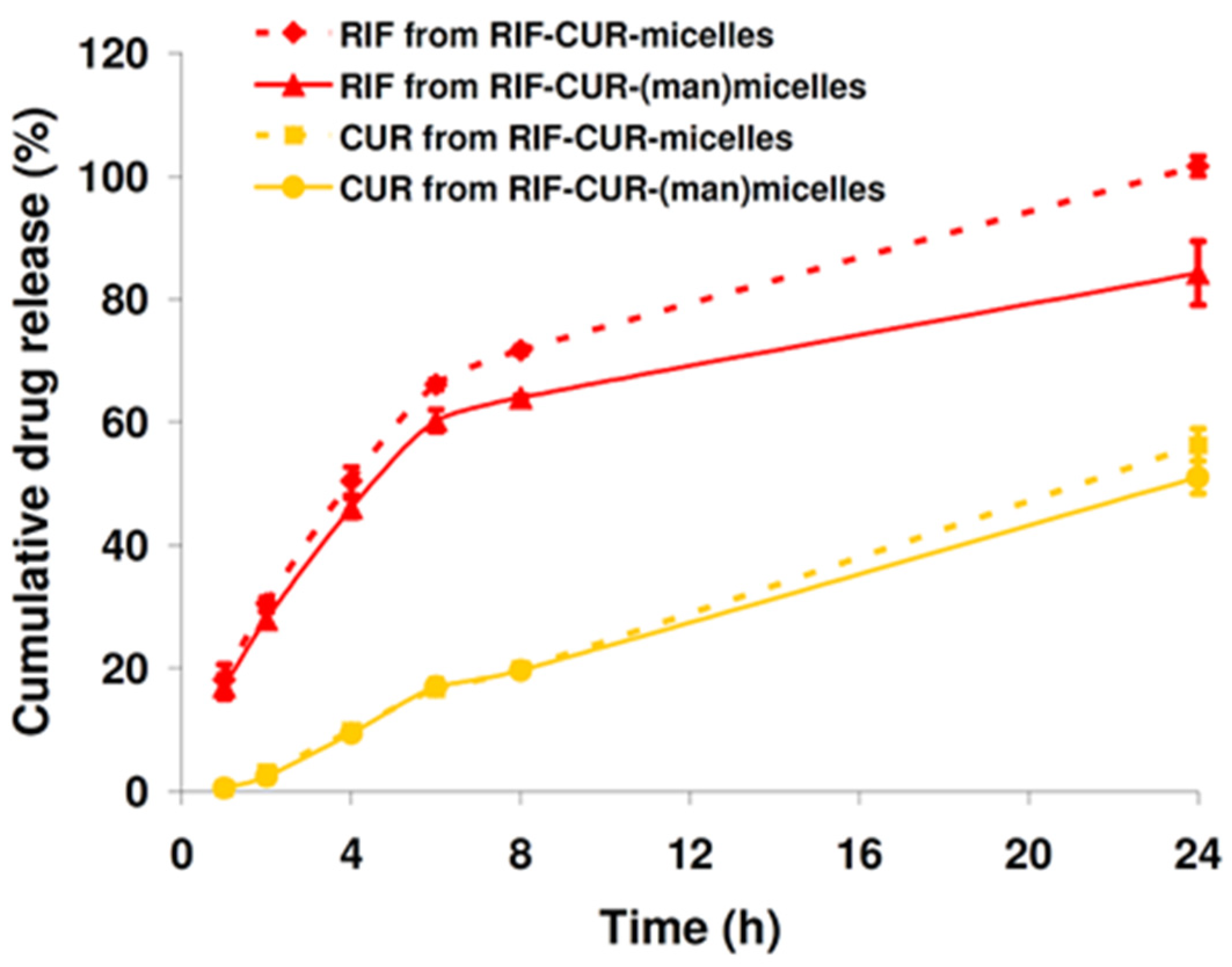
2.9. In Vitro Drug Release Study
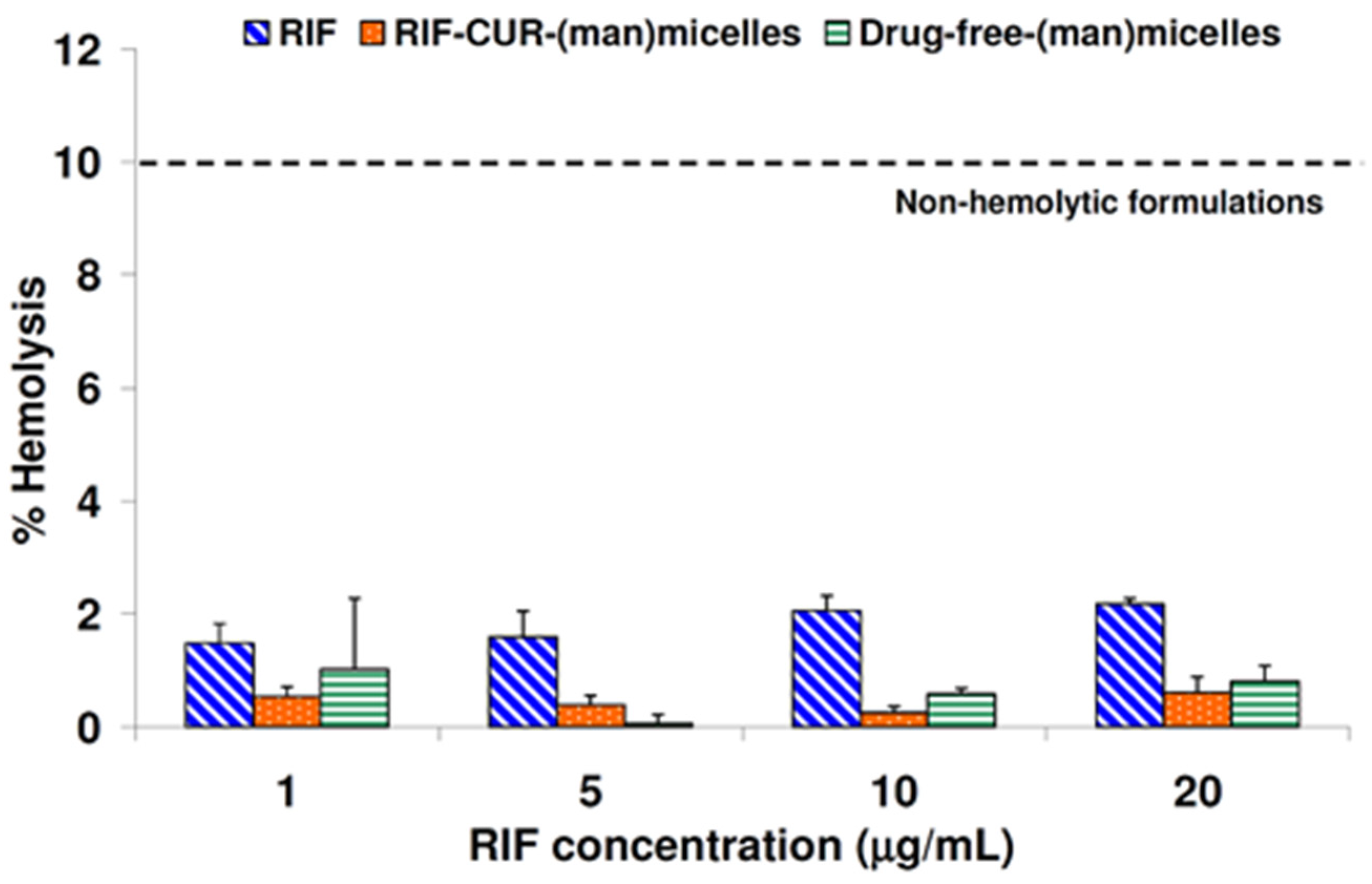
2.10. In Vitro Hemolytic Assay
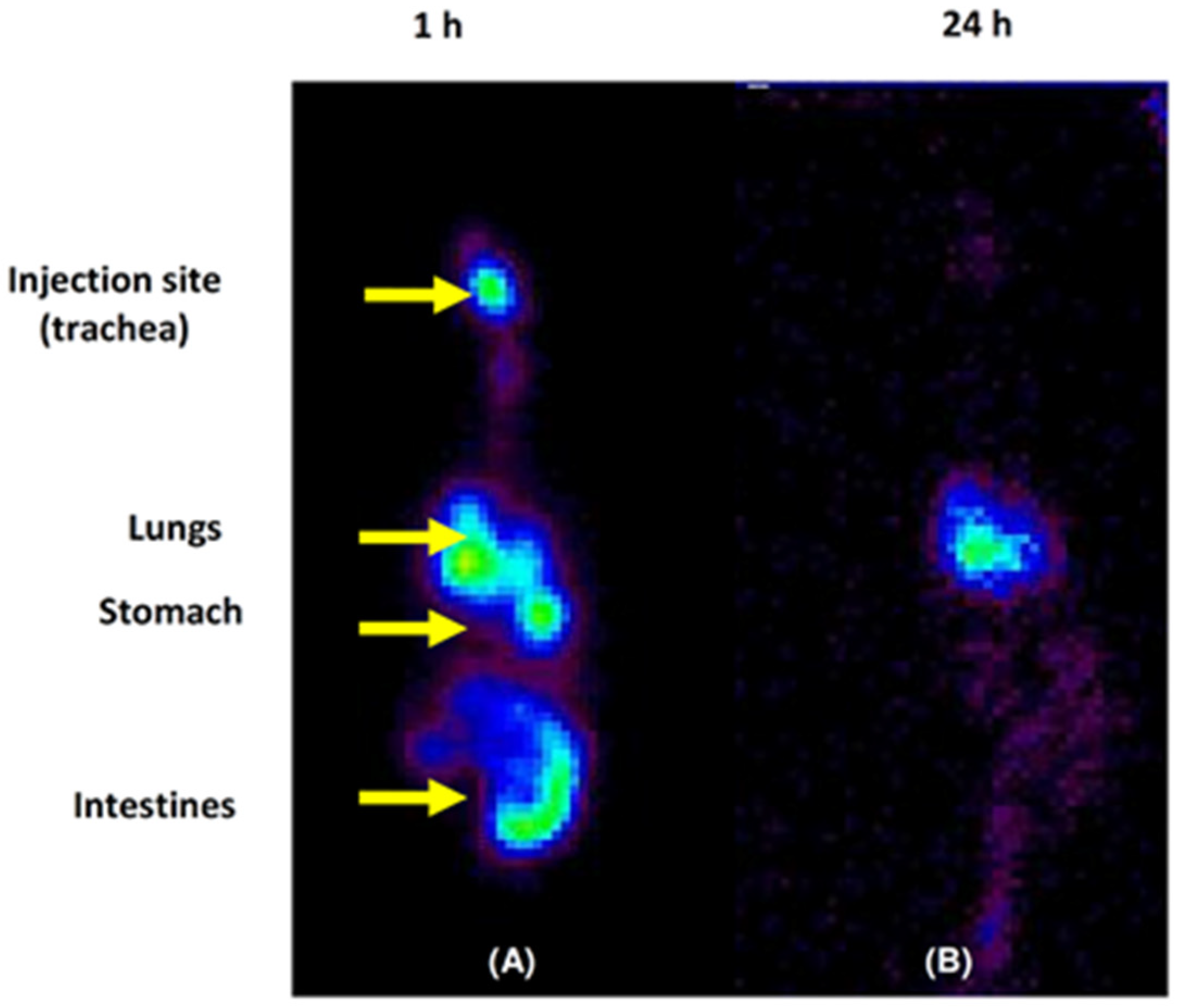
2.11. In Vivo Micellar Lung Accumulation Assay
2.11.1. Radiolabeling Procedure of Soluplus® (Man)Micelles
2.11.2. Radiolabeling Efficiency and Stability
2.11.3. Biodistribution Studies: Ex Vivo and Scintigraphic Procedures
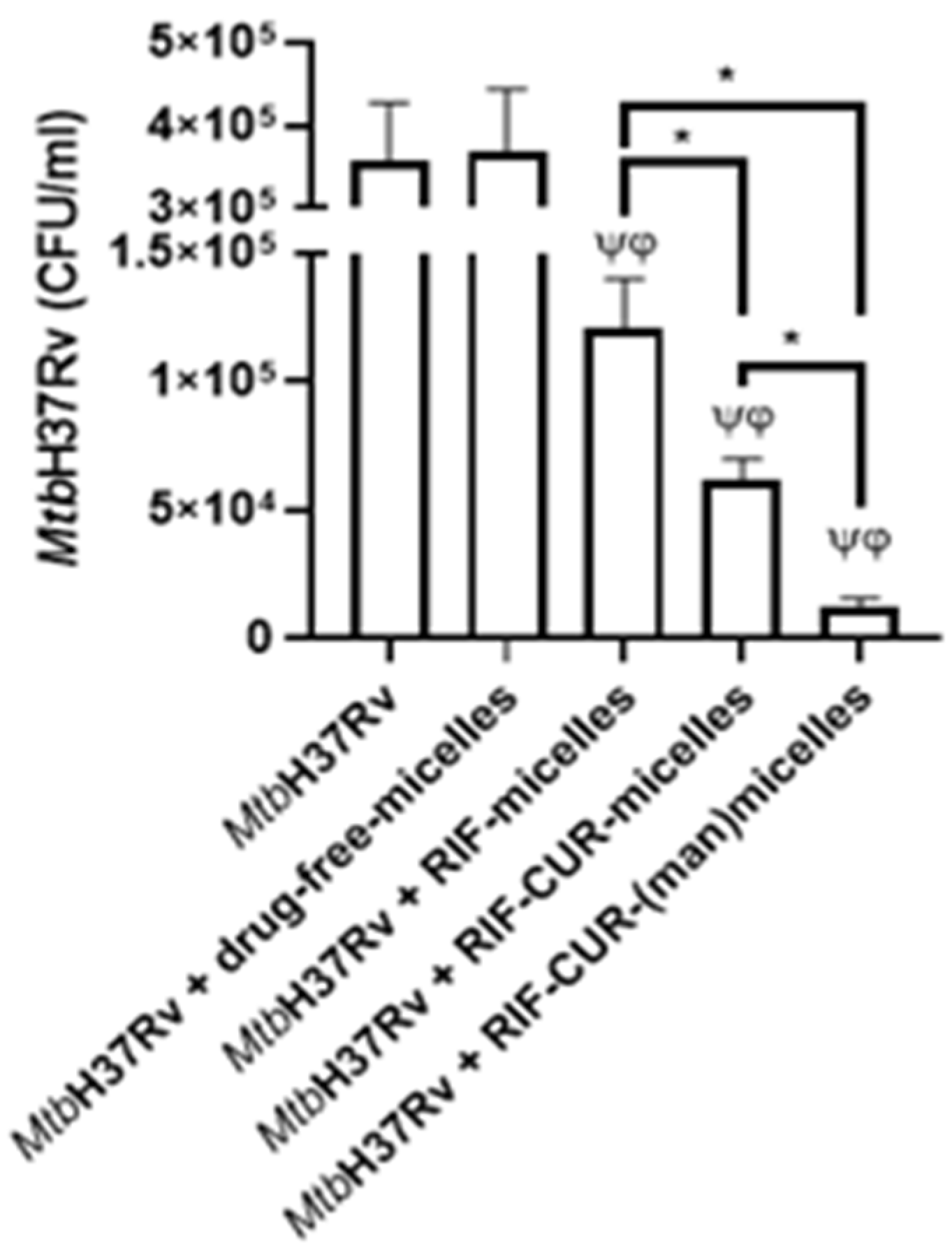
2.12. Microbicidal Efficacy against Mycobacterium tuberculosis
2.12.1. Bacterial Growth Conditions
2.12.2. Cell Culture and Infection
2.12.3. Colony-Forming Unit Assay
2.12.4. Statistical Analysis
3. Results and Discussion
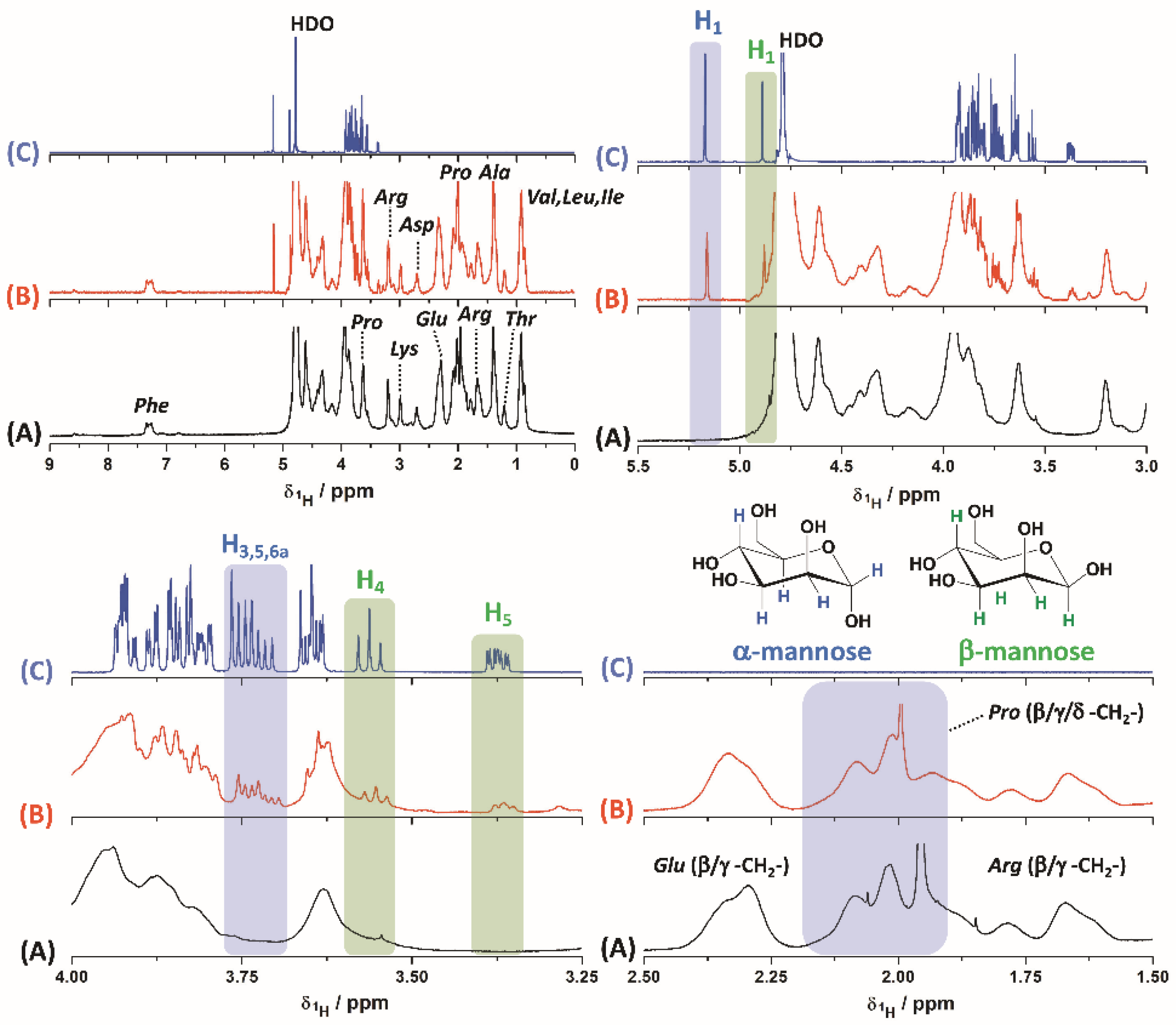
3.1. Gelatin–Mannose Preparation and Characterization
3.2. Micellar Preparation, Drug Encapsulation and Physicochemical Stability under Dilution
3.3. CMC Determination
3.4. Nebulization Studies
3.5. In Vitro Antioxidant Capacity
3.6. In Vitro Drug Release
3.7. Hemolysis Assay
3.8. Micellar Lung Accumulation
3.9. Microbicidal Efficacy against Mycobacterium tuberculosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- García-Fernández, A.; Sancenón, F.; Martínez-Máñez, R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2021, 177, 113953. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.; Wong, E. Afrezza (Insulin Human) Inhalation Powder: A New Inhaled Insulin for the Management of Type-1 or Type-2 Diabetes Mellitus. Pharm. Ther. 2015, 40, 735–741. [Google Scholar]
- United States Food and Drug Administration (FDA). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020148Orig1s025lbl.pdf (accessed on 12 March 2022).
- Mukhtar, M.; Pallagi, E.; Csóka, I.; Benke, E.; Farkas, Á.; Zeeshan, M.; Burián, K.; Kókai, D.; Ambrus, R. Aerodynamic properties and in silico deposition of isoniazid loaded chitosan/thiolated chitosan and hyaluronic acid hybrid nanoplex DPIs as a potential TB treatment. Int. J. Biol. Macromol. 2020, 165, 3007–3019. [Google Scholar] [CrossRef] [PubMed]
- Al Hajj, N.; Chee, C.F.; Wong, T.W.; Rahman, N.A.; Abu Kasim, N.H.; Colombo, P. Lung cancer: Active therapeutic targeting and inhalational nanoproduct design. Expert Opin. Drug Deliv. 2018, 15, 1223–1247. [Google Scholar] [CrossRef]
- Dogbe, M.G.; Mafilaza, A.Y.; Eleutério, C.V.; Cabral-Marques, H.; Simões, S.; Gaspar, M.M. Pharmaceutical Benefits of Fluticasone Propionate Association to Delivery Systems: In Vitro and In Vivo Evaluation. Pharmaceutics 2019, 11, 521. [Google Scholar] [CrossRef] [Green Version]
- Eedara, B.B.; Alabsi, W.; Encinas-Basurto, D.; Polt, R.; Mansour, H.M. Spray-Dried Inhalable Powder Formulations of Therapeutic Proteins and Peptides. AAPS PharmSciTech 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Thakur, A.K.; Chellappan, D.K.; Dua, K.; Mehta, M.; Satija, S.; Singh, I. Patented therapeutic drug delivery strategies for targeting pulmonary diseases. Expert Opin. Ther. Patents 2020, 30, 375–387. [Google Scholar] [CrossRef]
- Sanna, V.; Satta, S.; Hsiai, T.; Sechi, M. Development of targeted nanoparticles loaded with antiviral drugs for SARS-CoV-2 inhibition. Eur. J. Med. Chem. 2022, 231, 114121. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Tuberculosis Report 2021. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021 (accessed on 12 March 2022).
- Grotz, E.; Tateosian, N.; Amiano, N.; Cagel, M.; Bernabeu, E.; Chiappetta, D.A.; Moretton, M.A. Nanotechnology in Tuberculosis: State of the Art and the Challenges Ahead. Pharm. Res. 2018, 35, 213. [Google Scholar] [CrossRef]
- Goutelle, S.; Bourguignon, L.; Maire, P.H.; Van Guilder, M.; Conte, J.E.; Jelliffe, R.W. Population Modeling and Monte Carlo Simulation Study of the Pharmacokinetics and Antituberculosis Pharmacodynamics of Rifampin in Lungs. Antimicrob. Agents Chemother. 2009, 53, 2974–2981. [Google Scholar] [CrossRef] [Green Version]
- Misra, A.; Hickey, A.J.; Rossi, C.; Borchard, G.; Terada, H.; Makino, K.; Fourie, P.B.; Colombo, P. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis 2011, 91, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Gazi, U.; Martinez-Pomares, L. Influence of the mannose receptor in host immune responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Shirai, M.; Asada, K.; Yasui, H.; Karayama, M.; Hozumi, H.; Furuhashi, K.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; et al. Macrophage mannose receptor, CD206, predict prognosis in patients with pulmonary tuberculosis. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, A. Highly bioavailable curcumin (Theracurmin): Its development and clinical application. PharmaNutrition 2015, 3, 123–130. [Google Scholar] [CrossRef]
- Riedel, J.; Calienni, M.N.; Bernabeu, E.; Calabro, V.; Lázaro-Martinez, J.M.; Prieto, M.J.; Gonzalez, L.; Martinez, C.S.; Alonso, S.D.V.; Montanari, J.; et al. Paclitaxel and curcumin co-loaded mixed micelles: Improving in vitro efficacy and reducing toxicity against Abraxane®. J. Drug Deliv. Sci. Technol. 2021, 62, 102343. [Google Scholar] [CrossRef]
- Barua, N.; Buragohain, A.K. Therapeutic Potential of Curcumin as an Antimycobacterial Agent. Biomolecules 2021, 11, 1278. [Google Scholar] [CrossRef]
- Jahagirdar, P.S.; Gupta, P.K.; Kulkarni, S.P.; Devarajan, P.V. Intramacrophage Delivery of Dual Drug Loaded Nanoparticles for Effective Clearance of Mycobacterium tuberculosis. J. Pharm. Sci. 2020, 109, 2262–2270. [Google Scholar] [CrossRef]
- Grotz, E.; Tateosian, N.L.; Salgueiro, J.; Bernabeu, E.; Gonzalez, L.; Manca, M.L.; Amiano, N.; Valenti, D.; Manconi, M.; García, V.; et al. Pulmonary delivery of rifampicin-loaded soluplus micelles against Mycobacterium tuberculosis. J. Drug Deliv. Sci. Technol. 2019, 53, 101170. [Google Scholar] [CrossRef]
- Costa, A.M.M.M.; Sarmento, B.; Seabra, V. Mannose-functionalized solid lipid nanoparticles are effective in targeting alveolar macrophages. Eur. J. Pharm. Sci. 2018, 114, 103–113. [Google Scholar] [CrossRef]
- Moretton, M.A.; Bernabeu, E.; Grotz, E.; González, L.; Zubillaga, M.; Chiappetta, D.A. A glucose-targeted mixed micellar formulation outperforms Genexol in breast cancer cells. Eur. J. Pharm. Biopharm. 2017, 114, 305–316. [Google Scholar] [CrossRef]
- Dobrecky, C.; Marchini, T.; Ricco, R.; Garcés, M.; Gadano, A.; Carballo, M.; Wagner, M.L.; Lucangioli, S.; Evelson, P. Antioxidant activity of flavonoid rich fraction of Ligaria cuneifolia. Chem. Biodivers 2020, 17, e2000302. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, M.L.; Valenti, D.; Sales, O.D.; Nacher, A.; Fadda, A.M.; Manconi, M. Fabrication of polyelectrolyte multilayered vesicles as inhalable dry powder for lung administration of rifampicin. Int. J. Pharm. 2014, 472, 102–109. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Valenti, D.; Escribano, E.; Hillaireau, H.; Fadda, A.M.; Fattal, E. Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. Int. J. Pharm. 2017, 525, 203–210. [Google Scholar] [CrossRef]
- Manca, M.; Ferraro, M.; Pace, E.; Di Vincenzo, S.; Valenti, D.; Fernàndez-Busquets, X.; Peptu, C.; Manconi, M. Loading of Beclomethasone in Liposomes and Hyalurosomes Improved with Mucin as Effective Approach to Counteract the Oxidative Stress Generated by Cigarette Smoke Extract. Nanomaterials 2021, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Derbali, R.M.; Aoun, V.; Moussa, G.; Frei, G.; Tehrani, S.F.; Del’Orto, J.C.; Hildgen, P.; Roullin, V.G.; Chain, J.L. Tailored Nanocarriers for the Pulmonary Delivery of Levofloxacin against Pseudomonas aeruginosa: A Comparative Study. Mol. Pharm. 2019, 16, 1906–1916. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Qiao, M.; Chen, Q.; Tian, C.; Long, M.; Wang, M.; Li, Z.; Hu, W.; Li, G.; Cheng, L.; et al. Enhanced effect of pH-sensitive mixed copolymer micelles for overcoming multidrug resistance of doxorubicin. Biomaterials 2014, 35, 9877–9887. [Google Scholar] [CrossRef]
- United States Food and Drud Administration (FDA). Available online: http://wayback.archive-it.org/7993/20171031062708/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm261307.htm (accessed on 12 March 2022).
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Lv, F.; Wang, J.; Xu, P.; Han, Y.; Ma, H.; Xu, H.; Chen, S.; Chang, J.; Ke, Q.; Liu, M.; et al. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater. 2017, 60, 128–143. [Google Scholar] [CrossRef]
- Patil, K.; Bagade, S.; Bonde, S. In-vitro and ex-vivo characterization of novel mannosylated gelatin nanoparticles of linezolid by Quality-by-Design approach. J. Drug Deliv. Sci. Technol. 2020, 60, 101976. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, W.; Guo, Y.; Zhu, Y.; Lu, H.; Wu, Y. Superhydrophobic coatings on gelatin-based films: Fabrication, characterization and cytotoxicity studies. RSC Adv. 2018, 8, 23712–23719. [Google Scholar] [CrossRef] [Green Version]
- Pană, A.-M.; Ştefan, L.-M.; Bandur, G.; Sfîrloagă, P.; Gherman, V.; Silion, M.; Popa, M.; Rusnac, L.-M. Novel Glycopolymers Based on d-Mannose and Methacrylates: Synthesis, Thermal Stability and Biodegradability Testing. J. Polym. Environ. 2013, 21, 981–994. [Google Scholar] [CrossRef]
- Kosaka, A.; Aida, M.; Katsumoto, Y. Reconsidering the activation entropy for anomerization of glucose and mannose in water studied by NMR spectroscopy. J. Mol. Struct. 2015, 1093, 195–200. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Liu, M. One-step synthesis of mannose-modified polyethyleneimine copolymer particles as fluorescent probes for the detection of Escherichia coli. Sens. Actuators B Chem. 2018, 280, 171–176. [Google Scholar] [CrossRef]
- Ding, W.; Sun, J.; Lian, H.; Xu, C.; Liu, X.; Zheng, S.; Zhang, D.; Han, X.; Liu, Y.; Chen, X.; et al. The Influence of Shuttle-Shape Emodin Nanoparticles on the Streptococcus suis Biofilm. Front. Pharmacol. 2018, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Polymer Synthesis and Processing, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Štimac, A.; Šegota, S.; Sikirić, M.D.; Ribić, R.; Frkanec, L.; Svetličić, V.; Tomić, S.; Vranešić, B.; Frkanec, R. Surface modified liposomes by mannosylated conjugates anchored via the adamantly moiety in the lipid bilayer. Biochim. Biophys. Acta 2012, 1818, 2252. [Google Scholar] [CrossRef] [Green Version]
- Moretton, M.A.; Chiappetta, D.A.; Andrade, F.; das Neves, J.; Ferreira, D.; Sarmento, B.; Sosnik, A. Hydrolyzed Galactomannan-Modified Nanoparticles and Flower-Like Polymeric Micelles for the Active Targeting of Rifampicin to Macrophages. J. Biomed. Nanotechnol. 2013, 9, 1076–1087. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Prakash, S.; Katiyar, S.; Bihari, S. Low-dose inhaled versus standard dose oral form of anti-tubercular drugs: Concentrations in bronchial epithelial lining fluid, alveolar macrophage and serum. J. Postgrad. Med. 2008, 54, 245–246. [Google Scholar] [CrossRef]
- Truzzi, E.; Leite Nascimento, T.; Iannuccelli, V.; Costantino, L.; Martins Lima, E.; Leo, E.; Siligardi, C.; Lassinantti Gualtieri, M.; Maretti, E. In Vivo Biodistribution of Respirable Solid Lipid Nanoparticles Surface-Decorated with a Mannose-Based Surfactant: A Promising Tool for Pulmonary Tuberculosis Treatment? Nanomaterials 2020, 10, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretton, M.A.; Cohen, L.; Lepera, L.; Bernabeu, E.; Taira, C.; Höcht, C.; Chiappetta, D.A. Enhanced oral bioavailability of nevirapine within micellar nanocarriers compared with Viramune ®. Colloids Surf. B Biointerfaces 2014, 122, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Bonde, G.V.; Ajmal, G.; Yadav, S.K.; Mittal, P.; Singh, J.; Bakde, B.V.; Mishra, B. Assessing the viability of Soluplus® self-assembled nanocolloids for sustained delivery of highly hydrophobic lapatinib (anticancer agent): Optimisation and in-vitro characterisation. Colloids Surf. B Biointerfaces 2019, 185, 110611. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yu, J. The effect of particle shape and size on cellular uptake. Drug Deliv. Transl. Res. 2015, 6, 67–72. [Google Scholar] [CrossRef]
- Melis, V.; Manca, M.L.; Bullita, E.; Tamburini, E.; Castangia, I.; Cardia, M.C.; Valenti, D.; Fadda, A.M.; Peris, J.E.; Manconi, M. Inhalable polymer-glycerosomes as safe and effective carriers for rifampicin delivery to the lungs. Colloids Surf. B Biointerfaces 2016, 143, 301–308. [Google Scholar] [CrossRef]
- Palanisamy, G.S.; Kirk, N.M.; Ackart, D.F.; Shanley, C.A.; Orme, I.M.; Basaraba, R.J. Evidence for Oxidative Stress and Defective Antioxidant Response in Guinea Pigs with Tuberculosis. PLoS ONE 2011, 6, e26254. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Niu, B.; Wu, B.; Luo, S.; Fu, J.; Zhao, Y.; Quan, G.; Pan, X.; Wu, C. A homogenous nanoporous pulmonary drug delivery system based on metal-organic frameworks with fine aerosolization performance and good compatibility. Acta Pharm. Sin. B 2020, 10, 2404–2416. [Google Scholar] [CrossRef]
- Clemens, D.L.; Lee, B.-Y.; Xue, M.; Thomas, C.R.; Meng, H.; Ferris, D.; Nel, A.E.; Zink, J.I.; Horwitz, M.A. Targeted Intracellular Delivery of Antituberculosis Drugs to Mycobacterium tuberculosis-Infected Macrophages via Functionalized Mesoporous Silica Nanoparticles. Antimicrob. Agents Chemother. 2012, 56, 2535–2545. [Google Scholar] [CrossRef] [Green Version]
- Amin, K.; Dannenfelser, R.-M. In vitro hemolysis: Guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef]
- Bringhammar, T. Quality assurance of radiopharmaceuticals. In Technetium-99 M Pharmaceuticals. Preparation and Quality Control in Nuclear Medicine; Zolle, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 67–71. [Google Scholar]
- Bai, X.; Oberley-Deegan, R.E.; Bai, A.; Ovrutsky, A.R.; Kinney, W.H.; Weaver, M.; Zhang, G.; Honda, J.R.; Chan, E.D. Curcumin enhances human macrophage control of Mycobacterium tuberculosis infection. Respirology 2016, 21, 951–957. [Google Scholar] [CrossRef]
- Lunn, A.M.; Unnikrishnan, M.; Perrier, S. Dual pH-Responsive Macrophage-Targeted Isoniazid Glycoparticles for Intracellular Tuberculosis Therapy. Biomacromolecules 2021, 22, 3756–3768. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, M.; Ye, W.; Huang, Z.; Ma, Z.; Wang, W.; Wang, W.; Huang, Y.; Pan, X.; Wu, C. Inhalable solid lipid nanoparticles for intracellular tuberculosis infection therapy: Macrophage-targeting and pH-sensitive properties. Drug Deliv. Transl. Res. 2021, 11, 1218–1235. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.C.C.; Chaves, L.L.; Pinheiro, M.; Lima, S.; Ferreira, D.; Sarmento, B.; Reis, S. Mannosylated solid lipid nanoparticles for the selective delivery of rifampicin to macrophages. Artif. Cells Nanomed. Biotechnol. 2018, 46, 653–663. [Google Scholar] [CrossRef] [PubMed]

| Dh (nm) (±SD) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Con A Presence | PDI (±SD) | Peak 1 | Intensity (%) | Peak 2 | Intensity (%) | Peak 3 | Intensity (%) |
| Soluplus® micelles | - | 0.205 (0.006) | 158.1 (3.5) | 100.0 | - | - | - | - |
| + | 0.201 (0.005) | 161.8 (4.6) | 100.0 | |||||
| Soluplus® (man)micelles | - | 0.275 (0.013) | 270.1 (31.3) | 100.0 | - | - | - | - |
| + | 0.359 (0.008) | 246.5 (5.1) | 94.2 | 4370 (392) | 5.3 | 11.2 (19.4) | 0.6 | |
| Nanoformulation | Gel(man) Concentration (%) | Before Lyophilization | After Lyophilization | ||
|---|---|---|---|---|---|
| Size (nm) (±S.D.) | PDI (±S.D.) | Size (nm) (±S.D.) | PDI (±S.D.) | ||
| Drug-free-micelles | - | 114.1 (1.2) | 0.194 (0.002) | 124.0 (1.3) | 0.217 (0.006) |
| Drug-free-(man)micelles | 0.25 | 137.2 (4.1) | 0.213 (0.009) | 127.4 (0.3) | 0.257 (0.006) |
| 0.5 | 149.1 (1.8) | 0.226 (0.003) | 145.6 (4.4) | 0.264 (0.007) | |
| 0.7 | 287.6 (28.3) | 0.414 (0.015) | 164.5 (6.7) | 0.257 (0.009) | |
| 1.0 | 345.0 (68.7) | 0.444 (0.003) | 120.6 (4.7) | 0.207 (0.009) | |
| RIF–CUR-micelles | - | 82.2 (1.7) | 0.221 (0.007) | 81.2 (2.1) | 0.138 (0.020) |
| RIF–CUR-(man)micelles | 0.25 | 122.2 (3.7) | 0.224 (0.003) | 113.7 (1.7) | 0.215 (0.011) |
| 0.5 | 147.8 (4.6) | 0.244 (0.010) | 101.8 (3.8) | 0.218 (0.009) | |
| 0.7 | 197.6 (7.4) | 0.312 (0.042) | 108.1 (0.9) | 0.208 (0.001) | |
| 1.0 | 360.2 (11.2) | 0.288 (0.004) | 366.7 (5.5) | 0.424 (0.011) | |
| Nanoformulation | Time (h) | Size (nm) (±S.D.) | PDI (±S.D.) | |
|---|---|---|---|---|
| Peak 1 | Intensity (%) | |||
| RIF–CUR-micelles | 0 | 56.04 (0.48) | 100.0 | 0.044 (0.007) |
| 1 | 58.15 (0.51) | 100.0 | 0.040 (0.008) | |
| 2 | 58.09 (0.08) | 100.0 | 0.042 (0.009) | |
| 3 | 57.49 (0.51) | 100.0 | 0.037 (0.011) | |
| 24 | 62.69 (0.45) | 100.0 | 0.056 (0.008) | |
| RIF–CUR-(man)micelles | 0 | 61.26 (0.65) | 100.0 | 0.103 (0.016) |
| 1 | 61.35 (0.27) | 100.0 | 0.068 (0.008) | |
| 2 | 61.44 (0.36) | 100.0 | 0.075 (0.011) | |
| 3 | 60.08 (0.37) | 100.0 | 0.063 (0.004) | |
| 24 | 68.13 (1.10) | 100.0 | 0.080 (0.009) | |
| Sample | Total Mass Output (%) | Fine Particle Dose (FPD) (mg) | Fine Particle Fraction (FPF) (%) | Aerodynamic Diameter (± Geometric Standard Deviation) |
|---|---|---|---|---|
| RIF | 86 ± 6 | 8 ± 3 | 46 ± 4 | 1.27 ± 1.18 |
| CUR | 88 ± 7 | 2 ± 0.5 | 19 ± 3 | 2.18 ± 1.70 |
| RIF + CUR | 89 ± 12 | 8 ± 2 | 30 ± 4 | 2.52 ± 1.88 |
| RIF-micelles | 100 ± 3 | 15 ± 2 | 75 ± 15 | 1.26 ± 1.17 |
| CUR-micelles | 89 ± 2 | 5 ± 1 | 57 ± 6 | 2.14 ± 1.68 |
| RIF–CUR-micelles | 83 ± 6 | 15 ± 3 | 62 ± 9 | 1.65 ± 1.41 |
| RIF–CUR-(man)micelles | 81 ± 7 | 13 ± 2 | 52 ± 6 | 2.10 ± 1.66 |
| Sample | Total Antioxidant Capacity (nmol Trolox Eq/mg Sample) | ABTS˙ Inhibition (%) |
|---|---|---|
| RIF | 938.0 ± 212.0 | 5.5 ± 0.6 |
| CUR | 2239.0 ± 153.0 | 18.6 ± 1.6 |
| RIF+ CUR | 4523.0 ± 214.0 | 35.5 ± 1.7 |
| Drug-free micelles | ND | ND |
| RIF-micelles | 3918.0 ± 104.0 # | 16.0 ± 0.4 |
| RIF–CUR-micelles | 23,075.0 ± 205.0 * | 99.0 ± 1.0 |
| RIF–CUR-(man)micelles | 20,404.0 ± 663.0 * | 99.0 ± 6.0 |
| Sample | Total Antioxidant Capacity (nmol Trolox Eq/mg Sample) | DPPH˙ Inhibition (%) |
|---|---|---|
| RIF | 595.0 ± 76.0 | 18.6 ± 2.9 |
| CUR | 2678.0 ± 193.0 # | 49.7 ± 7.4 |
| RIF + CUR | 3440.0 ± 5.7 | 96.2 ± 0.7 |
| Drug-free micelles | ND | 1.0 ± 0.4 |
| RIF-micelles | 120.0 ± 37.0 | 25.2 ± 5.6 |
| RIF–CUR-micelles | 3633.0 ± 68.0 * | 75.6 ± 1.3 |
| RIF–CUR-(man)micelles | 3302.0 ± 83.0 * | 61.6 ± 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galdopórpora, J.M.; Martinena, C.; Bernabeu, E.; Riedel, J.; Palmas, L.; Castangia, I.; Manca, M.L.; Garcés, M.; Lázaro-Martinez, J.; Salgueiro, M.J.; et al. Inhalable Mannosylated Rifampicin–Curcumin Co-Loaded Nanomicelles with Enhanced In Vitro Antimicrobial Efficacy for an Optimized Pulmonary Tuberculosis Therapy. Pharmaceutics 2022, 14, 959. https://doi.org/10.3390/pharmaceutics14050959
Galdopórpora JM, Martinena C, Bernabeu E, Riedel J, Palmas L, Castangia I, Manca ML, Garcés M, Lázaro-Martinez J, Salgueiro MJ, et al. Inhalable Mannosylated Rifampicin–Curcumin Co-Loaded Nanomicelles with Enhanced In Vitro Antimicrobial Efficacy for an Optimized Pulmonary Tuberculosis Therapy. Pharmaceutics. 2022; 14(5):959. https://doi.org/10.3390/pharmaceutics14050959
Chicago/Turabian StyleGaldopórpora, Juan M., Camila Martinena, Ezequiel Bernabeu, Jennifer Riedel, Lucia Palmas, Ines Castangia, Maria Letizia Manca, Mariana Garcés, Juan Lázaro-Martinez, Maria Jimena Salgueiro, and et al. 2022. "Inhalable Mannosylated Rifampicin–Curcumin Co-Loaded Nanomicelles with Enhanced In Vitro Antimicrobial Efficacy for an Optimized Pulmonary Tuberculosis Therapy" Pharmaceutics 14, no. 5: 959. https://doi.org/10.3390/pharmaceutics14050959
APA StyleGaldopórpora, J. M., Martinena, C., Bernabeu, E., Riedel, J., Palmas, L., Castangia, I., Manca, M. L., Garcés, M., Lázaro-Martinez, J., Salgueiro, M. J., Evelson, P., Tateosian, N. L., Chiappetta, D. A., & Moretton, M. A. (2022). Inhalable Mannosylated Rifampicin–Curcumin Co-Loaded Nanomicelles with Enhanced In Vitro Antimicrobial Efficacy for an Optimized Pulmonary Tuberculosis Therapy. Pharmaceutics, 14(5), 959. https://doi.org/10.3390/pharmaceutics14050959